Abstract
Objectives:
To develop a prediction algorithm for soft tissue changes after orthognathic surgery that would result in accurate predictions (1) regardless of types or complexity of operations and (2) with a minimum number of input variables.
Materials and Methods:
The subjects consisted of 318 patients who had undergone the surgical correction of Class II or Class III malocclusions. Two multivariate methods—the partial least squares (PLS) and the sparse partial least squares (SPLS) methods—were used to construct prediction equations. While the PLS prediction model included 232 input variables, the SPLS method included a reduced number of variables generated by a handicapping algorithm via the sparsity control. The accuracy between the PLS and SPLS models was compared.
Results:
There were no significant differences in prediction accuracy depending on surgical movements, the sex of the subjects, or additional surgeries. The predictive performance with a reduced set of 34 input variables chosen using the SPLS method was statistically indistinguishable from the full set of variables with the original PLS prediction model.
Conclusions:
The prediction method proposed in the present study was accurate for a wide range of orthognathic surgeries. A reduced set of input variables could be selected through the SPLS method while simultaneously maintaining a prediction level that was as accurate as that of the original PLS prediction model.
Keywords: Predicting soft tissue changes; Sparse partial least squares, Sparsity control
INTRODUCTION
Prediction of postoperative soft tissue changes has been a critical step in the planning of surgical-orthodontic treatment. Although previous studies and current simulation programs offer a practical guideline for everyday practice, the prediction of treatment results has yet to be improved. For example, there is no single prediction algorithm that can be applied to various surgical movements simultaneously. Surgical movements differ for Class II and Class III skeletal discrepancies, and prior studies1–7 derived a different prediction equation for each skeletal discrepancy. Prediction results for bimaxillary surgery were reported8,9 to be less accurate than those for single-jaw surgeries. To achieve a more esthetic and stable result, there has been an increase in bimaxillary surgeries and other additional operations, such as genioplasties.10,11 In this regard, formulating one universally applicable prediction model seems appealing. With such a model, clinicians can accurately predict soft tissue changes following a wide variety of surgeries considered.
Recent studies3,4,6,7 showed that the prediction equation based on the partial least squares (PLS) method resulted in increased accuracy and enabled consideration of a variety of input variables. PLS prediction results, in comparison with those associated with conventional, ordinary least squares (OLS) approaches such as regression analyses, demonstrated that PLS was more accurate than were OLS statistical methods. However, the PLS prediction method should be improved in two aspects to be practical enough for use under normal clinical circumstances: (1) it had separate prediction equations according to each specific orthognathic surgery procedure, and (2) it demanded hundreds of input variables, including 78 cephalometric landmarks. These landmarks should be manually obtained by clinicians, and identifying a considerable number of landmarks might be a very laborious process for clinicians. From a clinical perspective, developing not only a universally applicable single equation but also a convenient prediction model that would produce results that are as useful as the same large data set analyzed with even more predictors might be desirable. However, the number of variables decisively affects the accuracy of the prediction. These two goals—improving clinical convenience and prediction accuracy simultaneously—are at cross-purposes and offset each other.
The present study aimed to develop a single prediction algorithm for soft tissue change after orthognathic surgery that results in accurate predictions (1) regardless of types or complexity of operations (Class II, Class III correction, mandibular surgery and/or maxillary surgery, and additional genioplasty) and (2) with a minimum number of input variables through an objective variable selection by modifying the original PLS prediction model.
MATERIALS AND METHODS
Subjects
The subjects consisted of 318 patients who had undergone the surgical correction of severe Class II or Class III malocclusion (Table 1). They underwent a wide variety of surgical movements. Table 2 shows surgeries that were performed to correct skeletal discrepancies: mandibular setback surgery, mandibular advancement surgery, Le Fort I surgery, anterior segmental osteotomy in the maxilla, and additional genioplasty. Two-thirds of the subjects (204 patients) received surgeries to correct Class III skeletal discrepancies. The remaining 114 Class II patients underwent surgical repositioning to correct mandibular insufficiencies. Among Class III patients, 71 patients had single-jaw mandibular surgery, 133 patients had bimaxillary surgery, and 81 patients had additional genioplasty. The patients had single- or double-jaw surgery with or without genioplasty. All patients received fixed orthodontic treatment. Patients who had a cleft lip and palate, severe asymmetry, or facial deformity due to a syndrome were not included. The institutional review board for the protection of human subjects of Seoul National University School of Dentistry reviewed and approved the research protocol (S-D 20140025).
