Abstract
Objective
To investigate whether a simplified inflammation-based risk scoring system comprising three readily available biomarkers (albumin, C-reactive protein, and leukocytes) may predict major adverse outcomes in patients with COVID-19.
Methods
Upon admission to the emergency room, the inflammation-based risk scoring system was applied and patients were classified as having mild, moderate, or severe inflammation. In-hospital occurrence of thrombosis, need for mechanical ventilation, and death were recorded.
Results
One-hundred patients (55 ± 13 years; 71% men) were included and classified as having mild (29%), moderate (12%), or severe (59%) inflammation. The need for mechanical ventilation differed among patients in each group (16%, 50%, and 71%, respectively; P < 0.0001), yielding a 4.1-fold increased risk of requiring mechanical ventilation in patients with moderate inflammation and 5.4 for those with severe inflammation. On the contrary, there were no differences for the occurrence of thrombosis (10%, 8%, and 22%, respectively; P = 0.142) or death (21%, 42%, and 39%, respectively; P = 0.106). In the multivariate analysis, only severe inflammation (hazard ratio [HR] = 4.1), D-dimer > 574 ng/mL (HR = 3.0), and troponin I ≥ 6.7 ng/mL (HR = 2.4) at hospital admission were independent predictors of the need for mechanical ventilation.
Conclusion
The inflammation-based risk scoring system predicts the need for mechanical ventilation in patients with severe COVID-19.
Keywords: COVID-19, Inflammation, C-reactive protein, Albumin, Leukocytes, Mechanical ventilation
Introduction
In December 2019, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection occurred in Wuhan, China, and rapidly spread around the world. In February 2020, the World Health Organization (WHO) officially named the disease caused by the new virus as coronavirus disease 2019 (COVID-19), which is now considered to be the largest public health emergency in the last century, with more than 130 million confirmed cases to date. Although most patients with COVID-19 are asymptomatic or develop only mild disease, a significant proportion of patients develop severe disease, which can lead to adverse outcomes, such as pneumonia and acute respiratory distress syndrome, coagulopathy and immune-mediated thrombosis, organ failure, and death [1, 2].
These manifestations are the consequence of acute hyper-inflammation during SARS-COV-2 infection, an entity that is reminiscent of several syndromes grouped under the umbrella term “cytokine storm syndrome,” in which hyper-inflammation and multi-organ disease arise from excessive cytokine release in response to uncontrolled immune activation. The conditions characterized by cytokine storm are diverse and include macrophage activation syndromes associated with autoimmune diseases (e.g., systemic juvenile idiopathic arthritis, adult-onset Still’s disease, and systemic lupus erythematosus) or with infectious diseases (e.g., viruses of the influenza and herpes families), as well as mutations in genes that mediate the release of cytotoxic granules from natural killer cells and CD8 + T cells (e.g., familial hemophagocytic lymphohistiocytosis) [3, 4].
Given the magnitude of the COVID-19 pandemic, identifying laboratory test results that reliably predict the progression to severe and fatal forms of the disease has become a top priority. Studies that have evaluated many molecules as potential early prognostic markers have shown that elevated levels of D-dimer, troponin I (cTnI), C-reactive protein (CRP), serum amyloid A, interleukin-6 (IL-6), IL-2 receptor alpha chain (also called soluble CD25), interferon gamma (IFN-γ), and IL-1β are associated with disease severity, development of acute respiratory distress syndrome, and mortality [5–7]. Unfortunately, most of these tests are available only for research or their availability is limited to highly specialized hospital centers. This means that most health-care personnel do not have access to a simple and clinically accessible tool for identifying patients at high risk of developing serious or fatal in-hospital complications [8].
In a previous report, we derived and validated a simplified score comprising three readily available biomarkers in the setting of acute coronary syndrome (ACS): serum levels of albumin and CRP, and white blood cell (WBC) count. ACS is an entity in which inflammation has recently emerged as a critical player, and understanding the role of inflammation may help further understanding of its pathogenesis and be useful for clinical stratification purposes [9]. When performed at the time of hospital admission, this simple inflammation-based risk scoring system provides a reliable prediction of in-hospital mortality and identifies patients at high risk of developing adverse outcomes during hospitalization. Consequently, the present study aimed to investigate whether this inflammation-based risk scoring system assessed on patient admission to the emergency room can predict the occurrence of significant adverse outcomes during hospitalization, specifically the need for invasive mechanical ventilation, occurrence of thrombosis, and death in patients with COVID-19.
Methods
Study design
This was a single-center cohort study to assess the utility of a simple inflammation-based risk scoring system as an early predictor of significant adverse outcomes during hospitalization of patients critically ill with COVID-19. This study was performed at the National Institute of Cardiology in Mexico City, Mexico, an academic center for tertiary care that is devoted to the study and management of cardiovascular diseases and allied conditions. As the COVID-19 pandemic evolved, our hospital converted the emergency room and cardiovascular critical care unit into areas dedicated to the critical care of patients with COVID-19.
At hospital admission, patients were classified according to the WHO guidelines as having moderate or severe disease. Moderate disease was defined as clinical signs of pneumonia, such as fever, cough, dyspnea, and/or tachypnea, but no signs of severe pneumonia and, in particular, an oxygen saturation (SaO2) ≥ 90% on room air. Severe disease was defined as clinical signs of pneumonia plus one of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or SaO2 < 90% on room air [10].
All patients gave their consent authorizing the use of their clinical data and biological samples for research. The study was approved by the local committee created ad hoc for studies on COVID-19. All procedures were carried out in accordance with the 2013 Declaration of Helsinki, its addenda, and local regulations.
Study participants
Patients older than 18 years who were admitted to the emergency room with a diagnosis of COVID-19 between April 12 and July 20, 2020 and required hospitalization were included. Our hospital received only seriously ill patients during this time. Patients with milder forms of the disease were transferred to less specialized medical centers and therefore were not included in the present analysis. At admission, a nasopharyngeal swab tested positive for SARS-CoV-2 in a test involving RT-PCR, although no serial tests were performed to assess viral clearance. A negative rapid influenza test result was also obtained for all patients. Finally, only patients from whom we received blood samples (obtained upon arrival at the emergency room) for the measurement of the levels of IL-6 and other inflammatory/thrombosis markers were included [11]. To assess comorbid conditions, the Charlson Comorbidity Index was calculated for all patients [12]. Clinical and laboratory data were obtained from the electronic medical record by two independent investigators (JG-F, CG-A), the resulting databases were compared and reviewed by a third investigator (LMA-G), and discrepancies were resolved by reviewing each discordant medical record again.
