Abstract
Background
Non‐invasive respiratory support is increasingly used for the management of respiratory dysfunction in preterm infants. This approach runs the risk of under‐treating those with respiratory distress syndrome (RDS), for whom surfactant administration is of paramount importance. Several techniques of minimally invasive surfactant therapy have been described. This review focuses on surfactant administration to spontaneously breathing infants via a thin catheter briefly inserted into the trachea.
Objectives
Primary objectives
In non‐intubated preterm infants with established RDS or at risk of developing RDS to compare surfactant administration via thin catheter with:
1. intubation and surfactant administration through an endotracheal tube (ETT); or
2. continuation of non‐invasive respiratory support without surfactant administration or intubation.
Secondary objective
1. To compare different methods of surfactant administration via thin catheter
Planned subgroup analyses included gestational age, timing of intervention, and use of sedating pre‐medication during the intervention.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), on 30 September 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐randomised trials.
Selection criteria
We included randomised trials comparing surfactant administration via thin catheter (S‐TC) with (1) surfactant administration through an ETT (S‐ETT), or (2) continuation of non‐invasive respiratory support without surfactant administration or intubation. We also included trials comparing different methods/strategies of surfactant administration via thin catheter. We included preterm infants (at < 37 weeks' gestation) with or at risk of RDS.
Data collection and analysis
Review authors independently assessed study quality and risk of bias and extracted data. Authors of all studies were contacted regarding study design and/or missing or unpublished data. We used the GRADE approach to assess the certainty of evidence.
Main results
We included 16 studies (18 publications; 2164 neonates) in this review. These studies compared surfactant administration via thin catheter with surfactant administration through an ETT with early extubation (Intubate, Surfactant, Extubate technique ‐ InSurE) (12 studies) or with delayed extubation (2 studies), or with continuation of continuous positive airway pressure (CPAP) and rescue surfactant administration at pre‐specified criteria (1 study), or compared different strategies of surfactant administration via thin catheter (1 study). Two trials reported neurosensory outcomes of of surviving participants at two years of age. Eight studies were of moderate certainty with low risk of bias, and eight studies were of lower certainty with unclear risk of bias.
S‐TC versus S‐ETT in preterm infants with or at risk of RDS
Meta‐analyses of 14 studies in which S‐TC was compared with S‐ETT as a control demonstrated a significant decrease in risk of the composite outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (risk ratio (RR) 0.59, 95% confidence interval (CI) 0.48 to 0.73; risk difference (RD) ‐0.11, 95% CI ‐0.15 to ‐0.07; number needed to treat for an additional beneficial outcome (NNTB) 9, 95% CI 7 to 16; 10 studies; 1324 infants; moderate‐certainty evidence); the need for intubation within 72 hours (RR 0.63, 95% CI 0.54 to 0.74; RD ‐0.14, 95% CI ‐0.18 to ‐0.09; NNTB 8, 95% CI; 6 to 12; 12 studies, 1422 infants; moderate‐certainty evidence); severe intraventricular haemorrhage (RR 0.63, 95% CI 0.42 to 0.96; RD ‐0.04, 95% CI ‐0.08 to ‐0.00; NNTB 22, 95% CI 12 to 193; 5 studies, 857 infants; low‐certainty evidence); death during first hospitalisation (RR 0.63, 95% CI 0.47 to 0.84; RD ‐0.02, 95% CI ‐0.10 to 0.06; NNTB 20, 95% CI 12 to 58; 11 studies, 1424 infants; low‐certainty evidence); and BPD among survivors (RR 0.57, 95% CI 0.45 to 0.74; RD ‐0.08, 95% CI ‐0.11 to ‐0.04; NNTB 13, 95% CI 9 to 24; 11 studies, 1567 infants; moderate‐certainty evidence). There was no significant difference in risk of air leak requiring drainage (RR 0.58, 95% CI 0.33 to 1.02; RD ‐0.03, 95% CI ‐0.05 to 0.00; 6 studies, 1036 infants; low‐certainty evidence). None of the studies reported on the outcome of death or survival with neurosensory disability.
Only one trial compared surfactant delivery via thin catheter with continuation of CPAP, and one trial compared different strategies of surfactant delivery via thin catheter, precluding meta‐analysis.
Authors' conclusions
Administration of surfactant via thin catheter compared with administration via an ETT is associated with reduced risk of death or BPD, less intubation in the first 72 hours, and reduced incidence of major complications and in‐hospital mortality. This procedure had a similar rate of adverse effects as surfactant administration through an ETT. Data suggest that treatment with surfactant via thin catheter may be preferable to surfactant therapy by ETT. Further well‐designed studies of adequate size and power, as well as ongoing studies, will help confirm and refine these findings, clarify whether surfactant therapy via thin tracheal catheter provides benefits over continuation of non‐invasive respiratory support without surfactant, address uncertainties within important subgroups, and clarify the role of sedation.
Plain language summary
Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome
Review question
Is giving surfactant via a minimally invasive technique involving placement of a thin catheter in the trachea of a spontaneously breathing infant effective and safe?
Background
Respiratory distress syndrome (RDS) is an important cause of disease and death in preterm infants. It is commonly treated with a medication called surfactant, which is given by a tube (called an endotracheal tube, or ETT). The ETT is placed in the windpipe (trachea). However, more infants with RDS are now being treated from the onset with non‐invasive respiratory support (through a mask) without use of an ETT. This means that the usual means of administering surfactant is not available. In such infants, surfactant therapy requires placement of an ETT, with or without the intent to remove it soon after the procedure. Surfactant improves clinical outcomes, but insertion of the ETT and mechanical ventilation (assisted breathing) can cause lung injury. This can contribute to development of a chronic lung disease known as bronchopulmonary dysplasia (BPD) and other problems. Alternatives to ETT insertion have been developed. The most popular method is the use of a thin catheter (tube) that is briefly inserted into the windpipe.
Study characteristics
We searched the electronic databases and found 16 randomised trials (18 publications) that met our selection criteria. These trials involved delivery of surfactant via a thin catheter. Evidence is up‐to‐date as of 30 September 2020.
Key results
Surfactant delivery via a thin catheter to spontaneously breathing preterm infants compared with surfactant administration through an ETT was associated with a decrease in the following: risk of death or BPD, need for assisted breathing in the first 72 hours of life, severe brain bleeding, death during first hospitalisation, and BPD among survivors. We are uncertain as to whether the intervention has an important effect on air leak requiring drainage because the results are imprecise. None of the studies reported on the outcome of death or survival with disability. The procedure had rates of adverse effects similar to surfactant administration through an ETT. These data suggest that treatment with surfactant via a thin catheter is preferable to surfactant therapy through an ETT. Further well‐designed studies of adequate size and power, as well as ongoing studies, are required to confirm and refine these findings, and to clarify whether surfactant therapy via a thin catheter provides benefits over continuation of non‐invasive respiratory support without surfactant.
Certainty of evidence
Most of the studies had important methodological weaknesses. We used the GRADE approach to assess the certainty of evidence. We downgraded the evidence to 'moderate to low'. More good quality studies are urgently needed to address uncertainties within important subgroups.
Summary of findings
Summary of findings 1. Surfactant administration via thin catheter (S‐TC) vs surfactant administration through an endotracheal tube (S‐ETT) in preterm infants with or at risk of respiratory distress syndrome.
| Surfactant administration via thin catheter (S‐TC) vs surfactant administration through an endotracheal tube (S‐ETT) in preterm infants with or at risk of respiratory distress syndrome | ||||||
| Patient or population: preterm infants with or at risk of respiratory distress syndrome Setting: neonatal intensive care units. Countries: Germany, Turkey, Canada, China, India, Iran, and Pakistan Intervention: surfactant administration through thin catheter (S‐TC) Comparison: surfactant administration through endotracheal tube (S‐ETT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with surfactant administration through endotracheal tube (S‐ETT) | Risk with surfactant administration through thin catheter (S‐TC) | |||||
| Death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age | Study population | RR 0.59 (0.48 to 0.73) | 1324 (10 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 26 per 100 | 16 per 100 (13 to 19) | |||||
| Need for intubation within the first 72 hours | Study population | RR 0.63 (0.54 to 0.74) | 1422 (12 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 36 per 100 | 23 per 100 (20 to 27) | |||||
| Air leak requiring drainage | Study population | RR 0.58 (0.33 to 1.02) | 1036 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 6 per 100 | 3 per 100 (2 to 6) | |||||
| Severe intraventricular haemorrhage (grade III or IV) | Study population | RR 0.63 (0.42 to 0.96) | 857 (5 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 12 per 100 | 7 per 100 (5 to 11) | |||||
| Death during first hospitalisation (all causes) | Study population | RR 0.63 (0.47 to 0.84) | 1424 (11 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 13 per 100 | 8 per 100 (6 to 11) | |||||
| Bronchopulmonary dysplasia (BPD) among survivors at 36 weeks' postmenstrual age | Study population | RR 0.57 (0.45 to 0.74) | 1567 (11 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 18 per 100 | 10 per 100 (8 to 13) | |||||
| Death or survival with neurosensory disability ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | None of the studies reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for serious study limitations (high risk of bias due to uncertainty about methods used to generate random sequence, conceal allocation, and mask outcome assessments) in many trials.
bDowngraded by one level for serious imprecision of effect estimate (inadequate optimal effect size and/or 95% CI around estimate consistent with substantial harm or benefit).
