Abstract
Triple-negative breast cancer (TNBC) is pathologically defined by lack of expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) amplification and portends an aggressive clinical course with worse outcomes compared to other breast cancers. Until recently, standard treatment options consisted of sequential cytotoxic chemotherapies for both early and metastatic disease. Advances in sequencing technology have led to the identification of four main subtypes of TNBC based on recurrent genetic alterations, transcriptional patterns, and molecular features: basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), and luminal androgen receptor (LAR). Frequent alterations found in DNA damage response pathways, germline and somatic BRCA1/2 genes, PI3K signaling pathways as well as the presence of androgen receptors and infiltrating immune cells could serve as actionable targets to optimize treatments and improve outcomes for patients with TNBC. Recent approvals for immune checkpoint inhibitors and the antibody drug conjugate, sacituzumab govitecan-hziy, for advanced TNBC illustrate the advances in treatment that can result from these molecular discoveries. This review will explore the molecular subtypes of TNBC and their distinct characteristics, as well as highlight the molecular features and potential “drivers” that have been identified as promising targets for new treatment strategies.
Keywords: Triple-negative breast cancer, molecular, subtypes, basal-like, mesenchymal, luminal androgen receptor
Introduction
Triple-negative breast cancer (TNBC) is a heterogenous disease comprised of breast tumors that lack expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) amplification. TNBC cases account for approximately 15% of all invasive breast cancers and are more commonly diagnosed in younger patients. They are also more common in black patients compared to other races.1,2 TNBCs have a poorer prognosis compared to other types of breast cancer and an increased risk of early distant recurrence and death.1 Unlike breast tumors that are ER, PR, or HER2 positive, there are very limited targeted treatment options for TNBC. Two PARP inhibitors, olaparib and talazoparib, have been approved in the metastatic setting for any breast cancer patients with germline BRCA1/2 mutations. The FDA also recently granted approval for the immune checkpoint inhibitor, atezolizumab, for the treatment of unresectable locally advanced and metastatic TNBC with PD-L1 stromal cell positivity in 2019 based on results of the IMpassion 130 phase III trial, and the antibody-drug conjugate sacituzumab govitecan-hziy was also approved by the FDA in April 2020 for heavily pre-treated metastatic TNBC.3,4 Even with these recent approvals, cytotoxic chemotherapy remains the primary treatment for TNBC in the neoadjuvant, adjuvant, and metastatic setting, and overall survival for patients with TNBC has not changed over the last 20 years.5 Extensive research efforts in genomic and transcriptomic profiling to identify molecular subtypes of TNBC have been made in recent years with the goal of discovering actionable molecular targets to improve treatment strategies for patients with TNBC.
Definition and Clinical Features of TNBC
The classification of a breast cancer as “triple-negative” is a pathologic definition that is determined by the absence of ER, PR, and HER2 protein expression by immunohistochemistry (IHC) and/or ERBB2 (HER2/neu) gene amplification by fluorescence in situ hybridization (FISH). The definition of ER/PR positivity has varied over the years, but it has most recently been defined as ER or PR protein expression greater than or equal to 1% by IHC according to the American Society of Clinical Oncology Guidelines.6 HER2-positivity is defined as protein expression 3+ and/or HER2/neu gene amplification greater than or equal to 2.0 by FISH.7 Accordingly, TNBC is currently defined as ER/PR expression of 0 and HER2 negative by either IHC expression of 0–1+ or lack of HER2/neu gene amplification by FISH (FISH < 2.0).
TNBC has a more aggressive clinical course compared to other types of breast cancer. In a study of approximately 1,600 patients with early-stage breast cancer, women with TNBC were more likely to develop a distant recurrence and had inferior survival within 5 years of diagnosis.8 Despite the aggressive biology of TNBC, high-grade TNBC tends to be more responsive to neoadjuvant chemotherapy, known as the “triple-negative paradox.”9 Approximately 30–40% of patients achieve pathological complete response (pCR) at surgery following neoadjuvant chemotherapy, and patients who achieve pCR have higher rates of survival.10,11 Patients who have evidence of residual disease at the time of surgery following neoadjuvant chemotherapy are six times more likely to have a recurrence and twelve times more likely to die of metastatic disease.11,12 These differences in response to neoadjuvant chemotherapy and survival have long suggested that there are inherent, biological differences amongst TNBC tumors that render some tumors more sensitive to cytotoxic chemotherapy. Thus, these observations have invigorated esearch efforts to better understand the biology of these tumors and identify recurrent molecular subtypes through gene expression and sequencing analysis.
