Abstract
BACKGROUND
Stress cardiac magnetic resonance imaging (CMR) has demonstrated excellent diagnostic and prognostic value in single-center studies.
OBJECTIVES
This study sought to investigate the prognostic value of stress CMR and downstream costs from subsequent cardiac testing in a retrospective multicenter study in the United States.
METHODS
In this retrospective study, consecutive patients from 13 centers across 11 states who presented with a chest pain syndrome and were referred for stress CMR were followed for a target period of 4 years. The authors associated CMR findings with a primary outcome of cardiovascular death or nonfatal myocardial infarction using competing risk-adjusted regression models and downstream costs of ischemia testing using published Medicare national payment rates.
RESULTS
In this study, 2,349 patients (63 ± 11 years of age, 47% female) were followed for a median of 5.4 years. Patients with no ischemia or late gadolinium enhancement (LGE) by CMR, observed in 1,583 patients (67%), experienced low annualized rates of primary outcome (<1%) and coronary revascularization (1% to 3%), across all years of study follow-up. In contrast, patients with ischemia+/LGE+ experienced a >4-fold higher annual primary outcome rate and a >10-fold higher rate of coronary revascularization during the first year after CMR. Patients with ischemia and LGE both negative had low average annual cost spent on ischemia testing across all years of follow-up, and this pattern was similar across the 4 practice environments of the participating centers.
CONCLUSIONS
In a multicenter U.S. cohort with stable chest pain syndromes, stress CMR performed at experienced centers offers effective cardiac prognostication. Patients without CMR ischemia or LGE experienced a low incidence of cardiac events, little need for coronary revascularization, and low spending on subsequent ischemia testing. (Stress CMR Perfusion Imaging in the United States [SPINS]: A Society for Cardiovascular Resonance Registry Study; NCT03192891)
Keywords: cost of care, prognosis, stress cardiac magnetic resonance imaging
Randomized multicenter studies (1–3) have demonstrated the high accuracy of vasodilator stress cardiac magnetic resonance imaging (CMR) in detecting coronary stenoses and in estimating impaired flow reserve (4) in coronary artery disease (CAD). Stress CMR has also been shown in many studies to be an effective cardiac prognosticating method for patients presenting with chest pain syndromes (5–8). The American College of Cardiology Foundation and American Heart Association have recommended stress CMR as an appropriate test for evaluation of symptomatic patients with intermediate to high pre-test probability for CAD (9). However, stress CMR remains an underutilized method in the United States. Here we present the results of the SPINS (Stress CMR Perfusion Imaging in the United States: A Society for Cardiovascular Resonance Registry Study) SCMR (Study of the Society for Cardiovascular Magnetic Resonance) registry. SPINS is a multicenter observational study of patients with stable chest pain syndromes designed to evaluate the long-term performance of stress CMR for cardiovascular prognosis and to investigate the cost of additional downstream cardiac testing following the index stress CMR.
METHODS
REGISTRY DESIGN AND PATIENT POPULATION.
Goals and infrastructures of the SCMR registry have been described previously (10). SPINS is a study of the SCMR registry and is retrospective in design. SPINS aimed to test the primary hypothesis that evidence of ischemia or infarction characterized by CMR provides effective cardiovascular risk stratification in patients with chest pain syndromes who are at intermediate to high pre-test likelihood of significant coronary disease. The study aimed to enroll consecutive patients who underwent a clinical vasodilator stress CMR in the United States. Inclusion criteria were: 1) age between 35 and 85 years at the time of CMR; 2) referral for evaluation of chest pain, dyspnea, abnormal electrocardiogram, or other clinical presentation that raised a suspicion of myocardial ischemia as determined by the treating clinician; and 3) presence of at least 2 of the following coronary risk factors: age >50 years for male or >60 years for female subjects; diabetes mellitus requiring medical treatment; chronic hypertension requiring treatment; hypercholesterolemia on medical treatment; family history of premature CAD defined as diagnosis in a first-degree male relative ≤55 years old or female relative ≤65 years old; body mass index ≥30 kg/m2; medically documented peripheral vascular disease; and history of percutaneous coronary intervention (PCI) or myocardial infarction (MI). Exclusion criteria were history of coronary artery bypass graft (CABG), recent MI within 30 days preceding the index CMR study, severe-grade valvular heart disease, nonischemic cardiomyopathy with a left ventricular ejection fraction <40%, infiltrative or hypertrophic cardiomyopathy, constrictive pericarditis, active pregnancy, competing medical illnesses with expected survival <2 years, and known inability to follow-up. Vasodilator stress included the use of intravenous infusion of adenosine, intravenous bolus of regadenoson, or dipyridamole.
SELECTION OF ENROLLING CENTERS AND CMR METHODS.
An enrolling center was required to: 1) have an active clinical vasodilator stress CMR perfusion imaging program ongoing for at least 10 years; 2) contribute between 100 and 500 consecutive patients who underwent a vasodilator stress CMR perfusion study between January 1, 2008, and December 31, 2013, so that at least 4 years of clinical follow-up could be achieved at study conclusion; and 3) have access to electronic medical records. Each center was also required to have all CMR scans interpreted by a Core Cardiology Training Symposium level II or III reader, with at least 1 Core Cardiology Training Symposium level III supervising reader. Enrolling centers must have performed CMR studies using either a 1.5-T or a 3-T scanner and pulse sequences for stress perfusion, cine, and late gadolinium enhancement (LGE) imaging of infarction. Enrolling centers were also required to have reported the myocardial extent of abnormal stress perfusion and LGE according to the 16-segment or 17-segment American Heart Association nomenclature. At each participating site, local institutional review board approval was obtained to conduct this clinical follow-up study with a waiver of written informed consent.
SAMPLE SIZE CALCULATION.
Sample size of the study was calculated to accrue at least 150 cases of all-cause death or acute MI for the purpose of determining the prognostic value of ischemia by stress CMR with adjustment for up to 10 known clinical risk variables. Based on a prior publication (8), prevalence of ischemia on stress CMR for the study cohort was estimated to be 23%. Incidence rates of all-cause death or acute MI were estimated to be 0.5% and 6.0% per year in patients with absence and presence of ischemia, respectively. Over a 4-year follow-up, at least 124 of 506 patients with ischemia and 36 of 1,694 patients without ischemia, were expected to have died or experienced an acute MI, thus an estimated target sample size of approximately 2,200 patients was needed to yield a power of 80% with an alpha error of 5% to detect a difference of >20% for the primary outcome between the patient groups with a normal versus abnormal stress CMR.
DATA COLLECTION.
Enrolling centers entered all study-related protected health information–free data into the CMR Cooperative encrypted web-based database (CMRcoop) for GCMR (Global CMR Registry). Clinical variables collected included patient demographics, clinical history (prior heart disease and coronary risk factors), and study indication. CMR variables included left ventricular volumes and dimensions, and stress perfusion and LGE (both reported as presence or absence on a segmental basis) using the American Heart Association segmental models. A stress perfusion defect was considered present if it was densest in the endocardium with a transmural gradient across the wall thickness, persisted beyond peak myocardial enhancement and for several R-R intervals, and conformed to a coronary arterial distribution. Inducible ischemia (ischemia+) was defined as the presence of a stress perfusion defect, in absence of matching LGE, in ≥1 segment (11). MI was defined as the presence of LGE (LGE+) conforming to infarction in ≥1 segment. Mild, moderate, and severe defects were defined as the involvement of 1 to 2, 3 to 5, and ≥6 myocardial segments, respectively. To determine the diagnostic value of stress CMR, we collected the CMR interpretations as reported by center readers at the time of study performance. For quality assurance, each center randomly selected 10% of its CMR studies and submitted the images for blinded interpretation by the CMR core lab at the Brigham and Women’s Hospital to evaluate core lab versus center agreement. Information regarding performance of all subsequent noninvasive tests for CAD during the follow-up period, including exercise stress testing, stress echocardiography, nuclear perfusion imaging, coronary computed tomographic angiography, repeat stress CMR, and invasive x-ray coronary angiography (XCA) was collected.
STUDY ENDPOINTS.