Background
Description of the condition
Respiratory distress syndrome (RDS) and its complications are major contributors to morbidity and mortality in preterm infants. Recognition that surfactant deficiency is an important cause of RDS (as reported in Avery 1959) ultimately led to the development of surfactant replacement therapy for RDS (Jobe 1993). Administration of exogenous surfactant is known to reduce mortality and risk of air leak, and has become a mainstay of therapy for preterm infants with RDS (Suresh 2005; Sweet 2016).
In recent years, non‐invasive respiratory support has become more popular for the management of respiratory dysfunction in preterm infants (Berger 2013; Soll 2013). Three large randomised controlled trials (RCTs) found that applying nasal continuous positive airway pressure (CPAP) from birth is at least as effective as intubation and ventilation among infants at < 30 weeks' gestation (Dunn 2011; Finer 2010; Morley 2008). Applying CPAP from the outset in an unselected population of preterm infants does, however, run the risk of under‐treating those with RDS, for whom CPAP may fail to provide adequate respiratory support. Absence of an endotracheal tube means the usual conduit for exogenous surfactant administration is unavailable. The risks and consequences of CPAP failure under these circumstances are now being appreciated (Ammari 2005; Dargaville 2013b; Dargaville 2016). Such infants, once intubated, receive surfactant at a later than ideal time and have increased risk of adverse outcomes compared to like‐gestation infants managed by CPAP alone (Ammari 2005; Dargaville 2013b; Dargaville 2016).
One approach to resolving the CPAP‐surfactant dilemma has been to briefly insert an endotracheal tube (ETT) to administer surfactant to infants on CPAP, followed by rapid extubation back to CPAP (InSurE; Intubate, Surfactant, Extubate procedure) (Stevens 2007; Verder 1994; Victorin 1990). This technique provides benefits over continuation of CPAP, but most often it requires sedating pre‐medication, and extubation may be delayed due to respiratory suppression.
Numerous investigators have sought an alternative solution to the problem of administering surfactant to infants on non‐invasive respiratory support. Several techniques of minimally invasive surfactant therapy have been described and are the topic of other Cochrane Reviews, including surfactant administration by aerosolisation (Abdel‐Latif 2012), by pharyngeal deposition (Abdel‐Latif 2011a), and through a laryngeal mask (Abdel‐Latif 2011b). The topic of this review, which has not previously been systematically reviewed, is administration of surfactant via a thin catheter briefly inserted into the trachea (Dargaville 2011; Kribs 2007).
Description of the intervention
Surfactant administration via thin catheter (S‐TC) encompasses any method in which a thin catheter, expected to be narrower than a standard endotracheal tube (ETT), is passed through the vocal cords to allow surfactant instillation. The most commonly used methods are:
flexible thin catheter and Magill's forceps (Cologne method), as described by Kribs and colleagues (Kribs 2007);
flexible thin feeding tube without Magill's forceps (take care method), as described by Kanmaz and colleagues (Kanmaz 2013);
semi‐rigid thin catheter (Hobart method), as described by Dargaville and colleagues (Dargaville 2011); and
modifications of the above methods.
Variation may be encountered in (1) the pre‐medication used, (2) the means of laryngoscopy used, including videolaryngoscopy, (3) the type of catheter, (4) the method used to guide the catheter through the vocal cords, (5) the approach to surfactant delivery (bolus versus infusion, rapid versus slow), (6) the surfactant preparation, (7) the surfactant dose, and (8) the approach to respiratory management before, during, and after the technique, including the type of non‐invasive respiratory support used. It is expected that infants are spontaneously breathing, and therefore positive‐pressure inflations are not required for surfactant dispersal. Unlike an ETT, a thin catheter is unsuitable for delivery of positive‐pressure inflations.
Several different acronyms may be used for the above methods, including:
MIST (minimally invasive surfactant therapy);
LISA (less invasive surfactant administration);
SurE (surfactant without endotracheal tube);
MISA (minimally invasive surfactant administration); and
NISA (non‐invasive surfactant administration).
For this review, we elected to not use any of these in preference to others, instead using a term capturing the essence of the method: surfactant administration via thin catheter (S‐TC).
How the intervention might work
For infants with RDS managed by non‐invasive respiratory support, administering surfactant directly into the trachea using a minimally invasive approach has the potential to overcome surfactant deficiency and replenish the endogenous surfactant pool. Progressive respiratory deterioration culminating in CPAP failure may thus be avoided, and along with it, the known associated adverse outcomes. Non‐randomised studies have demonstrated that surfactant administration via tracheal catheterisation is feasible (Kribs 2007; Kribs 2008; Kribs 2009; Kribs 2010; Dargaville 2011; Dargaville 2013a), and it appears to be safe (Aguar 2014; Porth 2011), and that a reduction in the need for subsequent ventilation or supplemental oxygen, or both, may be achievable. These short‐term clinical benefits have the potential to lead to improvement in longer‐term outcomes.
Why it is important to do this review
Surfactant administration via thin catheter is a promising, feasible therapy that is being adopted in many sites around the world (Bhayat 2020; Heiring 2017; Klotz 2017; Jeffreys 2019; Roberts 2020). Therefore it is important to determine whether this treatment is safe and effective. This technique has not been the topic of a previous Cochrane Review.
Objectives
Primary objectives
In non‐intubated preterm infants with established RDS or at risk of developing RDS to compare surfactant administration via thin catheter with:
intubation and surfactant administration through an endotracheal tube (ETT); or
continuation of non‐invasive respiratory support without surfactant administration.
Secondary objective
To compare different methods of surfactant administration via thin catheter
Planned subgroup analyses included gestational age, timing of intervention, and use of sedating pre‐medication during the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel interventional trials, randomised or quasi‐randomised, regardless of the unit of allocation (individual or cluster).
Types of participants
We included preterm infants (< 37 weeks' gestation) with or at risk of RDS.
Types of interventions
We included the following methods of surfactant administration via thin catheter.
A flexible catheter and Magill's forceps (Kribs 2007).
A flexible catheter without Magill's forceps (Kanmaz 2013).
A semi‐rigid catheter without Magill's forceps (Dargaville 2011).
Variations or modifications of the above methods, including use of videolaryngoscopy for catheter placement.
We included studies that compared different tracheal catheterisation techniques (e.g. semi‐rigid versus flexible catheter, sedation versus no sedation). We included trials using any surfactant formulation, including animal‐derived and synthetic surfactants (with or without surfactant protein activity).
Types of comparisons
In accordance with the objectives of this review, we categorised trials by the form of intervention used in the comparator (control) group, as below. Given the fundamental difference in the three therapeutic approaches for control infants (see later), we analysed data from trials within these categories separately and did not pool data together in a meta‐analysis.
The three comparisons are discussed below.
Comparison of surfactant administration via thin catheter (S‐TC) with surfactant administration via ETT (S‐ETT)
In this category, infants in the comparison (control) group were intubated and received surfactant by ETT. We further divided these trials into two groups.
S‐TC versus surfactant administration via ETT with the intent to rapidly extubate (InSurE)
In these trials, for controls, there was the intent to extubate soon after surfactant delivery, as in Haberman 2002 (i.e. the INtubate‐SURfactant‐Extubate (InSurE) procedure; Reininger 2005; Victorin 1990).
S‐TC versus surfactant administration via ETT with delayed extubation
In these trials, control infants remained intubated after surfactant delivery, with delayed extubation after a period of mechanical ventilation.
Comparison of S‐TC with continuation of non‐invasive respiratory support
In this category, management in the comparison (control) group consisted of continuation of non‐invasive respiratory support (CPAP, high‐flow (HF), variations thereof) without surfactant administration, unless pre‐specified failure criteria were met.
Comparison of different methods of surfactant delivery via thin catheter
In this category, a thin catheter was used for surfactant delivery to all participants, with comparison of different methods, including different approaches to the use of sedation.
Types of outcome measures
Primary outcomes
The following were recognised as critical outcomes for this review.
Death or bronchopulmonary dysplasia (BPD): the composite outcome of death or BPD, defined as the need for oxygen or respiratory support at 36 weeks' postmenstrual age (PMA) (Shennan 1988).
Need for intubation within the first 72 hours of life.
Air leak requiring drainage (during first hospitalisation).
Severe intraventricular haemorrhage (IVH), including grades III and IV (Papile 1978).
Death during first hospitalisation (all causes).
BPD (clinical definition) among survivors to 36 weeks' PMA.
Death or survival with neurosensory disability, with the latter measured beyond one year PMA and defined as any of (1) cerebral palsy by clinical examination or other means; (2) developmental delay more than two standard deviations below the population mean on standardised testing; (3) blindness (visual acuity < 6/60); or (4) deafness (hearing impairment requiring amplification).
