Abstract
Objective:
To compare changes in pharyngeal airway volume and minimal cross-sectional area (MCA) between patients undergoing rapid maxillary expansion (RME) and a matched control group and to identify markers for predicting airway changes using cone-beam computed tomography (CBCT).
Materials and Methods:
Pre- and posttreatment CBCT scans were selected of children who had RME (14 girls and 12 boys; mean age, 12.4 years) along with scans of a control group (matched for chronological age, skeletal age, gender, mandibular inclination) who underwent orthodontic treatment for minor malocclusions without RME. Changes in airway volume and MCA were evaluated using a standardized, previously validated method and analyzed by a mixed-effects linear regression model.
Results:
Upper airway volume and MCA increased significantly over time for both the RME and matched control groups (P < .01 and P = .05, respectively). Although the RME group showed a greater increase when compared with the matched controls, this difference was not statistically significant. A reduced skeletal age before treatment was a significant marker for a positive effect on the upper airway volume and MCA changes (P < .01).
Conclusions:
Tooth-borne RME is not associated with a significant change in upper airway volume or MCA in children when compared with controls. The younger the skeletal age before treatment, the more positive the effect on the upper airway changes. The results may prove valuable, especially in RME of young children.
Keywords: Maxillary expansion, Upper airway volume, Children, CBCT
INTRODUCTION
The dentofacial changes that develop as a result of upper airway constriction have been well documented in the literature, and early diagnosis and treatment are desirable to encourage normal craniofacial development.1 More recently, the association between upper airway morphology, sleep-disordered breathing, and obstructive sleep apnea (OSA) has been studied, and there is a general agreement that early management of these conditions may lead to better long-term medical and dental outcomes for patients.2
Rapid maxillary expansion (RME) is a commonly used orthodontic treatment to correct transverse dental and skeletal discrepancies while providing an increase in arch width to resolve mild to moderate crowding.3 Using an orthodontic appliance, force is exerted laterally against the posterior teeth or palatal mucosa, which in turn places force on the midpalatal suture. As the suture is usually patent in children and adolescents, the application of force perpendicular to it may lead to transverse maxillary growth.4
Although the primary aim of RME is to exert force on the maxilla, studies have shown that the skeletal effects are much more extensive, with displacement occurring in all bones articulating with the maxilla, except for the sphenoid bone, as well as the airway.5 Cistulli et al.6 investigated the effects of RME in a sample of 10 patients with mild to moderate OSA. Nine of these patients reported an improvement in snoring and daytime sleepiness, and all patients demonstrated a reduction in the Respiratory Distress Index, which returned to normal in seven patients. The authors concluded that RME may be a useful treatment alternative for selected patients with OSA.6
Understanding the effects of RME on the upper airway in three dimensions has historically been problematic. Acoustic rhinometry has been used; however, this technique is limited to the nasal passages.7,8 More recently, the use of medical computed tomography has been described; however, the limitations of this technique are that the radiation dose is relatively high and patients must be in the supine position.9,10 It has been shown that placement in these positions affects the volume of the airways.11–14
Cone-beam computed tomography (CBCT) has been shown to be an accurate and reliable method of assessing the upper airway in the upright position15 and is able to define the boundaries between the airway spaces and soft tissues accurately in both adults and children with easily identifiable landmarks and negligible magnification.16 A recent systematic review of previous CBCT studies investigating the changes in airway before and after treatment with RME found conflicting results and a lack of homogeneity among the measurement protocols used.17 Anandarajah18 proposed and validated a standardized method of upper airway assessment using CBCT and has used this technique to demonstrate an association between maxillary and mandibular width and airway volume in healthy untreated children.19
The aims of this study were (1) to compare changes in pharyngeal airway volume and minimal cross-sectional area (MCA) in an RME group with that of a control group matched in age, skeletal age, gender, and mandibular inclination using a validated method of airway volume measurement18 and (2) to identify pretreatment markers for predicting airway change in the two groups combined.
MATERIALS AND METHODS
This was a retrospective study involving two groups: an RME group and a pair-matched control group. The expansion protocol for the RME group was to turn the expansion screw on a tooth-borne Hyrax-type expander 0.25 mm per day for a minimum of 2 weeks. There was a retention period of 6 months, after which some of the RME patients continued with fixed appliances. The matched control group underwent nonextraction orthodontic treatment with fixed appliances only (no RME) for minor malocclusions.