All centers were instructed to obtain clinical follow-up data on all enrolled patients for at least 4 years after the index stress CMR. Clinical follow-up used both electronic medical records and direct patient contact with either a standardized checklist questionnaire or scripted telephone interview. Study investigators were trained during the initiation period, by group webinars and study documents, on specific definitions of all key variables required on the web-based database. All outcome variables and their standardized published clinical trial definitions were posted on the web database (12). Follow-up data was verified by each site’s principal investigator. In the final 6 months of the study period, a data quality report was generated by the data-coordinating center in Boston and sent weekly to each site. Primary outcome was defined as cardiovascular death or nonfatal MI. Secondary outcome was defined by a composite of cardiovascular death, nonfatal MI, hospitalization for unstable angina or congestive heart failure, and late unplanned CABG. Deaths were categorized as cardiovascular, cancer, or cause unknown. Cardiovascular deaths were deaths preceded by an acute MI, malignant ventricular arrhythmia, or decompensated heart failure. Acute MI diagnosis required chest pain or anginal equivalent and abnormal troponins with temporal changes consistent with myocardial injury. The cutoff levels of troponins were according to the specifications at the individual centers. Hospitalization for unstable angina was defined as an unscheduled hospitalization due to worsening chest pain or anginal equivalent, combined with evidence of ischemia by imaging or significant coronary stenosis by computed tomography or XCA. Heart failure hospitalization was defined as an unscheduled hospitalization due to worsening or new symptoms and/or signs of heart failure, >24 h of in-hospital stay, and intensification of heart failure treatment. Late unplanned CABG was defined as CABG performed >6 months after the index stress CMR. CABG was included as an event because, in general, it signifies the discovery of a life-threatening high-risk CAD state (e.g., left-main or multivessel disease) where CABG is used as a life-saving procedure; this is in contrast to elective PCI procedures, which are often performed to treat non–life-threatening CAD (e.g., relief of angina). For either primary or secondary outcome, only the first event was counted when multiple events occurred in a subject. A successful follow-up was defined as achieving an assessment of all outcome events for ≥4 years after the index CMR. End of follow-up data collection and locking of database occurred on May 25, 2018.
COSTS OF CARDIAC ISCHEMIA TESTING AFTER INDEX STRESS CMR.
All enrolling centers collected performance of all ischemia testing including stress single-photon emission computed tomography, coronary computed tomographic angiography, stress echocardiography, exercise treadmill test, repeat stress CMR, and XCA during the study follow-up period. As shown in Online Table 1, the corresponding costs of these procedures were determined based on published average national payment rates from the Medicare Hospital Outpatient Prospective Payment System, specific to the technical component of the most common Healthcare Common Procedure Coding System code and the year of the procedure. For payment rates of any procedures that were not published in 2008 to 2010, the corresponding 2011 payment rates were used. Procedure-specific costs and total cardiac testing cost were calculated by adding up the estimated Medicare payments for each procedure and from all procedures, respectively, then expressing them as costs per patient-years. Costs due to complications of test procedures, including cancers related to medical radiation, were not collected. Patients with <90 days of follow-up were excluded from this analysis.
STATISTICAL ANALYSIS.
Continuous variables were compared by Student’s t-test or analysis of variance and categorical variables by chi-square test as appropriate. Annualized event rates were calculated by dividing the number of patients who experienced the event by patient-years of follow-up. We used a Fine and Gray competing risk model to characterize all cumulative incidence functions for the primary and secondary outcomes, accounting for the effects of competing risks from noncardiovascular deaths (13). We first constructed multivariable clinical models, for primary and secondary outcomes, respectively, by including all clinical covariates with p < 0.1 on univariable screening and <10% imputed or missing data. Presence (+) or absence (−) of ischemia and LGE were then added separately to each clinical model to determine whether they each provided incremental and independent prognostic value adjusted to the covariates in the models. Cumulative incidence curves were generated by plotting cumulative incidence of primary or secondary outcome by time of follow-up. Proportional hazards assumption was then evaluated using visual inspection of the log-log survival curves and the Schoenfeld residuals test. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and p < 0.05 was used to establish statistical significance.
RESULTS
BASELINE PATIENT DEMOGRAPHICS AND CMR CHARACTERISTICS.
A total of 2,370 patients from 13 participating centers and 11 states met inclusion and exclusion criteria. Of these, 21 patients (0.9%) had incomplete studies (missing or nondiagnostic perfusion or LGE images), and the remaining 2,349 patients formed the cohort. Patient demographics are summarized in Table 1. Practice environments of the participating centers included university hospitals (n = 7), cardiovascular group practices (n = 2), multispecialty practices (n = 2), and U.S. government or military hospitals (n = 2). Primary indication for stress CMR included chest pain (55%), dyspnea (22%), changes on resting electrocardiogram suspicious of ischemia (7%), syncope or cardiac dysrhythmias (9%), and others (7%). Symptoms of patients with changes on resting electrocardiogram as the primary indication were not known. The mean age in the cohort was 63 ± 11 years with 47% of the cohort was female. There was a high prevalence of hypertension (78%) and dyslipidemia (70%). Median number of cardiac risk factors was 3 (interquartile range: 2 to 4). A history of MI and PCI were present in 15% and 23% of the cohort, respectively. Median basic CAD Consortium Score (14) was 34% (mean 38 ± 20%), which is indicative of an average intermediate pre-test likelihood of CAD. A 3-T scanner was used in 35% and magnetic resonance imaging vendors included all 3 top manufacturers (Siemens Healthineers, Erlangen, Germany: 69%; General Electric Healthcare, Princeton, New Jersey: 22%; and Philips Medical Systems, Amsterdam, the Netherlands: 9%). Gadolinium-based contrast agents included gadopentetate–diethylenetriamine pentaacetic acid (Magnevist, Bayer AG, Leverkusen, Germany) in 1,457 (62%), gadodiamide (Omniscan, GE Healthcare) in 400 (17%), gadobenate (Multihance, Bracco Diagnostics, Milan, Italy) in 246 (10.5%), gadoversetamide (Optimark, Guerbet, Villepinte, France) in 150 (6.4%), gadoteridol (Prohance, Bracco Diagnostics) in 91 (3.9%), and gadobutrol (Gadavist, Bayer AG) in 5 patients (0.2%). Average left ventricular size and function were within normal limits. Ischemia and LGE were present in 17% and 24% of patients, respectively, and 14% (of 24%) of the patients with LGE had no clinical history of MI. Overall, 766 patients (33%) had an abnormal stress CMR, defined as the presence of either ischemia or LGE. Within the 766 patients, 194 (8%) had ischemia but no LGE, 361 (15%) had LGE but no ischemia, and 211 (9%) had both ischemia and LGE. In this cohort, 40 patients (1.7%) were diagnosed to have non-CAD cardiac conditions on CMR. These included 3 new cases of cardiac amyloidosis, 5 cases of hypertrophic cardiomyopathy, 5 cases of myocarditis, 5 cases of nonischemic dilated cardiomyopathy, 8 cases of pericardial disease, 1 case of cardiac sarcoidosis, and 13 cases of nonspecific myocardial fibrosis. Apart from these conditions, 339 (14%) were found to have LGE consistent with unrecognized MI.
TABLE 1.
Demographics and Baseline Characteristics
| Overall (N = 2,349) | No Ischemia or LGE (n = 1,583) | Ischemia or LGE (n = 766) | p Value | |
|---|---|---|---|---|
| Clinical data | ||||
| Follow-up, yrs | 5.4 (4.6–6.8) | 5.5 (4.6–6.9) | 5.3 (4.3–6.5) | <0.001 |
| Age, yrs | 63 ± 11 | 62 ± 11 | 63 ± 11 | 0.61 |
| Female | 1,104 (47.0) | 821 (52.0) | 283 (37.0) | <0.001 |
| BMI, kg/m2 | 31 ± 7 | 31 ± 7 | 30 ± 7 | <0.001 |
| No. of cardiac risk factors | 3 (2–4) | 3 (2–4) | 4 (3–5) | <0.001 |
| Risk factors* | ||||
| Hypertension | 1,843 (78.0) | 1,196 (76.0) | 647 (85.0) | <0.001 |
| Hypercholesterolemia | 1,647 (70.0) | 1,053 (67.0) | 594 (78.0) | <0.001 |
| Diabetes mellitus | 664 (28.0) | 409 (26.0) | 255 (33.0) | <0.001 |
| Significant smoking, >10 pack-yrs | 757 (32.0) | 457 (29.0) | 300 (40.0) | <0.001 |
| History of premature CAD in first-degree relative | 761 (34.0) | 495 (32.0) | 266 (37.0) | 0.03 |
| CAD Consortium score, basic† | 34 (24–54) | 34 (17–47) | 44 (28–54) | <0.001 |
| History of PCI‡ | 538 (23.0) | 244 (15.0) | 294 (38.0) | <0.001 |
| History of MI‡ | 358 (15.0) | 110 (7.0) | 248 (33.0) | <0.001 |
| History of heart failure‡ | 245 (10.0) | 113 (7.0) | 132 (17.0) | <0.001 |
| Presenting reasons | ||||
| Chest pain | 1,303 (55.0) | 885 (56.0) | 418 (55.0) | 0.60 |
| Dyspnea or fatigue on exertion | 509 (22.0) | 338 (21.0) | 171 (22.0) | 0.59 |
| Abnormal ECG | 159 (6.8) | 99 (6.3) | 60 (7.8) | 0.16 |
| Arrhythmias | 154 (6.6) | 122 (7.7) | 32 (4.2) | 0.001 |
| Syncope | 50 (2.0) | 30 (1.9) | 20 (2.6) | 0.29 |
| Peripheral edema | 6 (0.3) | 4 (0.3) | 2 (0.3) | 1.00 |
| Abnormal stress nuclear imaging | 16 (0.7) | 12 (0.8) | 4 (0.5) | 0.60 |
| Abnormal stress echocardiography | 29 (1.2) | 19 (1.2) | 10 (1.3) | 0.84 |
| Abnormal exercise stress test | 10 (0.4) | 8 (0.5) | 2 (0.3) | 0.51 |
| Other symptoms and/or reasons | 113 (4.8) | 67 (4.2) | 46 (6.0) | 0.06 |
| Stress CMR | ||||
| Scanner field strength | ||||
| 1.5-T | 1,535 (65.0) | 1,054 (67.0) | 481 (63.0) | 0.07 |
| 3.0-T | 814 (35.0) | 529 (33.0) | 285 (37.0) | |
| CMR manufacturers§ | ||||
| Siemens | 1,615 (69.0) | 996 (63.0) | 619 (81.0) | <0.001 |
| General Electric | 512 (22.0) | 427 (27.0) | 85 (11.0) | |
| Phillips | 221 (9.0) | 159 (10.0) | 62 (8.0) | |
| LVEF, % | 63 (54–70) | 65 (58–72) | 57 (46–66) | <0.001 |
| LVEDVI, ml/m2 | 64 (50–79) | 60 (48–73) | 71 (57–89) | <0.001 |
| LVESVI, ml/m2 | 22 (16–32) | 20 (15–28) | 28 (20–44) | <0.001 |
| Inducible ischemia | 405 (17.0) | 0 (0.0) | 405 (53.0) | <0.001 |
| MI | 571 (24.0) | 0 (0.0) | 572 (75.0) | <0.001 |
Values are median (IQR), mean ± SD, or n (%).