All treatments, imaging and laboratory studies, admission to the intensive care unit, and the decision to provide mechanical ventilatory support were performed at the discretion of each of the treating physicians. Similarly, the decision to discharge the patient to home was made solely by the treating physician according to the clinical status of each patient.
Definition of the inflammation-based risk scoring system
The complete process used for the derivation and validation of the inflammation-based risk scoring system has been published elsewhere [9]. Briefly, in a cohort of 7,396 patients with ACS, elevated CRP level and WBC count, and low serum albumin level were found to be individually associated with a higher mortality rate. After that observation, a risk score was created by assigning weighted values to each of the inflammation biomarkers based on the odds ratios (ORs) for in-hospital mortality as follows: 1 point for WBC count ≥ 9.3 × 103 cells/μL, 2 points for CRP level ≥ 13.0 mg/L, and 3 points for serum albumin level ≤ 3.6 g/dL. Finally, four categories of systemic inflammation were created based on the total score: 0 points, no signs of systemic inflammation; 1–2 points, mild inflammation; 3–4 points, moderate inflammation; and 5–6 points, severe inflammation. Notably, after adjusting for multiple potential confounders in the multivariate analysis, the category of severe inflammation remained the most powerful predictor of mortality in patients with ACS.
For the purposes of the present study, the aforementioned inflammation-based risk scoring system was applied to each of the patients with COVID-19 whose condition was graded according to the serum levels of CRP and albumin, and WBC count measured upon arrival at the emergency room. In our hospital, serum albumin (reference range 3.5 to 5.7 g/dL) and high sensitivity CRP (reference range < 5 mg/L) are measured by photometry using the AU680 clinical chemistry analyzer (Beckman Coulter, Fullerton, CA), while WBCs are measured using the DxH900 automated hematology analyzer (Beckman Coulter, Miami, FL).
Statistical analysis
The data distribution was evaluated using the D’Agostino–Pearson K-squared test. Frequencies and percentages were used to describe categorical variables, and differences were assessed using the chi-square test (for trend as appropriate). To describe numerical variables, the mean ± 1 standard deviation (SD) or median with interquartile range (IQR) was used, and differences were evaluated using the Mann–Whitney U test or Student’s t test (two groups) and the Kruskal–Wallis test (post hoc test by Dunn) or one-way ANOVA (Tukey’s multiple comparison post hoc test) for linear trend (> 2 groups) as appropriate. The area under the receiver operating characteristic (ROC) curve (AUC) with 95% confidence intervals (95% CIs) was used to assess the potential of the inflammation-based risk scoring system to discriminate the risk of having a major adverse outcome: that is, thrombosis, need for invasive mechanical ventilation, or death during hospitalization.
For subsequent analyses, the cohort of patients with COVID-19 was stratified according to the inflammation-based risk scoring system. Only two patients had no systemic signs of inflammation (score, 0) and they were added to the mild inflammation group for the statistical calculations. Thus, the total cohort was stratified into patients with mild, moderate, or severe inflammation. Cumulative survival curves for the occurrence of each major adverse outcome during the hospital stay were constructed using the Kaplan–Meier method, and differences were assessed using the log-rank test for trends. Hazard ratios (HRs) with 95% CIs were obtained using a Cox proportional hazards model and the log-rank test. The length of survival was defined as the time of entry into the study (day of admission to the emergency room) until the occurrence of each major adverse outcome or discharge from the hospital. Discharged patients were considered censored observations at the time of their last day of hospital stay. The Kaplan–Meier curves were truncated at 30 days of follow-up.
Multivariate Cox proportional hazards models were used to assess the contribution of different variables in the occurrence of major adverse outcomes. The selected variables included those showing significance in the univariate analysis as well as others that are biologically or clinically relevant or that have been described as significant in other studies. Adjusted and unadjusted HRs with 95% CIs were used to describe significant associations. In the multivariate modeling, the main numerical variables were grouped into terciles to facilitate the clinical interpretation through predetermined ranges, and the first tercile was used as the reference value.
All analyses were two-tailed and a P value ≤ 0.05 was accepted as significant. GraphPad Prism statistical software (v. 6.01; GraphPad Software, La Jolla, CA) and IBM SPSS Statistics (v. 20; IBM Corp, Armonk, NY) were used for the calculations.
Results
During the study period, a total of 100 patients (71% male) with a mean age of 55 ± 13 years were included. Ninety patients were classified as having severe disease at admission and the rest with moderate disease. Table 1 summarizes the main clinical and demographic characteristics. An unusually high frequency of comorbidities was observed; the prevalence rates were 47% for hypertension, 37% for diabetes, 78% for overweight or obesity, and 13% for chronic kidney disease. The median Charlson Comorbidity Index was 2 (IQR, 1–3).
Table 1.
Demographic and clinical features of patients with COVID
| All patients (n = 100) | IRS system | P | Post-hoc test* | |||
|---|---|---|---|---|---|---|
| Mild (n = 29) |
Moderate (n = 12) |
Severe (n = 59) |
||||
| Age, years | 55 ± 13 | 49 ± 13 | 54 ± 13 | 57 ± 12 | 0.030 |
a: 0.999 b: 0.026 c: 0.999 |
| Male, n (%) | 71 (71) | 20 (69) | 8 (67) | 43 (73) | 0.874 | |
| BMI, kg/m2 | 28.6 ± 4.8 | 27.9 ± 3.6 | 29.3 ± 5.0 | 28.8 ± 5.3 | 0.611 | |
| Hypertension, n (%) | 47 (47) | 11 (38) | 7 (58) | 29 (49) | 0.430 | |
| Diabetes, n (%) | 37 (37) | 8 (28) | 3 (25) | 26 (44) | 0.211 | |
| Dyslipidemia, n (%) | 17 (17) | 5 (17) | 1 (8) | 11 (19) | 0.686 | |
| Smoking, n (%) | 21 (21) | 6 (21) | 3 (25) | 12 (20) | 0.935 | |
| Cancer, n (%) | 2 (2) | 0 | 0 | 2 (3) | 0.492 | |
| Previous MI, n (%) | 10 (10) | 2 (7) | 1 (8) | 7 (12) | 0.750 | |
| CHD, n (%) | 5 (5) | 3 (10) | 0 | 2 (3) | 0.259 | |
| CKD, n (%) | 13 (13) | 2 (7) | 0 | 11 (19) | 0.110 | |
| Organ transplant, n (%) | 2 (2) | 1 (3) | 0 | 1 (2) | 0.463 | |
| CCI, median (IQR) | 2 (1–3) | 1 (0–3) | 1 (0.7–3.2) | 3 (1–3.5) | 0.032 |
a: 0.999 b: 0.034 c: 0.640 |
Data are presented as mean ± standard deviation unless otherwise specified. Significant P values are in bold
Definitions: IRS system, Inflammation-based risk scoring system; BMI, body mass index; MI, myocardial infarction; CHD, chronic heart disease; CKD, chronic kidney disease; CCI, Charlson Comorbidity Index; IQR, interquartile range
* a: Mild versus Moderate; b: Mild versus Severe; c: Moderate versus Severe
The entire cohort of patients with COVID-19 was classified according to the inflammation-based risk score as follows: two patients showed no signs of systemic inflammation (total score, 0), 27 had mild inflammation (total score, 1–2), 12 had moderate inflammation (total score, 3–4), and 59 had severe inflammation (total score, 5–6). As stated above, for ease of analysis, patients with no signs of inflammation were grouped together with patients with mild inflammation because no differences were observed between them (data not shown). As can be seen in Table 1, patients with severe inflammation were significantly older and exhibited a higher burden of comorbidities according to the Charlson Comorbidity Index compared to patients with mild inflammation.