The CBCT scans were obtained from a database of 784 orthodontic patients who attended a private practice in Victoria, Australia, for orthodontic treatment between 2006 and 2012. Before they were entered into the database, all images were anonymized. Sex, age, morphological occlusion according to Angle's classification, and type of orthodontic treatment were also obtained from the database. The inclusion criteria were (1) RME treatment with a tooth-borne Hyrax expander due to a unilateral or bilateral crossbite, followed by fixed appliances; (2) a minimum increase of 3 mm in the intermolar width between pre- and posttreatment scans, which would result in a minimum expected orthopedic change in the maxilla of 1.5 mm20; (3) pretreatment and progress CBCT scans with complete imaging of the cranial base, maxilla, mandible, and first four cervical vertebrae and associated airways; (4) children between 8 and 15 years of age; and (5) biting in habitual intercuspal position with an Angle Class I molar relationship. The exclusion criteria were (1) previous orthodontic treatment, (2) previous adenotonsillectomy, (3) known syndromic conditions, (4) movement artifacts, (5) swallowing during scan acquisition, and (6) treatment plan requiring orthodontic extractions. Once inclusion and exclusion criteria had been applied, the final sample consisted of 26 patients. The power of the sample size was calculated, and it was determined that 21 subjects would be needed to achieve a power of 80% (α = .05).
A control group of 26 Angle Class I patients who had nonextraction treatment with fixed appliances only was randomly selected to match the RME group for chronological age (mean, 12 years, 4 months ± 2 years, 4 months), skeletal age using the Cervical Vertebral Maturation index according to Baccetti et al.21 (cervical vertebral maturation: stage 1 = 3, 2 = 8, 3 = 6, 4 = 7, 5 = 2), gender (male = 12, female = 14), and time interval between the pretreatment and progress scans (2 years ± 11 months). Patients were also matched for the mandibular inclination according to Björk22 (nasion-sella line/mandibular line 33° ± 6°), which was determined using digital tracings of lateral cephalometric radiographs generated from the CBCT scans (Total Interactive Orthodontic Planning System (TIOPS 4, Roskilde, Denmark). The study was approved by the Danish Data Protection Board (ref. SUND-2015-57-0121).
Scan Protocol
All images were acquired using an iCAT Next Generation CBCT machine (Imaging Sciences International, Hatfield, Pa) by the same operator. The following parameters were used: 120 Kv, 5 mA, 0.4-mm voxel resolution, 8.9-second scan time, and 13-cm (height) × 16-cm (diameter) scan volume. Patients were seated and restrained with a headrest and head strap but no chin rest to allow the Frankfort horizontal plane to be positioned parallel with the floor. Patients were instructed to occlude in the intercuspal position, relax their lips and tongue, breathe gently, and not swallow or move during acquisition.
Image Preparation and Airway Assessment
The Digital Imaging and Communications in Medicine (DICOM) data were processed using Dolphin Imaging Software (version 11.5; Dolphin Imaging and Management Solutions, Chatsworth, Calif). Images were analyzed under the same lighting conditions and by the same investigator using a previously validated protocol,18 which is briefly outlined below and illustrated in Figure 1.
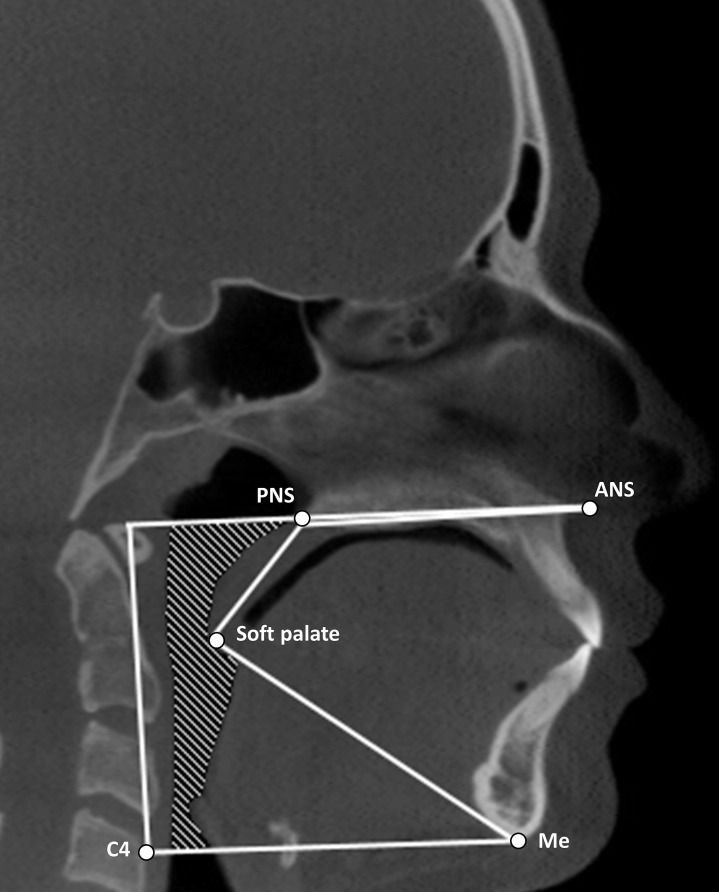
Figure 1.
