Abstract
Background
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are a family of intracellular Ca2+ release channels located on the membrane of endoplasmic reticulum, which have been shown to play critical roles in various cellular and physiological functions. However, their function in regulating gastrointestinal (GI) tract motility in vivo remains unknown. Here, we investigated the physiological function of IP3R1 in the GI tract using genetically engineered mouse models.
Methods
Pdgfrb-Cre mice were bred with homozygous Itpr1 floxed (Itpr1f/f) mice to generate conditional IP3R1 knockout (pcR1KO) mice. Cell lineage tracing was used to determine where Pdgfrb-Cre-mediated gene deletion occurred in the GI tract. Isometric tension recording was used to measure the effects of IP3R1 deletion on muscle contraction.
Results
In the mouse GI tract, Itpr1 gene deletion by Pdgfrb-Cre occurred in smooth muscle cells, enteric neurons, and interstitial cells of Cajal. pcR1KO mice developed impaired GI motility, with prolonged whole-gut transit time and abdominal distention. pcR1KO mice also exhibited lethality as early as 8 weeks of age and 50% of pcR1KO mice were dead by 40 weeks after birth. The frequency of spontaneous contractions in colonic circular muscles was dramatically decreased and the amplitude of spontaneous contractions was increased in pcR1KO mice. Deletion of IP3R1 in the GI tract also reduced the contractile response to the muscarinic agonist, carbachol, as well as to electrical field stimulation. However, KCl-induced contraction and expression of smooth muscle-specific contractile genes were not significantly altered in pcR1KO mice.
Conclusions
Here, we provided a novel mouse model for impaired GI motility and demonstrated that IP3R1 plays a critical role in regulating physiological function of GI tract in vivo.
Keywords: IP3 receptor, Ca2+ release channel, Gut motility, Intestinal pseudo-obstruction
Introduction
The gastrointestinal (GI) tract has the tasks of ingesting food, digesting and absorbing nutrients, and removing waste products. To accomplish these critical functions, the GI tract generates motility in either a tonic or a phasic fashion to efficiently move food from one chamber to the next or to retain food in one region until digestion and absorption are accomplished [1, 2]. GI motility disorders are very common, affecting approximately 20–30% of people in Western populations [3], and generate a wide spectrum of clinical symptoms that may affect the entire GI tract that can vary in severity, typically including chronic pain, nausea, vomiting, bloating and sever constipation. These symptoms markedly impair the patients’ quality of life and cause considerable health-care costs [4]. Multiple approaches, including radiological and physiological techniques, have been developed to assess GI motility disorders in these patients. Damages to smooth muscle effector cells, the control system or the interfacing system may contribute to the pathophysiology of GI motility disorders. However, our fundamental mechanistic understanding of GI motility disorders remains inadequate.
Inositol 1, 4, 5-trisphosphate (IP3) receptors (IP3Rs) are a family of Ca2+ channels located on the membrane of endoplasmic reticulum (ER), which mediates Ca2+ release from the ER to the cytoplasm in response to the binding of a second messenger, IP3. In mammals, three different IP3R subtypes are encoded by three distinct genes, Itpr1, Itpr2, and Itpr3, respectively [5]. Using genetically engineered mouse models, IP3 R-mediated Ca2+ signaling has been shown to play a critical role in regulating embryonic survival [6, 7], brain function [8], exocrine secretion [9], angiotensin II-induced hypertension [10], T cell development [11], and B-cell function [12]. In the GI tract, the musculature is well arranged into two layers separated by a neural network known as myenteric plexus, a component of the enteric nervous system: a longitudinal layer in which the muscle cells are arranged along the long axis of the gut, and an inner circular layer in which the muscle cells are arranged transversally. Additionally, the interstitial cells of Cajal (ICC), which generate spontaneous electrical activity and are electrically coupled to the smooth muscle cells, are found in the myenteric plexus, the intramuscular space, the submucosal layer, and the subserosal layer in different segments of the GI tract [13]. In normal individuals, smooth muscle cells integrate the inputs from all levels of control including the central nervous system, the enteric nervous system, hormonal stimuli, and paracrine factors to contract appropriately. In the GI tract, IP3 R-mediated Ca2+ mobilization and contraction of circular muscle cells in response to various contractile agonists are conserved across different species including rat and human [14, 15]. Furthermore, slow waves and pacemaker activity generated by the ICCs may also rely on IP3R-dependent intracellular Ca2+ release [16, 17]. In addition, IP3 R-mediated Ca2+ signals have also been observed in enteric neurons [18]. However, the in vivo function of IP3Rs in regulating GI motility remains unclear. Genetically engineered mouse models have been used to characterize the physiological function of each IP3R subtype in vivo. However, no obvious GI phenotypes were mentioned in IP3R2 knockout or IP3R3 knockout mice [9, 19–21]. By contrast, gastric muscles obtained from global IP3R1 knockout mice exhibit altered mechanical responses and lack slow waves [22, 23], implicating that IP3R1 might play a role in regulating GI physiology. However, global deletion of IP3R1 in mice also resulted in dramatic growth retardation and severe brain defects such as ataxia and seizures, leading to early lethality before weaning [8], which confounds further investigation of IP3R1 function in the adult mouse GI tract. Furthermore, it also remains unclear whether the in vitro phenotype observed in isolated muscles was a direct consequence of IP3R1 deletion in the GI tract or just a secondary effect resulting from the global abnormalities developed in IP3R1 knockout mice. Therefore, we generated an IP3R1-floxed mouse model and deleted IP3R1 in the GI tract of adult mice using Cre recombinase driven by platelet-derived growth factor receptor-β (Pdgfrb-Cre). We found that deletion of IP3R1 using Pdgfrb-Cre resulted in abdominal distention, prolonged whole-gut transit, and premature mouse mortality.
Materials and methods
Mice
The generation of IP3R1 floxed mice has been previously described [11]. The Pdgfrb-Cre mice expressing Cre recombinase under the control of a fragment of the gene encoding PDGFRβ have also been previously described [24]. The B6.129X1-Gt(ROSA) 26Sortm1(EYFP)Cos/J (Rosa-EYFP) reporter mice were purchased from the Jackson laboratory (Bar Harbor, ME). All mice were housed under a 12-h-day/night cycle at a temperature of 25.0 °C, with free access to a standard diet and clean water. For cell lineage tracing, Pdgfrb-Cre mice were crossed with Rosa-EYFP mice to generate Pdgfrb-Cre+Rosa-EYFP+ mice. Itpr1f/f mice were bred with Pdgfrb-Cre+ mice to generate the double heterozygous Pdgfrb-Cre+Itpr1f/+ mice, which were further crossed with Itpr1f/f mice to generate the tissue-specific IP3R1 knockout Pdgfrb-Cre+Itpr1f/f (pcR1KO) mice. Littermate Pdgfrb-Cre−Itpr1f/f mice were used as controls.
DNA analysis
Offspring from intercrosses were genotyped by PCR analysis using mouse-tail DNA, as previously described [25]. Gene-specific primer sequences were presented as follows: Itpr1, wildtype/flox allele (forward: 5′-GAGAAAGGTTAACAGCACTGGATT-3′; reverse: 5′-ACTGGGCAGGCATATATAGTTAGC-3′), Rosa-EYFP, wildtype/flox allele (forward: 5′-AAAGTCGCTCTGAGTTGTTAT-3′; reverse 1: reverse 1: 5′-GGAGCGGGAGAAATGGATATG-3′; reverse 2: 5′-AAGACCGCGAAGAGTTTGTC-3′), and Pdgfrb-Cre allele (forward: 5′-GTTCGCAAGAACCTGATGGACA; reverse: CTAGAGCCTGTTTTGCACGTTC). PCR products were visualized by ethidium bromide staining.