Molecular Subtypes of TNBC
Over the last 20 years, transcriptional, genetic and epigenetic analyses have led to the classification of breast cancer tumors into distinct ‘intrinsic’ subtypes: luminal A, luminal B, HER2-enriched, basal-like, and claudin-low.13,14 Each of these subtypes have unique differences in prognosis and treatment sensitivity. The majority of TNBCs fall into the basal-like class or the rarer claudin-low class, but it has been recognized that nearly all intrinsic subtypes can be represented in any given pathological classification group.15–17 Nonetheless, given the heterogeneity observed in TNBC, it is reasonable to postulate that enhanced molecular scrutiny could further delineate nuances and subtypes within TNBC. Lehmann and colleagues were the first to apply similar gene expression and sequencing techniques to only TNBC tumors to investigate the wide heterogeneity seen within this single type of breast cancer. They analyzed 587 TNBC cases in 21 publicly available data sets to identify molecular subtypes.17 Initially, 6 different stable TNBC subtypes were characterized based on unique gene expression profiles: basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL), and luminal androgen receptor (LAR). In parallel, cell-line models were identified for each subtype using the same signatures.17
Lehmann’s group found that BL1 tumors are enriched in cell cycle, cell division, and DNA damage response (ATR/BRCA) pathways. Increased expression of DNA damage response genes, such as CHEK1, MSH2, were molecularly linked to enhanced proliferation and cell-cycle checkpoint loss. Consistent with these observations, BL1 tumors have high nuclear Ki-67 staining assessed by IHC, and high MKI67 mRNA.17 These findings suggest that BL1 tumors may be more responsive to antimitotic and DNA-damaging treatments which correlates with the significantly higher pCR rates seen when these tumors are treated with neoadjuvant taxane-based therapies.18,19 In contrast, BL2 tumors displayed gene expression patterns indicative of increased growth factor signaling (EGFR, NGF, MET, and Wnt/B-catenin pathways), providing a rationale for evaluating the addition of key molecularly targeted agents to therapy in these tumors.
Immunomodulatory (IM) tumors are enriched in immune cell signaling and cytokine signaling pathways, antigen processing and presentation, and core immune signal transduction pathways (JAK/STAT signaling, TNF). They have high expression of immune signaling genes with a gene expression pattern that overlaps with the gene signature found in medullary breast cancer.17 Medullary breast cancer is a rare, distinct form of TNBC that has a high-grade histology but associated