Illustration of margins for delineation of upper airway. Superior: line passing from the palatal plane (ANS – PNS) extending to the posterior wall of the pharynx. Inferior: line passing from the anterosuperior edge of the fourth cervical vertebra (C4) to menton (Me). Anterior: line passing from soft palate to menton (Me). Posterior: posterior wall of pharynx. Lateral: respective pharyngeal walls.18
To standardize the measurements, the skull was oriented in all three planes according to the following lines: coronal plane (horizontal line through orbitale), sagittal plane (Frankfort horizontal), and axial plane (Crista galli to basion). The airway margins are illustrated in Figure 1 and were outlined on a sagittal slice representing the midsagittal plane. The margins were superior (palatal plane), inferior (line passing from the antero-superior edge of C4 to menton), anterior (line passing from the soft palate to menton), posterior (posterior wall of the pharynx), and lateral (respective pharyngeal walls). The process of airway segmentation was systematized as follows:
The “seed point” was defined as a virtual marker for the region of interest demarcation and was placed centrally in the airway region immediately posterior to the soft palate to facilitate automated segmentation of the airway based on gray-scale values.
The most appropriate threshold value for each patient was determined automatically by the software. The sagittal view was then enlarged as much as possible while ensuring visualization of all previously determined margins. The threshold was manually adjusted if necessary for each data set (operator-adjusted threshold) until the airway volume adequately depicted the airway–soft tissue interface. Coronal and transverse views were then evaluated to ensure the manually adjusted threshold value was correct and there was no extension of the airway segmentation into the soft tissues.
The MCA was calculated by setting the upper and lower limits within the previously defined margins, which included both anterior and posterior margins of the airway. This was to ensure that a partial section created by the difference in airway boundary for volume and the plane for area calculation was not used to determine MCA. The software automatically calculated the MCA (mm2) within the defined margins. Both measurements were carried out at the beginning (T0) and the end of active treatment (T1).
Assessment of Transverse Effects of RME
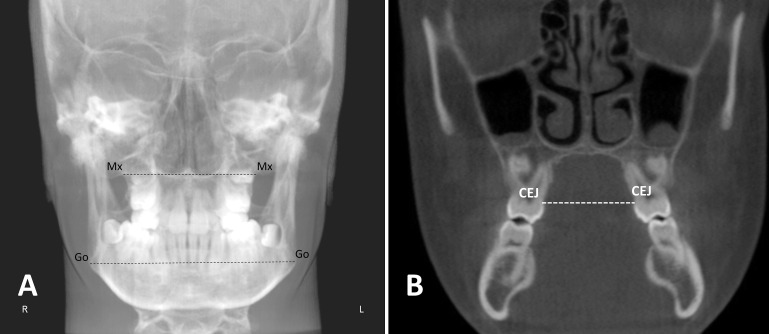
Maxillary and mandibular width at T0 and T1 was assessed for the RME group using posteroanterior cephalometric radiographs. These were generated automatically by the software at zero magnification with the scans in their previously standardized position and then compared with the matched control group to evaluate the skeletal treatment changes achieved with the RME appliance, according to the method described by Yoon et al.23 (Figure 2). The intermolar width was measured on the CBCT scans from the most palatal aspect of the upper first molars at the level of the cementoenamel junction, as described by Adkins et al.24 (Figure 2).
Figure 2.