Secondary outcomes
Measures of safety of the surfactant administration procedure
Catheter/ETT placement unsuccessful at first attempt (during trial‐related intervention)
Bradycardia (heart rate < 100 beats per minute (bpm)) during the intervention
Hypoxaemia (oxygen saturation < 80%) during the intervention
And for studies comparing thin catheter methods
Need for positive‐pressure ventilation during the intervention
Need for immediate intubation (within 15 minutes of the intervention)
Metrics of respiratory support
Need for intubation within the first 72 hours, or not intubated but reached failure criteria
Need for intubation at any time
Need for intratracheal surfactant therapy post intervention
Duration of mechanical ventilation via ETT (days; among survivors)
Duration of any respiratory support (mechanical ventilation, CPAP, heart failure (HF)) (days; among survivors)
Duration of oxygen therapy (days; among survivors)
Postnatal systemic corticosteroid therapy for BPD mitigation
Outcomes during first hospitalisation
BPD (physiological definition), evaluated when necessary by a room‐air challenge at 36 weeks' PMA for infants with borderline oxygen requirements (Walsh 2004)
IVH, any grade (Papile 1978)
Cystic periventricular leukomalacia (PVL)
Patent ductus arteriosus (PDA) requiring medical therapy
Necrotising enterocolitis (NEC): modified Bell stage 2 or greater (Bell 1978; Walsh 1988)
Spontaneous intestinal perforation
Retinopathy of prematurity (ROP), stage 3 or greater
Duration of hospitalisation (days; among survivors)
Postdischarge outcomes
Oxygen therapy at home
Number of hospital re‐admissions with respiratory illness in the first two years
Parent‐reported wheeze in the first two years
Bronchodilator use in the first two years
Neurosensory disability (defined per primary outcome above), among survivors
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register).
Electronic searches
We conducted a comprehensive search on 30 September 2020. This search included the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 30 September 2020); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1946 to September Week 2 2020).
We have included the search strategies for each database in Appendix 1. We did not apply language or date restrictions.
We searched clinical trial registries for ongoing or recently completed trials (ISRCTN Registry). We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/) and the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov) via Cochrane CENTRAL.
Searching other resources
We communicated with expert informants and searched bibliographies of reviews and trials for references to other trials. We also searched previous reviews including cross‐references, abstracts, and conference and symposia proceedings (as above) from 1990 to 30 September 2020. For unpublished trials, we contacted the contact investigator to request information. We considered unpublished studies and studies reported only as abstracts as eligible for review only if final trial data were reported (i.e. data from an interim analysis were not included). We contacted the corresponding authors of identified trials for additional information when needed. We searched clinical trial registries for ongoing and recently completed trials (as above). We searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We used the standard methods of Cochrane and Cochrane Neonatal. Two review authors independently conducted searches, assessed study eligibility, and extracted study results and risk of bias. We resolved discrepancies by discussion and consensus.
Selection of studies
Two review authors (MEA and PAD) independently reviewed the titles and abstracts of potentially relevant studies against inclusion and exclusion criteria. We (MEA and PAD) independently assessed the titles and abstracts of studies identified by the search strategy for eligibility for inclusion in this review. We obtained full‐text versions of studies for closer examination of eligibility, or when inadequate information was provided in the abstract.
Data extraction and management
We (MEA and PAD) independently extracted data from full‐text articles using a specifically designed spreadsheet to manage the information. We (MEA and PAD) resolved discrepancies through discussion and consensus, or, if required, by consultation with a third review author (PGD). We (MEA and PAD) entered data into Review Manager software (Review Manager 2020), and we checked it for accuracy.
Assessment of risk of bias in included studies
Two review authors (MEA and PAD) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed the results of included studies using the statistical package Review Manager 5 software (Review Manager 2020). We used the standard methods of Cochrane Neonatal. We used a fixed‐effect model for meta‐analysis. In assessing treatment effects for dichotomous data or categorical data, we reported the risk ratio (RR) or the risk difference (RD), respectively, along with the 95% confidence interval (CI). If the RD was statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) (1/RD). For outcomes measured on a continuous scale, we reported the mean difference (MD), along with the 95% CI.
Unit of analysis issues
We combined cluster‐randomised and individually randomised trials in a single meta‐analysis using the generic inverse variance method.
Dealing with missing data
In the case of missing data, we described the number of participants with missing data in the Results section and in the Characteristics of included studies table. When possible, we performed an intention‐to‐treat (ITT) meta‐analysis using reconstructed denominators. We discussed the implications of data missing from the review as appropriate.
Assessment of heterogeneity
We used Review Manager 5 to assess the heterogeneity of treatment effects between trials (Review Manager 2020). We used two formal statistical approaches to assess the presence of statistical heterogeneity.
The Chi² test for homogeneity: because this test has low power when the number of studies included in the meta‐analysis is small, we set the level of significance at 10% probability (P < 0.1) (Higgins 2019).
The I² statistic: the I² statistic describes the percentage of total variation across studies due to heterogeneity rather than to sampling error, and is thus a measure of the validity of data pooling for meta‐analysis. We graded the degree of heterogeneity as follows: ≤ 24%, no heterogeneity; 25% to 49%, low heterogeneity; 50% to 74%, moderate heterogeneity; and ≥ 75%, high heterogeneity.
When we noted evidence of apparent or statistical heterogeneity, we assessed the source of heterogeneity by using sensitivity and subgroup analyses to look for evidence of bias or methodological differences between trials.
Assessment of reporting biases
We made attempts to obtain the study protocols of all included studies and to compare outcomes reported in the protocol versus those reported in the findings for each of the included studies. If reporting bias was suspected (see Assessment of reporting biases), we made attempts to contact the study authors to ask them to provide further information. When this was not possible, and when missing data were thought to introduce serious bias, we examined the impact of including/excluding such studies in the overall assessment of results by performing a sensitivity analysis.
We investigated non‐reporting (including publication) bias by visually assessing funnel plot asymmetry, and by using Egger's test in meta‐analyses if data from at least 10 trials contributing events were available (Egger 1997).
Data synthesis
We performed meta‐analyses using the standard methods of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We used a fixed‐effect model. When studies were statistically heterogenous, we examined study characteristics including study design and quality. When appropriate, we performed sensitivity analysis including only trials with higher methodological rigour.
We did not pool trials that included different comparison groups (see Types of interventions).
Subgroup analysis and investigation of heterogeneity
We identified several factors that could influence the safety and efficacy of interventions examined in this review and therefore planned sub‐group analyses based on:
gestational age (≤ 28 weeks (extremely preterm), 29 to 32 weeks (very preterm), 33 to 36 weeks (preterm));
timing of surfactant administration (i.e. prophylaxis versus rescue). Here we defined prophylaxis trials as those in which surfactant treatment was administered soon after birth to infants at risk of RDS, and rescue trials as those that used treatment with surfactant selectively in infants demonstrating features of RDS; and
use of sedation and analgesia pre‐medication in the tracheal catheterisation group (i.e. sedation and analgesia used versus withheld).
Sensitivity analysis
We explored methodological heterogeneity through the use of sensitivity analysis. We assessed studies as having low risk of bias if sequence generation and allocation concealment were adequate, and if losses were less than 10% with ITT analysis.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook to assess the certainty of evidence for the following (clinically relevant) outcomes (Schünemann 2013).
Death or bronchopulmonary dysplasia (BPD) at 36 weeks' PMA.
Need for intubation within the first 72 hours of life.
Air leak requiring drainage.
Severe IVH (grade III or IV).
Death during first hospitalisation (all causes).
BPD among survivors to 36 weeks' PMA.
Death or survival with neurosensory disability.
Two review authors (MEA and PAD) independently assessed the certainty of evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence by one level for serious (or by two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create Table 1 to report the certainty of evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as belonging to one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We identified 6133 records from the initial search of PubMed, CENTRAL, MEDLINE, and CINAHL. We performed additional searches of clinicaltrials.gov and other registries and identified 202 further records that appeared to be relevant. Additional searches of reference lists and other Internet resources yielded 14 additional relevant articles. After we removed duplicates, there were 4849 records. Among these, 28 articles remained relevant after inspection of titles or abstract, or both. We evaluated the abstracts or full‐texts of articles and excluded 12 records. The diagram of the flow of studies from the initial search to the meta‐analysis is shown in Figure 1. A description of all included studies is displayed under Characteristics of included studies, and excluded studies with reasons for exclusion are given in the Characteristics of excluded studies table.
1.
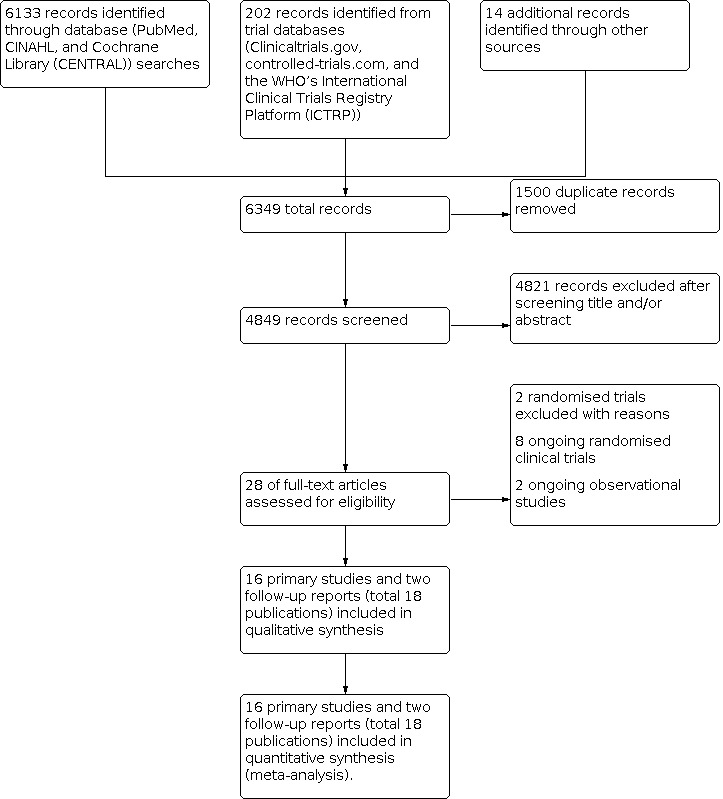
Study flow diagram.