The main clinical and laboratory characteristics evaluated upon admission to the emergency room are presented in Table 2. Body temperature, SaO2, heart rate, and respiratory rate did not differ between groups. By contrast, significant differences were observed in a variety of laboratory analytes. Specifically, patients with mild inflammation had lower neutrophil and total leukocyte counts than those with moderate and severe inflammation, while lower CRP levels but higher levels of albumin and hemoglobin were observed in patients with mild inflammation compared to their counterparts with severe inflammation (Table 2). No differences were found for other inflammatory markers, such as serum ferritin and IL-6 levels, and platelet count.
Table 2.
Clinical features and laboratory data at admission
| All patients (n = 100) | IRS system | P | Post-hoc test* | |||
|---|---|---|---|---|---|---|
| Mild (n = 29) |
Moderate (n = 12) |
Severe (n = 59) |
||||
| Temperature, °C | 37.1 ± 0.9 | 37.1 ± 0.9 | 37.1 ± 1.0 | 37.1 ± 0.9 | 0.988 | |
| SaO2, % at room air | 79.5 ± 13.9 | 83.5 ± 9.8 | 77.3 ± 12.9 | 78.0 ± 15.4 | 0.182 | |
| Heart rate, beats/min | 97.4 ± 20.0 | 96.5 ± 16.1 | 109.1 ± 14.1 | 95.3 ± 22.1 | 0.063 | |
| Respiratory rate, breaths/min | 24.7 ± 6.2 | 22.5 ± 5.4 | 26.3 ± 7.9 | 25.4 ± 6.0 | 0.079 | |
| WBC × 103/μL, median (IQR) | 8.5 (6.0–11.6) | 6.9 (4.9–7.9) | 13.6 (11.1–14.9) | 8.9 (6.0–11.9) | < 0.0001 |
a: < 0.0001 b: 0.005 c: 0.004 |
| Neutrophils × 103/μL, median (IQR) | 7.5 (4.6–10.5) | 5.2 (3.6–6.9) | 12.0 (9.8–13.6) | 8.2 (5.4–10.7) | < 0.0001 |
a: < 0.0001 b: 0.001 c: 0.006 |
| Lymphocytes × 103/μL, median (IQR) | 0.7 (0.6–1.0) | 0.8 (0.6–1.1) | 0.9 (0.7–1.0) | 0.7 (0.5–0.9) | 0.971 | |
| Hemoglobin, g/dL, median (IQR) | 14.8 (13.6–16.0) | 15.5 (14.7–16.0) | 14.8 (13.9–16.1) | 14.5 (12.9–16.1) | 0.031 |
a: 0.415 b: 0.023 c: 0.850 |
| Platelets × 103/μL, median (IQR) | 204 (163–278) | 179 (150–230) | 228 (170–253) | 221 (175–292) | 0.463 | |
| LDH, U/L, median (IQR) | 345 (262–452) | 295 (209–401) | 410 (344–482) | 357 (269–497) | 0.056 | |
| D-dimer, ng/mL, median (IQR) | 377 (215–665) | 172 (116–443) | 288 (253–1913) | 516 (256–861) | 0.156 | |
| Ferritin, μg/L, median (IQR) | 640 (274–1120) | 438 (198–848) | 598 (247–1848) | 704 (351–1184) | 0.233 | |
| C-reactive protein, mg/L, median (IQR) | 149 (71–257) | 109 (37–154) | 195 (102–257) | 181 (81–302) | 0.004 |
a: 0.074 b: 0.005 c: 0.999 |
| Fibrinogen, mg/dL, median (IQR) | 5.5 (4.6–6.1) | 5.0 (4.3–5.7) | 6.1 (5.5–6.6) | 5.6 (4.6–6.2) | 0.003 |
a: 0.002 b: 0.463 c: 0.013 |
| Albumin, g/dL, median (IQR) | 3.4 (3.1–3.8) | 3.9 (3.7–4.0) | 3.8 (3.6–3.8) | 3.1 (2.9–3.4) | < 0.0001 |
a: 0.317 b: < 0.0001 c: < 0.0001 |
| Troponin I, ng/mL, median (IQR) | 11.6 (5.6–39.0) | 8.2 (4.5–18.5) | 10.5 (5.2–27.2) | 18.3 (6.6–63.2) | 0.501 | |
| Interleukin-6, pg/mL, median (IQR) | 14.2 (4.5–57.0) | 13.3 (4.5–52.1) | 15.6 (4.5–25.2) | 11.5 (4.5–65.4) | 0.529 | |
| Creatine kinase, U/L, median (IQR) | 72 (49–12) | 106 (48–187) | 67 (55–212) | 71 (48–226) | 0.749 | |
| Serum creatinine, mg/dL, median (IQR) | 1.0 (0.8–1.4) | 0.9 (0.8–1.1) | 0.9 (0.8–1.8) | 1.0 (0.8–1.5) | 0.504 | |
Data are presented as mean ± standard deviation unless otherwise specified. Significant P values are in bold
Definitions: IRS system, Inflammation-based risk scoring system; SaO2, oxygen saturation; WBC, white blood cells; IQR, interquartile range; LDH, lactate dehydrogenase
* a: Mild versus Moderate; b: Mild versus Severe; c: Moderate versus Severe
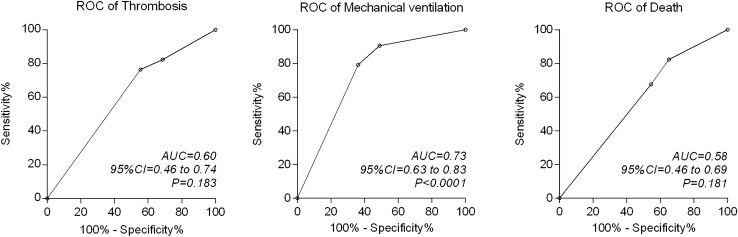
Beyond the basic and advanced life support used for these patients, we found no differences in the use of experimental drugs with potential utility against COVID-19 (see Table 3). Except for one, all patients received anticoagulation therapy with unfractionated or low-molecular-weight heparins during hospitalization. We observed a relatively low prevalence of thrombotic and embolic events (frequency of any event, 17%) and a high percentage of patients with bleeding (16%). The distribution of these adverse events did not differ between the study subgroups. Table 3 also summarizes the occurrence of other significant in-hospital outcomes. A total of 53% of our patients required endotracheal intubation and mechanical ventilatory support, and the frequency differed according to the degree of inflammation. Only five of the 29 patients (16%) with mild inflammation required mechanical ventilation, whereas 50% (6/12 patients) with moderate inflammation and 71% (42/59 patients) with severe inflammation required mechanical ventilation (P < 0.0001). A total of 34 patients eventually died and, although the difference in mortality was substantial between patients with mild, moderate, or severe inflammation, these figures did not reach significance in the analysis for trends (21%, 42%, and 39% mortality, respectively; P = 0.106). The AUC (Fig. 1) showed that the inflammation-based risk scoring system identified those patients who required mechanical ventilation (AUC, 0.73; 95% CI: 0.63 to 0.83; P < 0.0001), but was unable to predict the occurrence of thrombosis (AUC, 0.60; 95% CI: 0.46 to 0.74; P = 0.183) or death (AUC, 0.58; 95% CI: 0.46 to 0.69; P = 0.181).