with a favorable prognosis.20 Mesenchymal (M and MSL) subtypes are enriched in pathways involved in cell motility and cell differentiation such as the Wnt pathway, ALK pathway, and TGF-B signaling. Tumors in the MSL subtype share similar pathways to M tumors, but also express genes linked to growth factor signaling including EGFR signaling and adipocytokine signaling as well as elevated angiogenesis genes including VEGFR2, TEK, EPAS1.17 Both M and MSL subtypes have gene expression patterns that are similar to metaplastic breast cancer, a highly dedifferentiated type of breast cancer with mesenchymal/sarcomatoid or squamous features and is resistant to chemotherapy.21
The luminal androgen receptor (LAR) subtype is potentially the most targetable subtype identified. This unique subset of tumors is characterized by an ER/PR-negative phenotype with a paradoxical hormonally regulated transcriptional program and response to anti-androgens. This phenotype had originally been described previously, though not yet fully explored.22 While ER negative, LAR has enrichment in hormonal pathways including steroid synthesis and androgen/estrogen metabolism. AR mRNA was highly expressed, along with downstream AR targets and coactivators. Like ER+ positive tumors, tumors with higher AR expression have been found to have a strong association with the presence of PIK3CA mutations.23 Interestingly, the LAR subtype was mostly comprised of lobular carcinomas.17
These initial subtypes were identified using surgical specimens which contained stromal and immune components in addition to the tumor cells and normal cells. Lehmann and colleagues later conducted additional analysis with histopathological quantification and laser-capture microdissection to evaluate the contribution of these normal cells to the TNBC subtypes which led to refinement of their initial classification of 6 subtypes down to 4 tumor-specific subtypes: BL1, BL2, M, and LAR (Figure 1).24 They found that the previously identified MSL and IM subtypes were tumors with substantial infiltration of tumor-associated mesenchymal cells and lymphocytes, respectively. Approximately 20% of TNBCs are enriched in immune cell markers and signaling and were classified as IM subtype. However, pathological evaluation of lymphocytes on H&E sections found that the infiltrating lymphocytes drive the gene expression profile rather than tumor cells. With the refined 4 subtype (TNBCtype-4) classification, the IM and MSL classifications were removed and are more fitting as tumor cell-intrinsic subtype descriptors of cellular heterogeneity.24
Figure 1:
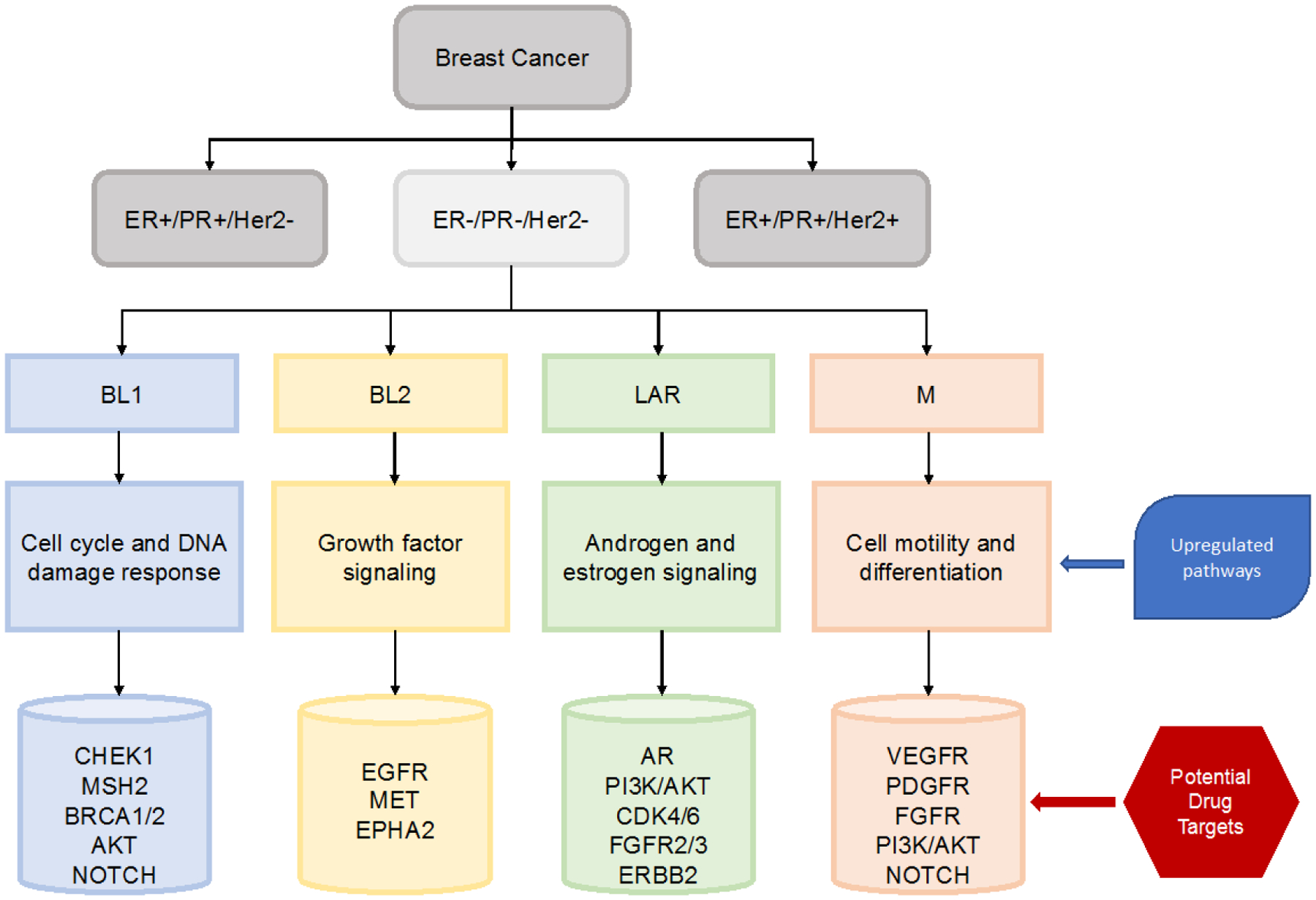
Molecular subtypes of TNBC and potential actionable targets.