Quantitative real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) and cDNA was synthesized using TransScript one-step gDNA Removal and cDNA Synthesis Supermix Kit (TransGen Biotech). Quantitative real-time PCR (RT-PCR) was performed using TransStart Tip Green qPCR Super-Mix (TransGen Biotech) according to the manufacturer’s instructions. The sequences for primers of Itpr1 and Gapdh were used as previously described [10]. The primer sequences for Acta2, Myh11, and Mlck are presented as follows: Acta2 (forward: 5′-GAGAAGCCCAGCCAGTCG-3′; reverse: 5′-CTCTTGCTCTGGGCTTCA-3′), Myh11 (forward: 5′-GAGG TGGTCGTGGAGTTGGT-3′; reverse: 5′-GTATCGCTCCCTCAGGTTGT-3′), Mlck (forward: 5′-ACATGCTA CTGAGTGGCCTCTCT-3′; reverse: 5′-GGCAGACAGGACATTGTTTAAGG-3′).
Immunoblot analysis
The colon or jejunum was isolated from the control and mutant mice, then muscles were removed from the mucosal layer and pulverized in liquid nitrogen. Protein lysates were prepared using a lysis buffer containing 8.0 M urea, 2.0 M thiourea, 3.0% SDS, 75.0 mM DTT, 0.05 M Tris–HCl (pH 6.8), and 0.03% bromophenol blue. After performing ultra-sonication four times (for 5 s each time), lysates were centrifuged for 10 min at 15,294 rpm at 4 °C. The super-natants were then heated at 95 °C for 5 min. To analyze IP3R1 protein levels, proteins were separated by 6% SDS-PAGE gel and transferred to a 0.45-μm PVDF membrane (Millipore). The primary antibody against mouse IP3R1 was raised in our lab as previously described [11]. The antibody against GAPDH (Santa Cruz Biotechnology, catalog sc-32233) was commercially purchased.
Immunostaining
Immunostaining was performed as previously described [26]. Briefly, tissues were collected and fixed in 4% paraformaldehyde (PFA). They were then incubated in an ascending series of sucrose concentrations from 10% to 20%, and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, USA). Cryosections were then prepared at 5 μm thickness. For immunolabelling, the sections were first permeabilized with solution 1 (0.2% Triton X-100 in 1X phosphate-buffered saline (PBS)) for 15 min and then blocked with 5% bovine serum album (BSA) and 2% horse serum in solution 1 for one-two hours at room temperature. The samples were then incubated with primary antibody overnight at 4 °C. Following three washes with solution 1 for 5 min each, samples were incubated with secondary antibody for one hour at room temperature. After washing with solution 1 four times (5 min each), samples were mounted with DAKO fluorescent mounting medium with cover slips. The primary antibodies including affinity purified rat monoclonal anti-mouse cKit (eBioscience, catalog 14–1171, at 1:100 dilution), affinity purified rabbit polyclonal anti-mouse Trans-genlin (SM22α; Abcam, catalog ab14106, at 1:1000 dilution), and rabbit polyclonal to Ubiquitin C-terminal hydrolase L1 (PGP9.5; Abcam, catalog ab10404, at 1:200 dilution) were commercially purchased. We used the following secondary antibodies: Alexa Fluor 594 donkey anti-rabbit IgG (Invitrogen, catalog A21207, at 1:300 dilution) and Alexa Fluor 594 donkey anti-rat (Invitrogen, catalog A21209, at 1:300 dilution).
Whole-mount staining for NADPH diaphorase
Whole-mount staining for NADPH diaphorase was performed using previously described protocols [27]. Briefly, the colons were collected, washed and then filled with PBS, tying the ends with suture thread. Tissues were subsequently fixed with 4% PFA for 15–30 min and rinsed with PBS (four times, 5 min each). After that, tissues were immersed in 0.3% Triton X-100 (diluted in PBS) for 20 min, followed by incubation in the NADPH diaphorase staining solution containing 0.1% β-NADPH (Sigma, catalog N1630), 0.05% Nitro blue Tetrazolium (Sigma, catalog N5514), and 0.3% Triton X-100. The reaction was performed at 37 °C and stopped when nerve elements were detected. The colons were washed, post-fixed with 4% PFA, and cut along the mesenteric attachment. The mucosal layer was peeled off under a dissection microscope and the specimens were visualized under a light microscope.
Whole-gut transit time test
To analyze intestinal function, a whole-gut transit time test was performed as previously described [28]. Carmine (50 μl; 3 mg of carmine in 0.5% methylcellulose) was orally administrated to each mouse. The mice were then put in individual cages lined with white sheets of paper. The whole-gut transit time was calculated from the time of carmine administration to the first excretion of colored feces.
Smooth muscle isometric tension recording
The colons were collected and the internal contents and connective tissues were quickly removed in oxygenated Kreb’s solution (in mM: NaCl 120.7, KCl 5.9, NaH2PO4-H2O 1.2, NaHCO3 15.5, CaCl2 2.5, MgCl2 1.2, Glucose 11.5, pH 7.4). For isometric tension recordings, approximately 0.5 cm muscle strips with mucosa were cut from lower colons and suspended in the circular direction in a 10-ml organ bath containing oxygenated Kreb’s solution at 37 °C. The tissues were allowed to equilibrate for one hour before stimulation was applied and resting tension was set to 0.5 g. After equilibration, transmural electrical field stimulation (2 Hz, 30 s), carbachol (10 μm), and KCl (60 mM) were applied to elicit muscle contraction, respectively. After recording, the tissue’s weight was measured for reference. The tension was expressed as the peak amplitude or the averaged tension during stimulation.
Statistics
Statistical analysis was performed using a 2-tailed, unpaired Student’s t-test in Microsoft Excel (Redmond, WA). All data were presented as mean ± SEM (error bars). P < 0.05 was considered statistically significant.
Study approval
All animal care and use procedures in this study were approved by Institutional Animal Care and Use committee at UC San Diego (San Diego, USA) and at Peking University Shenzhen Graduate School (Shenzhen, China), respectively. Periodic review of procedures was performed, and amendments were made as needed.
Results
Generation and characterization of conditional IP3R1 deletion by Pdgfrb-Cre in mice
Since we initially wanted to investigate the role of IP3R1-mediated Ca2+ release in vascular smooth muscle cells and pericytes, we used Pdgfrb-Cre to conditionally delete IP3R1 in mice. Here, we surprisingly found that Pdgfrb-Cre also targets multiple cell types in the GI tract of the adult mouse. We bred Pdgfrb-Cre mice with Rosa-EYFP reporter mice that contain an Enhanced Yellow Fluorescent Protein gene (EYFP) inserted into the Gt(ROSA)26Sor locus [29]. We employed antibodies against SM22α cKit and PGP9.5 to recognize smooth muscle cells, the interstitial cells of Cajal (ICCs), and enteric ganglionic neurons, respectively, to identify distinct cell types. In the muscular layers of the colon isolated from 3-month-old Pdgfrb-Cre+Rosa-EYFP+ mice, we observed EYFP fluorescence in almost all smooth muscle cells in both the longitudinal and circular muscular layers and in the majority of ICCs and enteric neurons (Fig. 1a), suggesting that Pdgfrb-Cre successfully executes gene recombination in all of these cell types.
Fig. 1.