Table 3.
In-hospital management and major outcomes
| All patients (n = 100) | IRS system | P | Post-hoc test* | |||
|---|---|---|---|---|---|---|
| Mild (n = 29) |
Moderate (n = 12) |
Severe (n = 59) |
||||
| Main therapies | ||||||
| Glucocorticoids, n (%) | 28 (28) | 5 (17) | 3 (25) | 20 (33) | 0.254 | |
| Hydroxychloroquine, n (%) | 22 (22) | 4 (13) | 2 (16) | 16 (27) | 0.326 | |
| Azithromycin, n (%) | 28 (28) | 5 (17) | 2 (16) | 21 (35) | 0.127 | |
| Lopinavir/ritonavir, n (%) | 68 (68) | 21 (72) | 7 (58) | 40 (67) | 0.678 | |
| Biologic drugs, n (%) | 16 (16) | 6 (20) | 2 (16) | 8 (13) | 0.690 | |
| Heparin, n (%) | 99 (99) | 28 (96) | 12 (100) | 59 (100) | 0.290 | |
| Major outcomes | ||||||
| Any thrombosis, n (%) | 17 (17) | 3 (10) | 1 (8) | 13 (22) | 0.142 | |
| PT, n (%) | 2 (2) | 1 (3) | 0 (0) | 1 (2) | 0.747 | |
| Stroke, n (%) | 1 (1) | 0 (0) | 0 (0) | 1 (2) | 0.704 | |
| Myocardial infarction, n (%) | 3 (3) | 1 (3) | 0 (0) | 2 (3) | 0.809 | |
| Bleeding, n (%) | 16 (16) | 1 (3) | 2 (16) | 13 (22) | 0.082 | |
| Mechanical ventilation, n (%) | 53 (53) | 5 (16) | 6 (50) | 42 (71) | < 0.0001 |
a: 0.031 b: < 0.0001 c: 0.152 |
| Death, n (%) | 34 (34) | 6 (21) | 5 (42) | 23 (39) | 0.106 | |
| In-hospital stay, days | 20 ± 18 | 14 ± 12 | 18 ± 16 | 23 ± 20 | 0.089 | |
Data are presented as mean ± standard deviation unless otherwise specified. Significant P value is in bold
Definitions: IRS system, Inflammation-based risk scoring system; PT, pulmonary thromboembolism. Biologic drugs denote the use of tocilizumab, ruxolitinib and/or intravenous immunoglobulin
* a: Mild versus Moderate; b: Mild versus Severe; c: Moderate versus Severe
Fig. 1.
Receiver operating characteristic (ROC) curves for the accuracy of the inflammation-based risk scoring system to predict the development of thrombosis, need for mechanical ventilation, and death during hospitalization of patients with COVID-19
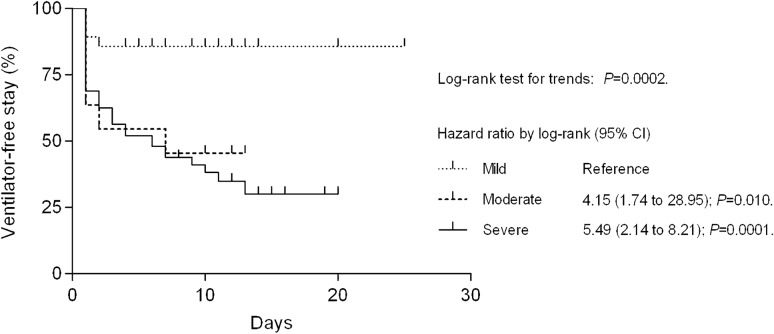
Once the usefulness of the inflammation-based risk scoring system in predicting the requirement for mechanical ventilation was identified, we estimated the percentage of patients who remained ventilator-free during hospitalization. As can be seen in Fig. 2, patients with moderate inflammation had an HR for mechanical ventilation of 4.15 (95% CI: 1.74 to 28.95; P = 0.010), whereas those with severe inflammation had an HR of 5.49 (95% CI: 2.14 to 8.21; P = 0.0001) compared with their counterparts with mild inflammation (reference group). The mean duration of in-hospital stay in patients with mild, moderate, or severe inflammation was 14 ± 12 days, 18 ± 16 days, and 23 ± 20 days, respectively (P = 0.089). For the entire study cohort, this value was 20 ± 18 days.
Fig. 2.
Estimates of ventilator-free survival for patients with COVID-19 categorized according to the inflammation-based risk scoring system at hospital admission. Patients with moderate inflammation had a 4.15 times greater risk of needing mechanical ventilation compared with those with mild inflammation (reference group). This number increased to 5.49 in patients with severe inflammation
The main clinical and laboratory characteristics of patients with COVID-19 grouped according to the need for mechanical ventilation are presented in Table 4. Patients who required mechanical ventilation were older, had lower SaO2 upon admission, and showed higher levels of inflammation and thrombosis markers than their counterparts who did not require mechanical ventilation. In addition, the severe inflammation-based risk score was overrepresented among patients who required mechanical ventilation and, in parallel, they received glucocorticoids more frequently and had a significantly longer hospital stay and higher mortality rate.