We included in our meta‐analysis 16 primary studies (18 publications including 16 primary studies and two reports of neurosensory outcomes in two primary trials among surviving participants at two years of age). Studies included in this review include those that examined effects of administration of surfactant via thin catheter on clinical outcomes among infants with or at risk of RDS. We divided these studies into groups based on treatment strategy (Figure 2).
2.
Primary and follow‐up studies included in the review categorised by comparison group.
Trials comparing S‐TC with S‐ETT
S‐TC versus InSurE
We identified 12 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Halim 2019; Han 2020; Jena 2019; Kanmaz 2013; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020).
S‐TC versus surfactant administration via ETT with delayed extubation
We identified two studies (Kribs 2015; Olivier 2017), along with a further report in Mehler 2020 detailing two‐year neurosensory outcomes for infants recruited in the NINSAPP trial (Kribs 2015).
Trials comparing S‐TC with continuation of non‐invasive respiratory support
We identified a single study (AMV trial; Göpel 2011). In this study, the comparison group (control) continued on CPAP respiratory support without surfactant administration unless certain failure criteria were met. Herting 2020 is a report of two‐year neurosensory outcomes for infants recruited in the AMV trial.
Trials comparing different methods or strategies of surfactant delivery via thin catheter
We identified a single study (Dekker 2019). This study compared two methods of performing MIST: one with sedation and one without sedation during the MIST procedure.
Included studies
Of the 16 included studies (18 publications), seven were multi‐centre (Göpel 2011; Han 2020; Jena 2019; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Olivier 2017), and nine involved a single centre (Bao 2015; Boskabadi 2019; Choupani 2018; Dekker 2019; Gupta 2020; Halim 2019; Kanmaz 2013; Mosayebi 2017; Yang 2020). Herting 2020 and Mehler 2020 are reports of two‐year neurosensory follow‐up of infants recruited in Göpel 2011 and Kribs 2015, respectively.
In total, we recruited 2164 preterm infants. The number of infants included in each trial and their gestational age are provided in Table 2. The description of all studies is summarised under Characteristics of included studies.
1. Number of infants recruited and gestational age ranges for included trials.
| Trial | Multicentre study | Country | Total number of infants recruited | Gestational age (weeks) | Age at surfactant administration | Sedation | ||
| Eligiblity criteria | Intervention group | Control group | ||||||
| Bao 2015 | No | China | 90 | 28 to 32 | 29.1 ± 1.5 | 29.3 ± 1.6 | Within 2 hours of birth | None |
| Boskabadi 2019 | No | Iran | 40 | < 32 | 29.1 ± 2.6 | 28.2 ± 2.1 | Not specified | Not specified |
| Choupani 2018 | No | Iran | 104 | 28 to 37 | 32.9 ± 2.6 | 33.1 ± 2.3 | Within 1 hour of birth | Not specified |
| Dekker 2019 | No | Netherlands | 78 | 26 to 37 | 29 + 0 (27 + 5 to 32 + 0) | 29 + 0 (28 + 0 to 31 + 0) | Within first 24 hours of life | Propofol |
| Gupta 2020 | No | India | 58 | 28 to 34 | 30.07 ± 1.51 | 29.90 ± 1.67 | Within 6 hours of birth | None |
|
Göpel 2011 (follow‐up reported in Herting 2020) |
Yes (n = 12) | Germany | 220 | 26 to 28 | 27.6 ± 0.8 | 27.5 ± 0.8 | Within 12 hours of birth | Sedation and analgesia were used at the discretion of attending neonatologist |
| Halim 2019 | No | Pakistan | 100 | ≤ 34 | 32 to 34 weeks = 26 (52%) 30 to 31 + 6 weeks = 11 (22%) 28 to 29 + 6 weeks = 8 (16%) < 28 weeks = 5 (10%) | 32 to 34 weeks 24 (48%) 30 to 31 + 6 weeks = 14 (28.6%) 28 to 29 + 6 weeks = 6 (12.2%) < 28 weeks = 5 (10.2%) | Within 12 hours of birth | None |
| Han 2020 | Yes (n = 8) | China | 344 | 25 + 0 to 31 + 6 | 30.6 ± 1.6 | 30.8 ± 1.3 | Within 6 hours of birth | None |
| Jena 2019 | Yes (n = 3) | India | 350 | ≤ 34 | 31.0 (29.0 to 33.0) | 31.0 (29.0 to 33.0) | Within 6 hours of birth | None |
| Kanmaz 2013 | No | Turkey | 200 | < 32 | 28 ± 2 | 28.3 ± 2 | Not specified | None |
|
Kribs 2015 (follow‐up reported in Mehler 2020) |
Yes (n = 13) | Germany | 211 | 23 to 26 | 25.3 ± 1.1 | 25.2 ± 0.91 | 10 to 120 minutes of age | None |
| Mirnia 2013a | Yes (n = 3) | Iran | 136 | 27 to 32 | 29.6 ± 1.7 | 29.6 ± 1.7 | Not specified | None |
| Mohammadizadeh 2015 | Yes (n = 2) | Iran | 38 | ≤ 34 | 30 ± 2 | 31 ± 2 | Within 1 hour of birth | Not specified |
| Mosayebi 2017 | No | Iran | 53 | 28 to 34 | 32.6 ± 1.1 | 31.9 ± 1.5 | Not specified | None |
| Olivier 2017 | Yes (n = 3) | Canada | 45 | 32 to 36 | 34 ± 1.4 | 33.8 ± 1.5 | Within first 24 hours of life | Fentanyl |
| Yang 2020 | No | China | 97 | 32 + 0 to 36 + 6 | 33.7 ± 1.0 | 34.1 ± 1.3 | Within 12 hours of birth | Not specified |
| TOTAL | 2164 | |||||||
Data reported as mean ± SD; median (interquartile range) or number (%).
Excluded studies
We excluded two randomised studies (Characteristics of excluded studies).
One single‐centre study ‐ Mirnia 2013b ‐ that was reported as part of another included multi‐centre randomised trial (Mirnia 2013a).
One randomised study comparing different ventilation strategies within the minimally invasive surfactant therapy approach (Oncel 2016).
Ongoing studies
We identified 11 ongoing studies (see Characteristics of ongoing studies).
Still recruiting (ChiCTR1900020970; NCT04016246; NCT04445571).
Finished recruiting but not analysed yet (ACTRN12611000916943).
Recruitment not yet started (ACTRN12611000917932; NCT01848262; NCT04073173).
Terminated or suspended (NCT01615016; NCT02772081).
Non‐randomised observational studies (NCT03989960; UMIN000021785).
Risk of bias in included studies
The risk of bias for studies included in this review based on the review authors' judgements is summarised in Figure 3 and Figure 4 and is discussed below.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was performed and was reported adequately in 10 studies (Bao 2015; Choupani 2018; Dekker 2019; Göpel 2011; Gupta 2020; Halim 2019; Jena 2019; Kanmaz 2013; Kribs 2015; Olivier 2017).
We considered randomisation as high risk in Choupani 2018, as it involved initial convenience sampling followed by random allocation. Five studies did not report adequately on the method of randomisation (Boskabadi 2019; Han 2020; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017). One study used a quasi‐randomised method (alternate allocation) (Yang 2020).
Allocation concealment was adequately reported and was appropriately performed in 11 studies (Bao 2015; Dekker 2019; Göpel 2011; Gupta 2020; Han 2020; Jena 2019; Kanmaz 2013, Kribs 2015; Mohammadizadeh 2015; Olivier 2017; Yang 2020). Five studies did not report on the method of allocation concealment (Boskabadi 2019; Choupani 2018; Halim 2019; Mirnia 2013a; Mosayebi 2017).
Blinding
Blinding of participants and personnel (performance bias) was achieved in one study (Yang 2020), and blinding of outcome assessment (to prevent detection bias) was achieved in two studies (Dekker 2019; Yang 2020). In all other studies, blinding was not performed or was unclear.
Incomplete outcome data
There was complete follow‐up of all enrolled participants with minimal risk of attrition bias, with the exception of two studies that did not report the outcome of 11% to 13% of randomised infants (Dekker 2019; Han 2020).
Selective reporting
There was no reporting bias in four studies (Göpel 2011; Gupta 2020; Kanmaz 2013; Kribs 2015). We obtained the study protocol from the authors of five studies (Bao 2015; Han 2020; Kanmaz 2013; Mosayebi 2017; Olivier 2017).
The study protocol was not available for 10 studies (Boskabadi 2019; Choupani 2018; Halim 2019; Jena 2019; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020; and Herting 2020 and Mehler 2020, which were reports of two‐year neurosensory follow‐up of infants recruited in the Göpel 2011 and Kribs 2015 trials, respectively).
Ten studies were not registered with an international trial registry (Boskabadi 2019; Choupani 2018; Halim 2019; Jena 2019; Mirnia 2013a; Mohammadizadeh 2015; Olivier 2017; Yang 2020; and Herting 2020 and Mehler 2020, which were reports of two‐year neurosensory follow‐up of infants recruited in the Göpel 2011 and Kribs 2015 trials, respectively). Four studies were registered with an international trials registry either retrospectively or after the start of patient recruitment (Bao 2015; Han 2020; Kanmaz 2013; Mosayebi 2017).