Full risk factor profile missing for 123 patients.
CAD Consortium score missing for 332 patients.
History of PCI, MI, and CHF missing for 5, 17, and 4 patients, respectively.
Manufacturer information missing for 1 patient.
BMI = body mass index; CAD = coronary artery disease; CMR = cardiac magnetic resonance imaging; ECG = electrocardiogram; IQR = interquartile range; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end-diastolic volume index; LVESVI = left ventricular end-systolic volume index; MI = myocardial infarction; PCI = percutaneous coronary intervention.
CMR PROGNOSIS FOR PRIMARY AND SECONDARY OUTCOME.
Successful follow-up of ≥4 years was achieved in 2,294 patients (97.7%) with a median follow-up of 5.5 years (interquartile range: 4.6 to 6.8 years). During study follow-up, 255 patients (11%) died with 74 (3.2%) due to cardiovascular causes and 181 (7.7%) noncardiovascular causes. Primary outcome occurred in 153 patients including, as a first event, 87 nonfatal MI and 66 cardiovascular deaths. Sixty-two of the nonfatal MI and 45 of the cardiovascular deaths occurred within the first 4 years of follow-up. Secondary outcome occurred in 374 patients including, as a first event, 77 nonfatal MI, 124 hospitalizations for unstable angina, 86 hospitalizations for heart failure, 44 cases of late unplanned CABG, and 43 cardiovascular deaths. Primary outcome rates, expressed as percentage per patient-year, stratified by CMR findings of ischemia and LGE, are shown in Figure 1. Among the 1,583 patients (67%) who had no ischemia and no LGE (ischemia−/LGE−), primary and secondary outcome occurred at low rates of 0.6% and 1.7% per patient-year, respectively. In contrast, those with ischemia+ and LGE+ experienced rates of 4.5% and 10.1% per patient-year, respectively. Patients with no, mild, moderate, and severe ischemia extent experienced primary outcome rates at 0.9%, 2.9%, 2.9%, and 3.1%, respectively; and secondary outcome rates at 2.3%, 6.8%, 9.7%, and 6.9%, respectively. During the first 4 years of follow-up, presence of ischemia was associated with an odds ratio of 4.2 for acute MI (p < 0.0001) and 2.4 for cardiovascular death (p = 0.004). Figure 2 demonstrates the primary outcome rates stratified by the time period of follow-up, year 1 through year 5. Patients with ischemia−/LGE− experienced low primary outcome rates from year 1 to year 4 (0.3% to 0.7%/year) and at 1.4% in year 5. In contrast, those with ischemia+/LGE+ experienced the highest primary outcome rates across all 5 years (ranging from 2.4% to 8.5%). Online Figure 1 demonstrates the secondary outcome rates by status of ischemia and LGE in a similar format. Patients with ischemia−/LGE− experienced the lowest secondary events rates. Figure 3 demonstrates the need for coronary revascularization in the whole cohort, stratified by the time periods of follow-up. In patients with ischemia−/LGE−, coronary revascularization was needed in 3% in year 1 and in 1% for each of the subsequent years. Most coronary revascularization in patients with ischemia+/LGE+ occurred in the first 90 days.
FIGURE 1. Primary and Secondary Outcome Event Rates.
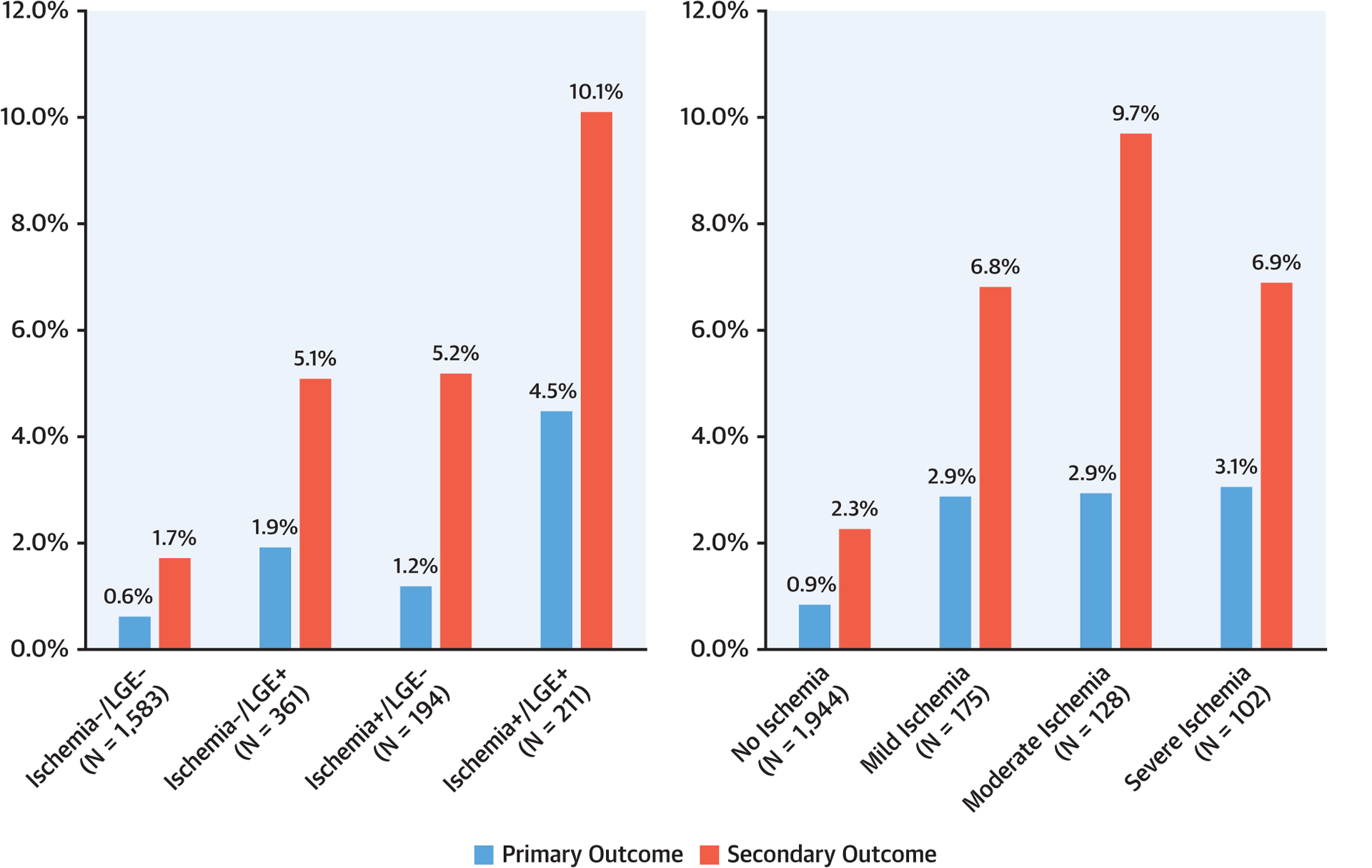
Annualized rates of primary and secondary outcomes, stratified by presence and/or absence of ischemia and left gadolinium enhancement (LGE) (left) and extent of ischemia (right).
FIGURE 2. Primary Outcome Over Years of Follow-Up.