Table 1.
Subject Characteristics
| Variables |
N |
Mean |
Standard Deviation |
Minimum |
Maximum |
| Age, y | |||||
| Female | 191 | 24.1 | 5.3 | 16.0 | 51.6 |
| Male | 127 | 23.6 | 3.5 | 18.8 | 39.1 |
| Time after surgery, mo | 9.5 | 4.1 | 3.7 | 30.5 | |
| Maxillary surgery | |||||
| No | 91 | ||||
| Yes | 227 | ||||
| Genioplasty | |||||
| No | 139 | ||||
| Yes | 179 | ||||
Table 2.
Features of Class II and Class III Subjects
| Variables |
Class II Subjects |
Class III Subjects |
||||
| n |
Mean |
SDa |
n |
Mean |
SDa |
|
| Maxillary surgery | ||||||
| No | 20 | 71 | ||||
| Yes | 94 | 133 | ||||
| Genioplasty | ||||||
| No | 16 | 123 | ||||
| Yes | 98 | 81 | ||||
| Overjet before surgery, mm | 7.7 | 2.4 | −5.8 | 3.8 | ||
| Overbite before surgery, mm | 2.7 | 3.1 | −0.2 | 1.8 | ||
| Amount of surgical repositioning at point A,b mm | ||||||
| Anteroposterior repositioning | −0.2 | 2.1 | 1.4 | 1.9 | ||
| Vertical repositioning | −1.5 | 3.1 | −1.1 | 2.3 | ||
| Amount of surgical repositioning at point B,b mm | ||||||
| Anteroposterior repositioning | 6.0 | 3.9 | −7.3 | 3.8 | ||
| Vertical repositioning | −1.0 | 4.7 | −2.9 | 4.2 | ||
SD indicates standard deviation.
A positive value indicates forward and downward in the horizontal and vertical directions, respectively; a negative value indicates either posterior direction or superior direction during surgical repositioning.
For every patient, lateral cephalograms were obtained before and after orthognathic surgery. Postoperative cephalograms were taken at least 3.7 months (average, 9.5 months) after the surgery to allow the resolution of postoperative swelling. Figure 1 illustrates the anatomical tracing, soft tissue outline, cephalometric landmarks, and their abbreviations. Forty-six skeletal landmarks and 32 soft tissue landmarks from the glabella to the terminal point were identified. With its origin at Sella, the vertical reference was established perpendicular to Sella-Nasion +7°. The x coordinates represented the horizontal distance from the vertical axis, and the y coordinates represented the vertical distance from the horizontal axis measured in millimeters, which followed the same superimposition method that appeared in previous PLS studies.3,4,6,7 The coordinates of every landmark on each tracing were sequentially computed relative to the x and y reference system with a custom digitizing program via Microsoft Visual C# 2010 (Microsoft, Redmond, Wash).
Figure 1.
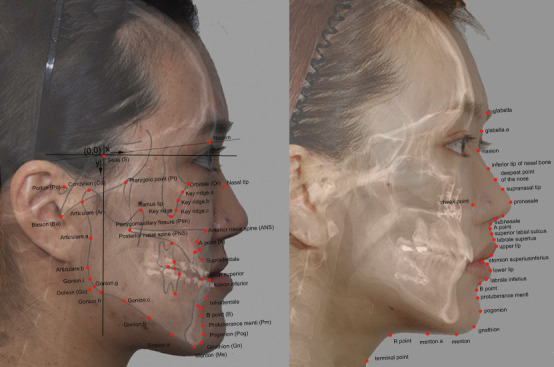
Reference planes and 78 cephalometric landmarks used in the present study. Left: preoperative image, with hard tissue landmarks in capital letters. Right: postoperative image, with soft tissue landmarks in lowercase letters.
Variables
The original prediction equation based on the PLS method included a total of 232 input variables. The input variables were the patient's age, sex, time after surgery, types of surgery, existence of genioplasty, presurgical x and y coordinates of 78 cephalometric landmarks, and the amount of the surgical skeletal repositioning at each landmark in both the anteroposterior and vertical directions. The output variables were the soft tissue responses at the 32 soft tissue landmarks both in x and y coordinates, totaling 64 output variables.