Other potential sources of bias
Seven trials were multi‐centre trials with no treatment standardisation between centres (e.g. caffeine use, surfactant dose, type of catheter used), leading to variability between centres (Göpel 2011; Han 2020; Jena 2019; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Olivier 2017). However, multi‐variate logistical regression analysis was implemented in some of these studies and showed no significant centre effect.
Nine trials were single‐centre studies (Bao 2015; Boskabadi 2019; Choupani 2018; Dekker 2019; Gupta 2020; Halim 2019; Kanmaz 2013; Mosayebi 2017; Yang 2020). In one trial, some infants who might have been eligible could not be enrolled because of concern for standardisation of the intervention (Take Care) (Kanmaz 2013). Seven studies were not reported according to CONSORT guidelines, hence it is difficult to judge their quality (e.g. randomisation, allocation concealment, blinding) (Boskabadi 2019; Choupani 2018; Han 2020; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020).
Effects of interventions
See: Table 1
See Table 1 for trials comparing S‐TC with S‐ETT in preterm infants with or at risk of RDS; see Data and analyses.
We have reported information in this section under three trial categories with different comparator groups.
-
A. Trials comparing S‐TC with S‐ETT.
S‐TC versus InSurE.
S‐TC versus surfactant via ETT with delayed extubation.
S‐TC versus S‐ETT (all studies).
B. Trials comparing S‐TC with continuation of non‐invasive respiratory support.
C. Trials comparing different methods or strategies of thin catheter surfactant delivery.
We reported the analyses relevant to each comparison under the following three subtitles.
Overall analysis (primary and secondary outcomes).
Sub‐group analyses.
Sensitivity analysis.
A. Trials comparing S‐TC with S‐ETT
1. S‐TC compared with S‐ETT ‐ overall analysis
Primary outcomes
1.1 Death or BPD
See Analysis 1.1; Figure 5; Figure 6.
1.1. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 1: Death or BPD
5.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.1 Death or BPD.
6.

Funnel plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.1 Death or BPD.
S‐TC versus InSurE
This outcome was reported by nine studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). One study showed a significant difference in the risk of this outcome (Jena 2019). The meta‐analysis of treatment trials showed significant differences in the risk of this outcome between S‐TC and InSurE (typical risk ratio (RR) 0.52, 95% confidence interval (CI) 0.40 to 0.68; typical risk difference (RD) ‐0.11, 95% CI ‐0.16 to ‐0.07; number needed to treat for an additional beneficial outcome (NNTB) 9, 95% CI 6 to 15; 9 studies, 1113 infants). Heterogeneity among the studies was low (I² = 2%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by one study (Kribs 2015). This study showed no significant differences in the risk of this outcome (RR 0.79, 95% CI 0.55 to 1.13; RD ‐0.09, 95% CI ‐0.22 to 0.04; 1 study, 211 infants).
S‐TC versus S‐ETT (all studies)
This outcome was reported by 10 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). The meta‐analysis of trials showed a significant decrease in the risk of this outcome with S‐TC compared to S‐ETT (typical RR 0.59, 95% CI 0.48 to 0.73; typical RD ‐0.11, 95% CI ‐0.15 to ‐0.07; NNTB 9, 95% CI 7 to 16); 10 studies, 1324 infants). Heterogeneity among the studies was low (I² = 19%). There was no statistically significant evidence of funnel plot asymmetry consistent with trials favouring controls missing from the meta‐analysis (Egger test for bias, P = 0.467).
1.2 Need for intubation within the first 72 hours
See Analysis 1.2; Figure 7; Figure 8.
1.2. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 2: Need for intubation within the first 72 hours
7.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.2 Need for intubation within the first 72 hours.
8.

Funnel plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.2 Need for intubation within the first 72 hours.
S‐TC versus InSurE
This outcome was reported by 10 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020). Two studies showed a significant difference in the risk of this outcome (Jena 2019; Kanmaz 2013). The meta‐analysis of treatment trials showed a significant decrease in the risk of this outcome with S‐TC compared to InSurE (typical RR 0.61, 95% CI 0.50 to 0.75; typical RD ‐0.12, 95% CI ‐0.17 to ‐0.07; NNTB 8, 95% CI 6 to 14; 10 studies, 1166 infants). There was no heterogeneity among these studies (I² = 0%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by two studies (Kribs 2015; Olivier 2017). These studies showed a significant difference in the risk of this outcome. The meta‐analysis of treatment trials showed a significant decrease in the risk of this outcome with S‐TC compared to surfactant via ETT with delayed extubation (typical RR 0.68, 95% CI 0.0.53 to 0.86; typical RD ‐0.21, 95% CI ‐0.32 to ‐0.09; NNTB 5, 95% CI 3 to 12; 2 studies, 256 infants). Heterogeneity among the studies was high (I² = 85%).
S‐TC versus S‐ETT (all studies)
This outcome was reported by 12 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Olivier 2017; Yang 2020). The meta‐analysis of trials showed a significant decrease in the risk of this outcome with S‐TC compared to S‐ETT (typical RR 0.63, 95% CI 0.54 to 0.74; typical RD ‐0.14, 95% CI ‐0.18 to ‐0.09; NNTB 8, 95% CI; 6 to 12; 12 studies, 1422 infants). Heterogeneity among the studies was low (I² = 31%). There was no statistically significant evidence of funnel plot asymmetry consistent with trials favouring controls missing from the meta‐analysis (Egger test for bias, P = 0.322).
1.3 Air leak requiring drainage (during first hospitalisation)
See Analysis 1.3; Figure 9.
1.3. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 3: Air leak requiring drainage
9.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.3 Air leak requiring drainage.
S‐TC versus InSurE
This outcome was reported by four studies (Jena 2019; Kanmaz 2013; Mirnia 2013a; Yang 2020). None of the individual studies showed significant differences in the risk of this outcome. The meta‐analysis of trials showed no significant differences in the risk of this outcome between S‐TC and InSurE (typical RR 0.72, 95% CI 0.35 to 1.48; typical RD ‐0.01, 95% CI ‐0.04 to 0.01; 4 studies, 783 infants). There was no heterogeneity among the studies (I² = 0%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by two studies (Kribs 2015; Olivier 2017). These two studies showed no significant differences in the risk of this outcome. The meta‐analysis of treatment trials showed no significant differences in the risk of this outcome between S‐TC and surfactant via ETT with delayed extubation (typical RR 0.41, 95% CI 0.16 to 1.05; typical RD ‐0.07, 95% CI CI‐0.13 to 0.00; 2 studies, 253 infants). There was no heterogeneity among the studies (I² = 0%).
S‐TC versus S‐ETT (all studies)
This outcome was reported by six studies (Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Olivier 2017; Yang 2020). The meta‐analysis of trials showed no significant differences in the risk of this outcome between S‐TC and S‐ETT (typical RR 0.58, 95% CI 0.33 to 1.02; typical RD ‐0.03, 95% CI ‐0.05 to 0.00; 6 studies, 1036 infants). There was no heterogeneity among the studies (I² = 0%).
1.4 Severe intraventricular haemorrhage (IVH), including grades III and IV
See Analysis 1.4; Figure 10.
1.4. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 4: Severe IVH
10.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.4 Severe IVH.
S‐TC versus InSurE
This outcome was reported by four studies (Bao 2015; Gupta 2020; Han 2020; Kanmaz 2013). None of the individual studies showed a significant difference in the risk of this outcome. The meta‐analysis of treatment trials showed no significant differences in the risk of this outcome between S‐TC and InSurE (typical RR 0.77, 95% CI 0.45 to 1.32; typical RD ‐0.02, 95% CI ‐0.06 to 0.02; 4 studies, 646 infants). There was no heterogeneity among the studies (I² = 0%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by one study (Kribs 2015). This study showed a significant difference in the risk of this outcome (RR 0.46, 95% CI 0.24 to 0.90; RD ‐0.12, 95% CI ‐0.22 to ‐0.02; NNTB 8, 95% CI 5 to 49; 1 study, 211 infants).
S‐TC versus S‐ETT (all studies)
This outcome was reported by five studies (Bao 2015; Gupta 2020; Han 2020; Kanmaz 2013; Kribs 2015). The meta‐analysis of trials showed a significant decrease in the risk of this outcome with S‐TC compared to S‐ETT (typical RR 0.63, 95% CI 0.42 to 0.96; typical RD ‐0.04, 95% CI ‐0.08 to ‐0.00; NNTB 22, 95% CI 12 to 193; 5 studies, 857 infants). There was no heterogeneity among the studies (I² = 0%).
1.5 Death during first hospitalisation (all causes)
See Analysis 1.5; Figure 11; Figure 12.
1.5. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 5: Death during first hospitalisation
11.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.5 Death during first hospitalisation.
12.

Funnel plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.5 Death during first hospitalisation.
S‐TC versus InSurE
This outcome was reported by nine studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). One study showed a significant difference in the risk of this outcome (Mirnia 2013a). The meta‐analysis of treatment trials showed significant differences in the risk of this outcome between S‐TC and InSurE (typical RR 0.60, 95% CI 0.44 to 0.82; typical RD ‐0.05, 95% CI ‐0.09 to ‐0.02; NNTB 19, 95% CI 11 to 52; 9 studies, 1213 infants). Heterogeneity among the studies was low (I² = 0%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by one study (Kribs 2015). This study showed no significant difference in the risk of this outcome (RR 0.81, 95% CI 0.37, 1.79; RD ‐0.02, 95% CI ‐0.10, 0.06; 1 study, 211 infants).