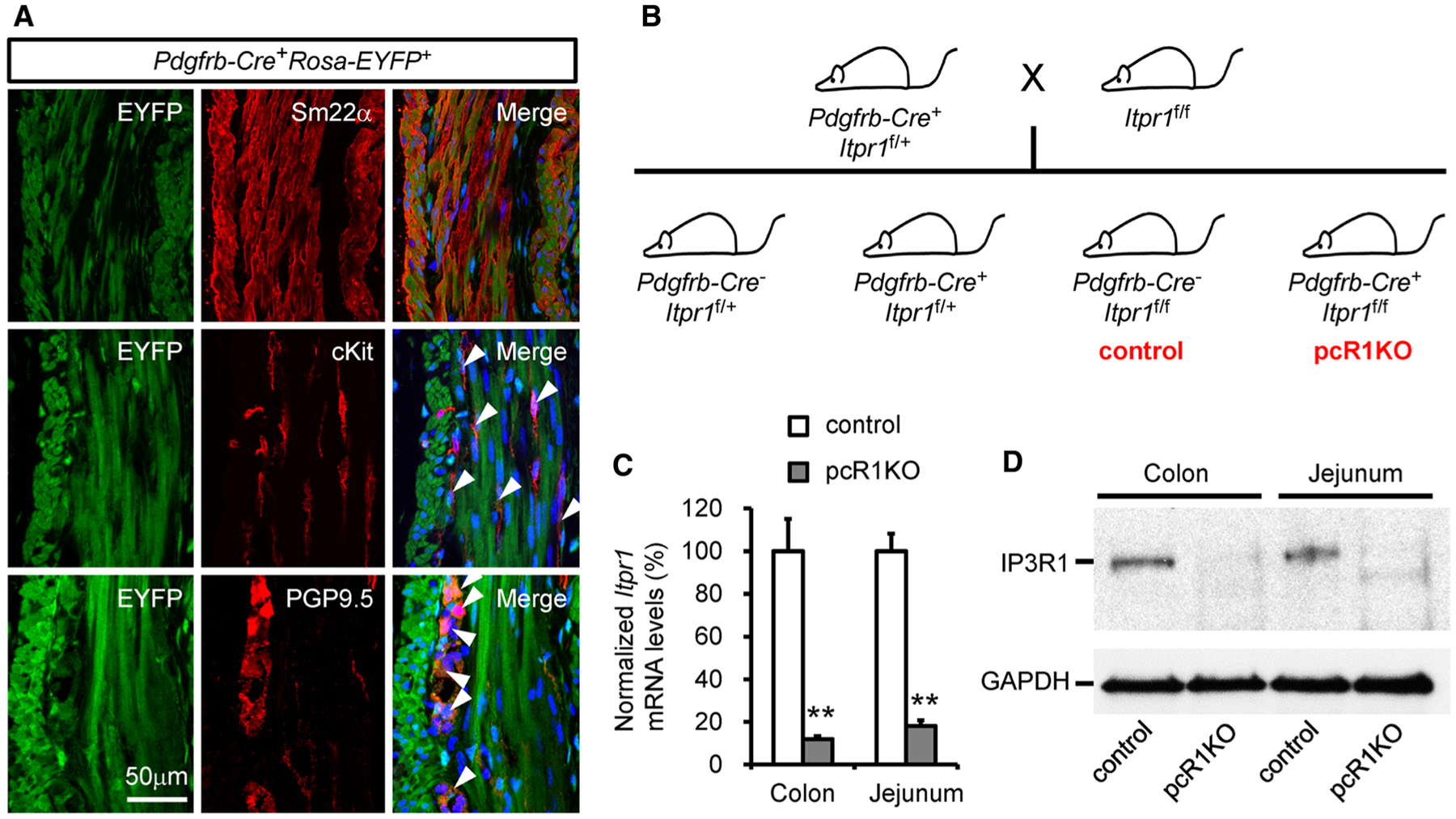
Generation and characterization of conditional IP3R1 knockout mice using Pdgfrb-Cre. a Cell lineage tracing showing Pdgfrb-Cre also targets ICCs and enteric neurons in addition to smooth muscle cells in the colon. Smooth muscle cells, ICCs, and enteric neurons were labeled by immunostaining cross-sections of Pdgfrb-Cre+Rosa-EYFP+ colon using antibodies against Sm22α, cKit, and PGP9.5, respectively. Arrowheads indicate the ICC or the enteric neuron that also expresses EYFP. Data are representative of at least three independent experiments. b Schematic diagram of the mouse breeding strategy used to generate conditional IP3R1 knockout mice with Pdgfrb-Cre. Pdgfrb-Cre+Itpr1f/f mice were considered pcR1KO mice whereas Pdgfrb-Cre−Itpr1f/f mice were used as controls. c and d Muscles were separated from the mucosal layers in the colon and jejunum isolated from control and pcR1KO mice at 1 month of age and were further used for RT-PCR and protein analysis. Both Itpr1 mRNA (c) and protein (d) levels were dramatically reduced in pcR1KO mice compared with control mice; n = 4 mice per group. Significance was determined using a 2-tailed, unpaired Student’s t test. **P < 0.01 versus control. Error bars represent mean ± SEM
Next, we crossed Pdgfrb-Cre+Itpr1f/+ mice with Iptr1f/f mice to generate Pdgfrb-Cre+Itpr1f/f (pcR1KO) animals (Fig. 1b). Once the pcR1KO mice were generated, we examined whether Pdgfrb-Cre efficiently deletes IP3R1 in the GI tract. For this purpose, we isolated the colon and jejunum from control and pcR1KO mice at 1 month of age and separated the muscular layer from the mucosal layer in each tissue. We then performed quantitative real-time PCR and western blot analysis to measure the mRNA and protein expression of IP3R1 in the muscular layer. We found that both mRNA and protein levels of IP3R1 in the muscular layers of the pcR1KO jejunum and colon were dramatically reduced relative to the control tissues (Fig. 1c, d), suggesting that deletion of IP3R1 by Pdgfrb-Cre was very efficient in the muscular layer of the GI tract.
Deletion of IP3R1 by Pdgfrb-Cre in mice resulted in abdominal distention and mortality
The pcR1KO mice were born in Mendelian ratios with no obvious abnormalities at birth and phenotypically undistinguishable from their control siblings at 3 weeks of age. However, mutant mice exhibited premature lethality after 8 weeks of age and about half of the mutant mice died before 40 weeks of age (Fig. 2a). The mutant mice exhibited ante-mortem weakness, fatigue and loss of body weight, indicative of malnutrition and cachexia. Furthermore, 3-month-old mutant mice exhibited considerable growth retardation, indicated by reduced body length, and developed apparent abdominal distension (Fig. 2b). The vast majority of the 3-month-old pcR1KO mice also had massively enlarged and dilated ileum, cecum and colons, all of which were abnormally filled with accumulated food and in most cases displaced from their normal positions (Fig. 2c). Additionally, the lengths of the small and large intestine were significantly increased in 3-month-old pcR1KO mice relative to control mice (Fig. 2d). Histological examination using hematoxylin & eosin staining revealed that the thickness of the jejunum mucosal layer, but not the muscular later, was significantly increased in pcR1KO mice compared to control jejunum (Fig. 2e, f). By contrast, the thicknesses of both the mucosal layer and the muscular layer of mutant colon were dramatically increased at 3 months of age (Fig. 2g, h).
Fig. 2.