Predicting Methods
First, a PLS model was built to construct the prediction equation. While the PLS method included all the landmarks as input variables, by imposing a penalty (sparsity) in a preprocessing step of the PLS algorithm, the sparse partial least squares (SPLS) method selected a reduced set of input variables. When the penalty term (eta) is zero, SPLS is equivalent to the original PLS algorithm. As the penalty term (eta) that has a value between 0 and 1 increases, the number of selected input variables decreases.12 Eta values are progressively raised until there is a significant difference in the prediction accuracy between the original PLS and SPLS methods.
Validation and Prediction Accuracy Comparisons
To assess prediction accuracy on a new subject, the leave-one-out cross-validation technique was applied since this method is known to be the most appropriate validation strategy in a clinical research framework.7,13 When applying the leave-one-out cross-validation technique, a researcher includes all of the subjects except one subject to build a model. The remaining subject's data can be used for validation because it wasn't used to build the model. The process is then repeated to use every subject's data as a validation data set.
The mean absolute error of prediction, |Yactual – Ypredicted|, was used to represent the accuracy of a prediction model. Since two-dimensional cephalometric images have two-dimensional errors, to compare and visualize the error pattern, scattergrams with 95% confidence ellipses were constructed.14,15 Language R (Vienna, Austria)16 was used to perform statistical analyses.
RESULTS
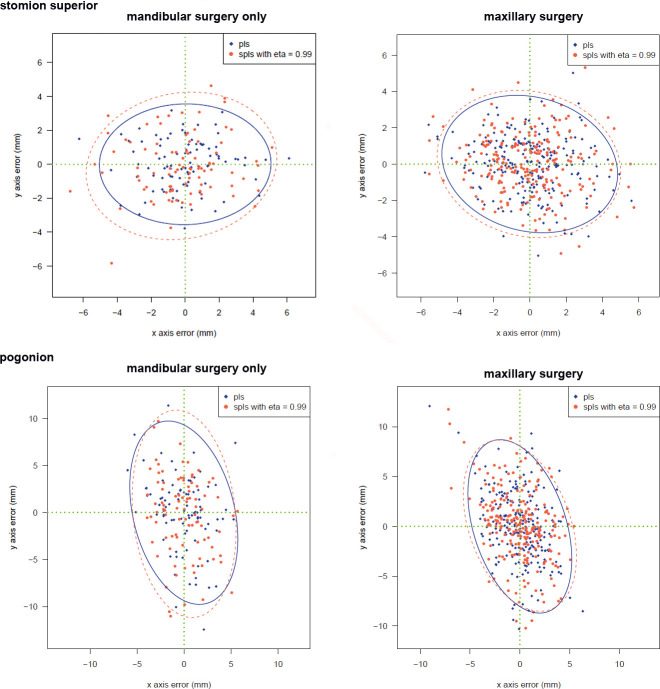
The prediction algorithms based on multivariate PLS and SPLS methods were accurate regardless of the type of surgery. There were no significant differences in the prediction accuracy depending on surgical movements, the gender of the subjects, or additional surgeries (Figure 2).
Figure 2.
Prediction errors from the PLS (blue) and SPLS with eta = 0.99 (red). Left: plots are drawn from patients who underwent mandibular surgery only; right: plots are drawn from patients who underwent maxillary surgery.
When the sparsity term, eta value, was increased from 0.1 to 0.99, the prediction errors by SPLS showed an increasing pattern according to the sparsity term (Table 3). However, even the SPLS with eta = 0.99 did not demonstrate a statistically significant difference in the prediction errors compared to the results from the original PLS method. At this moment of eta = 0.99, the number of input variables was reduced to 34 (Table 4). Figure 3 illustrates those selected landmarks superimposed on a patient's photographs.
Table 3.