S‐TC versus S‐ETT (all studies)
This outcome was reported by 10 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). The meta‐analysis of trials showed a significant difference in the risk of this outcome between S‐TC and S‐ETT (typical RR 0.63, 95% CI 0.47 to 0.84; typical RD ‐0.02, 95% CI ‐0.10 to 0.06; NNTB 20, 95% CI 12 to 58; 10 studies, 1424 infants). There was no heterogeneity among the studies (I² = 0%). There was no statistically significant evidence of funnel plot asymmetry consistent with trials favouring controls missing from the meta‐analysis (Egger test for bias, P = 0.217).
1.6 BPD (clinical definition) among survivors to 36 weeks' PMA
See Analysis 1.6; Figure 13; Figure 14.
1.6. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 6: BPD (clinical definition); in survivors to 36 weeks' PMA
13.

Forest plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.6 BPD (clinical definition); in survivors to 36 weeks' PMA.
14.

Funnel plot of comparison: 1 Trials comparing S‐TC with S‐ETT ‐ overall analysis, outcome: 1.6 BPD (clinical definition); in survivors to 36 weeks' PMA.
S‐TC versus InSurE
This outcome was reported by 10 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Han 2020; Jena 2019; Kanmaz 2013; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). One study showed a significant difference in the risk of this outcome (Jena 2019). The meta‐analysis of treatment trials showed a significant difference in the risk of this outcome between S‐TC and InSurE (typical RR 0.57, 95% CI 0.44 to 0.75; typical RD ‐0.07, 95% CI ‐0.11 to ‐0.04; NNTB 14, 95% CI 9 to 28; 10 studies, 1378 infants). Heterogeneity among the studies was low (I² = 15%).
S‐TC versus surfactant via ETT with delayed extubation
This outcome was reported by one study (Kribs 2015). This study showed no significant difference in the risk of this outcome (RR 0.58, 95% CI 0.32 to 1.05; RD ‐0.11, 95% CI ‐0.22 to 0.01; 1 study, 189 infants).
S‐TC versus S‐ETT (all studies)
This outcome was reported by 11 studies (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Han 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). The meta‐analysis of trials showed a significant difference in the risk of this outcome between S‐TC and S‐ETT (typical RR 0.57, 95% CI 0.45 to 0.74; typical RD ‐0.08, 95% CI ‐0.11 to ‐0.04; NNTB 13, 95% CI 9 to 24; 11 studies, 1567 infants). There was no heterogeneity among the studies (I² = 0%). There was no statistically significant evidence of funnel plot asymmetry consistent with trials favouring controls missing from the meta‐analysis (Egger test for bias, P = 0.373).
1.7 Death or survival with neurosensory disability (beyond one year)
None of the studies reported on this outcome.
Secondary outcomes
Different studies reported different sets of secondary outcomes (Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 1.23; Analysis 1.24).
1.7. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 7: Catheter/ETT placement unsuccessful at first attempt
1.8. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 8: Bradycardia (heart rate < 100 bpm) during the intervention
1.9. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 9: Hypoxaemia (oxygen saturation < 80%) during the intervention
1.10. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 10: Need for intubation within the first 72 hours or not intubated but reached failure criteria
1.11. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 11: Need for intubation at any time
1.12. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 12: Need for intratracheal surfactant therapy post intervention
1.13. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 13: Duration of mechanical ventilation (days; in survivors)
1.14. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 14: Duration of any respiratory support (days; in survivors)
1.15. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 15: Duration of oxygen therapy (days; in survivors)
1.16. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 16: Postnatal systemic corticosteroid therapy for BPD mitigation
1.17. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 17: BPD (physiological definition)
1.18. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 18: IVH, any grade
1.19. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 19: Cystic PVL
1.20. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 20: PDA requiring medical therapy
1.21. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 21: NEC, modified Bell stage ≥2
1.22. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 22: ROP stage ≥ 3
1.23. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 23: Duration of hospitalisation (days; in survivors)
1.24. Analysis.

Comparison 1: Trials comparing S‐TC with S‐ETT ‐ overall analysis, Outcome 24: Discharged home with oxygen
S‐TC versus InSurE
Meta‐analyses showed statistically significant differences in favour of S‐TC in the following outcomes.
Need for intubation within the first 72 hours, or not intubated but reached failure criteria (typical RR 0.72, 95% CI 0.53 to 0.96; typical RD ‐0.09, 95% CI ‐0.17 to ‐0.01, I² = 0%; NNTB 11. 95% CI 6 to 102; 4 studies, 464 infants; Analysis 1.10).
Need for intubation at any time (typical RR 0.70, 95% CI 0.54 to 0.90; typical RD ‐0.15, 95% CI ‐0.25 to ‐0.05, I² = 29%; NNTB 7, 95% CI 4 to 23; 3 studies, 338 infants; Analysis 1.11).
NEC: modified Bell stage ≥ 2 (typical RR 0.34, 95% CI 0.14 to 0.81; typical RD ‐0.04, 95% CI ‐0.07 to ‐0.01, I² = 50%; NNTB 26, 95% CI 15 to 99; 686 infants, 3 studies; Analysis 1.21).
S‐TC versus surfactant via ETT with delayed extubation
Meta‐analyses showed statistically significant differences in favour of S‐TC in the following outcomes.
Need for intubation within the first 72 hours, or not intubated but reached failure criteria (typical RR 0.68, 95% CI 0.53 to 0.86; typical RD ‐0.21, 95% CI ‐0.32 to ‐0.09, I² = 85%; NNTB 5, 95% CI 3 to 12; 2 studies, 135 infants; Analysis 1.10).
Need for intubation at any time (typical RR 0.75, 95% CI 0.68 to 0.84; typical RD ‐0.24, 95% CI ‐0.33 to ‐0.16, I² non‐applicable; NNTB 5, 95% CI 3 to 6; 183 infants, 1 study; Analysis 1.11).
S‐TC versus S‐ETT (all studies)
Meta‐analyses showed statistically significant differences in favour of S‐TC in the following outcomes.
Need for intubation within the first 72 hours, or not intubated but reached failure criteria (typical RR 0.70, 95% CI 0.58 to 0.84; typical RD ‐0.13, 95% CI ‐0.20 to ‐0.07, I² = 29%; NNTB 8, 95% CI 5 to 17; 6 studies, 720 infants; Analysis 1.10).
Need for intubation at any time (typical RR 0.73, 95% CI 0.64 to 0.83; typical RD ‐0.18, 95% CI‐0.25 to ‐0.11, I² = 8%; NNTB 6, 95% CI 4 to 10; 4 studies, 549 infants; Analysis 1.11).
NEC: modified Bell stage ≥ 2 (typical RR 0.34, 95% CI 0.14 to 0.81; typical RD ‐0.04, 95% CI ‐0.07 to ‐0.01, I² = 50%; NNTB 26, 95% CI 15 to 99; 3 studies, 686 infants; Analysis 1.21).
2. S‐TC compared with S‐ETT ‐ subgroup analyses
See Analysis 2.1.
2.1. Analysis.

Comparison 2: Trials comparing S‐TC with S‐ETT ‐ sub‐group analyses, Outcome 1: Death or BPD
2.1 Gestational age (≤ 28 weeks (extremely preterm), 29 to 32 weeks (very preterm), 33 to 36 weeks (preterm))
Table 2 shows the gestation range of infants recruited in each trial. Only one study exclusively recruited infants at ≤ 28 weeks' gestation (Kribs 2015). One trial recruited infants at < 32 weeks and provided stratified analysis for extremely preterm infants for some outcomes (Kanmaz 2013). Two trials included only infants at > 32 weeks (Olivier 2017; Yang 2020). Eleven trials included infants at ≤ 28 weeks but did not provide stratified analysis based on gestation (Bao 2015; Boskabadi 2019; Choupani 2018; Dekker 2019; Gupta 2020; Halim 2019; Han 2020; Jena 2019; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017). We did not perform meta‐analyses, as subgroup data were not available.
2.2 Trials examining surfactant administration in a prophylactic or rescue context
Table 2 shows the time of surfactant administration in each trial. None of the studies provided stratification based on prophylactic (intervention performed within 15 minutes of birth in infants) versus rescue (intervention performed beyond 15 minutes) surfactant administration. We did not perform meta‐analyses, as subgroup data were not available.
2.3 Trials with and without the use of sedating pre‐medication in the tracheal catheterisation group
Table 2 shows the use of sedation, if any, in each trial. In nine studies, sedating pre‐medication was not used before the intervention (Bao 2015; Gupta 2020; Halim 2019; Han 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mosayebi 2017). Only Olivier 2017 used sedating pre‐medication (fentanyl 1 mcg/kg) before S‐TC. Use of pre‐medication was not clear in four studies (Boskabadi 2019; Choupani 2018; Mohammadizadeh 2015; Yang 2020).
Analyses showed significant subgroup effects in favour of S‐TC for death or BPD in trials where no sedation was used in the S‐TC group (typical RR 0.58, 95% CI 0.46 to 0.73; typical RD ‐0.12, 95% CI ‐0.17 to ‐0.07, I² = 50%; NNTB 8, 95% CI 6 to 14; 6 studies, 1045 infants; Analysis 2.1). We did not perform meta‐analyses for the sub‐group of trials in which sedation was mandatory in the S‐TC group, as only one trial was of this type (Olivier 2017).