Illustrations of transverse skeletal and dental measurements. (A) posteroanterior cephalometric reference points (Mx: intersection of the lateral contour of the maxillary alveolar process and the lower contour of the maxillozygomatic process of the maxilla; Go: the most lateral point on the angle of the mandible) and lines (dotted) measuring the maxillary width (Mx-Mx) and mandibular width (Go-Go).23 (B) Intermolar width measurement from the most palatal aspects of the upper first molars at the level of the cementoenamel junction.24
Reliability
Twenty randomly selected patient measurements were repeated after 2 weeks to assess the method error and reliability of the upper airway measures, the transverse cephalometric measurements, and the mandibular inclination. No systematic error was found when tested by a t-test. The method error according to Dahlberg's formula25 ranged between 0.2% and 1.9%, and the reliability according to Houston26 was 1 for all measurements.
Statistical Analysis
Both the RME and control group data sets were normally distributed when tested by a Shapiro-Wilks test. The dentofacial and airway differences between the two groups at T0 were evaluated by a paired t-test. The intra- and intergroup changes in the dentofacial and airway measurements between T0 and T1 were evaluated separately using a linear mixed-effects model, which allowed for the longitudinal and nested structure of the data. The fixed effects part of the models included the dependent variable volume and MCA and independent variables group and time as well as their interaction. The random effects part of the model included the individual participants nested in pairs. A backward, stepwise regression model (P < .05) using bootstrap aggregation with replacement and 1000 repetitions determined whether any of the variables measured at T0 (Table 1) were effective markers for predicting the degree of change in airway volume and MCA. Statistical analysis was carried out using Stata version 15 (StataCorp LLC, College Station, Tex).
Table 1.
Changes Between T0 and T1 in the RME and Control Groups
| T0 |
T1 |
P Value |
95% Confidence Interval |
|
| Volume, mm3 | ||||
| Control group | 12216.05 | 15794.43 | .006 | 13,258.24 to 18,330.62 |
| RME group | 12873.73 | 17460.92 | <.001 | 15,028.06 to 19,893.78 |
| RME effect | +1008.81 | .56 | −2401.10 to 4418.73 | |
| MCA, mm2 | ||||
| Control group | 125.97 | 169.54 | .020 | 131.89 to 207.18 |
| RME group | 126.53 | 164.69 | .018 | 132.97 to 196.40 |
| RME effect | −5.41 | .83 | −56.02 to 45.19 | |
| Maxillary width, mm | ||||
| Control group | 58.90 | 60.17 | .004 | 59.31 to 61.03 |
| RME group | 58.78 | 61.70 | <.001 | 60.84 to 62.57 |
| RME effect | +1.65 | .005 | 0.50 to 2.80 | |
| Maxillary intermolar width, mm | ||||
| Control group | 31.74 | 32.62 | .001 | 32.28 to 33.75 |
| RME group | 29.23 | 33.83 | <.001 | 33.14 to 34.52 |
| RME effect | +3.72 | <.001 | 2.33 to 4.31 | |
| Mandibular width, mm | ||||
| Control group | 86.18 | 89.49 | <.001 | 88.61 to 90.38 |
| RME group | 86.50 | 89.31 | <.001 | 88.50 to 90.12 |
| RME effect | -0.50 | .41 | −1.67 to 0.69 | |
RESULTS
There were no significant differences in the skeletal and airway measurements between the RME and matched control group at T0. However, the maxillary intermolar width was significantly smaller in the RME group when compared with the matched control group (29.2 mm and 31.7 mm, respectively; P < .001).
Both groups showed a significant increase in the maxillary, mandibular, and molar widths as well as airway volume and MCA between T0 and T1 (Table 1). In the RME group, the increase in the maxillary and intermolar width was significantly greater compared with the controls (P = .05 and P < .001, respectively). No significant difference in the increase in the upper airway volume and MCA was found in the RME group when compared with the controls (Table 1).
Skeletal age was the only significant pretreatment marker for prediction of airway change during treatment. Patients with a younger skeletal age showed the greatest increase in airway volume and MCA (P = .01 and P = .02, respectively; Table 2).
Table 2.