Mean Absolute Error of Prediction from Partial Least Squares (PLS) and Sparse PLS (SPLS) Prediction Models According to Sparsity Term, eta Values, from 0.1 to 0.99. To Concisely Report Results, Only Five Landmarks Among the 32 Soft Tissue Landmarks Investigated Are Presented in this Table
| PLS eta | SPLS |
||||||||||||||
| 0.0 |
0.1 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.65 |
0.7 |
0.75 |
0.8 |
0.85 |
0.9 |
0.95 |
0.99 |
|
| Horizontal | |||||||||||||||
| Pronasale | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.91 | 0.91 | 0.91 | 0.90 | 0.87 | 0.88 | 0.97 |
| Upper lip | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.19 | 1.20 | 1.20 | 1.19 | 1.18 | 1.20 |
| Lower lip | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.08 | 1.08 | 1.20 |
| Pogonion | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.26 | 1.31 | 1.23 |
| Menton | 2.09 | 2.09 | 2.09 | 2.09 | 2.09 | 2.09 | 2.08 | 2.09 | 2.08 | 2.09 | 2.09 | 2.12 | 2.10 | 2.07 | 2.05 |
| Vertical | |||||||||||||||
| Pronasale | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 0.97 | 1.03 |
| Upper lip | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.14 | 1.16 | 1.19 |
| Lower lip | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.25 | 1.28 | 1.27 | 1.30 |
| Pogonion | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.59 | 1.60 | 1.59 | 1.58 | 1.59 |
| Menton | 1.27 | 1.27 | 1.27 | 1.27 | 1.27 | 1.27 | 1.26 | 1.27 | 1.27 | 1.29 | 1.27 | 1.28 | 1.33 | 1.33 | 1.33 |
Table 4.
The Number of Input Variables in the Prediction Method and the Number of Selected Input Variables According to Sparsity Term, eta Values, from 0.1 to 0.99a
| PLS eta |
Sparse PLS |
|||||||||||||
| 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.65 | 0.7 | 0.75 | 0.8 | 0.85 | 0.9 | 0.95 | 0.99 |
| 232 | 232 | 232 | 232 | 232 | 232 | 229 | 227 | 220 | 207 | 181 | 147 | 104 | 76 | 34 |
PLS indicates partial least squares.
Figure 3.
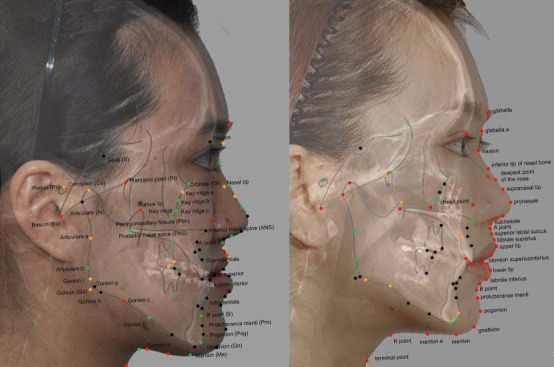
Selected landmark coordinates that were used in the SPLS with eta = 0.99. Selected x coordinates are indicated as orange dots; selected y coordinates are indicated as green dots; and selected landmarks (x and y coordinates) are indicated as red dots. Left: preoperative image with hard tissue landmarks in capital letters; right: postoperative image with soft tissue landmarks in lowercase letters.
DISCUSSION
The present study aimed to develop a practical prediction algorithm for soft tissue change after surgery that is applicable to a wide variety of surgical corrections. Both the PLS and SPLS methods proposed in the present study did not show a significant difference in the prediction accuracy depending on surgical movements, the gender of the subjects, or additional surgeries, which implied that a single equation could be applied to predict soft tissue changes after orthognathic surgery. Previous studies1,2 reported that the predictability of the upper lip position after mandibular setback or advancement was poor and highly variable in both directions. On the other hand, other articles8,17,18 reported that the main area of inaccuracy was the lower lip. In this study, however, both the PLS and SPLS predictions of the upper and lower lip areas showed accurate results.
Statistical methods such as regression analyses require the prerequisite condition of independence among the input variables. As maxillofacial landmarks are on adjacent or continuous structures, high correlation among the landmarks should be considered. In this regard, PLS algorithms that are capable of controlling for the collinearity among input variables are advantageous. PLS methods have gained popularity in a broad range of fields in science because of their competency in managing a large number of variables and highly correlated data.19 In the present study, one of the modifications on the PLS algorithm, the SPLS method, showed accuracy comparable with that of the original model, which had a much greater number of input variables.
In general, the best prediction model would be the simplest model that minimizes the error. To facilitate clinical applicability, it would be more practical to have a minimum number of input variables. When applying an OLS method such as multiple linear regression prediction, stepwise variable selection methods are commonly used to reduce the number of input variables in a parsimonious way and to find significantly influential input variables of the prediction equation. However, unlike OLS methods, the PLS method is incapable of applying the conventional stepwise variable selection technique. Instead, in reducing input variables, the PLS method should put sparsity on the matrix decomposition process, just as principal component analyses do when reducing the number of principal components.12,19–23 Setting sparsity may play a similar role in reducing input variables, as a stepwise variable selection technique provides for multiple regression prediction models. As such, to select a reduced set of cephalometric landmarks objectively, the present study applied a handicapping algorithm (ie, the SPLS method) by putting the sparsity on the original PLS method. Through SPLS, insights on key landmarks in predicting soft tissue changes after orthognathic surgery were expected.