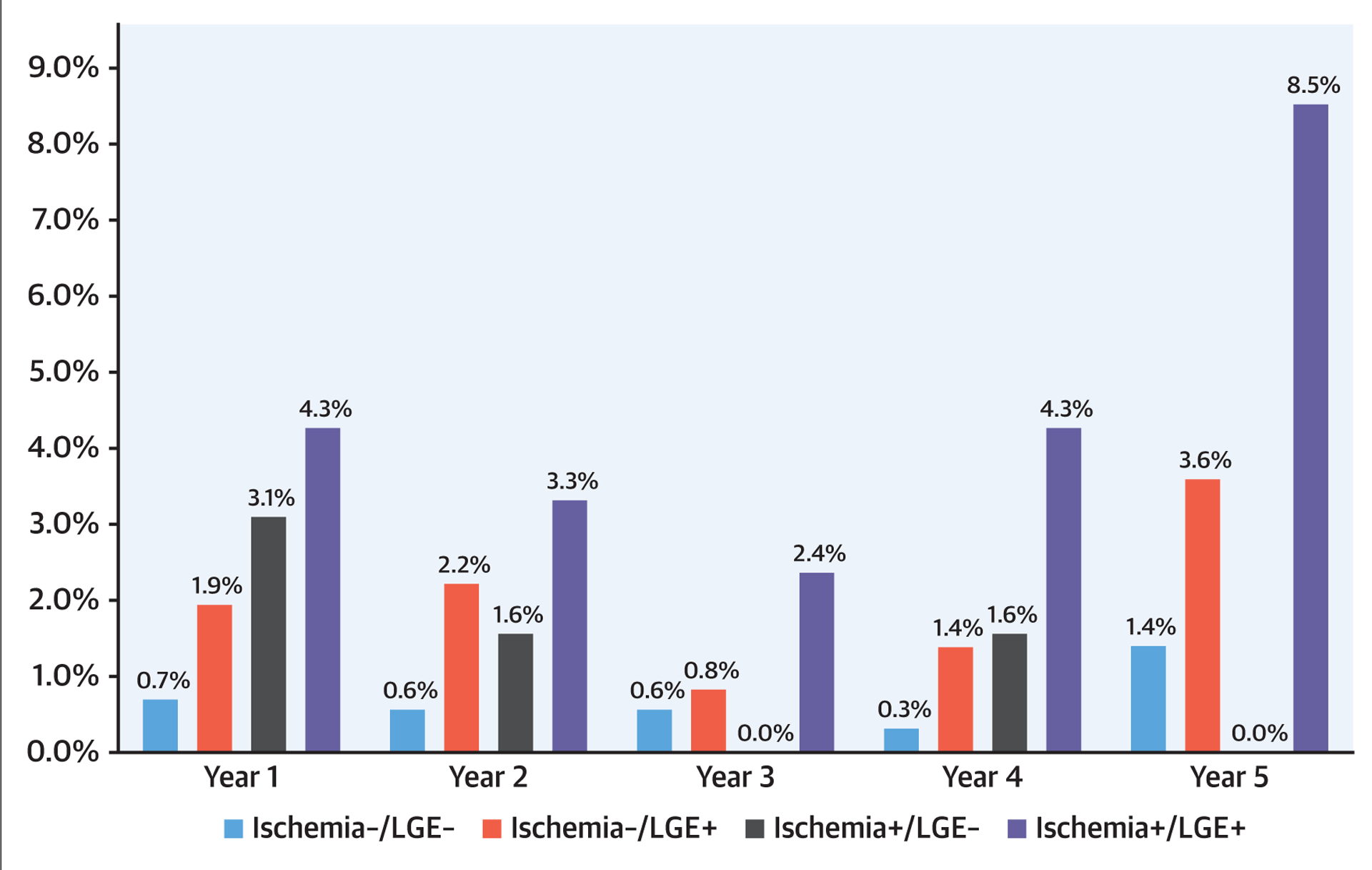
Occurrence of primary outcome across different years of study follow-up, stratified by presence and/or absence of ischemia and left gadolinium enhancement (LGE).
FIGURE 3. Need for Coronary Revascularization.
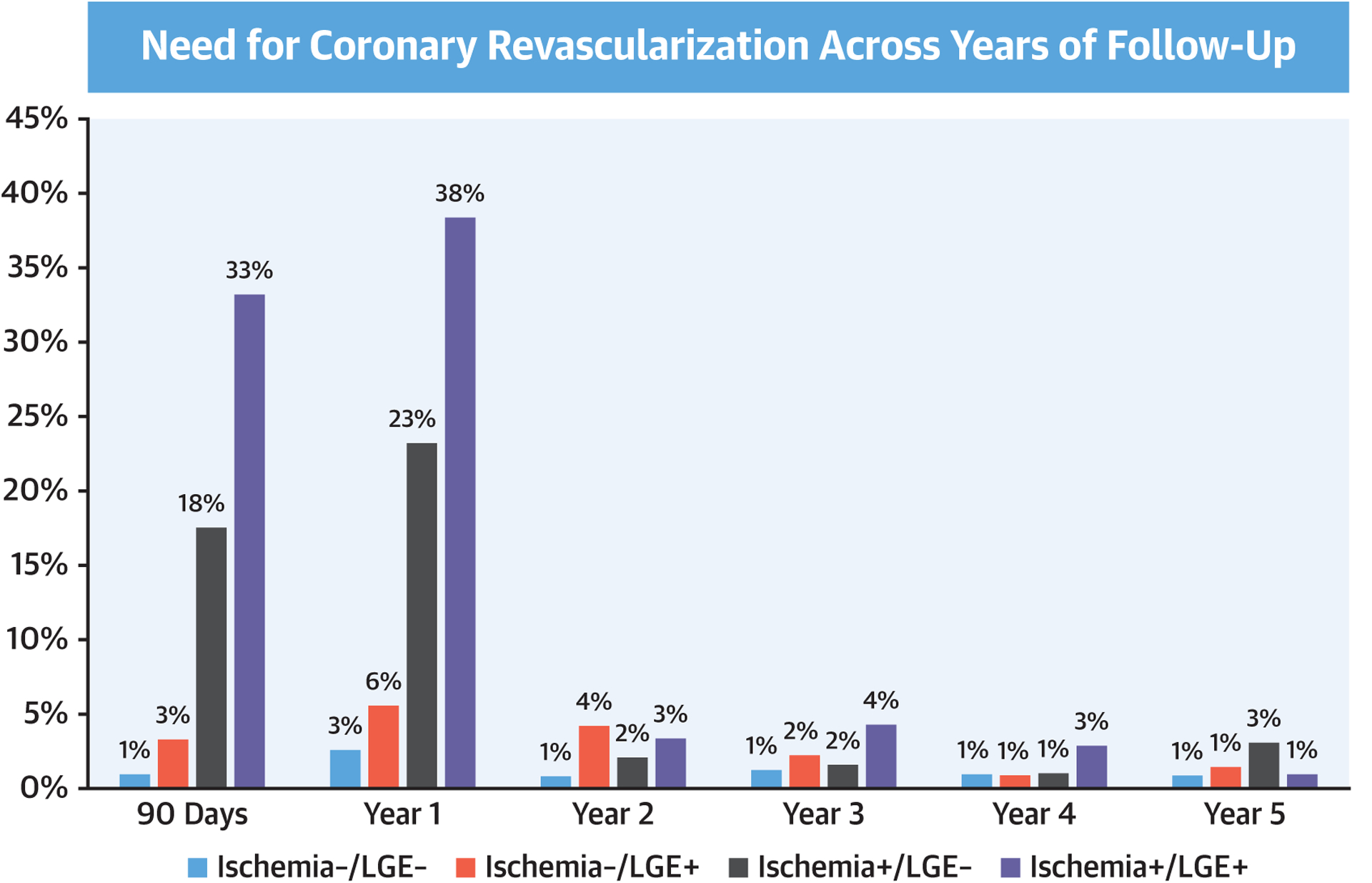
Occurrence of coronary revascularization across different years of study follow-up, stratified by presence and/or absence of ischemia and left gadolinium enhancement (LGE).
The Central Illustration demonstrates event-free survival with 95% confidence intervals, based on cumulative incidence function for primary and secondary outcome and stratified by ischemia and LGE. Stratified by ischemia and LGE, patients in the ischemia+/LGE+ group had the highest cumulative incidence of primary and secondary outcomes over time, whereas in contrast patients in the ischemia−/LGE− group had the lowest incidence. Patients with ischemia+/LGE− had incidence of primary and secondary outcomes over time that were not statistically different from patients with ischemia−/LGE+. There was progressively higher incidence over time with greater extent of ischemia, for either primary outcome or secondary outcome, although there was statistical overlap between the moderate and severe categories toward primary outcome. Univariable analyses associating patient and CMR characteristics with primary and secondary outcome are presented in Table 2. For primary outcome, age, smoking, history of hypertension, diabetes, history of MI, history of PCI, history of congestive heart failure, and left ventricular end-systolic volume index were the strongest set of clinical covariates selected from univariable screening in forming the clinical model for the primary outcome (−2 log L: 1,950). Table 3 demonstrates the multivariable clinical models of primary and secondary outcome. Presence of ischemia and presence of LGE independently improved this clinical model for primary outcome when they were separately added (−2 log L: 1,933 and 1,939, for ischemia and LGE, respectively, both p < 0.0001) or when both added (−2 log L: 1,927) to the model. Adjusted to the effects of the covariates in the clinical model and to each other, presence of ischemia and presence of LGE both maintained significant association with primary outcome (adjusted hazard ratio: 1.96; 95% confidence interval: 1.35 to 2.86; p = 0.0004; and adjusted hazard ratio: 1.64; 95% confidence interval: 1.08 to 2.51; p = 0.02, respectively). Similar significant association was observed for presence of ischemia and LGE with secondary outcome. Both ischemia presence and extent also demonstrated strong association with all-cause death or nonfatal MI (hazard ratios: 1.84 and 1.28, respectively, both p < 0.0001). Visual inspection of the log-log survival curves and calculation of the Schoenfeld residuals showed that the proportionality assumption was not violated.