Table 4.
Clinical and laboratory characteristics of COVID-19 patients according to the need for mechanical ventilation
| No ventilatory support (n = 47) | Mechanical ventilation (n = 53) | P | |
|---|---|---|---|
| Age, years | 52.2 ± 12.6 | 57.9 ± 12.8 | 0.030 |
| Male, n (%) | 31 (65) | 40 (75) | 0.378 |
| CCI, median (IQR) | 1 (1–3) | 3 (1–3) | 0.062 |
| SaO2, % at admission | 82.4 ± 10.6 | 76.9 ± 15.9 | 0.049 |
| WBC × 103/μL, median (IQR) | 7.0 (5.0–8.7) | 10.8 (7.8–12.4) | < 0.0001 |
| NLR, median (IQR) | 6.5 (3.9–10.0) | 13.1 (9.6–22.2) | < 0.0001 |
| D-dimer, ng/mL, median (IQR) | 241 (136–410) | 544 (290–965) | < 0.0001 |
| Ferritin, μg/L, median (IQR) | 438 (232–822) | 708 (425–1240) | 0.020 |
| Albumin, g/dL, median (IQR) | 3.8 (3.4–3.9) | 3.2 (2.9–3.5) | < 0.0001 |
| CRP, mg/L, median (IQR) | 113.4 (49.4–154.7) | 202.2 (107.3–330.9) | 0.0001 |
| cTnI, ng/mL, median (IQR) | 6.6 (4.3–18.3) | 23.1 (8.4–73.1) | 0.0001 |
| IL-6, pg/mL, median (IQR) | 4.5 (4.5–34.9) | 15.6 (4.5–75.9) | 0.073 |
| Inflammation-based risk scoring system | < 0.0001 | ||
| Mild inflammation, n (%) | 24 (51) | 5 (9) | |
| Moderate inflammation, n (%) | 6 (12) | 6 (11) | |
| Severe inflammation, n (%) | 17 (36) | 42 (79) | |
| Main therapies | |||
| Glucocorticoids, n (%) | 5 (10) | 23 (43) | 0.0003 |
| Hydroxychloroquine, n (%) | 8 (17) | 14 (26) | 0.332 |
| Lopinavir/ritonavir, n (%) | 34 (72) | 34 (64) | 0.400 |
| Anti-cytokine drugs, n (%) | 9 (19) | 7 (13) | 0.585 |
| Major outcomes | |||
| Any thrombosis, n (%) | 4 (8) | 13 (24) | 0.059 |
| Death, n (%) | 3 (6) | 31 (58) | < 0.0001 |
| In-hospital stay, days | 11.3 ± 4.1 | 27.8 ± 22.3 | < 0.0001 |
Data are presented as mean ± standard deviation unless otherwise specified. Significant P values are in bold
Anti-cytokine drugs denote the use of tocilizumab or ruxolitinib
Definitions: CCI, Charlson Comorbidity Index; IQR, interquartile range; SaO2, oxygen saturation; WBC, white blood cells; NLR, neutrophil-to-lymphocyte rate; CRP, C-reactive protein; cTnI, cardiac troponin I; IL-6, interleukin-6; IRS system, Inflammation-based risk scoring system
Finally, several variables in the unadjusted model, such as the inflammation-based risk scoring system and serum levels of D-dimer, ferritin, cTnI, and IL-6, predicted the need for mechanical ventilation (see Table 5). However, when the analyses were adjusted, the independent predictors that remained significant were a severe inflammation-based risk score (HR, 4.12; 95% CI: 1.59 to 10.70; P = 0.004), serum D-dimer level > 574 ng/mL (HR, 3.01; 95% CI: 1.28 to 7.05; P = 0.011), and serum cTnI level ≥ 6.7 ng/mL (HR, 2.42; 95% CI: 1.04 to 5.60; P = 0.039). As noted, the most powerful independent predictor of the need for mechanical ventilation was a severe inflammation-based risk score, even after adjustment for multiple clinical and laboratory variables, such as comorbidities and markers of inflammation and thrombosis, assessed at hospital admission.
Table 5.
Independent predictors at admission of the need for mechanical ventilation in the multivariate Cox proportional hazards model
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Inflammation score | ||||||
| Mild | Reference | Reference | ||||
| Moderate | 3.42 | 1.04 to 11.23 | 0.042 | 3.09 | 0.92 to 10.34 | 0.067 |
| Severe | 5.52 | 2.18 to 13.99 | < 0.001 | 4.12 | 1.59 to 10.70 | 0.004 |
| Age | 1.02 | 0.99 to 1.04 | 0.072 | |||
| Male gender | 1.30 | 0.69 to 2.44 | 0.403 | |||
| BMI | 1.01 | 0.96 to 1.07 | 0.540 | |||
| SaO2%, at admission | 0.97 | 0.96 to 0.99 | 0.023 | |||
| Charlson Comorbidity Index | ||||||
| 1 point | 0.92 | 0.37 to 2.31 | 0.870 | |||
| 2 points | 1.28 | 0.46 to 3.53 | 0.631 | |||
| 3 points | 1.62 | 00.67 to 3.86 | 0.272 | |||
| 4 points | 1.25 | 0.45 to 3.62 | 0.678 | |||
| 5 points | 1.31 | 0.45 to 3.79 | 0.611 | |||
| 6 points | 2.27 | 0.28 to 18.30 | 0.433 | |||
| Lymphocyte count | 0.93 | 0.72 to 1.20 | 0.584 | |||
| D-dimer | ||||||
| 1st tercile (0–246 ng/mL) | Reference | Reference | ||||
| 2nd tercile (247–574 ng/mL) | 2.61 | 1.14 to 5.97 | 0.023 | 2.24 | 0.95 to 5.26 | 0.063 |
| 3rd tercile (> 574 ng/mL) | 5.44 | 4.44 to 12.14 | < 0.001 | 3.01 | 1.28 to 7.05 | 0.011 |
| Ferritin | ||||||
| 1st tercile (0–356 μg/L) | Reference | |||||
| 2nd tercile (356–875 μg/L) | 1.78 | 0.86 to 3.68 | 0.116 | |||
| 3rd tercile (> 875 μg/L) | 2.39 | 1.18 to 4.85 | 0.016 | |||
| Troponin I | ||||||
| 1st tercile (0–6.6 ng/mL) | Reference | Reference | ||||
| 2nd tercile (6.7–24 ng/mL) | 2.46 | 1.08 to 5.60 | 0.032 | 2.42 | 1.04 to 5.60 | 0.039 |
| 3rd tercile (> 24 ng/mL) | 4.19 | 1.88 to 9.35 | < 0.001 | 2.69 | 1.06 to 6.82 | 0.037 |
| Interleukin-6 | ||||||
| 1st tercile (0–4.4 pg/ml) | Reference | |||||
| 2nd tercile (4.5–38.8 pg/ml) | 1.74 | 0.84 to 3.60 | 0.134 | |||
| 3rd tercile (> 38.8 pg/ml) | 2.03 | 1.09 to 3.76 | 0.025 | |||
Definitions: HR, hazard ratio; 95% CI, 95% confidence intervals; BMI, body mass index; SaO2, oxygen saturation
Significant P values are in bold
Discussion
This study was conducted to assess whether a risk scoring system based on serum albumin and CRP levels, and leukocyte counts rated at the time of admission to the emergency room can be used to predict adverse outcomes in patients hospitalized with severe COVID-19. Our results show that this simple and readily available score has a good discriminatory ability to identify patients who will require mechanical ventilation during hospitalization. Having severe inflammation according to this risk scoring system appears to be the most important independent predictor of the need for mechanical ventilatory support in patients with severe COVID-19.