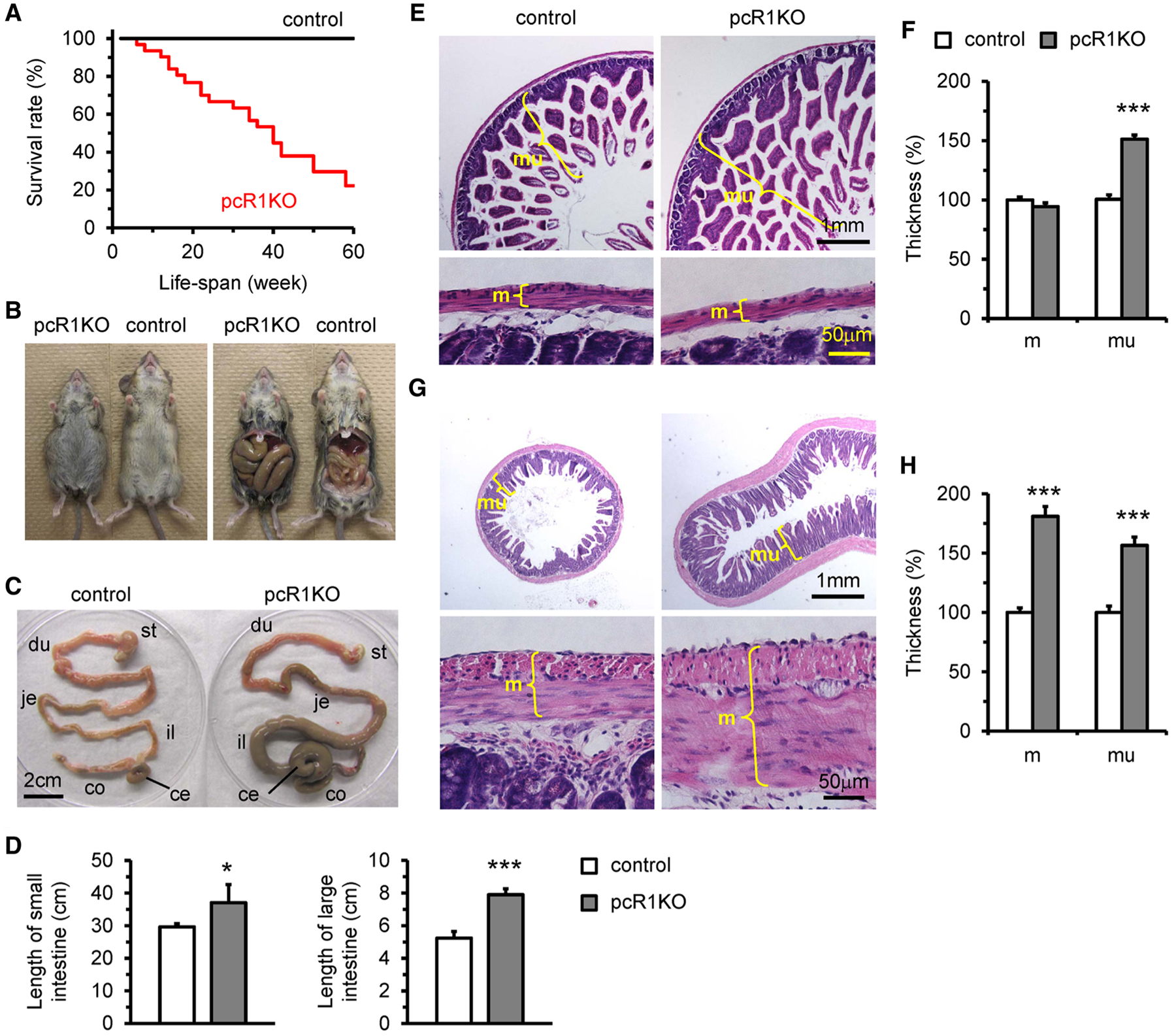
Deletion of IP3R1 by Pdgfrb-Cre resulted in abdominal distention and lethality. a Kaplan–Meier survival analysis of control (n = 12) and pcR1KO (n = 31) mice. b pcR1KO mice at 3 months of age exhibited abdominal distension. The ileum was dramatically enlarged and displaced from its regular position due to severe food accumulation. c The GI tracts isolated from control and pcR1KO mice shown in b. In addition to the ileum, the cecum and colon in pcR1KO mouse were also dramatically enlarged. st, stomach; du, duodenum; je, jejunum; il, ileum; ce, cecum; co, colon. d The length of the small and large intestines of control (n = 3) and pcR1KO (n = 4) mice at 3 months of age. e–h Transverse sections of jejunum (e) and colon (f) from control and pcR1KO mice at 3 months of age stained with hematoxylin & eosin. The thicknesses of muscular and mucosal layers were measured in sections of jejunum (f) and colon (h), respectively. m, muscular layer; mu, mucosal layer. n = 5 mice per group. For all data significance was determined using a 2-tailed, unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001 versus control. Error bars represent mean ± SEM
pcR1KO mice exhibited defects in gastrointestinal motility
We next investigated the effects of IP3R1 deletion on GI function in 1-month-old animals since most mutant mice displayed normal GI morphology without any obvious abdominal distention at this time point (Fig. 3a). The thicknesses of the muscular and mucosal layers in both jejunum and colon were also not significantly altered between pcR1KO and control mice at this stage (Fig. 3b). However, we observed that the stools collected from individual caged mutant mice were dramatically longer than those from control siblings (Fig. 3c, d). Moreover, we measured the whole-gut transit time as a marker of GI motility by monitoring the time until excretion after oral administration of the red dye carmine to control and mutant animals. We found that the whole-gut transit time was dramatically increased in pcR1KO mice compared with control mice (Fig. 3e). Taken together, IP3R1 was indispensable for physiological segmental and propulsive movements of the gut and the dysfunctional peristalsis observed in young pcR1KO mice could potentially account for the food accumulation and intestinal remodeling in elder pcR1KO mice, leading to abdominal distention and eventually mouse mortality.
Fig. 3.
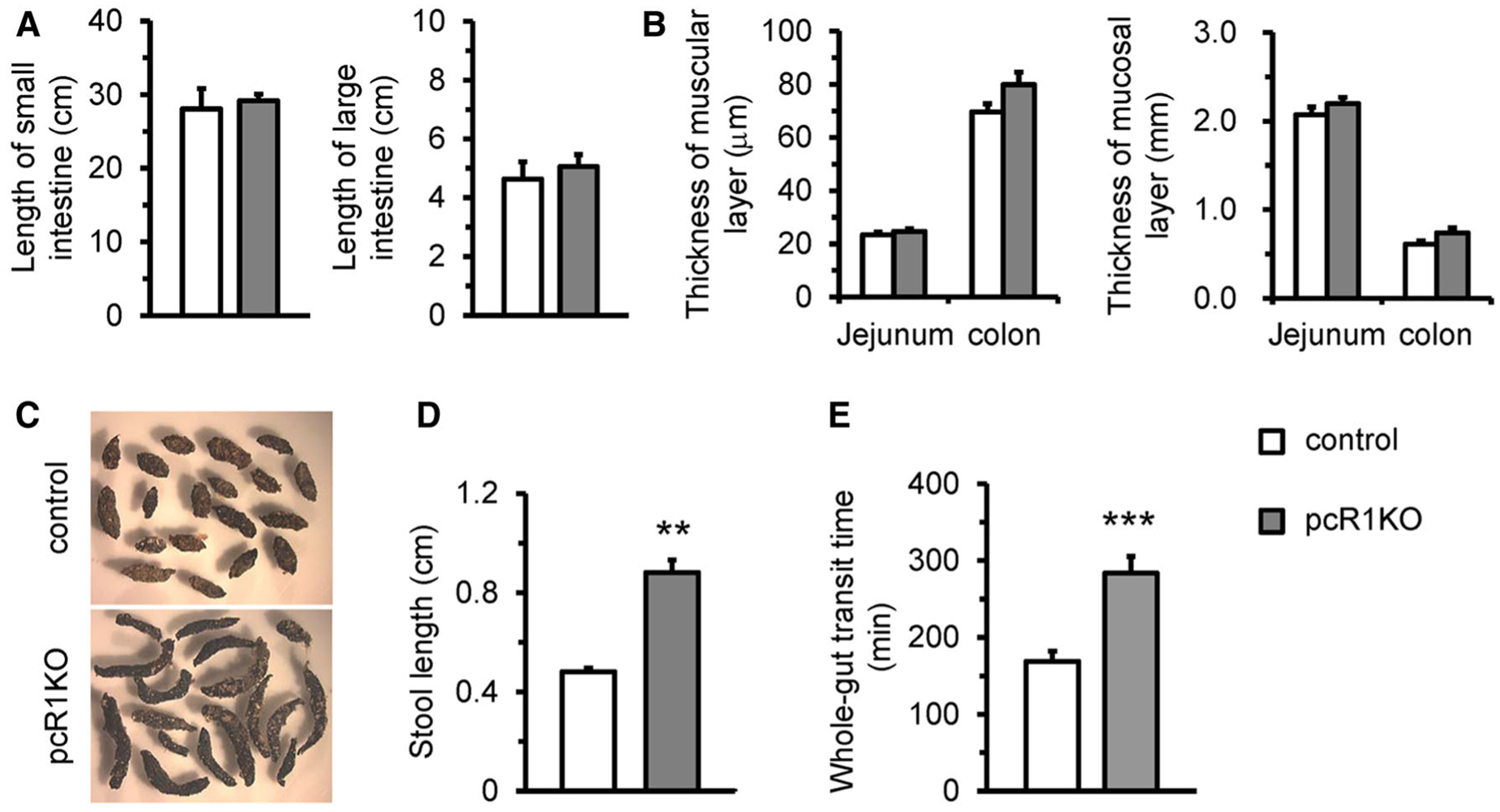
pcR1KO mice exhibited early-onset GI dysfunction. a Length of the small and large intestines in control (n = 6) and pcR1KO (n = 5) mice at 1 month of age. b The thicknesses of the muscular and mucosal layers were measured from transverse sections of the jejunum and colon collected from control and pcR1KO mice. n = 5 mice per group. c and d Stools collected from control and pcR1KO mice at 1 month of age, respectively. The stools from pcR1KO mice were significantly longer those from control mice; n = 4 mice per groups. At least 5 stools were collected from each mouse. e Measurement of whole-gut transit time in individual caged control and pcR1KO mice. The time taken for excretion of the first colored feces was recorded; n = 13 mice per group. For all data significance was determined using a 2-tailed, unpaired Student’s t test. **P < 0.01, ***P < 0.001 versus control. Error bars represent mean ± SEM
Contractile functional changes in the GI tract of pcR1KO mice
Smooth muscle cells are the primary contractile components of the GI tract and contractile dysfunction has been associated with various GI diseases including intestinal pseudo-obstruction and megacolon [30–34]. Therefore, we further assessed the effects of IP3R1 deletion on smooth muscle contractility. The colons were isolated from 1-month-old control and pcR1KO mice and muscle strips with mucosa were suspended in a circular direction. Contractions were measured using isometric tension recordings. We first investigated whether deletion of IP3R1 could affect spontaneous contractions, which are supposed to be generated by ICCs that are electrically coupled to smooth muscle cells [1]. We found that loss of IP3R1 