CENTRAL ILLUSTRATION. Stress Cardiac Magnetic Resonance Imaging Registry for Prognosis and Costs in the United States.
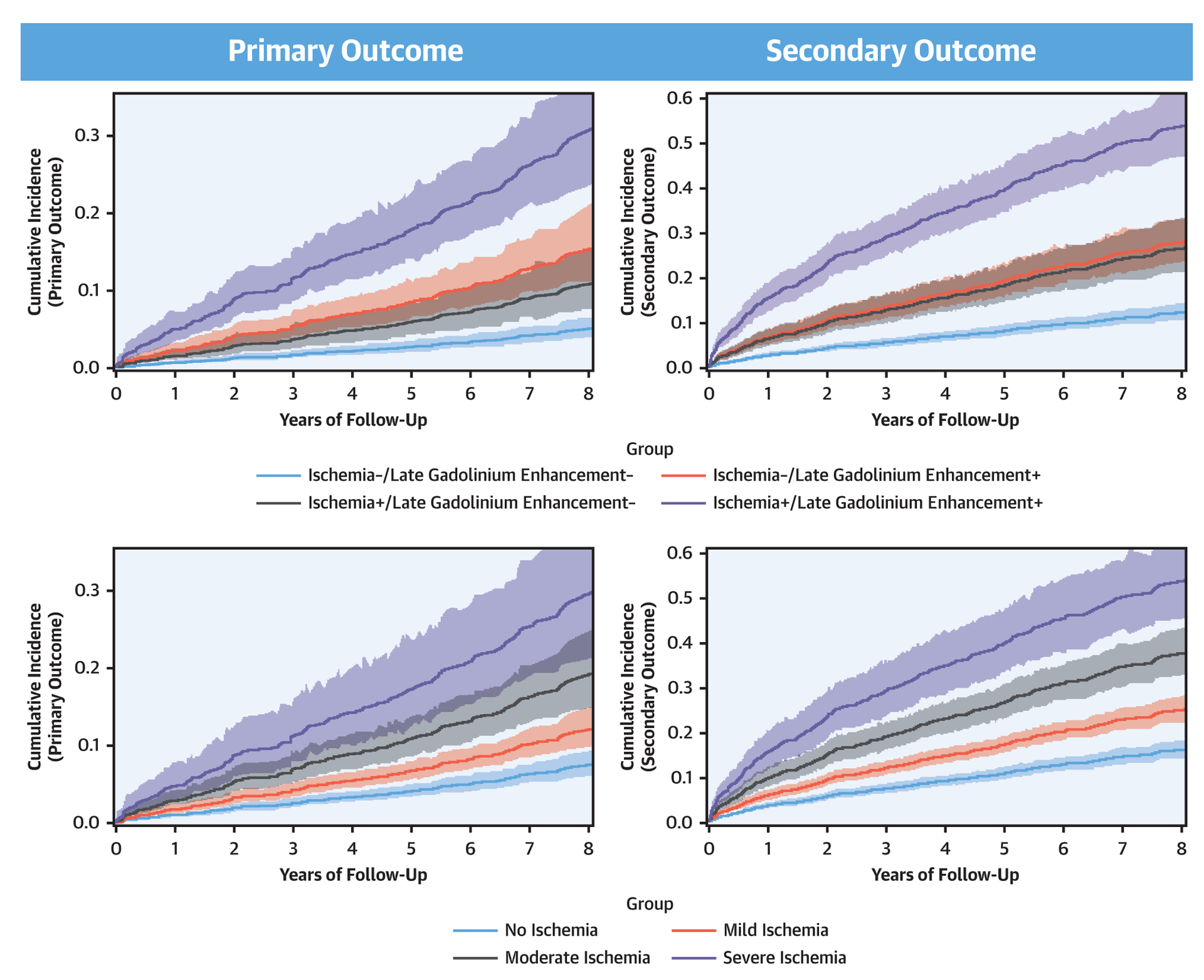
Cumulative incidence functions for primary and secondary outcomes derived from a Fine and Gray competing risk model accounting for noncardiovascular death as a competing risk event. The top panels were stratified by presence and/or absence of ischemia and late gadolinium enhancement, and the bottom panels were stratified by the extent of ischemia.
TABLE 2.
Univariable Association of Clinical and Stress CMR Indices With Outcome Using a Fine and Gray Competing Risk Model
| Primary Outcome | Secondary Outcome | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Demographics | ||||
| Age, per yr | 1.01 (1.00–1.03) | 0.06 | 1.01 (1.00–1.02) | 0.10 |
| Female | 0.58 (0.42–0.81) | 0.002 | 0.79 (0.64–0.97) | 0.02 |
| BMI, per kg/m2 | 1.00 (0.98–1.02) | 0.89 | 1.00 (0.99–1.02) | 0.76 |
| Cardiac risk factors | ||||
| Hypertension | 1.53 (0.98–2.39) | 0.06 | 2.05 (1.50–2.79) | <0.0001 |
| Hypercholesterolemia | 1.02 (0.72–1.45) | 0.90 | 1.23 (0.97–1.54) | 0.09 |
| Diabetes mellitus | 1.67 (1.21–2.31) | 0.002 | 1.51 (1.23–1.87) | <0.001 |
| Smoking | 1.82 (1.32–2.51) | <0.001 | 1.56 (1.27–1.93) | <0.0001 |
| Family history of CAD | 0.75 (0.52–1.08) | 0.12 | 1.00 (0.80–1.25) | 0.98 |
| Number of cardiac risk factors | 1.36 (1.19–1.56) | <0.0001 | 1.40 (1.29–1.53) | <0.0001 |
| CAD Consortium score, basic | 1.02 (1.01–1.03) | <0.0001 | 1.01 (1.01–1.02) | <0.0001 |
| History of PCI | 2.73 (1.98–3.76) | <0.0001 | 2.48 (2.02–3.06) | <0.0001 |
| History of MI | 4.26 (3.08–5.88) | <0.0001 | 2.79 (2.23–3.48) | <0.0001 |
| History of CHF | 3.71 (2.60–5.30) | <0.0001 | 2.72 (2.12–3.48) | <0.0001 |
| Stress CMR | ||||
| LVEF, per % Δ | 0.97 (0.96–0.98) | <0.0001 | 0.97 (0.96–0.98) | <0.0001 |
| LVEDVI, per ml/m2 Δ | 1.01 (1.01–1.02) | <0.0001 | 1.01 (1.01–1.02) | <0.0001 |
| LVESVI, per ml/m2 Δ | 1.02 (1.01–1.02) | <0.0001 | 1.01 (1.01–1.02) | <0.0001 |
| Ischemia+ | 3.41 (2.46–4.73) | <0.0001 | 3.30 (2.67–4.08) | <0.0001 |
| Extent of ischemia, per segment | 1.10 (1.07–1.14) | <0.0001 | 1.11 (1.08–1.14) | <0.0001 |
| LGE+ | 4.10 (2.97–5.65) | <0.0001 | 3.24 (2.64–3.97) | <0.0001 |
| Extent of LGE, per segment | 1.11 (1.08–1.14) | <0.0001 | 1.09 (1.07–1.11) | <0.0001 |
| Abnormal CMR, ischemia or MI | 3.85 (2.77–5.36) | <0.0001 | 3.59 (2.91–4.42) | <0.0001 |
Primary outcome was defined as cardiovascular death or nonfatal MI. Secondary outcome was defined by a composite of cardiovascular death, nonfatal MI, hospitalization for unstable angina or CHF, and late unplanned coronary arterial bypass surgery.
present;
difference;
CHF = congestive heart failure; CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
TABLE 3.