Uncontrolled inflammation is recognized to play an important role in the development of tissue damage, regardless of the type of trigger for the inflammatory response. This is the reason why different acute-phase markers are used to assess disease severity and monitor response to treatment in patients with an inflammatory condition [13]. In parallel, a variety of inflammatory biomarkers are included in risk scoring systems for cardiovascular, neoplastic, and autoimmune diseases [9, 14, 15]. A classic example is the Glasgow Prognostic Score, an inflammation-based clinimetric instrument composed of elevated serum CRP (cutoff 10 mg/L) and decreased albumin (cutoff 3.5 g/L) concentration that has been shown to be accurate in predicting survival in different types of cancer, including colon–rectum, stomach, cervical, kidney, and lung [14]. The Glasgow Prognostic Score was used as the basis for planning and developing the inflammation-based risk scoring system for ACS [9]. Hyper-inflammation is a hallmark of COVID-19, but it can be distinguished from other forms of viral-induced cytokine storm because the elevation of serum ferritin level is moderate and end-organ disease is found mainly in the lungs [3]. Additionally, the hyper-inflammation seen in patients with COVID-19 is characterized by profound abnormalities in the number and function of blood cells, immune-mediated coagulopathy, tissue damage, hepatitis, and activation of hepatocytes and macrophages. Therefore, the type of markers used to assess disease severity and to predict significant outcomes cannot be extrapolated from other forms of cytokine storm syndrome.
Measurement of cytokine levels is an interesting theoretical approach in COVID-19. However, several pro-inflammatory interleukins, such as IFN-γ or IL-1β, are not easily detected in serum or plasma samples, whereas other more stable serum proteins, such as chemokines that are produced in response to IFNs, have not been consistently shown to reliably predict significant outcomes in patients with COVID-19 [3]. Tests for cytokines that may reflect the severity of COVID-19, such as IL-6 and soluble CD25, are still predominantly performed for research and are not available in most medical centers. Unlike the limitations of the aforementioned biomarkers, components of the proposed inflammation-based risk scoring system are highly standardized and available analytes in laboratories around the world [9]. Furthermore, the levels of each reactant reflect selected components of the inflammatory process. CRP is synthesized and released by hepatocytes and other cells in response to various inflammatory cytokines, mainly IL-6. The decrease in albumin synthesis during inflammation is mediated by IL-6, IL-1β, and tumor necrosis factor, all of which are involved in the cytokine storm [16]. The damage to hepatocytes that occurs during hyper-inflammation could cause further decrease in the serum albumin level [17]. Finally, an elevated WBC count may exacerbate inflammatory signaling and facilitate chemotactic gradients, which would guide selected leukocyte subpopulations to sites of tissue and organ injury, such as the lung [18].
Notably, each of the biomarkers included in the inflammation-based risk scoring system is independently associated with the occurrence of adverse outcomes of COVID-19, including acute respiratory distress syndrome, disease severity, and death. CRP level has been shown to correlate with the extent of lung lesions on computed tomography and appears to reflect disease severity [19]. In a study of patients with early-stage COVID-19 (within 7 days of the onset of clinical symptoms), elevated CRP level was an important risk factor for progression to severe pneumonia (OR, 4.77; 95% CI: 1.92 to 11.87; P = 0.001) [20]. A retrospective analysis showed that a CRP level > 10 mg/L at admission was associated with a higher probability of admission to the intensive care unit in COVID-19 patients with cardiovascular disease (OR, 8.12; 95% CI: 1.63 to 40.49; P = 0.011), which suggests that monitoring CRP level could help in risk stratification in patients with early-stage disease [21]. Researchers have validated the observation that CRP level is highly predictive of the need for mechanical ventilation. In one study, a CRP level > 97 mg/L correctly classified 80% of patients with COVID-19 who went on to develop respiratory failure [22]. Consistent with this earlier report, 67 of our 100 patients had CRP values > 97 mg/L, 41 of whom required mechanical ventilation, whereas only 12 of the 33 with a CRP level below that level required ventilatory support (61% vs 36%; P = 0.032). Finally, a recent meta-analysis identified a significant and consistent difference in CRP levels between patients with severe and non-severe COVID-19. In addition to cTnI, D-dimer, procalcitonin, and a few other biochemical markers, CRP emerged as a biomarker with high potential for prognostic risk stratification in patients with COVID-19 [23].
Hypoalbuminemia occurs in about one-third of patients with COVID-19, particularly in those with severe disease [24]. Several studies have found a consistent association between low albumin level and an increase in both mortality and length of hospital stay [24]. A serum albumin level < 3.5 g/dL is an independent predictor of death and increases the OR for in-hospital mortality by up to six times [25]. Interestingly, serum albumin level on admission also predicts the need for mechanical ventilation in patients with influenza A H1N1 infection, and hypoalbuminemia is associated with hyper-inflammation, hyper-coagulation, and atherosclerosis in patients with human immunodeficiency virus infection [26, 27]. Hypoalbuminemia in patients with COVID-19 may be partially explained by liver dysfunction, as reflected by elevated levels of aminotransferases and lactic dehydrogenase. Liver dysfunction may be secondary to angiotensin-converting enzyme 2 (ACE2)-mediated hepatitis, although a recent study detected active viremia in only 7% of patients, which means that liver dysfunction caused by ACE2-mediated hepatitis does not seem to account for the liver disorders commonly observed in patients with COVID-19 [28]. By contrast, a dysregulated immune response may be a crucial pathogenic contributor to liver disorders in the early stages of COVID-19, which could lead to increased capillary permeability and the release of albumin into the interstitial space [24]. A causative role of hyper-inflammation is supported by the extremely high levels of CRP, IL-6, and macrophage colony-stimulating factor found in patients with COVID-19 and liver injury and hypoalbuminemia [28].