significantly reduced the frequency of spontaneous contractions in colonic muscles, while the amplitude of spontaneous contractions actually increased in pcR1KO muscles compared with control muscles (Fig. 4a, b). We then measured the contractile responses of colons from either genotype to the muscarinic agonist (carbachol, CCh), electrical field stimulation (EFS), and KCl. Treatment with CCh (10 μm) induced phasic contractions in both control and pcR1KO muscles, but deletion of IP3R1 significantly decreased both the peak amplitudes and the averaged tensions induced by CCh (Fig. 4c, d). Furthermore, in both control and pcR1KO colonic muscles, the cessation of EFS elicited a transient contraction, also named the off-contraction, but the amplitude of this off-contraction in pcR1KO muscle was significantly reduced (Fig. 4e, f). However, high concentration of KCl (60 mM) was able to elicit a quick and sustained contraction in colonic circular muscles of both control and pcR1KO mice, but the amplitude of the tension induced by KCl was not significantly altered in mutant muscles (Fig. 4g, h), suggesting the contractile capacity of colonic smooth muscles was not affected by loss of IP3R1. Consistently, the expression of smooth muscle-specific contractile genes, including Acta2, Myh11, and Mlck, was not significantly changed between control and pcR1KO colonic smooth muscles (Fig. 4i).
Fig. 4.
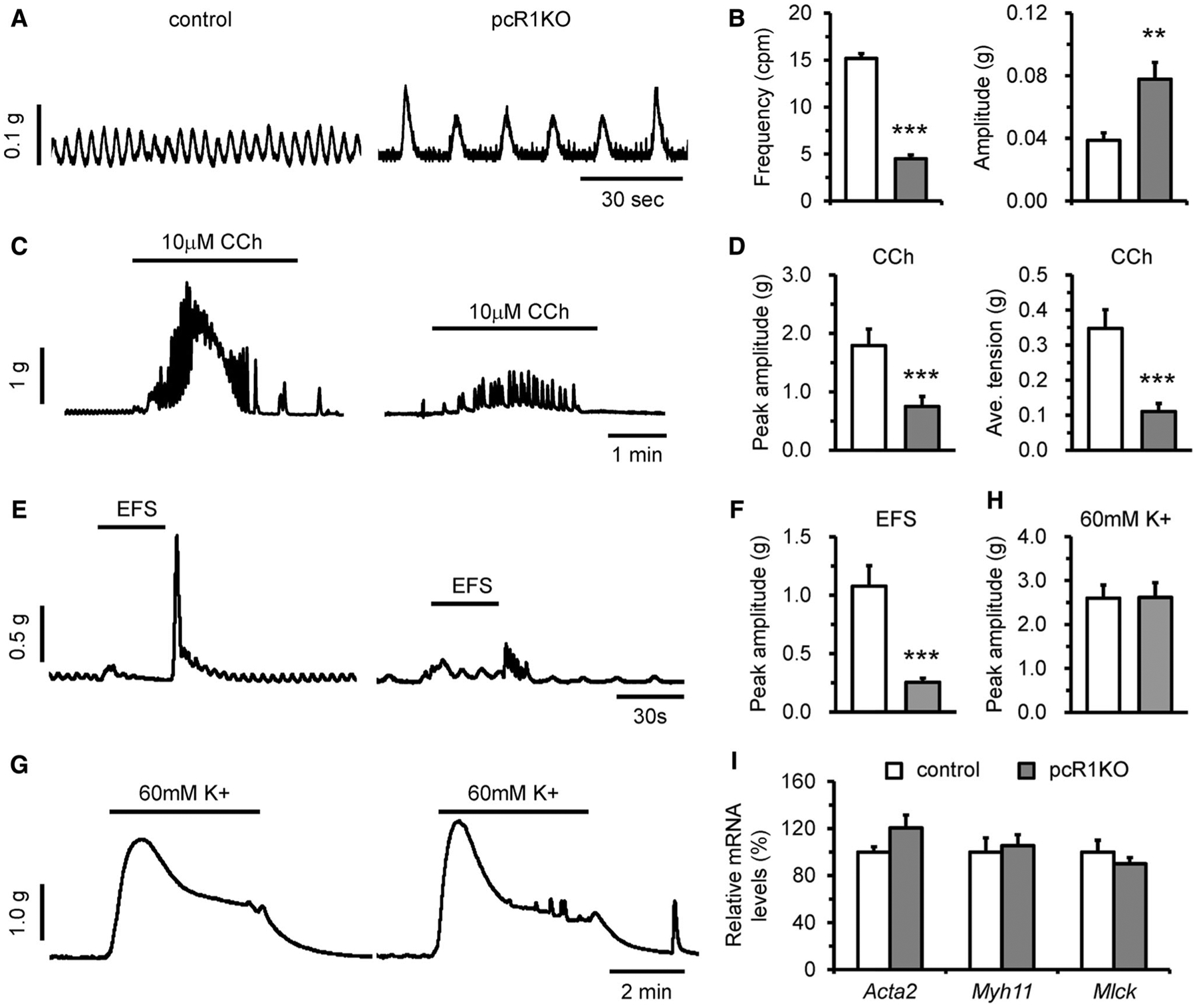
Deletion of IP3R1 caused defects in colonic circular muscle contractility. The colons were isolated from 1-month-old control and pcR1KO mice, respectively. Muscle strips with mucosa were suspended in the circular direction, and contractility was measured using isometric tension recording. a Representative spontaneous contractions in colonic segments from control and pcR1KO mice. b Statistical analysis showing that the frequency of spontaneous contractions was dramatically reduced, whereas the amplitude was increased in pcR1KO colons; n = 19–20 muscle strips per group. c Representative recordings of contraction induced by 10 μm carbachol (CCh). d Quantitative analysis of peak amplitude and averaged tension showing contraction induced by CCh in pcR1KO colons was significantly reduced; n = 19–20 muscle strips per group. e Representative recordings of contraction induced by electrical field stimulation (EFS). f Quantitative analysis of peak amplitude demonstrating contractions induced by EFS in pcR1KO colons was significantly reduced; n = 17–18 muscle strips per group. g Representative recordings of contraction induced by 60 mM KCl (K +). h Quantitative analysis of peak amplitudes in control and pcR1KO colons; n = 18 muscle strips per group. i Quantitative RT-PCR analysis of the expression of Acta2, Myh11, and Mlck in control and pcR1KO colonic muscles; n = 4 mice per group. For all data significance was determined using a 2-tailed, unpaired Student’s t test. **P < 0.01, ***P < 0.001 versus control. Error bars represent mean ± SEM
Distribution of ICCs and enteric neurons was not obviously altered after deletion of IP3R1
ICCs play an important role in regulating generation of slow waves in the GI tract, and loss of ICCs or disruption of ICC networks has been associated with abnormal GI function [35–37]. Therefore, we performed immunostaining using an antibody against cKit to assess whether the ICC network was affected by deletion of IP3R1. Depending on the distribution and function, ICCs in the colon can be divided into three major subgroups, ICC-MY at the layer of the myenteric plexus, ICC-IM in the intramusculature of the longitudinal and circular muscle layers, and ICC-SM on the submucosal surface of the circular muscle layer [13]. However, we did not observe differences in the distribution of cKit-positive cells in between pcR1KO and control colons at 1 month of age. In fact, ICC-MY, ICC-IM, and ICC-SM were clearly observed in the corresponding area in both control and pcR1KO colons (Fig. 5a). Since gene recombination mediated by Pdgfrb-Cre was also observed in enteric neurons, we next investigated the effect of IP3R1 deletion on the density of enteric ganglions. Therefore, we