Backward Stepwise Regression Evaluating the Effect of Pretreatment Markers on Airway Volume and MCA
|
P Value |
Regression Coefficient |
95% Confidence Interval |
|
| Volume, mm3 | |||
| Mandibular inclination | .97 | Removed from model | |
| Mandibular width | .94 | Removed from model | |
| Maxillary width | .43 | Removed from model | |
| Intermolar width | .42 | Removed from model | |
| Gender | .10 | Removed from model | |
| Age | .07 | Removed from model | |
| Skeletal age | .01 | −3204.66 | −5657.78 to −751.60 |
| MCA, mm2 | |||
| Mandibular inclination | .98 | Removed from model | |
| Mandibular width | .95 | Removed from model | |
| Maxillary width | .71 | Removed from model | |
| Inter-molar width | .58 | Removed from model | |
| Gender | .08 | Removed from model | |
| Age | .06 | Removed from model | |
| Skeletal age | .02 | −48.95 | −89.68 to −8.22 |
DISCUSSION
The present study investigated the airway volume and MCA in children who had tooth-borne RME treatment followed by fixed appliances compared with a nonextraction fixed appliance control group, evaluated by standardized three-dimensional analysis of CBCT scans.18 Gender, age, skeletal age, and mandibular inclination were also controlled for, since these have previously been shown to be closely associated with upper airway dimensions.19,27,28
In the present study, both groups showed a significant increase in the dental and skeletal maxillary width, which was in agreement with previous studies.29–31 There was also a significant increase in airway volume and MCA in both groups between T0 and T1, which was in disagreement with some previous studies29,32 but not others.30,33 The increase in airway volume and MCA over time in both groups was likely due to growth. A CBCT airway growth study of 1300 patients found that there was a consistent increase in upper airway volume from age 6–20 years.34
Despite the significant increase in the maxillary width in the RME group, no significant increase in the airway volume or MCA was found in the RME group when compared with the controls in this study. This was consistent with most previous studies, which have also found that, although RME significantly increased the volume of the nasal airway, there was no effect on the pharyngeal airway volume.29,30,32,35,36 However, one study did find that RME increased pharyngeal airway volume.37 There are conflicting findings from previous studies on the effects of RME on MCA, with some studies finding no effect33,35 and another finding a positive effect32; however, the latter study did not compare to a control group. Studies in which miniscrew-assisted RME was applied have also shown no significant effect on the pharyngeal airway dimensions38 despite having a considerable skeletal effect.39
An explanation as to why both tooth-borne and miniscrew-assisted RME had no effect on the pharyngeal airway may be the compensatory mechanism of the head posture due to obstruction of the nasal and/or the pharyngeal airway.40 In response to a reduced airway volume, there is an increase in the craniocervical angle in order to maintain an adequate airway volume.27 It is therefore possible that RME increases the airway volume and MCA; however, there is also a corresponding reduction in the compensatory head posture leading to no overall significant net gain in airway volume and MCA.
No association between mandibular inclination and change in airway volume or MCA was found, which was consistent with findings from another CBCT study.41 The fact that skeletal age, rather than chronological age, was found to be a predictive marker for the amount of airway change in the present study may be explained by the significant increase in the airway volume during the pubertal growth spurt.42 Growth studies have shown that skeletal age is more closely associated with the pubertal growth spurt than chronological age.43
CONCLUSIONS
Despite increasing intermolar and maxillary widths, the tooth-borne rapid maxillary expander is not associated with a significant change in upper pharyngeal airway volume or minimum cross-sectional area when used in children.
Skeletal age was found to be a predictive marker for airway changes.
The results may prove valuable, especially in understanding the broader effects of RME in young children.
ACKNOWLEDGMENTS
The authors wish to thank Dr Simon Freezer and Dr Paul Bucholtz for providing the data used in this study and Professor Adrian Esterman, Professor of Biostatistics, for statistical advice.