At the beginning of the pandemic, a pivotal study characterized 41 patients admitted to hospital for SARS-CoV-2 infection in Wuhan, China. These patients presented with dyspnea, fever, myalgia, abnormalities in blood cells, elevated plasma levels of several cytokines and chemokines, as well as radiographic evidence of pneumonia [29]. Later studies around the world have shown that patients with COVID-19 frequently present with leukocytosis despite the existence of lymphopenia at the expense of an increase in the absolute number of neutrophils [30–33]. An increase in neutrophil count was also observed in our cohort along with a decrease in the total number of circulating lymphocytes, which was more pronounced in patients with a high score on the inflammation-based risk scoring system. Moreover, WBC count is often significantly higher in patients who do not survive to SARS-CoV-2 infection than in survivors [33]. A recent meta-analysis of 1,289 COVID-19 cases identified that severe disease is associated with lower lymphocyte and higher leukocyte counts, which suggests that the neutrophil-to-lymphocyte ratio (NLR) may be useful for early identification of patients with a serious illness [34]. In our cohort, the NLR upon admission was double in patients who eventually required mechanical ventilation, further supporting the value of this marker in the early identification of patients with more aggressive COVID-19. Furthermore, the NLR and the inflammation-based risk scoring system showed high collinearity (Spearman’s rho coefficient 0.467; P < 0.0001), and thus similar performance in terms of predicting each of the main outcomes.
At this time, risk stratification systems are just emerging to predict major hospital outcomes in COVID-19. In fact, the Ventilation in COVID Estimator (VICE) risk score was recently derived and validated in a large single-center cohort, including four factors (diabetes, SaO2/FiO2 ratio, CRP, and lactate dehydrogenase) [35]. Overall, the VICE risk score predicts the need for mechanical ventilation (AUC, 0.84) in a manner quite similar to that observed in our study, although it only included one acute-phase reactant (namely CRP). In the present study, the inflammation-based risk scoring system also demonstrated an adequate capacity to identify these high-risk patients at hospital admission. We believe that this scoring system can be easily implemented in virtually any medical facility dedicated to the care of patients with COVID-19, particularly in those with limited access to high-tech laboratory tests or located in low-income countries. Experience from hyper-inflammation in other forms of cytokine storm syndrome suggests that early intervention is essential to avoid life-threatening complications [36]. In patients with COVID-19, anti-inflammatory treatment with dexamethasone resulted in lower mortality at 28 days among patients receiving invasive mechanical ventilation or oxygen without invasive mechanical ventilation but not in those without this respiratory support [37]. This paramount observation supports the idea that hyper-inflammation, which plays a critical role in COVID-19-associated lung injury, could be tamed by the use of glucocorticoids, resulting in less lung damage and perhaps less need for mechanical ventilatory support. In this sense, it is attractive to assume that the inflammation-based risk scoring system may be useful for identifying patients who might be particularly susceptible and would therefore benefit from dexamethasone administration. In a similar way, the divergence of results of studies that have evaluated the efficacy of tocilizumab, a monoclonal antibody directed against IL-6, in improving adverse outcomes in patients with COVID-19 is notable. Although some studies have not shown a decrease in mortality after tocilizumab administration [38], other studies have suggested that tocilizumab may be beneficial in improving hospital outcomes, provided that early intervention is performed and patients are selected accordingly to some clinical (e.g., Sequential Organ Failure Assessment [SOFA] score) and laboratory (IL-6, CRP, D-dimer, and cTnI levels, WBC counts) features [39]. Therefore, it is conceivable to assume that the inflammation-based risk scoring system may be useful for preselecting patients who have an appropriate inflammatory profile and may be candidates for anti-cytokine therapies.
The results of the present study should be tempered by its limitations. First, the sample size was limited because we included only patients admitted to a single highly specialized center, and the results must be externally validated before they can be generalized to other hospitals. Second, our study included mostly critically ill patients, and the results cannot be extrapolated to patients with milder forms of the disease without further research. Third, it is increasingly recognized that frailty is relevant in the prognosis of COVID-19, either because patients with poorer baseline function (greater frailty) are placed in less proactive treatment streams or because frailty by itself, specifically in patients older than 65 years, leads to higher in-hospital mortality [40]. Unfortunately, we do not rate the frailty of our patients upon admission. Finally, the inflammation-based risk scoring system was not efficient enough to discriminate patients with COVID-19 who would eventually die, and it is evident that factors other than those associated with the need for mechanical ventilation cannot be evaluated using the inflammatory markers in our scoring system. In particular, the administration of therapies, such as glucocorticoids and anti-cytokine therapies during hospitalization, could modify mortality, but this was outside the scope of our study. More in-depth studies are urgently required to identify these factors to be able to develop more efficient risk rating systems than the current one. Once the present study has demonstrated the efficacy of the inflammation-based risk scoring system in predicting the need for mechanical ventilation, it remains to evaluate its efficiency against other risk scoring systems, which often do not consider inflammation as highly relevant.
In conclusion, this study identified that a simplified risk scoring system based on the levels of three inflammatory biomarkers available routinely in any hospital setting is useful for predicting the need for mechanical ventilation in patients with severe COVID-19. Such a risk categorization method is urgently needed given the global spread of COVID-19 during the northern hemisphere autumn of 2020, as well as the uncertainly about the dual outbreaks of COVID-19 and influenza that are expected during the upcoming colder months in the northern hemisphere. In hospitals with limited medical resources, this simple risk scoring system may be helpful for prioritizing patients quickly from admission to the emergency room, especially when limited health-care resources must be allocated.
Acknowledgements
Sources of support: none to be acknowledged.
Funding
None to be declared.