Multivariable Models for Primary and Secondary Outcomes Using a Fine and Gray Competing Risk Model
| Primary Outcome | Secondary Outcome | |||||
|---|---|---|---|---|---|---|
| Parameter Estimate | p Value | HR (95% CI) | Parameter Estimate | p Value | HR (95% CI) | |
| Age, per yr | 0.02 | 0.08 | 1.02 (1.00–1.03) | 0.01 | 0.12 | 1.01 (1.00–1.02) |
| History of | ||||||
| PCI | 0.37 | 0.09 | 1.45 (0.95–2.22) | 0.49 | 0.0004 | 1.63 (1.25–2.14) |
| MI | 0.72 | 0.003 | 2.05 (1.28–3.28) | 0.29 | 0.07 | 1.34 (0.98–1.82) |
| CHF | 0.65 | 0.01 | 1.92 (1.17–3.15) | 0.50 | 0.004 | 1.65 (1.17–2.32) |
| Diabetes | 0.30 | 0.10 | 1.35 (0.94–1.94) | 0.22 | 0.07 | 1.24 (0.99–1.57) |
| Hypertension | 0.17 | 0.46 | 1.19 (0.75–1.89) | 0.55 | 0.001 | 1.73 (1.24–2.42) |
| Tobacco use | 0.48 | 0.006 | 1.62 (1.15–2.28) | 0.31 | 0.006 | 1.37 (1.09–1.71) |
| LVESVI, ml/m2 | 0.01 | 0.09 | 1.01 (1.00–1.01) | 0.01 | 0.02 | 1.01 (1.00–1.01) |
| LGE+ | 0.50 | 0.02 | 1.64 (1.08–2.51) | 0.48 | 0.0005 | 1.62 (1.23–2.12) |
| Ischemia+ | 0.67 | 0.0004 | 1.96 (1.35–2.86) | 0.73 | <0.0001 | 2.08 (1.62–2.67) |
UTILIZATION OF INVASIVE TESTING AND CORONARY REVASCULARIZATION AFTER CMR.
Referrals to invasive XCA and performance of coronary revascularization at 90 days after CMR per discretion of the primary caring team, stratified by ischemia and LGE and ischemia extent, are shown in Figure 4. Only 4% of patients with ischemia−/LGE− were referred to undergo CA, which compared with 46% among patients with ischemia+/LGE+, with the presence of ischemia being a key factor for referral to CA. Probabilities of coronary revascularization procedure (either PCI or CABG), once referred to CA, ranged from 24% in the ischemia−/LGE− group to 73% in the ischemia+/LGE+ group. Increasing extent of ischemia was associated with stepwise higher likelihood of referral to CA and coronary revascularization.
FIGURE 4. Invasive XCA and Revascularization at 90 Days.
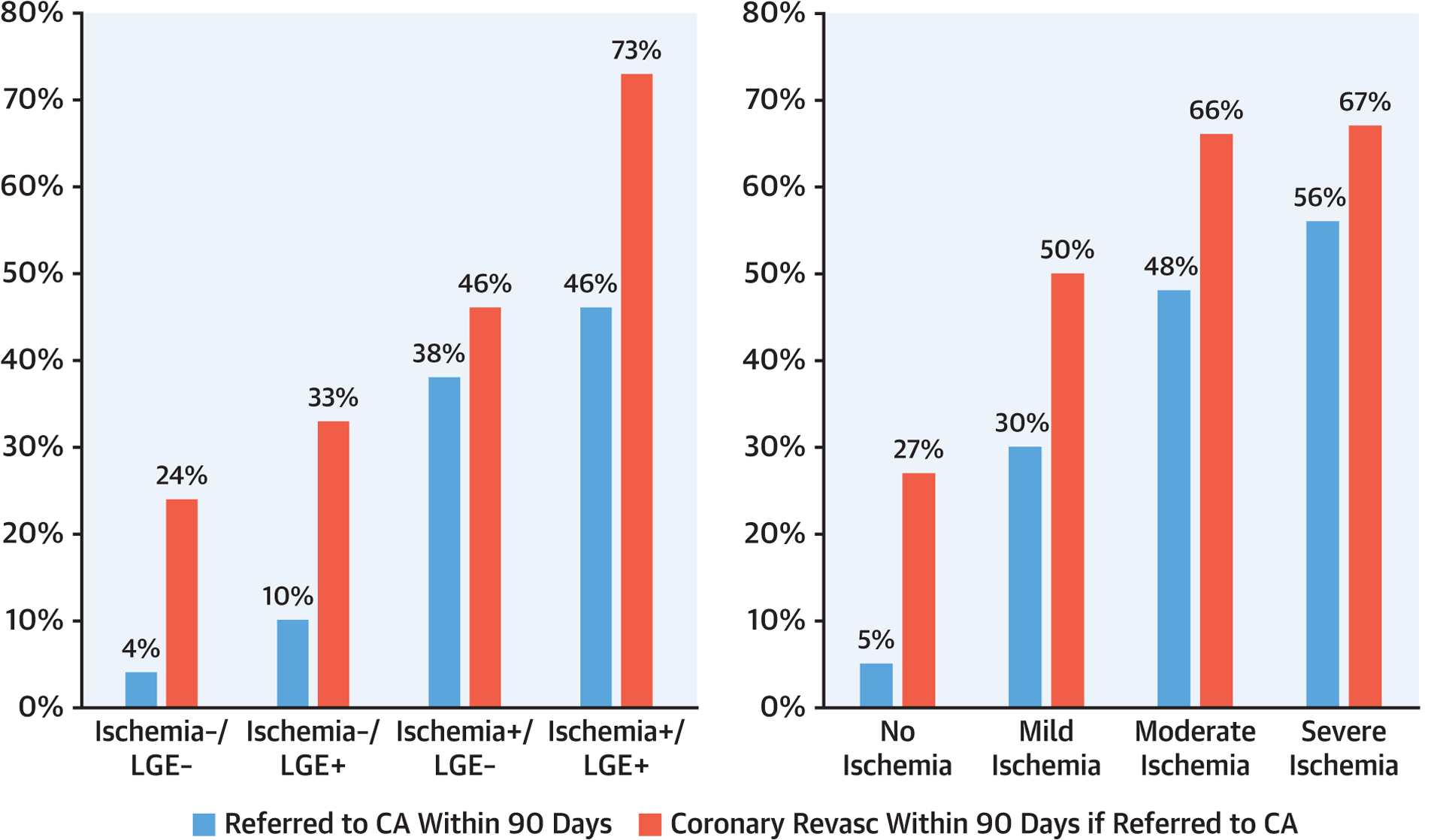
Referral to invasive coronary angiography (XCA) at 90-day post-stress cardiac magnetic resonance imaging with corresponding proportion of patients undergoing revascularization (Revasc), stratified by presence and/or absence of ischemia and left gadolinium enhancement (LGE) (left) and extent of ischemia (right).
COSTS SPENT ON ISCHEMIA TESTING DURING STUDY FOLLOW-UP.
During follow-up, 142 stress echocardiography, 808 stress single-photon emission computed tomography, 199 coronary computed tomographic angiography, 149 repeat stress CMR, 215 exercise treadmill, and 915 XCA clinical examinations were performed. Figure 5 illustrates the average cost (US$/patient) spent on ischemic testing according to follow-up periods. Between the 4 CMR result groups, those with ischemia−/LGE− had the lowest cost spending across all periods of follow-up. During the first 90 days after CMR, patients with ischemia+/LGE+ incurred approximately 10-fold higher costs than did those with ischemia−/LGE− in the same time period ($585 vs. $54, p < 0.0001) due to the referral to XCA. Whereas XCA contributed the most to overall costs during the first year in patients with ischemia+ and to a lesser degree those with ischemia−/LGE+, stress single-photon emission computed tomography contributed the most in later years across all groups.
FIGURE 5. Costs of Ischemia Testing.