Following the analysis by Lehmann et al., Burstein and colleagues also identified TNBC subtypes with specific molecular markers and targets in their analysis published in 2015.25 They performed DNA and RNA sequencing on 198 TNBC tumors collected at Baylor College of Medicine and validated their findings in an external dataset of 7 publicly available TNBC studies. They identified 4 stable subtypes which divided into stromal, immune, and basal signatures consistent with the TNBCtype model. Luminal-AR (LAR) subtype tumors expressed the androgen receptor, estrogen receptor, and prolactin but remain ER-negative by IHC staining. Gene expression in this subtype showed expression of ESR1 and estrogen-regulated genes. This subtype was consistent with LAR subtype described by Lehmann.24 The mesenchymal (MES) subtype had high expression of cell cycle, mismatch repair and DNA damage networks, as well as hereditary breast cancer signaling pathways. Basal-like immune-suppressed (BLIS) is one of two basal-like subtypes identified. BLIS tumors display downregulation of B cell, T cell, and NK cell immune-regulating pathways and cytokine pathways. Basal-like immune-activated (BLIA) tumors are the second of the basal-like subtypes and have upregulation of genes known to modulate B cell, T cell, and NK cell functions.25
Genomic Features of Molecular Subtypes
TNBCs are significantly associated with high genomic instability with frequent somatic mutations in TP53 (82%) and PIK3CA (10%) as well as BRCA1 germline mutations, but it has been unclear until more recent genomic analyses whether specific mutations are associated with molecular TNBC subtypes.26 The Cancer Genome Atlas (TCGA) project16 found that approximately 20% of basal-like breast cancers had a germline or somatic BRCA mutation. Copy number analysis also found significant amplifications including PIK3CA (49%), KRAS (32%), BRAF (30%), and EGFR (23%) as well as deletions in PTEN and INPP4B.27
Bareche and colleagues further investigated Lehmann’s molecular subtypes to better understand molecular drivers within each subtype as well as differences in survival and response to therapy.17,24,27 They conducted an analysis that combined somatic mutation, copy number aberrations (CNAs), and gene expression profiles of 550 TNBCs in publicly available datasets. BL1 subtype was the most genomically unstable with high rate of TP53 mutation (92%) and copy-number deletion in genes involved in DNA repair (BRCA12, PTEN, MDM2, RB1, TP53). Up to 90% of BL1 tumors had copy number gains for KRAS, NRAS, and BRAF. M subtype was more genetically stable.27 LAR tumors had a higher mutational burden with significantly increased number of mutations in PIK3CA (55%), AKT1 (13%), and CDH1 (13%) genes.17,27 Higher rates of ERBB2 mutations have also been reported in LAR tumors compared to other subtypes.28
Heterogeneity of Tumor-Immune Microenvironment
The microenvironment of breast cancer consists of the extracellular matrix and other stromal cell types including immune cells, tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), cancer-associated adipocytes (CAAs), and endothelial cells.29,30 Alterations in the microenvironment significantly influence tumorigenesis, disease progression, and response to treatment.29,31 Studies have shown that the presence of tumor infiltrating lymphocytes (TILs) in TNBC are associated with greater response to both neoadjuvant and adjuvant chemotherapy.32,33 These findings have spurred further investigation into the immune infiltration of TNBCs as a biomarker to help guide treatment and predict clinical outcomes.