Figure 3 illustrates the landmark coordinates that were shown to be critical for soft tissue profile prediction. It was notable that the points selected by SPLS were inconsistent with key landmarks. For example, the variable selection process excluded well-known anatomical landmarks such as Pogonion and Menton. It is conjectured that the selected points might have reflected the correlation among the landmarks. For instance, when predicting the profile below labium inferius, the landmark required was the y coordinate of preoperative Gnathion, and the x coordinate of postoperative Pogonion. These points may seem insufficient, yet they still brought about a more accurate prediction result than that associated with the conventional methods. This might have resulted from the highly intercorrelated nature of the landmarks on the facial profile.
After applying the sparsity term, eta = 0.99, the number of input variables was dramatically reduced from 232 to 34. The SPLS method considerably simplified the prediction model. Although the resultant error values from the SPLS were increasing over the sparsity values, there was still no statistically significant difference. Therefore, this SPLS prediction model seemed to be more pragmatic for use in clinical image software.
The present study proposed how to produce a useful model that is as accurate as the model developed with a higher number of input variables. The future goal might be the application of this method to three-dimensional (3D) data. While 3D photogrammetry and 3D cephalometrics look promising in the near future,21,24–26 one of the major challenges might be that a 3D prediction algorithm will demand more massive data than would be required in a two-dimensional situation. Going to 3D would present even more of a burden on measurement and computational resources. In 3D images of faces, for example, the number of input variables may reach into the hundreds.21 This is because a single landmark includes coordinate information for all three planes of space as well as the additional variables for 3D surface curvatures.21,27 The variable selection methods applied in the present study might be useful in dealing with a vast number of 3D variables and might facilitate the development of treatment prediction algorithms in 3D.
CONCLUSIONS
The prediction algorithms based on PLS and SPLS methods were accurate regardless of the type of surgery.
By applying the SPLS method, the number of input variables could objectively be reduced while maintaining a prediction level that was as accurate as that associated with the original PLS prediction model.
ACKNOWLEDGMENTS
This study was a part of a doctoral dissertation (HYS) and was supported by grant 02-2014-0003 from the Seoul National University Dental Hospital Research Fund. The authors also thank the anonymous reviewers who read and commented on this article. The authors also express gratitude to the copyeditor of The Angle Orthodontist for her invaluable assistance in improving this manuscript.
REFERENCES
- 1.Joss CU, Joss-Vassalli IM, Berge SJ, Kuijpers-Jagtman AM. Soft tissue profile changes after bilateral sagittal split osteotomy for mandibular setback: a systematic review. J Oral Maxillofac Surg. 2010;68:2792–2801. doi: 10.1016/j.joms.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Joss CU, Joss-Vassalli IM, Kiliaridis S, Kuijpers-Jagtman AM. Soft tissue profile changes after bilateral sagittal split osteotomy for mandibular advancement: a systematic review. J Oral Maxillofac Surg. 2010;68:1260–1269. doi: 10.1016/j.joms.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Suh HY, Lee YS, et al. A better statistical method of predicting postsurgery soft tissue response in Class II patients. Angle Orthod. 2014;84:322–328. doi: 10.2319/050313-338.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YS, Suh HY, Lee SJ, Donatelli RE. A more accurate soft-tissue prediction model for Class III 2-jaw surgeries. Am J Orthod Dentofacial Orthop. 2014;146:724–733. doi: 10.1016/j.ajodo.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Nadjmi N, Tehranchi A, Azami N, Saedi B, Mollemans W. Comparison of soft-tissue profiles in Le Fort I osteotomy patients with Dolphin and Maxilim softwares. Am J Orthod Dentofacial Orthop. 2013;144:654–662. doi: 10.1016/j.ajodo.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Suh HY, Lee SJ, Lee YS, et al. A more accurate method of predicting soft tissue changes after mandibular setback surgery. J Oral Maxillofac Surg. 2012;70:e553–e562. doi: 10.1016/j.joms.2012.06.187. [DOI] [PubMed] [Google Scholar]