3. S‐TC compared with S‐ETT ‐ sensitivity analysis
See Analysis 3.1Analysis 3.2Analysis 3.3Analysis 3.4Analysis 3.5 and Analysis 3.6.
3.1. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 1: Death or BPD
3.2. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 2: Need for intubation within the first 72 hours
3.3. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 3: Air leak requiring drainage
3.4. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 4: Severe IVH
3.5. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 5: Death during first hospitalisation
3.6. Analysis.

Comparison 3: Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis, Outcome 6: BPD (clinical definition); in survivors to 36 weeks' PMA
We performed sensitivity analysis by excluding eight studies of low or unknown quality based on lack of any of the following: adequate randomisation, allocation concealment, less than 10% loss to follow‐up with ITT analysis (Boskabadi 2019; Choupani 2018; Halim 2019; Han 2020; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020; see Figure 4). We identified eight trials of moderate quality (Bao 2015; Dekker 2019; Gupta 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Olivier 2017; and Herting 2020, which reported two‐year neurosensory follow‐up of infants recruited in Kribs 2015). The results of this analysis are shown in Analysis 3.1, Analysis 3.2, Analysis 3.3, Analysis 3.4, Analysis 3.5, and Analysis 3.6.
Meta‐analyses showed statistically significant differences in favour of S‐TC in the following outcomes.
Death or BPD (typical RR 0.60, 95% CI 0.46 to 0.78; typical RD ‐0.11, 95% CI ‐0.17 to ‐0.06, I² = 62%; NNTB 9, 95% CI 6 to 18; 5 studies, 799 infants; Analysis 3.1).
Need for intubation within the first 72 hours (typical RR 0.60, 95% CI 0.51 to 0.72; typical RD ‐0.18, 95% CI ‐0.23 to ‐0.12, I² = 51%; NNTB 6, 95% CI 4 to 9; 6 studies, 954 infants; Analysis 3.2).
Severe IVH (typical RR 0.55, 95% CI 0.34 to 0.89; typical RD ‐0.07, 95% CI ‐0.12 to ‐0.01, I² = 0%; NNTB 15, 95% CI 8 to 64; 3 studies, 559 infants; Analysis 3.4).
BPD (clinical definition) in survivors to 36 weeks' PMA (typical RR 0.45, 95% CI 0.31 to 0.64; typical RD ‐0.11, 95% CI ‐0.16 to ‐0.06, I² = 34%; NNTB 9, 95% CI 6 to 16; 5 studies, 858 infants; Analysis 3.6).
B. Trials comparing S‐TC with continuation of non‐invasive respiratory support (CPAP)
One study compared S‐TC with continuation of CPAP: the Avoid Mechanical Ventilation (AMV) study (Göpel 2011). Two‐year outcome data for the AMV trial were published in Herting 2020. Given that there was only one study, meta‐analysis was not applicable.
Findings from the AMV trial are summarised in Analysis 4.1Analysis 4.2, Analysis 4.3, Analysis 4.4, Analysis 4.5, Analysis 4.6, Analysis 4.7, Analysis 4.8, Analysis 4.9, Analysis 4.10, Analysis 4.11, Analysis 4.12, and Analysis 4.13.
4.1. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 1: Death or BPD
4.2. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 2: Incidence of air leak requiring drainage
4.3. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 3: Severe IVH
4.4. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 4: Death during first hospitalisation
4.5. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 5: BPD (clinical definition); in survivors to 36 weeks' PMA
4.6. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 6: Catheter/ETT placement unsuccessful at first attempt
4.7. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 7: Bradycardia (heart rate < 100 bpm) during the intervention
4.8. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 8: Need for intubation within the first 72 hours or not intubated but reached failure criteria
4.9. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 9: Need for intubation at any time
4.10. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 10: Postnatal systemic corticosteroid therapy for BPD mitigation
4.11. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 11: Cystic PVL
4.12. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 12: ROP ≥ stage 3
4.13. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 13: Discharged home with oxygen
We were unable to undertake planned subgroup and sensitivity analyses to determine whether findings are affected by including only studies using adequate methods (low risk of bias), as there was only one study (Göpel 2011), and its two‐year neurosensory follow‐up data are reported in Herting 2020.
C. Trials comparing different methods or strategies of thin catheter surfactant delivery
One study of sedation during MIST (PROMISES) reported a comparison of surfactant via thin catheter with and without sedating pre‐medication (propofol) (Dekker 2019). Given that there was only one study, meta‐analysis was not applicable.
Findings from the above study are summarised in Analysis 5.1, Analysis 5.2, Analysis 5.3, Analysis 5.4, Analysis 5.5, Analysis 5.6, Analysis 5.7, Analysis 5.8, and Analysis 5.9.
5.1. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 1: Air leak
5.2. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 2: Severe IVH
5.3. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 3: Need for intubation during the procedure
5.4. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 4: Need for intubation within the first 24 hours
5.5. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 5: Death during first hospitalisation
5.6. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 6: Need for positive‐pressure ventilation during the intervention
5.7. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 7: Duration of the procedure (seconds)
5.8. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 8: Pain score using a validated instrument for measuring discomfort/pain during the procedure (e.g. COMFORTneo score)
5.9. Analysis.

Comparison 5: Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis, Outcome 9: Hypotension requiring treatment
We were unable to undertake planned subgroup and sensitivity analyses to determine whether findings are affected by including only studies using adequate methods (low risk of bias), as there was only one study (Dekker 2019).
Discussion
Summary of main results
Sixteen primary studies ‐ Bao 2015; Boskabadi 2019; Choupani 2018; Dekker 2019; Göpel 2011; Gupta 2020; Halim 2019; Han 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Olivier 2017; Yang 2020 ‐ and two neurosensory follow‐up reports of infants recruited in two of the primary studies ‐ Herting 2020 (primary study: Göpel 2011) and Mehler 2020 (primary study: Kribs 2015), including 2164 patients, met the inclusion criteria for this review (i.e. total 18 publications).
Evidence from 10 studies including 1324 infants and contributing data to the primary outcomes of this review shows that surfactant therapy via thin catheter (S‐TC) compared to surfactant via endotracheal tube (ETT) reduced the incidence of the combined outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (PMA) (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Yang 2020). Furthermore, S‐TC was associated with a reduced need for mechanical ventilation within the first 72 hours, and at any time, fewer cases of severe intraventricular haemorrhage (IVH), less BPD among survivors at 36 weeks' PMA, and lower mortality before hospital discharge (Bao 2015; Boskabadi 2019; Choupani 2018; Gupta 2020; Halim 2019; Han 2020; Jena 2019; Kanmaz 2013; Kribs 2015; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Olivier 2017; Yang 2020). The procedure was generally safe and well tolerated with comparable incidences of bradycardia, hypoxaemia, and procedural complications when compared to surfactant dosing via ETT. There was no significant difference in the need for more than one attempt to instrument the trachea with a thin catheter compared to an ETT. A higher rate of surfactant reflux was reported with surfactant administration via a thin catheter. Incidences of other hospital outcomes such as patent ductus arteriosus (PDA), any IVH, and retinopathy of prematurity (ROP) were similar.
Sensitivity analysis after exclusion of low‐quality studies showed similar results.
Only one trial compared surfactant therapy via thin catheter with continuation of continuous positive airway pressure (CPAP) (Göpel 2011). This study did not detect a difference in the incidence of death or BPD but did find a reduction in the need for mechanical ventilation in the first 72 hours of life, and at any time. A report of two‐year follow‐up of participants in this study showed that surfactant therapy via thin catheter appeared to be safe, with comparable two‐year outcomes (Herting 2020).
One study examined surfactant therapy via thin catheter with and without sedation (Dekker 2019). This study showed that low‐dose sedation increased comfort during surfactant administration via thin catheter in preterm infants but increased the need for transient non‐invasive ventilation. Sedation remains a controversial issue, given the uncertainty regarding its benefits and risks in this population (Mehler 2013; Zwicker 2016).
Neurosensory follow‐up data were limited. Two of the primary studies ‐ Göpel 2011 and Kribs 2015 ‐ reported neurosensory outcomes data (Herting 2020 and Mehler 2020, respectively).
Findings of this Cochrane Review suggest that in preterm infants with respiratory distress syndrome (RDS), surfactant administration via thin catheter in spontaneously breathing infants on CPAP may be preferable to surfactant dosing via ETT. Limited or no data were available for subgroup analyses, with no prophylaxis trials, limited numbers of infants at ≤ 28 weeks' gestation, and only one study mandating the use of sedating pre‐medication. Findings within these subgroups should thus be interpreted with caution.
Pulmonary benefits seen with tracheal catheterisation techniques may be related to many factors. These techniques not only avoid endotracheal intubation and associated positive‐pressure ventilation (PPV), they also combine surfactant administration with spontaneous breathing. There is potential for synergy arising from the combination of an effective treatment for RDS and avoidance of lung injury associated with PPV (Björklund 1997). Furthermore, these techniques allow continuation of CPAP, thus avoiding damage from temporary loss of functional lung capacity and atelectasis during the process of intubation (Sinclair 2009), while allowing spontaneous breathing to distribute surfactant within the lungs.