REFERENCES
- 1.McNamara JA., Jr Influence of respiratory pattern on craniofacial growth. Angle Orthod. 1981;51:269–300. doi: 10.1043/0003-3219(1981)051<0269:IORPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Magliocca KR, Helman JI. Obstructive sleep apnea: diagnosis, medical management and dental implications. J Am Dent Assoc. 2005;136:1121–1129. doi: 10.14219/jada.archive.2005.0316. [DOI] [PubMed] [Google Scholar]
- 3.McNamara JA. Early intervention in the transverse dimension: is it worth the effort? Am J Orthod Dentofacial Orthop. 2002;121:572–574. doi: 10.1067/mod.2002.124167. [DOI] [PubMed] [Google Scholar]
- 4.Işeri H, Tekkaya AE, Öztan Ö, Bilgic S. Biomechanical effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element method. Eur J Orthod. 1998;20:347–356. doi: 10.1093/ejo/20.4.347. [DOI] [PubMed] [Google Scholar]
- 5.Bishara SE, Staley RN. Maxillary expansion: clinical implications. Am J Orthod Dentofacial Orthop. 1987;91:3–14. doi: 10.1016/0889-5406(87)90202-2. [DOI] [PubMed] [Google Scholar]
- 6.Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998;21:831–835. doi: 10.1093/sleep/21.8.831. [DOI] [PubMed] [Google Scholar]
- 7.Doruk C, Sökücü O, Sezer H, Canbay EI. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur J Orthod. 2004;26:397–401. doi: 10.1093/ejo/26.4.397. [DOI] [PubMed] [Google Scholar]
- 8.Compadretti GC, Tasca I, Alessandri-Bonetti G, Peri S, D'Addario A. Acoustic rhinometric measurements in children undergoing rapid maxillary expansion. Int J Pediatr Otorhinolaryngol. 2006;70:27–34. doi: 10.1016/j.ijporl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Doruk C, Sökücü O, Biçakçi AA, Yilmaz U, Taş F. Comparison of nasal volume changes during rapid maxillary expansion using acoustic rhinometry and computed tomography. Eur J Orthod. 2007;29:251–255. doi: 10.1093/ejo/cjl069. [DOI] [PubMed] [Google Scholar]
- 10.Garib DG, Henriques JFC, Janson G, Freitas MR, Coelho RA. Rapid maxillary expansion—tooth tissue-borne versus tooth-borne expanders: a computed tomography evaluation of dentoskeletal effects. Angle Orthod. 2005;75:548–557. doi: 10.1043/0003-3219(2005)75[548:RMETVT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 12.Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim N, Fitzpatrick MF, Whyte KF, Jalleh R, Wightman AJ, Douglas NJ. The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1991;144:845–847. doi: 10.1164/ajrccm/144.4.845. [DOI] [PubMed] [Google Scholar]
- 14.Jan MA, Marshall I, Douglas NJ. Effect of posture on upper airway dimensions in normal human. Am J Respir Crit Care Med. 1994;149:145–148. doi: 10.1164/ajrccm.149.1.8111573. [DOI] [PubMed] [Google Scholar]
- 15.Guijarro-Martinez R, Swennen GRJ. Cone-beam computerized tomography imaging and analysis of the upper airway: a systematic review of the literature. Int J Oral Maxillofac Surg. 2011;40:1227–1237. doi: 10.1016/j.ijom.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Weissheimer A, de Menezes LM, Sameshima GT, Enciso R, Pham J, Grauer D. Imaging software accuracy for 3-dimensional analysis of the upper airway. Am J Orthod Dentofacial Orthop. 2012;142:801–813. doi: 10.1016/j.ajodo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Di Carlo G, Saccucci M, Ierardo G, et al. Rapid maxillary expansion and upper airway morphology: a systematic review on the role of cone beam computed tomography. BioMed Res Int. 2017;2017:5460429. doi: 10.1155/2017/5460429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anandarajah S, Abdalla Y, Dudhia R, Sonnesen L. Proposal of new upper airway margins in children assessed by CBCT. Dento Maxillofacial Radiol. 2015;44:20140438. doi: 10.1259/dmfr.20140438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anandarajah S, Dudhia R, Sandham A, Sonnesen L. Risk factors for small pharyngeal airway dimensions in preorthodontic children: a three-dimensional study. Angle Orthod. 2017;87:138–146. doi: 10.2319/012616-71.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett BJ, Caruso JM, Rungcharassaeng K, Farrage JR, Kim JS, Taylor GD. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2008;134:8–e1. doi: 10.1016/j.ajodo.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Baccetti T, Franchi L, McNamara JA., Jr An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002;72:316–323. doi: 10.1043/0003-3219(2002)072<0316:AIVOTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Björk A. The face in profile: an anthropological X-ray investigation on Swedish children and conscripts. Sven Tandlak Tidskr. 1947;40:6. [Google Scholar]