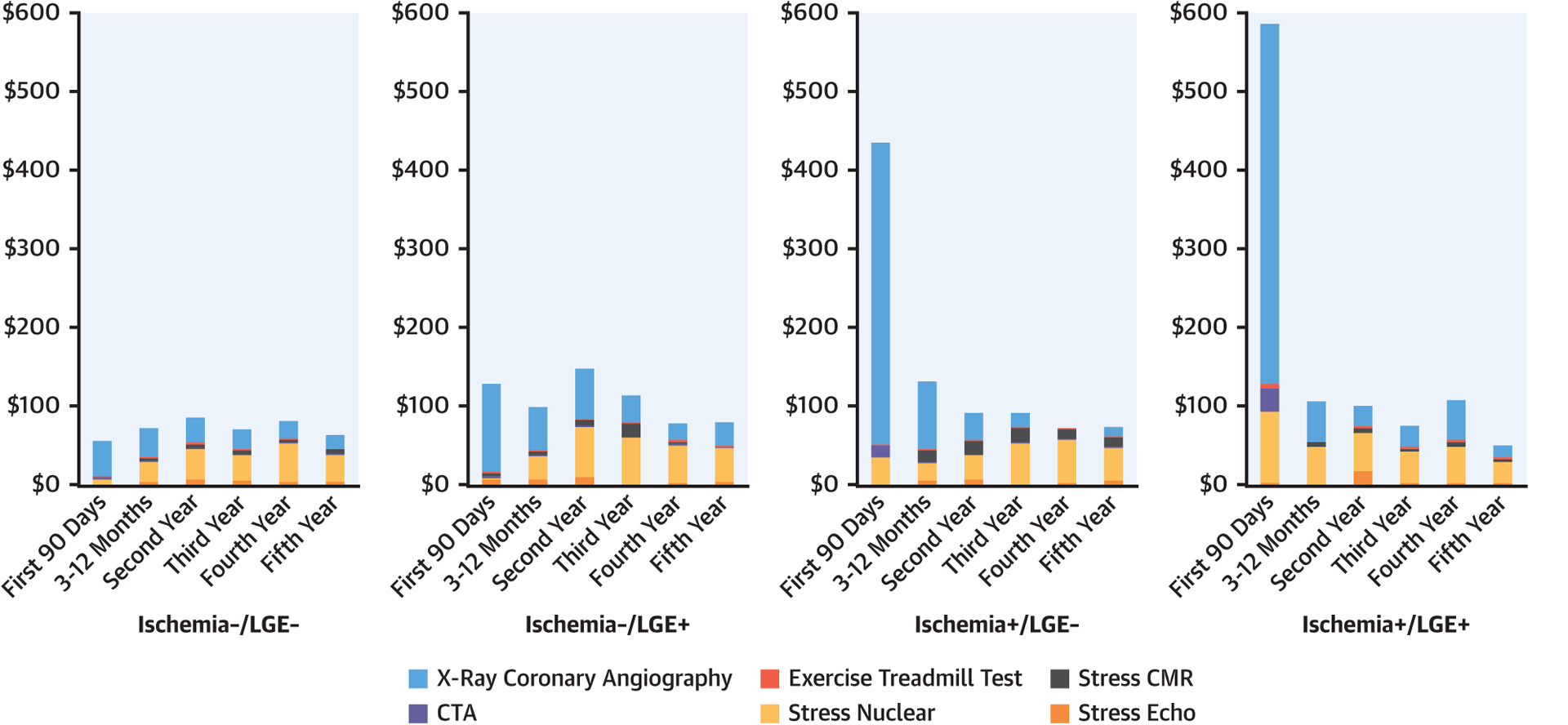
Costs of downstream cardiac tests incurred during follow-up, stratified by stress cardiac magnetic resonance imaging (CMR) findings with breakdown by modality. Costs are in U.S. dollars spent per patient. CTA = computed tomography angiography; LGE = late gadolinium enhancement.
PATTERNS ACROSS DIFFERENT PRACTICE SETTINGS.
Characteristics of the enrolling centers are shown in Online Table 2. University hospitals (n = 7), cardiovascular group practices (n = 2), multispecialty practices (n = 2), and U.S. government or military hospitals (n = 2) enrolled 1,019 patients (43%), 464 (20%), 610 (26%), and 256 (11%), respectively. Over 4 years after CMR, 188 patients (8%), 81 (3.5%), and 15 (0.6%) had PCI, CABG, and both, respectively. Figure 6 illustrates the performance of invasive XCA at 90 days, by practice types and CMR findings. Across all practice types, patients with ischemia−/LGE− were referred to undergo XCA at 90 days at low rates (2.8% to 4.9%). Patients with ischemia+ underwent XCA at substantially higher rates across all practice types, the highest at 62% by the government/military hospital group. As illustrated in Online Figure 2, costs spent at 1 year demonstrated a similar pattern across the practice types and CMR findings.
FIGURE 6. Invasive XCA at 90 Days, Stratified by Practice Types.
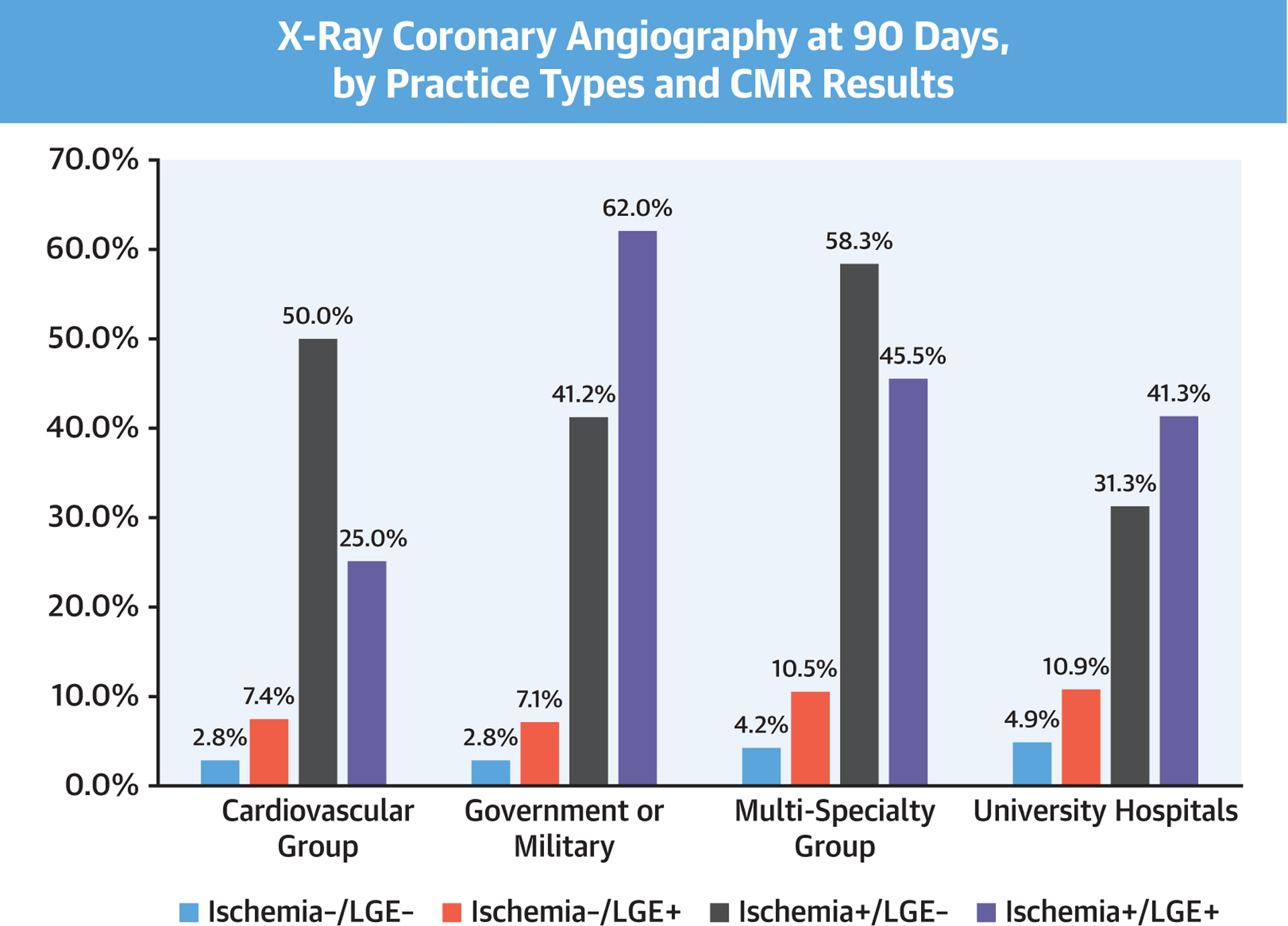
Referral to invasive XCA at 90-day post stress CMR, stratified by presence and/or absence of ischemia and LGE, according to practice environment. Abbreviations as in Figures 1, 4, and 5.
CONCORDANCE RATES BETWEEN ENROLLING CENTERS AND THE IMAGING CORE LABORATORY.
Images from 235 studies (10%) were interpreted by the CMR core lab blinded to clinical characteristics and outcomes. The concordance rates of centers versus core lab interpretation on ischemia presence, ischemia grade, LGE presence, and LGE grade were 82%, 86%, 90%, and 92%, respectively.
DISCUSSION
SPINS is the largest multicenter study in the United States to date evaluating the prognostic value of stress CMR in patients presenting with stable chest pain syndromes. The study comprised a consecutive cohort from centers with diverse practice settings with a follow-up target of 4 years achieved in >97% of patients. There are 3 key findings (Central Illustration). First, in this cohort with an intermediate pre-test likelihood of CAD and a median basic consortium score of 34%, 67% of the study cohort had ischemia−/LGE− and experienced low annual rate of primary and secondary outcomes after CMR (0.6% and 1.7%, respectively), which is in contrast to the patients with ischemia+/LGE+ (4.5% and 10.1%, respectively). Second, the need for referral to coronary revascularization was low for patients with ischemia−/LGE−, at 3% in the first year and <1% in each of the subsequent 3 years, compared with 38% and 3% for patients with ischemia+/LGE+. Third, patients with ischemia−/LGE− had low average annual costs spent on downstream ischemia testing across all years of follow-up, and this finding is consistent across practice types of the participating sites in the United States.