Bareche et al. investigated the tumor microenvironment heterogeneity (TME) in the molecular subtypes of TNBC. They examined immune infiltrate localization, composition, and expression of targetable immune pathways in 1512 TNBC samples from 3 different publicly available transcriptomic and genomic datasets.34 IM TNBCs had the highest expression of adaptive immune-related gene signatures and a fully inflamed spatial pattern suggesting that these tumors are the optimal candidate for treatment with immune checkpoint inhibitors (ICIs). M and LAR subtypes were considered to have an immune cold phenotype with high expression of stromal signatures. BL tumors were also associated with an immunosuppressed microenvironment. M, LAR, and BL subtypes displayed low expression of immune targets. TNBC subtype was associated with unique immune localization patterns, and similar results were also reported by Gruosso et al. They found that TME patterns were significantly associated with 10-year OS, and patients with fully-inflamed tumors had the best prognosis.35 The differential expression of immune targets and the tumor microenvironment across TNBC molecular subtypes could allow for tailoring of treatment with ICIs in TNBC patients in the future.
Prognostic Implications of Molecular Subtypes
In both large analyses conducted by Lehmann et al., molecular subtypes were associated with significantly different relapse-free survival (RFS) rates.17,24 BL1 tumors were higher grade but lower stage with increased relapse-free survival and overall survival. BL1 tumors were also most likely to achieve pCR with standard neoadjuvant chemotherapy. LAR tumors had higher regional spread and regional lymph node involvement and preferentially metastasized to the bone consistent with the disease course of ER+ positive tumors while M tumors had higher propensity to metastasize to the lung.24 Burstein et al. found that their BLIS subtype had the worst disease-free survival while the BLIA subtype had the best DFS.25
A retrospective analysis showed that TNBC subtype correlated with response to neoadjuvant treatment with anthracycline and taxane (A-T).36 BL1 subtype had the highest pCR rate while BL2 and LAR had the lowest. TNBC subtype was a better predictor of pCR status than intrinsic breast cancer subtypes of basal-like vs non basal-like.36 Lehmann and colleagues re-examined this same cohort with the refined TNBCtype-4 to determine if these differences in outcome still held true.24 Analysis of a combined cohort of 306 TNBC patients found that the majority of TNBC tumors classified as basal (80%) by PAM50 compared to 20% nonbasal, consistent with previous reports.24,37 Basal tumors had a greater response to neoadjuvant chemotherapy than non-basal tumors, and stratification of pre-treatment biopsies by TNBCtype also showed significant differences in response to neoadjuvant chemotherapy. BL1 tumors had the highest pCR rate of 41% compared to 29% for LAR and 18% for BL2.24 Bareche et al. also found that each subtype had distinct and statistically significant clinicopathological differences when using multivariate model. IM tumors were associated with better prognosis compared to all other subtypes, consistent with the well-established knowledge that highly immune-infiltrated TNBCs are both more responsive to chemotherapy and perform better prognostically. In contrast, the LAR subtype was associated with worse prognosis of all subtypes, likely reflecting more luminal/chemo-refractory biology without clinical intervention toward a hormonal target.27 The results of these multiple analyses show that patients with BL1 tumors are most likely to achieve pCR with neoadjuvant chemotherapy and furthermore, illustrate the importance of molecular subtyping research and its potential predictive power in identifying the subsets of patients most likely to benefit from neoadjuvant treatment in clinical practice. Further investigation into targeted treatments or novel combination therapies for subtypes that are less responsive to neoadjuvant chemotherapy and likely to have residual disease are warranted with future clinical trials.