performed immunostaining against PGP9.5, a neuronal marker, to identify enteric ganglions, and found ganglions could be clearly observed in the myenteric plexuses of both control and pcR1KO colons at 1 month of age (Fig. 5b). Moreover, we also calculated the number of ganglions in each section and found that the density of ganglions in the colon was not significantly different in pcR1KO mice compared with control mice (Fig. 5c). We next performed whole-mount staining for NADPH diaphorase to examine the structure of the myenteric plexus in control and pcR1KO colons and found that loss of IP3R1 did not disrupt the networks of the myenteric plexus in either the middle or distal segments of the pcR1KO colons. Since NADPH diaphorase staining labels neuronal nitric oxide synthase (nNOS)-positive inhibitory neurons in the myenteric plexus [38, 39], our data also indicate that the density of nNOS-positive neurons was not significantly altered by deletion of IP3R1 (Fig. 5d, e). Taken together, our results implicated no major disruption of ICCs and enteric neurodevelopment in the colon of pcR1KO mice. However, it remains to be determined whether loss of IP3R1 results in more subtle malformations or defects in cellular differentiation and function of these neuronal structures.
Fig. 5.
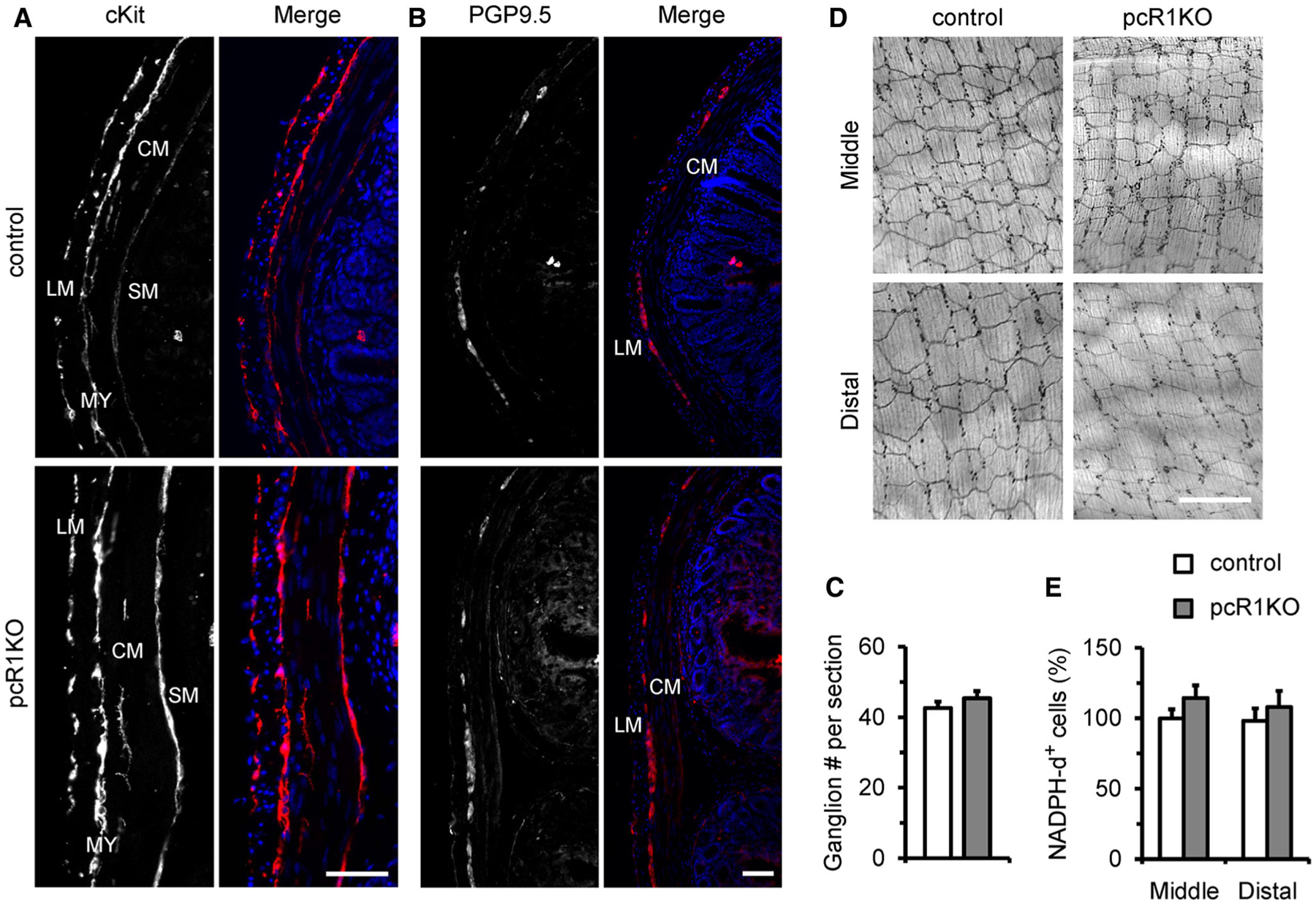
Distribution of ICCs and enteric neurons were not altered in pcR1KO colons. a Representative transverse sections of control and pcR1KO colons labeled with cKit to identify ICC distribution. In both control and pcR1KO sections, cKit-positive cells could be found in the longitudinal muscle (LM) layer, circular muscle (CM) layer, myenteric plexus (MY), and submucosal surface (SM). Data are representative of at least three independent experiments. Scale bar, 50 μm. b Representative transverse sections of control and pcR1KO colons labeled with the neuronal marker, PGP9.5. PGP9.5-positive cells were observed in the myenteric plexus between the longitudinal (LM) and circular (CM) muscle layers. Data are representative of at least three independent experiments. Scale bar, 50 μm. c The number of ganglions per section were calculated in control and pcR1KO mice. At least three independent sections were used for each mouse. n = 5 mice per group. d Whole-mount staining for NADPH diaphorase showing the distribution of nNOS-positive inhibitory neurons in middle and distal colons from control and pcR1KO mice. e Normalization of the densities of NADPH diaphorase-positive (NADPH-d+) cells in control and pcR1KO colons. Scale bar, 400 μm; n = 4 mice per group. Significance was determined using a 2-tailed, unpaired Student’s t test. Error bars represent mean ± SEM
Discussion
In this study, we used Pdgfrb-Cre, a widely utilized model to target vascular smooth muscle cells and pericytes, to delete IP3R1 in mice. We initially wanted to investigate whether deletion of IP3R1 by Pdgfrb-Cre affects vascular development and blood pressure regulation. The Pdgfrb-Cre mouse line [24], where the expression of Cre recombinase is driven by a transgenic fragment of the Pdgfrb gene, has been used extensively to target vascular smooth muscle cells and pericytes [24, 40]. In addition, Pdgfrb-Cre has also been used to target hepatic stellate cells [41] and embryonic lymphatic endothelial cells in the developing mesentery and the heart [42, 43]. Here, we provided data to show that Pdgfrb-Cre targeted not only smooth muscle cells in the GI tract, but also the enteric neurons and ICCs although it remains to be determined if the Cre is also expressed in enteric glial cells. Furthermore, it turned out that mutant mice developed early onset GI motility disorder and prolonged whole-gut transit times. These deficits led to food stasis in the lumen and abdominal distension and were associated with histological changes in the length of small and large intestines as well as in the muscular and mucosal layers, which is likely an adaptive response that somehow differs in some degree due to different workloads of smooth muscle in the GI tract. In the end, all of these defects resulted in malnutrition, cachexia, and even lethality in the mutant mice.