Declairations
Conflict of interest
None to be declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O, Hoyt KJ, Han J, Grom AA, Gattorno M, Ravelli A, De Benedetti F, Behrens EM, Cron RQ, Nigrovic PA. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amezcua-Guerra LM. Brief annotations on cytokine release syndrome and interleukin-6 therapeutic blockage in SARS-CoV-2/COVID-19. Arch Cardiol Mex. 2020;90:84–87. doi: 10.24875/ACM.M20000067. [DOI] [PubMed] [Google Scholar]
- 5.Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: a joint american College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper. J Glob Infect Dis. 2020;12:47–93. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipworth B, Chan R, Lipworth S, RuiWen KC. Weathering the cytokine storm in susceptible patients with severe SARS-CoV-2 infection. J Allergy Clin Immunol Pract. 2020;8:1798–1801. doi: 10.1016/j.jaip.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Li R, Wang J, Jiang Q, Gao C, Yang J, Ge L, Hu Q. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. 2020;20:519. doi: 10.1186/s12879-020-05242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Pacheco H, Bojalil R, Amezcua-Guerra LM, Sandoval J, Eid-Lidt G, Arias-Mendoza A, Azar-Manzur F, Álvarez-Sangabriel A, Altamirano-Castillo A, Briseño-Cruz JL, Carrillo-Vega J, Vazquez-Rangel A, Abbate A, Gomez-Arroyo J, Martínez-Sánchez C. Derivation and validation of a simple inflammation-based risk score system for predicting in-hospital mortality in acute coronary syndrome patients. J Cardiol. 2019;73:416–424. doi: 10.1016/j.jjcc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. (2020). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331446. License: CC BY-NC-SA 3.0 IGO.
- 11.Amezcua-Guerra LM, Rojas-Velasco G, Brianza-Padilla M, Vázquez-Rangel A, Márquez-Velasco R, Baranda-Tovar F, Springall R, Gonzalez-Pacheco H, Juárez-Vicuña Y, Tavera-Alonso C, Sanchez-Muñoz F, Hernández-Salas M. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann Rheum Dis. 2021;80:e73. doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Amezcua-Guerra LM, et al. C-reactive protein and complement components but not other acute-phase reactants discriminate between clinical subsets and organ damage in systemic lupus erythematosus. Clin Lab. 2011;57:607–613. [PubMed] [Google Scholar]
- 14.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 15.Inoue E, et al. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–409. doi: 10.1136/ard.2006.054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded "Cardiovascular Continuum". J Am Coll Cardiol. 2016;67:1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Huang X, Gu H, Lou L, Xu Z. Predictive criteria of severe cases in COVID-19 patients of early stage: A retrospective observational study. J Clin Lab Anal. 2020:e23562. [DOI] [PMC free article] [PubMed]
- 21.He F, Quan Y, Lei M, Liu R, Qin S, Zeng J, Zhao Z, Yu, Yang L, Cao J. Clinical features and risk factors for ICU admission in COVID-19 patients with cardiovascular diseases. Aging Dis. 2020;11:763–769. doi: 10.14336/AD.2020.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(e4):128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danwang C, Endomba FT, Nkeck JR, Wouna DLA, Robert A, Noubiap JJ. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19) Biomark Res. 2020;8:37. doi: 10.1186/s40364-020-00217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020 doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Rica R, Borges M, Aranda M, Del Castillo A, Socias A, Payeras A, Rialp G, Socias L, Masmiquel L, Gonzalez-Freire M. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain: a retrospective cohort study. Microorganisms. 2020;8:1106. doi: 10.3390/microorganisms8081106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wi YM, Kim JM, Peck KR. Serum albumin level as a predictor of intensive respiratory or vasopressor support in influenza A (H1N1) virus infection. Int J Clin Pract. 2014;68:222–229. doi: 10.1111/ijcp.12249. [DOI] [PubMed] [Google Scholar]
- 27.Dirajlal-Fargo S, Kulkarni M, Bowman E, Shan L, Sattar A, Funderburg N, McComsey GA. Serum Albumin Is Associated With Higher Inflammation and Carotid Atherosclerosis in Treated Human Immunodeficiency Virus Infection. Open Forum Infect Dis. 2018;5:ofy291. [DOI] [PMC free article] [PubMed]
- 28.Gao Y, Li Q, Shi H, Feng Y, Zhang T, Chen Y, Liang L, Chen D, Wu H, Jin R, Huang X. Preliminary exploration of the cause of liver disorders during early stages in COVID-19 patients. Front Med (Lausanne) 2020;7:501. doi: 10.3389/fmed.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Ye D, Wang M, Zhao M, Li D, Ye J, Liu J, Xu Y, Zhang J, Pan W, Liu M, Luo Z, Wan J. Clinical features of COVID-19 patients with different outcomes in Wuhan: a retrospective observational study. Biomed Res Int. 2020;2020:2138387. doi: 10.1155/2020/2138387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homayounieh F, Zhang EW, Babaei R, Karimi Mobin H, Sharifian M, Mohseni I, Kuo A, Arru C, Kalra MK, Digumarthy SR. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PLoS One. 2020;15:e0239519. doi: 10.1371/journal.pone.0239519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar MS, Haider Kazmi SJ, Khan NA, Akram M, Jawed R, Rafaey W, Hassan M, Rasheed U, Khan M, Khan AR. Role of Biochemical Markers in Invasive Ventilation of Coronavirus Disease 2019 Patients: Multinomial Regression and Survival Analysis. Cureus. 2020;12:e10054. doi: 10.7759/cureus.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Guzman PN, Daunt A, Mukherjee S, Crook P, Forlano R, Kont MD, Løchen A, Vollmer M, Middleton P, Judge R, Harlow C, Soubieres A, Cooke G, White PJ, Hallett TB, Aylin P, Ferguson, Hauck K, Thursz MR, Nayagam S. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang G, Kovalic AJ, Graber CJ. Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerg Infect Dis. 2020;26:1839–1841. doi: 10.3201/eid2608.201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson CJ, Wooster L, Sigurslid HH, Li RH, Jiang W, Tian W, Lino Cardenas CL, Malhotra R. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to Mass General Brigham: The VICE and DICE scores. EClinicalMedicine. 2021;33:100765. doi: 10.1016/j.eclinm.2021.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar B, Aleem S, Saleh H, Petts J, Ballas ZK. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37:638–643. doi: 10.1007/s10875-017-0439-x. [DOI] [PubMed] [Google Scholar]
- 37.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. n Engl. J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda T, Obata R, Rizk DOD, Kuno T. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J Med Virol. 2020 doi: 10.1002/jmv.26365. [DOI] [PubMed] [Google Scholar]
- 39.Guillén L, Padilla S, Fernández M, Agulló V, García JA, Telenti G, García-Abellán J, Botella Á, Gutiérrez F, Masiá M. Preemptive interleukin-6 blockade in patients with COVID-19. Sci Rep. 2020;10:16826. doi: 10.1038/s41598-020-74001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sablerolles RSG, Lafeber M, van Kempen JAL, van de Loo BPA, Boersma E, Rietdijk WJR, Polinder-Bos HA, Mooijaart SP, van der Kuy H, Versmissen J, Faes MC. COMET research team. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021;2:e163–e170. doi: 10.1016/S2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]