Future actionable targets in TNBC
Molecular analysis and genomic profiling of TNBC has led to the identification of genetic and cellular pathway alterations that offer promising opportunities for targeted therapies. However, these discoveries have yet to show significant success in clinical trials. BL1 tumors have high genomic instability and high copy number losses for TP53, BRCA1/2, RB1 genes and high copy number gains for PPAR1 gene, so-called “BRCAness,” which support the idea that these tumors may be sensitive to PARP inhibitors.17,27 Their high copy number gains in KRAS, NRAS, and BRAF suggest they might be sensitive to MEK1/2 inhibitors. BL1 tumors also have high frequency of PIK3CA copy number gains and overexpression of PIK3CA, AKT2, and AKT3 genes providing rationale for future treatment with PI3K/AKT pathway inhibitors. Cell lines representative of the M subtype were responsive to the tyrosine kinase inhibitor, dasatinib, and PI3K/mTOR inhibitors.17 A phase II trial of dasatinib in patients with heavily pretreated metastatic breast cancer did not find any significant antitumor activity and was stopped early.38 Another phase II trial of dasatinib in unselected patients with TNBC again found limited activity with an overall response rate of 4.7% and high rates of adverse events.39
LAR cell lines are dependent on AR signaling and displayed sensitivity to the androgen receptor antagonist, bicalutamide, suggesting that androgen receptor antagonists could be effective treatments for LAR subtypes.17,25,40 A phase II trial of bicalutamide in patients with metastatic AR-positive TNBC showed modest benefit with a clinical benefit rate (CBR) of 19%.41 A subsequent trial of the second-generation AR-antagonist, enzalutamide, found slightly higher CBR of 25% .41,42 LAR tumors have high rates of somatic mutations in the PI3K signaling pathway, and preclinical models have suggested benefit from treatment with PI3K/AKT pathway inhibitors.27,28,43 The recent phase Ib/II study, TBCR032, investigated the combination of both enzalutamide with the PI3K inhibitor, taselisib, in patients with AR+ TNBC.44 Results trended toward higher CBR with combination treatment, but the trial was terminated early after results of a phase III trial of taselisib showed limited benefit of the drug in metastatic breast cancer.44,45 Genomic analyses in TBCR032 discovered novel FGFR2 fusions and AR splice variants in LAR tumors which could be explored as targetable mutations in the future.44
Other studies have found overexpression of PDGFR and EGFR in LAR, M, and some basal-like tumors suggesting that IGF, PDGFR, and EGFR inhibitors could be effective treatments for these tumors.25,27 M subtype also has increased expression for angiogenesis pathways with overexpression of mRNA for VEGFR.27 Phase II and phase III trials targeting VEGFR and EGFR had disappointing results. A phase III trial of paclitaxel with or without the VEGF inhibitor, bevacizumab, for patients with metastatic breast cancer found that patients with VEGFA amplification had inferior PFS and OS when treated with bevacizumab compared to those without VEGFA amplification.46 A phase II clinical trial evaluating the VEGF inhibitor, cetuximab, in combination with carboplatin in metastatic TNBC produced responses in fever than 20% of patients. Genomic patterns showed that cetuximab blocked expression in the EGFR pathway in only a minority of patients suggesting alternate mechanisms for pathway activation.47 While targeting these genetic alterations has not translated to significant clinical benefit, it is important to note that these trials included an unselected population of patients with TNBC. Clinical trials selecting patients for targeted therapies based on the molecular subtype or genetic mutations of their tumors could yield more promising results in the future.
Recent advances in the treatment of TNBC
While therapies designed to target the alterations of specific TNBC subtypes are still lacking, advances in the treatment of TNBC have been made recently with the FDA approvals for sacituzumab govitecan-hziy and atezolizumab in the treatment of metastatic disease. Sacituzumab govitecan-hziy is an antibody-drug conjugate that combines a humanized monoclonal antibody targeting human trophoblast cell-surface antigen 2 (Trop-2) with SN-38, the active metabolite of irinotecan, to allow delivery of high concentrations of SN-38 directly to tumor cells. In a phase II, multicenter trial of 108 patients with heavily pre-treated TNBC, there was a 33.3% response rate (95% CI, 24.6 to 43.1) and a median duration of response of 7.7 months (95% CI, 4.9 to 10.8).4 These clinical trial results led to the accelerated FDA approval of this drug for refractory metastatic TNBC in April 2020 becoming the first antibody-drug conjugate approved for TNBC. The confirmatory phase III ASCENT trial (NCT02574455) was stopped early by the independent data safety monitoring committee due to significant improvement in PFS and OS compared to standard chemotherapy.48 Median PFS with sacituzumab govitecan-hziy was 5.6 months compared to 1.7 months with standard chemotherapy (HR 0.41, p<0.001), and median OS was significantly longer at 12.1 months with sacituzumab govitecan-hziy compared to 6.7 months with standard chemotherapy (HR 0.48, p<0.001).48
Early molecular subtyping found that tumors with high numbers of infiltrating immune components, initially classified as IM tumors, have high mRNA expression of PD1, PDL1, and CTLA4 supporting the idea that these tumors might be especially responsive to immune checkpoint inhibitors, but highly immune-infiltrated TNBCs are also known to be more responsive to chemotherapy alone (cite), providing challenges to use of this biomarker to delineate patients specifically benefiting from immunotherapy. Since the initial discovery of the IM subtype, treatment of TNBC with immune checkpoint inhibitors has moved through clinical trials. The PD-L1 inhibitor, atezolizumab, combined with nab-paclitaxel was approved in 2019 as treatment for unresectable or metastatic TNBC with PD-L1 positivity based on results from the IMpassion 130 phase III trial.3 The phase III clinical trial, KEYNOTE-522, investigating pembrolizumab, a PD-1 inhibitor, in combination with chemotherapy in the neoadjuvant setting for early TNBC showed significantly increased pCR rates and will likely lead to its approval for neoadjuvant treatment.49 The benefit of immunotherapy in the metastatic setting seems restricted to those tumors that have expression of PD-L1 in stromal cells, and only 40% of patients in the Impassion 130 trial had tumors with PD-L1+ status.3 Further exploration of biomarkers to identify tumors most likely to benefit from ICI therapy as well as other molecular targets in TNBC remains an area of ongoing research.