As a gate-keeping noninvasive test, it is important that a “low-risk” population be identified thereby avoiding unnecessary downstream tests and invasive treatment. From a cohort of 3,647 patients, the multinational EuroCMR (European Cardiovascular Magnetic Resonance) registry reported a negative cardiovascular event rate as low as 1% per year, demonstrating that stress CMR was effective in obviating the need for invasive angiography (15,16). The Italian STRATEGY (Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Revascularized Patients) study observed that stress CMR has higher cost-effectiveness than coronary computed tomography angiography in assessing symptomatic patients with a history of coronary revascularization (17). SPINS extended current knowledge by examining the roles of stress CMR in the U.S. health care system. Apart from low incidence of primary and secondary outcomes, patients without ischemia or LGE by CMR had low downstream need for coronary revascularization and incurred low costs for CAD testing throughout study follow-up. Stress CMR is currently underutilized for chest pain assessment compared with other noninvasive methods in the United States; however, the performance characteristics observed in SPINS strongly support the use of stress CMR as an effective gatekeeping strategy for invasive angiography.
It is increasingly recognized that the presence of scar independently predicts adverse outcomes in CAD (18). Studies have shown that CMR has excellent sensitivity in detecting subendocardial infarctions (19). In SPINS, the presence of either inducible ischemia or LGE was independently associated with higher primary and secondary events. In addition, the effects of inducible ischemia and LGE were additive, such that patients with both findings were at the highest risk. CMR also allows for the detection of unrecognized MI, which is of prognostic importance. In a large study of older community dwellers in Iceland, the rate of unrecognized MI by CMR was 17% (20). Over long-term follow-up, unrecognized MI by CMR was associated with increased all-cause mortality. In our study, although the prevalence of MI by LGE was 24%, the majority (14%) did not have any prior history of MI, highlighting the diagnostic importance of CMR.
In the current era of intense debate between anatomical and functional testing in stable CAD, 2 large randomized trials have compared coronary computed tomographic angiography to stress testing (21,22). The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) and SCOT-HEART (Scottish Computed Tomography of the Heart Trial) studies, however, included relatively low-risk patients and did not include stress CMR as part of their diagnostic strategies. Given its consistent negative predictive value demonstrated in SPINS and EuroCMR (16) and its lack of ionizing radiation exposure, CMR is a practical choice when considering stress testing. The MR-IMPACT (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial) I and II studies are currently the largest prospective multicenter trials that have included stress CMR (1,3). These studies and CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease) focused on the diagnostic accuracy of CMR (23), whereas CE-MARC 2 examined CMR’s impact on downstream angiography use (24). The recently presented MR-INFORM (MR Perfusion Imaging to Guide Management of Patients With Stable Coronary Artery Disease) study compared stress CMR with anatomic assessment using XCA with fractional flow reserve in 918 symptomatic patients at high pre-test probability of CAD (25). In this 1:1 randomized control trial, the major adverse cardiac event rate was similar in both strategies at 1-year follow-up.
Health care payers and patients are increasingly aware of the cost burden from repeat cardiac testing in noninvasive cardiovascular imaging. With the current focus on value-based care, few studies have thus far examined the downstream clinical and economic values of stress CMR. In the current SPINS cohort, downstream rate of coronary revascularization by either PCI or CABG was the highest among patients with ischemia+/LGE+. On the other hand, those with ischemia−/LGE− by CMR had very low spending rates for ischemia testing or coronary revascularization. Our results are congruent with the cost-minimization results of the EuroCMR registry (16).
STUDY LIMITATIONS.
First, given the retrospective design of this study, we could not capture all the direct therapeutic and management decisions made at the time of the CMR study. Second, CMR studies were performed in a clinical setting so we cannot determine whether any knowledge of coronary anatomy from prior angiography could have influenced the CMR interpretations. Third, our participating sites were predominantly tertiary-care experienced centers, therefore, there may have been a local referral bias of higher-risk patients to CMR, and uncertainty exists whether the current results generalize to less experienced centers. Fourth, the SPINS study was conducted at a time when quantitation of CMR perfusion, LGE size, and invasive fractional flow reserve were not performed as a clinical routine, thus, these factors, which are relevant to today’s practice, therefore could not be accounted for. Fifth, core lab assessment of 10% of the images for ischemia and LGE presence resulted in only a modest concordance rate. Given the retrospective study design aimed at capturing the clinical consequences of local interpretation at time of CMR performance, there was no attempt to standardize reading or interpretation procedures between the enrolling centers and the core lab. Finally, our study is not able to assess CMR guidance of coronary revascularization toward improving patient outcome, given its nonrandomized study design and limited study power. This nonrandomized study design without a comparative imaging-based strategy also prohibited any conclusions in causal estimates or comparison against key alternative methods in this setting. These limitations will need to be addressed in prospective randomized trials.
CONCLUSIONS
Among patients with stable intermediate-risk chest pain syndromes, a stress CMR without evidence of ischemia or LGE was associated with very low incidence of adverse cardiac events and low health care costs spent on downstream cardiac testing.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
CMR stress perfusion imaging can identify patients with chest pain who are at risk of ischemic events and guide referral for coronary revascularization. Implementation of stress CMR as an initial diagnostic modality may prove less costly than conventional strategies.
TRANSLATIONAL OUTLOOK:
Future studies should compare the cost and value of stress CMR with other noninvasive modalities in the evaluation of patients with suspected ischemic heart disease.
Acknowledgments
The SPINS Registry was funded by the Society for Cardiovascular Magnetic Resonance, using a research grant jointly sponsored by Siemens Healthineers (Erlangen, Germany) and Bayer AG (Leverkusen, Germany). These sponsors to the Society for Cardiovascular Magnetic Resonance provided financial support for the study but did not play a role in study design, data collection, analysis, interpretation, or manuscript drafting. Dr. Arai has research agreements with Siemens, Bayer, and Circle Cardiovascular Imaging. Dr. Bandettini is the principal investigator of one of the Bayer-sponsored GadaCAD2 (Gadavist-Enhanced Cardiac Magnetic Resonance Imaging to Detect Coronary Artery Disease) sites. Dr. Patel has received a research grant from and served on the Speakers Bureau of Astellas. Dr. Schulz-Menger has research agreements with Siemens; and serves on the Advisory Board of Bayer. Dr. Stuber has received nonmonetary research support form Siemens Healthineers. Drs. Raman and Simonetti both receive institutional research support from Siemens. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CA
coronary angiography
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance imaging
- LGE
late gadolinium enhancement
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- XCA
x-ray coronary angiography
Footnotes
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Schwitter J, Wacker CM, Wilke N, et al. for the MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 2013;34:775–81. [DOI] [PubMed] [Google Scholar]
- 2.Schwitter J, Wacker CM, Wilke N, et al. for the MR-IMPACT Investigators. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: the secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson 2012;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008;29:480–9. [DOI] [PubMed] [Google Scholar]
- 4.Watkins S, McGeoch R, Lyne J, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation 2009;120:2207–13. [DOI] [PubMed] [Google Scholar]
- 5.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;50:1174–9. [DOI] [PubMed] [Google Scholar]
- 6.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation 2011;123: 1509–18. [DOI] [PubMed] [Google Scholar]
- 7.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah R, Heydari B, Coelho-Filho O, et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation 2013;128:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63:380–406. [DOI] [PubMed] [Google Scholar]
- 10.Kwong RY, Petersen SE, Schulz-Menger J, et al. The Global Cardiovascular Magnetic Resonance Registry (GCMR) of the Society for Cardiovascular Magnetic Resonance (SCMR): its goals, rationale, data infrastructure, and current developments. J Cardiovasc Magn Reson 2017; 19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 2012;94:496–509. [Google Scholar]
- 14.Genders TS, Steyerberg EW, Hunink MG, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ 2012;344:e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruder O, Wagner A, Lombardi M, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry—multinational results from 57 centers in 15 countries. J Cardiovasc Magn Reson 2013;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moschetti K, Petersen SE, Pilz G, et al. Cost-minimization analysis of three decision strategies for cardiac revascularization: results of the “suspected CAD” cohort of the European Cardiovascular Magnetic Resonance Registry. J Cardiovasc Magn Reson 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreini D, Pontone G, Bogaert J, et al. Long-term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol 2016;68:2166–81. [DOI] [PubMed] [Google Scholar]
- 18.Vincenti G, Masci PG, Monney P, et al. Stress perfusion CMR in patients with known and suspected CAD: prognostic value and optimal ischemic threshold for revascularization. J Am Coll Cardiol Img 2017;10:526–37. [DOI] [PubMed] [Google Scholar]
- 19.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374–9. [DOI] [PubMed] [Google Scholar]
- 20.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012; 308:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas PS, Hoffmann U, Patel MR, et al. for the PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012; 379:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood JP, Ripley DP, Berry C, et al. for the CE-MARC 2 Investigators. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 Randomized Clinical Trial. JAMA 2016;316:1051–60. [DOI] [PubMed] [Google Scholar]
- 25.Hussain ST, Paul M, Plein S, et al. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J Cardiovasc Magn Reson 2012;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