- 7.Yoon KS, Lee HJ, Lee SJ, Donatelli RE. Testing a better method of predicting postsurgery soft tissue response in Class II patients: a prospective study and validity assessment. Angle Orthod. 2015;85:597–603. doi: 10.2319/052514-370.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaipatur NR, Flores-Mir C. Accuracy of computer programs in predicting orthognathic surgery soft tissue response. J Oral Maxillofac Surg. 2009;67:751–759. doi: 10.1016/j.joms.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Eckhardt CE, Cunningham SJ. How predictable is orthognathic surgery? Eur J Orthod. 2004;26:303–309. doi: 10.1093/ejo/26.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Lim HW, Park JH, Park HH, Lee SJ. Time series analysis of patients seeking orthodontic treatment at Seoul National University Dental Hospital over the past decade. Korean J Orthod. 2017;47:298–305. doi: 10.4041/kjod.2017.47.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CH, Park HH, Seo BM, Lee SJ. Modern trends in Class III orthognathic treatment: a time series analysis. Angle Orthod. 2017;87:269–278. doi: 10.2319/043016-349.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun H, Keles S. Sparse partial least squares regression for simultaneous dimension reduction and variable selection. J R Stat Soc Series B Stat Methodol. 2010;72:3–25. doi: 10.1111/j.1467-9868.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donatelli RE, Lee SJ. How to test validity in orthodontic research: a mixed dentition analysis example. Am J Orthod Dentofacial Orthop. 2015;147:272–279. doi: 10.1016/j.ajodo.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Donatelli RE, Lee SJ. How to report reliability in orthodontic research: part 2. Am J Orthod Dentofacial Orthop. 2013;144:315–318. doi: 10.1016/j.ajodo.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Donatelli RE, Lee SJ. How to report reliability in orthodontic research: part 1. Am J Orthod Dentofacial Orthop. 2013;144:156–161. doi: 10.1016/j.ajodo.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 17.Ksiezycki-Ostoya BK, McCollum AGH, Becker PJ. Sagittal soft-tissue changes of the lower lip and chin associated with surgical maxillary impaction and consequent mandibular autorotation. Semin Orthod. 2009;15:185–195. [Google Scholar]
- 18.Donatsky O, Bjorn-Jorgensen J, Hermund NU, Nielsen H, Holmqvist-Larsen M, Nerder PH. Immediate postoperative outcome of orthognathic surgical planning, and prediction of positional changes in hard and soft tissue, independently of the extent and direction of the surgical corrections required. Br J Oral Maxillofac Surg. 2011;49:386–391. doi: 10.1016/j.bjoms.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Mevik B-H, Wehrens R. The pls package: principal component and partial least squares regression in R. J Stat Softw. 2007;18(23) [Google Scholar]
- 20.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. Data Mining, Inference, and Prediction 2nd ed. New York, NY: Springer Verlag; 2009. [Google Scholar]
- 21.Kang TJ, Eo SH, Cho HJ, Donatelli RE, Lee SJ. A sparse principal component analysis of Class III malocclusions. Angle Orthod. Available online since March 21, 2019. [DOI] [PMC free article] [PubMed]
- 22.Witten DM, Tibshirani R, Hastie T. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics. 2009;10:515–534. doi: 10.1093/biostatistics/kxp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun H, Keles S. Expression quantitative trait loci mapping with multivariate sparse partial least squares regression. Genetics. 2009;182:79–90. doi: 10.1534/genetics.109.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo JC, Gianneschi G, Azer D, et al. The relationship between 3D dentofacial photogrammetry measurements and traditional cephalometric measurements. Angle Orthod. 2019;89:275–283. doi: 10.2319/120317-825.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J, Yu H, Yin Y, et al. Esthetic evaluation of facial cheek volume: a study using 3D stereophotogrammetry. Angle Orthod. 2019;89:129–137. doi: 10.2319/020418-97.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponce-Garcia C, Lagravere-Vich M, Cevidanes LHS, de Olivera Ruellas AC, Carey J, Flores-Mir C. Reliability of three-dimensional anterior cranial base superimposition methods for assessment of overall hard tissue changes: a systematic review. Angle Orthod. 2018;88:233–245. doi: 10.2319/071217-468.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sam A, Currie K, Oh H, Flores-Mir C, Lagravere-Vich M. Reliability of different three-dimensional cephalometric landmarks in cone-beam computed tomography: a systematic review. Angle Orthod. 2019;39:317–332. doi: 10.2319/042018-302.1. [DOI] [PMC free article] [PubMed] [Google Scholar]