Conclusion
Genomic profiling and other sequencing technologies have led to better understanding of the heterogenous biology of TNBC and allowed for the identification of molecular subtypes of TNBC. A variety of genetic alterations found in TP53, BRCA1/2, KRAS, NRAS, and PIK3CA genes amongst many others and the disruption in their associated cellular pathways provide opportunities for novel targeted treatments. Molecular studies have also uncovered the heterogeneity of the tumor microenvironment within TNBC subtypes including vast differences in immune infiltrate localization, composition, and expression of targetable immune pathways. The success of immune checkpoint inhibitors in clinical trials have led to the approval of atezolizumab in combination with nab-paclitaxel in the metastatic setting3 and likely forthcoming approval of pembrolizumab in the neoadjuvant setting.49 The first Trop-2 antibody drug conjugate, sacituzumab govitecan-hziy, was also approved in early 2020 for refractory metastatic TNBC. These new approvals signify a long-awaited change in the treatment landscape for TNBC, but nevertheless these treatments remain restricted to a small subset of TNBC tumors. Novel treatment strategies for TNBC such as combination treatments with both molecular targets and ICIs as well as ICIs in combination with vaccines to enhance T-cell priming and activation are underway.50–52
References
- 1.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007;110(4):876–84. (In eng). DOI: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 2.Plasilova ML, Hayse B, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine (Baltimore) 2016;95(35):e4614. (In eng). DOI: 10.1097/MD.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379(22):2108–2121. (In eng). DOI: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 4.Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019;380(8):741–751. (In eng). DOI: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 5.Zeichner SB, Terawaki H, Gogineni K. A Review of Systemic Treatment in Metastatic Triple-Negative Breast Cancer. Breast Cancer (Auckl) 2016;10:25–36. (In eng). DOI: 10.4137/BCBCR.S32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010;6(4):195–7. (In eng). DOI: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25(1):118–45. (In eng). DOI: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 8.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13(15 Pt 1):4429–34. (In eng). DOI: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13(8):2329–34. (In eng). DOI: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 10.Glück S, Ross JS, Royce M, et al. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat 2012;132(3):781–91. (In eng). DOI: 10.1007/s10549-011-1412-7. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26(8):1275–81. (In eng). DOI: JCO.2007.14.4147 [pii] 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 12.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 2012;132(3):1049–62. (In eng). DOI: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406(6797):747–52. (In eng). DOI: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5(1):5–23. (In eng). DOI: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 2006;7:96. (In eng). DOI: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70. (In eng). DOI: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121(7):2750–67. (In eng). DOI: 10.1172/JCI45014 45014 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer JA, Chakravarthy AB, Rosenbluth JM, et al. Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation. Clin Cancer Res 2010;16(2):681–90. (In eng). DOI: 10.1158/1078-0432.CCR-09-1091 1078–0432.CCR-09–1091 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juul N, Szallasi Z, Eklund AC, et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: a retrospective analysis of five clinical trials. Lancet Oncol 2010;11(4):358–65. (In eng). DOI: 10.1016/S1470-2045(10)70018-8. [DOI] [PubMed] [Google Scholar]
- 20.Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res 2006;66(9):4636–44. (In eng). DOI: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 21.Gibson GR, Qian D, Ku JK, Lai LL. Metaplastic breast cancer: clinical features and outcomes. Am Surg 2005;71(9):725–30. (In eng). [PubMed] [Google Scholar]