In the GI tract, the musculature is composed of longitudinal and circular muscular layers that are separated by the myenteric plexus, a component of the enteric nervous system. In addition, like other motor systems in the body, GI muscles exhibit spontaneous electrical and contractile activity, which is normally controlled by ICCs [1]. Therefore, in normal individuals, enteric neurons, ICCs, and smooth muscle cells are histologically well arranged and functionally coordinated in the GI tract to generate appropriate contractile patterns. Any neuropathic, mes-enchymopathic (loss of ICCs or disrupted ICC network) or myopathic changes separately or in combination may lead to GI dysfunction. As an intracellular ER Ca2+ release channel, IP3R may mediate various roles in regulating GI functions. First, IP3 R-mediated Ca2+ release is able to regulate smooth muscle contraction. Smooth muscle cells are the primary contractile components of the GI tract, and contractile dysfunction of smooth muscle cells is associated with various diseases, including hypertension, atherosclerosis, asthma as well as GI motility disorders [44]. Using genetically engineered mouse models, the essential role of smooth muscle contractile function in the pathogenesis of GI motility disorders has been greatly highlighted. For example, global deletion of smoothelin-A, smooth muscle-specific deletion of the α subunit of the heterotrimeric G stimulatory protein (Gsα) or smooth muscle-specific deletion of the serum response factor (SRF) in mice results in severe intestinal pseudo-obstruction, accompanied with reduced expression of smooth muscle contractile proteins and diminished contraction [30, 31, 33, 34]. IP3-dependent Ca2+ mobilization and contraction were described in single isolated intestinal circular muscle cells about 30 years ago [45, 46]. Furthermore, it has been shown that IP3R1 is predominantly expressed in muscular cells of the GI tract [47]. Consistently, our results also demonstrate that loss of IP3R1 dramatically decreased the contractile response induced by CCh in colonic circular muscles and caused a phenotype similar to intestinal pseudo-obstruction. Considering that KCl-induced contraction and expression of smooth muscle contractile genes were not significantly changed in pcR1KO colons, further investigation using a smooth muscle cell-specific IP3R1 knockout mouse model should be performed to validate whether reduced circular muscle contractile responses directly results in GI motility disorders and abdominal distention in mice.
Damage to the functional and/or structural integrity of the enteric neural system may play a critical role in regulating gut motility. It has been shown by immunohisto-chemistry that IP3R1 was expressed in enteric neurons [18]. Functionally, histamine induces intracellular Ca2+ mobilization in these cells, which is blocked by 2-aminoethoxydiphenyl borate (2-APB), an IP3R inhibitor [18]. Our results showed that the distribution and density of enteric neurons in pcR1KO colon were not significantly altered, suggesting that IP3R1 is dispensable for the development of enteric neural system. Although we observed that the off-contraction induced by EFS was significantly reduced in pcR1KO colonic muscles, it remains unclear whether reduced contractile responses are a result of inhibition of excitatory signaling or activation of inhibitory signaling in the myenteric plexus, making this an important issue for future studies. Another interesting question that needs to be addressed in the future is whether deletion of IP3R1 in autonomous or central nervous system could also influence GI motility.
On the other hand, IP3R-mediated Ca2+ release may also regulate the activity of ICCs in the GI tract. ICCs are pacemaker cells that generate electrical slow waves in the GI tract. Slow waves drive phasic contractions that are the basic contractions of segmentation and gastric peristalsis. Loss of ICCs or disruption of ICC networks has been associated with abnormal GI function [35–37]. We did not find obvious changes in the distribution of ICCs in pcR1KO colons, suggesting that IP3R1 is not required for the development and formation of ICC networks, which is consistent with what have been found in IP3R1 global knockout mice [22]. The importance of IP3R-mediated Ca2+ release in the generation of slow waves and phasic contractions has been previously investigated mostly using pharmacological approaches. For example, Xestospongin C, a membrane-permeable blocker of IP3R, disrupts slow wave activity [48, 49]. 2-APB, another IP3R blocker with lower specificity, also reduces slow wave frequency and eventually blocks slow waves in guinea pig antral muscles [50]. Furthermore, it has been reported that slow waves were totally lost in the gastric smooth muscles obtained from IP3R1 global knockout mice [22]. However, premature lethality of IP3R1 global knockout mice before weaning prevented further studies on whether and how loss of IP3R1 affects GI morphology and function in adult mice. In the colons isolated from young pcR1KO mice without abdominal distention, the frequency of spontaneous contractions was dramatically reduced but not totally abolished. Although we did not perform electrophysiological experiments to record slow waves directly in control and pcR1KO colons, our results strongly implicate that IP3R1 regulates ICC activities in adult GI tract. However, whether reduced ICC activity is the primary abnormality in the pcR1KO GI tract needs to be further investigated especially in an ICC-specific IP3R1 knockout mouse model.
In summary, we demonstrated that IP3R1, a type of intracellular ER Ca2+ release channel, is important for maintaining normal GI function in vivo. Our pcR1KO mice, in which IP3R1 was deleted by Pdgfrb-Cre, exhibited slower spontaneous contractions and decreased contractile response to CCh, leading to prolonged GI transit time, abdominal distention and lethality. Therefore, we provided a novel mouse model for GI dysmotility and demonstrated that IP3R1 plays a critical role in regulating physiological function of GI tract in vivo.