- 23.Yoon Y-J, Perkiomaki MR, Tallents RH, et al. Transverse craniofacial features and their genetic predisposition in families with nonsyndromic unilateral cleft lip and palate. Cleft Palate Craniofac J. 2004;41:256–261. doi: 10.1597/02-134.1. [DOI] [PubMed] [Google Scholar]
- 24.Adkins MD, Nanda RS, Currier GF. Arch perimeter changes on rapid palatal expansion. Am J Orthod Dentofacial Orthop. 1990;97:194–199. doi: 10.1016/S0889-5406(05)80051-4. [DOI] [PubMed] [Google Scholar]
- 25.Dahlberg G. Statistical Methods for Medical and Biological Students. New York: Interscience Publications; 1940. [Google Scholar]
- 26.Houston WJB. The analysis of errors in orthodontic measurements. Am J Orthod. 1983;83:382–390. doi: 10.1016/0002-9416(83)90322-6. [DOI] [PubMed] [Google Scholar]
- 27.Solow B, Siersbæk-Nielsen S, Greve E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984;86:214–223. doi: 10.1016/0002-9416(84)90373-7. [DOI] [PubMed] [Google Scholar]
- 28.Muto T, Yamazaki A, Takeda S, et al. Relationship between the pharyngeal airway space and craniofacial morphology, taking into account head posture. Int J Oral Maxillofac Surg. 2006;35:132–136. doi: 10.1016/j.ijom.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Nguyen M, Gohl E, Mah JK, Sameshima G, Enciso R. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010;137:S71–S78. doi: 10.1016/j.ajodo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 30.El H, Palomo JM. Three-dimensional evaluation of upper airway following rapid maxillary expansion: a CBCT study. Angle Orthod. 2013;84:265–273. doi: 10.2319/012313-71.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baratieri CdL, Alves M, Jr, Mattos CT, Lau GWT, Nojima LI, de Souza MMG. Transverse effects on the nasomaxillary complex one year after rapid maxillary expansion as the only intervention: a controlled study. Dent Press J Orthod. 2014;19:79–87. doi: 10.1590/2176-9451.19.5.079-087.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Y, Koenig LJ, Pruszynski JE, Bradley TG, Bosio JA, Liu D. Dimensional changes of upper airway after rapid maxillary expansion: a prospective cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013;143:462–470. doi: 10.1016/j.ajodo.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghian S, Ghafari R, Feizbakhsh M, Dadgar S. Dimensional changes of upper airway after rapid maxillary expansion evaluated with cone beam computed tomography. Orthod Waves. 2016;75:10–17. [Google Scholar]
- 34.Schendel SA, Jacobson R, Khalessi S. Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg. 2012;70:2174–2183. doi: 10.1016/j.joms.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Zeng J, Gao X. A prospective CBCT study of upper airway changes after rapid maxillary expansion. Int J Pediatr Otorhinolaryngol. 2013;77:1805–1810. doi: 10.1016/j.ijporl.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Pangrazio-Kulbersh V, Wine P, Haughey M, Pajtas B, Kaczynski R. Cone beam computed tomography evaluation of changes in the naso-maxillary complex associated with two types of maxillary expanders. Angle Orthod. 2011;82:448–457. doi: 10.2319/072211-464.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwasaki T, Takemoto Y, Inada E, et al. Three-dimensional cone-beam computed tomography analysis of enlargement of the pharyngeal airway by the Herbst appliance. Am J Orthod Dentofacial Orthop. 2014;146:776–785. doi: 10.1016/j.ajodo.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Kim S-Y, Park Y-C, Lee K-J, et al. Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod. 2018;88:435–441. doi: 10.2319/092917-656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantarella D, Dominguez-Mompell R, Moschik C, et al. Midfacial changes in the coronal plane induced by microimplant-supported skeletal expander, studied with cone-beam computed tomography images. Am J Orthod Dentofacial Orthop. 2018;154:337–345. doi: 10.1016/j.ajodo.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 40.Sonnesen L, Petersson A, Berg S, Svanholt P. Pharyngeal airway dimensions and head posture in obstructive sleep apnea patients with and without morphological deviations in the upper cervical spine. J Oral Maxillofac Res. 2017;8(3):e4. doi: 10.5037/jomr.2017.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grauer D, Cevidanes LS, Styner MA, Ackerman JL, Proffit WR. Pharyngeal airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop. 2009;136:805–814. doi: 10.1016/j.ajodo.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fishman LS. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod. 1979;49:181–189. doi: 10.1043/0003-3219(1979)049<0181:CVSAAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]