- 22.Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006;25(28):3994–4008. (In eng). DOI: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 2009;15(7):2472–8. (In eng). DOI: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann BD, Jovanović B, Chen X, et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One 2016;11(6):e0157368. (In eng). DOI: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21(7):1688–98. (In eng). DOI: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann BD, Pietenpol JA. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015;24 Suppl 2:S36–40. (In eng). DOI: 10.1016/j.breast.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bareche Y, Venet D, Ignatiadis M, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol 2018;29(4):895–902. (In eng). DOI: 10.1093/annonc/mdy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang YZ, Ma D, Suo C, et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019;35(3):428–440.e5. (In eng). DOI: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res 2011;13(6):227. (In eng). DOI: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu T, Di G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin J Cancer Res 2017;29(3):237–252. (In eng). DOI: 10.21147/j.issn.1000-9604.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74. (In eng). DOI: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32(27):2959–66. (In eng). DOI: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33(9):983–91. (In eng). DOI: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 34.Bareche Y, Buisseret L, Gruosso T, et al. Unraveling Triple-Negative Breast Cancer Tumor Microenvironment Heterogeneity: Towards an Optimized Treatment Approach. J Natl Cancer Inst 2020;112(7):708–719. (In eng). DOI: 10.1093/jnci/djz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruosso T, Gigoux M, Manem VSK, et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest 2019;129(4):1785–1800. (In eng). DOI: 10.1172/JCI96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 2013;19(19):5533–40. (In eng). DOI: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 2014;232(2):142–50. (In eng). DOI: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herold CI, Chadaram V, Peterson BL, et al. Phase II trial of dasatinib in patients with metastatic breast cancer using real-time pharmacodynamic tissue biomarkers of Src inhibition to escalate dosing. Clin Cancer Res 2011;17(18):6061–70. (In eng). DOI: 10.1158/1078-0432.CCR-11-1071. [DOI] [PubMed] [Google Scholar]
- 39.Finn RS, Bengala C, Ibrahim N, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res 2011;17(21):6905–13. (In eng). DOI: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014;16(4):406. (In eng). DOI: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 2013;19(19):5505–12. (In eng). DOI: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol 2018;36(9):884–890. (In eng). DOI: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asghar US, Barr AR, Cutts R, et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin Cancer Res 2017;23(18):5561–5572. (In eng). DOI: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann BD, Abramson VG, Sanders ME, et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR. Clin Cancer Res 2020;26(9):2111–2123. (In eng). DOI: 10.1158/1078-0432.CCR-19-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baselga J, Dent S, Cortes J. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. Journal of Clinical Oncology 2018;36:no. 18_suppl. DOI: 10.1200/JCO.2018.36.18_suppl.LBA1006. [DOI] [Google Scholar]
- 46.Schneider BP, Gray RJ, Radovich M, et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res 2013;19(5):1281–9. (In eng). DOI: 10.1158/1078-0432.CCR-12-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012;30(21):2615–23. (In eng). DOI: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardia A, et al. ASCENT: A randomized phase 3 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer. ESMO Virtual Congress 2020, LBA17. [Google Scholar]
- 49.Schmid P, Cortes J, Bergh JCS, et al. KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple-negative breast cancer (TNBC). Journal of Clinical Oncology 2018;36(15_suppl):TPS602–TPS602. DOI: 10.1200/JCO.2018.36.15_suppl.TPS602. [DOI] [Google Scholar]
- 50.Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018;124(10):2086–2103. (In eng). DOI: 10.1002/cncr.31272. [DOI] [PubMed] [Google Scholar]
- 51.Isakoff S, Tolaney SM, Tung NM, et al. A phase 1b study of safety and immune response to PVX-410 vaccine alone and in combination with durvalumab (MEDI4736) in HLA-A2+ patients following adjuvant therapy for stage 2/3 triple negative breast cancer [abstract]. J Clin Oncol. 2017; 35 (15 suppl): TPS1126. [Google Scholar]
- 52.Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res 2016;22(6):1499–509. (In eng). DOI: 10.1158/1078-0432.CCR-15-1125 1078–0432.CCR-15–1125 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]