Acknowledgements
The work was supported by the National Science Foundation of China (31370823, 81700289, 31800767), the Guangdong Province Basic Research Foundation (2018A030310012), the Shenzhen Basic Research Foundation (KCYJ20160428154108239, KQJSCX20170330155020267, JCYJ20170818090044949, KQTD2015032709315529), and the National Institutes of Health (J.C. and F.X.). J.C. is the American Heart Association (AHA) Endowed Chair in Cardiovascular Research.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. [DOI] [PubMed] [Google Scholar]
- 2.Knowles CH, Lindberg G, Panza E, et al. New perspectives in the diagnosis and management of enteric neuropathies. Nat Rev Gastroenterol Hepatol. 2013;10:206–18. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keller J, Bassotti G, Clarke J, et al. Expert consensus document: advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foskett JK, White C, Cheung KH, et al. Inositol trisphosphate receptor Ca2 + release channels. Physiol Rev. 2007;87:593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakazawa M, Uchida K, Aramaki M, et al. Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J Mol Cell Cardiol. 2011;51:58–66. [DOI] [PubMed] [Google Scholar]
- 7.Uchida K, Aramaki M, Nakazawa M, et al. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS ONE. 2010;5:e12500. 10.1371/journal.pone.0012500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto M, Nakagawa T, Inoue T, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–71. [DOI] [PubMed] [Google Scholar]
- 9.Futatsugi A, Nakamura T, Yamada MK, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–4. [DOI] [PubMed] [Google Scholar]
- 10.Lin Q, Zhao G, Fang X, et al. IP3 receptors regulate vascular smooth muscle contractility and hypertension. JCI Insight. 2016. 10.1172/jci.insight.89402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang K, Leandro Gomez-Amaro R, Stachura DL, et al. Loss of IP3R-dependent Ca2 + signalling in thymocytes leads to aberrant development and acute lymphoblastic leukemia. Nat Commun. 2014. 10.1038/ncomms5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H, Wang H, Lin Q, et al. Loss of IP3 receptor-mediated Ca(2 +) release in mouse B cells results in abnormal B cell development and function. J Immunol. 2017;199:570–80. [DOI] [PubMed] [Google Scholar]
- 13.Iino S, Horiguchi K. Interstitial cells of cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem. 2006;39:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makhlouf GM, Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–76. [DOI] [PubMed] [Google Scholar]
- 15.Kuemmerle JF, Murthy KS, Makhlouf GM. Longitudinal smooth muscle of the mammalian intestine. A model for Ca2 + signaling by cADPR. Cell Biochem Biophys. 1998;28:31–44. [DOI] [PubMed] [Google Scholar]
- 16.Ward SM, Baker SA, de Faoite A, et al. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2 + uptake in canine colonic muscles. J Physiol. 2003;549:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu MH, Sung TS, O’Driscoll K, et al. Intracellular Ca(2 +) release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol. 2015;308:C608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehn M, Bader S, Bell A, et al. Distribution of voltage-dependent and intracellular Ca2 + channels in submucosal neurons from rat distal colon. Cell Tissue Res. 2013;353:355–66. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zima AV, Sheikh F, et al. Endothelin-1-induced arrhyth-mogenic Ca2 + signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–81. [DOI] [PubMed] [Google Scholar]
- 20.Cooley N, Ouyang K, McMullen JR, et al. No contribution of IP3-R(2) to disease phenotype in models of dilated cardiomyopathy or pressure overload hypertrophy. Circ Heart Fail. 2013;6:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato-Miyaoka M, Hisatsune C, Ebisui E, et al. Regulation of hair shedding by the type 3 IP3 receptor. J Invest Dermatol. 2012;132:2137–47. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Takano H, Yamamoto Y, et al. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525(Pt 1):105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano H, Imaeda K, Yamamoto Y, et al. Mechanical responses evoked by nerve stimulation in gastric muscles of mouse lacking inositol trisphosphate receptor. Auton Neurosci. 2001;87:249–57. [DOI] [PubMed] [Google Scholar]
- 24.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73. [DOI] [PubMed] [Google Scholar]
- 25.Fang X, Stroud MJ, Ouyang K, et al. Adipocyte-specific loss of PPARgamma attenuates cardiac hypertrophy. JCI Insight. 2016. 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange S, Ouyang K, Meyer G, et al. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci. 2009;122:2640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamada H, Kiyama H. Suppression of c-Kit signaling induces adult neurogenesis in the mouse intestine after myenteric plexus ablation with benzalkonium chloride. Sci Rep. 2016. 10.1038/srep32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friebe A, Mergia E, Dangel O, et al. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci U S A. 2007;104:7699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001. 10.1186/1471-213x-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mericskay M, Blanc J, Tritsch E, et al. Inducible mouse model of chronic intestinal pseudo-obstruction by smooth muscle-specific inactivation of the SRF gene. Gastroenterology. 2007;133:1960–70. [DOI] [PubMed] [Google Scholar]
- 31.Angstenberger M, Wegener JW, Pichler BJ, et al. Severe intestinal obstruction on induced smooth muscle-specific ablation of the transcription factor SRF in adult mice. Gastroenterology. 2007;133:1948–59. [DOI] [PubMed] [Google Scholar]
- 32.Parish IA, Stamp LA, Lorenzo AM, et al. A novel mutation in nucleoporin 35 causes murine degenerative colonic smooth muscle myopathy. Am J Pathol. 2016;186:2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niessen P, Rensen S, van Deursen J, et al. Smoothelin-a is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–601. [DOI] [PubMed] [Google Scholar]
- 34.Qin X, Liu S, Lu Q, et al. Heterotrimeric G stimulatory protein alpha subunit is required for intestinal smooth muscle contraction in mice. Gastroenterology. 2017;152(1114–25):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isozaki K, Hirota S, Miyagawa J, et al. Deficiency of c-kit + cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol. 1997;92:332–4. [PubMed] [Google Scholar]
- 36.Boeckxstaens GE, Rumessen JJ, de Wit L, et al. Abnormal distribution of the interstitial cells of cajal in an adult patient with pseudo-obstruction and megaduodenum. Am J Gastroenterol. 2002;97:2120–6. [DOI] [PubMed] [Google Scholar]
- 37.Feldstein AE, Miller SM, El-Youssef M, et al. Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J Pediatr Gastroenterol Nutr. 2003;36:492–7. [DOI] [PubMed] [Google Scholar]
- 38.Santer RM. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994;49:115–21. [DOI] [PubMed] [Google Scholar]
- 39.Fu M, Landreville S, Agapova OA, et al. Retinoblastoma protein prevents enteric nervous system defects and intestinal pseudo-obstruction. J Clin Invest. 2013;123:5152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenzel D, Nye E, Nisancioglu M, et al. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–24. [DOI] [PubMed] [Google Scholar]
- 41.Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klotz L, Norman S, Vieira JM, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanczuk L, Martinez-Corral I, Ulvmar MH, et al. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 2015. 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 45.Murthy KS, Grider JR, Makhlouf GM. InsP3-dependent Ca2 + mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol. 1991;261:G937–44. [DOI] [PubMed] [Google Scholar]
- 46.Grider JR, Makhlouf GM. Suppression of inhibitory neural input to colonic circular muscle by opioid peptides. J Pharmacol Exp Ther. 1987;243:205–10. [PubMed] [Google Scholar]
- 47.Siefjediers A, Hardt M, Prinz G, et al. Characterization of inositol 1,4,5-trisphosphate (IP3) receptor subtypes at rat colonic epithelium. Cell Calcium. 2007;41:303–15. [DOI] [PubMed] [Google Scholar]
- 48.Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP(3)-sensitive calcium release in the murine small intestine. Am J Physiol Gastrointest Liver Physiol. 2001;280:G439–48. [DOI] [PubMed] [Google Scholar]
- 49.Ward SM, Ordog T, Koh SD, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickens EJ, Edwards FR, Hirst GD. Vagal inhibition in the antral region of guinea pig stomach. Am J Physiol Gastrointest Liver Physiol. 2000;279:G388–99. [DOI] [PubMed] [Google Scholar]