Overall completeness and applicability of evidence
Through a comprehensive search strategy, we identified 16 randomised controlled trials (RCTs) that matched our selection criteria in terms of population, intervention, comparison, and outcomes (16 studies in 18 publications). We excluded one trial report ‐ Mirnia 2013b ‐ due to overlap with a more complete report of the same trial, detailing findings at all three study centres (Mirnia 2013a). Eleven studies are ongoing (Characteristics of ongoing studies); the recruitment status of these studies is as follows.
Active recruiting (ACTRN12611000916943; ChiCTR1900020970; NCT04016246; NCT04445571).
Active not recruiting (ACTRN12611000917932; NCT03989960; NCT04073173; UMIN000021785).
Unknown (NCT01848262).
Terminated (NCT01615016).
Suspended (NCT02772081).
A total of 2164 newborn infants were assessed in all 16 trials (fewer for some outcomes) (Table 2). We believe the studies gathered in this review represent the best available evidence to answer the questions posed in this review. We were unable to undertake most of the subgroup analyses to further determine applicability of study findings to infants with different clinical and demographic characteristics due to insufficient data.
Quality of the evidence
Risk of bias in the studies included in this review varied (Figure 3), with eight trials having an overall low or unknown risk of bias (Boskabadi 2019; Choupani 2018; Halim 2019; Han 2020; Mirnia 2013a; Mohammadizadeh 2015; Mosayebi 2017; Yang 2020; see Figure 4).
The certainty of evidence, assessed according to GRADE, was moderate to low (Table 1). Although we judged the studies to be at varying risks of bias overall, evidence for our three main outcomes is drawn from studies at low risk of bias. We downgraded the quality of evidence to moderate for the main outcomes below mainly due to serious study limitations in many trials.
Death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age.
Need for intubation within the first 72 hours.
Bronchopulmonary dysplasia (BPD) among survivors at 36 weeks' postmenstrual age.
We also downgraded the certainty of evidence to low for the main outcomes below mainly due to serious study limitations and serious imprecision in many trials.
Air leak requiring drainage.
Severe intraventricular haemorrhage (grade III or IV).
Death during first hospitalisation (all causes).
We did not perform subgroup analysis, as subgroup data were limited or were not available.
Potential biases in the review process
Only one study exclusively studied extremely preterm infants at ≤ 28 weeks' gestation (Kribs 2015). Other studies included some infants at ≤ 28 weeks, but it was difficult to extract these data. Further studies are needed to address this high‐risk group. Several review outcomes were not available or could not be definitively addressed due to lack of data. Outcomes such as long‐term neurosensory outcomes are important, and data are scarce (Herting 2020; Mehler 2020). Furthermore, data based on factors such as sedation or analgesia are very important and will need to be addressed in future studies (Dekker 2016).
Agreements and disagreements with other studies or reviews
The findings of our systematic review are consistent in part with meta‐analyses published recently (Aldana‐Aguirre 2016; Gupta 2012; Isayama 2016; Lau 2017; Panza 2020; Wu 2017). They differ from the findings of a previously published meta‐narrative review, which did not include more recent studies (More 2014).
Authors' conclusions
Implications for practice.
Sixteen randomised clinical trials (18 publications) of surfactant administration with a thin catheter have been conducted using different thresholds for surfactant replacement and different comparator groups in preterm infants of varying gestational ages. Evidence from the studies included in this review indicates that infants with RDS treated with surfactant via thin catheter appear to be less likely to need mechanical ventilation, less likely to develop BPD, and less likely to develop severe IVH than are infants receiving surfactant via endotracheal tube. It is unclear whether delivery of surfactant via thin catheter is superior to continuation of CPAP without surfactant therapy. Surfactant administration via thin catheter was generally safe and well tolerated. Although the incidence of failure to catheterise the trachea at first attempt was not significantly different from intubation, training of healthcare personnel is recommended.
Implications for research.
Further research is needed to address high‐risk infants born at ≤ 28 weeks' gestation and to examine whether methods of S‐TC are effective when used for surfactant delivery as delivery room prophylaxis. Data on safety, use of sedating pre‐medication, and long term neurosensory outcomes are also needed. Procedural aspects such as catheter type (flexible versus semi‐rigid), use or not of Magill's forceps, and mode of laryngoscopy also require further investigation. Finally, thin catheter surfactant delivery should be compared with other minimally invasive methods, including aerosolisation and laryngeal mask administration.
What's new
| Date | Event | Description |
|---|---|---|
| 11 May 2021 | Amended | Minor edit. |
History
Protocol first published: Issue 5, 2015 Review first published: Issue 5, 2021
Acknowledgements
The methods section of the review is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating Editor; and Bill McGuire, Co‐coordinating Editor; who provided editorial and administrative support. Carol Friesen, Information Specialist, designed and ran the literature searches.
Georg Schmölzer and Kanekal Suresh Gautham have peer‐reviewed and offered feedback for this review.
Appendices
Appendix 1. Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web
Date of search: search completed on 30 September 2020 Terms: ID Search
#1 (pulmonary or lung or tracheal or catheter or less invasive or minimally invasive or non‐invasive or noninvasive) AND CENTRAL:TARGET #2 MESH DESCRIPTOR Pulmonary Surfactants EXPLODE ALL AND CENTRAL:TARGET #3 (surfactant* or Beractant or Poractant or Curosurf or Survanta or Exosurf or Lucinactant) AND CENTRAL:TARGET #4 #3 or #2 #5 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET #6 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET #7 #6 OR #5 AND CENTRAL:TARGET #8 #1 AND #4 AND #7
MEDLINE via Ovid
Date of search: search completed on 30 September 2020 Terms: 1. (pulmonary or lung or tracheal or catheter or less invasive or minimally invasive or non‐invasive or noninvasive).mp. 2. exp Pulmonary Surfactants/ 3. (surfactant* or Beractant or Poractant or Curosurf or Survanta or Exosurf or Lucinactant).mp. 4. 2 or 3 5. exp infant, newborn/ 6. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 7. 5 or 6 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. drug therapy.fs. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. or/8‐15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 7 and 18 20. randomi?ed.ti,ab. 21. randomly.ti,ab. 22. trial.ti,ab. 23. groups.ti,ab. 24. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 25. placebo*.ti,ab. 26. 20 or 21 or 22 or 23 or 24 or 25 27. 6 and 26 28. 19 or 27 29. 1 and 4 and 28
CINAHL via EBSCOhost
Date of search: search completed on 30 September 2020 Terms: (pulmonary OR lung OR tracheal OR catheter OR (less invasive) OR (minimally invasive) OR non‐invasive OR noninvasive) AND (surfactant* OR Beractant OR Poractant OR Curosurf OR Survanta OR Exosurf OR Lucinactant) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
ISRCTN
Date of search: search completed on 30 September 2020 Terms: 1. Interventions: Surfactant* AND Participant age range: Neonate
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies for which study protocols were published in advance, we compared pre‐specified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (when it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (when not all of the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Trials comparing S‐TC with S‐ETT ‐ overall analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death or BPD | 10 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.48, 0.73] |
| 1.1.1 S‐TC vs INSURE | 9 | 1113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.40, 0.68] |
| 1.1.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.13] |
| 1.2 Need for intubation within the first 72 hours | 12 | 1422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.54, 0.74] |
| 1.2.1 S‐TC vs INSURE | 10 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.50, 0.75] |
| 1.2.2 S‐TC vs surfactant via ETT with delayed extubation | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.86] |
| 1.3 Air leak requiring drainage | 6 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 1.02] |
| 1.3.1 S‐TC vs INSURE | 4 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.48] |
| 1.3.2 S‐TC vs surfactant via ETT with delayed extubation | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.16, 1.05] |
| 1.4 Severe IVH | 5 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.42, 0.96] |
| 1.4.1 S‐TC vs INSURE | 4 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.32] |
| 1.4.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 1.5 Death during first hospitalisation | 11 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.84] |
| 1.5.1 S‐TC vs INSURE | 10 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.82] |
| 1.5.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.37, 1.79] |
| 1.6 BPD (clinical definition); in survivors to 36 weeks' PMA | 11 | 1567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.74] |
| 1.6.1 S‐TC vs INSURE | 10 | 1378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.44, 0.75] |
| 1.6.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.05] |
| 1.7 Catheter/ETT placement unsuccessful at first attempt | 6 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.26] |
| 1.7.1 S‐TC vs INSURE | 5 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] |
| 1.7.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.57] |
| 1.8 Bradycardia (heart rate < 100 bpm) during the intervention | 5 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.71, 1.83] |
| 1.8.1 S‐TC vs INSURE | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.39] |
| 1.8.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.89 [1.13, 13.38] |
| 1.9 Hypoxaemia (oxygen saturation < 80%) during the intervention | 4 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.99, 1.71] |
| 1.9.1 S‐TC vs INSURE | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.08] |
| 1.9.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.50, 3.11] |
| 1.10 Need for intubation within the first 72 hours or not intubated but reached failure criteria | 6 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.84] |
| 1.10.1 S‐TC vs INSURE | 4 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.96] |
| 1.10.2 S‐TC vs surfactant via ETT with delayed extubation | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.86] |
| 1.11 Need for intubation at any time | 4 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 1.11.1 S‐TC vs INSURE | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 1.11.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.84] |
| 1.12 Need for intratracheal surfactant therapy post intervention | 7 | 963 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.36] |
| 1.12.1 S‐TC vs INSURE | 6 | 918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.73, 1.32] |
| 1.12.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.63, 3.96] |
| 1.13 Duration of mechanical ventilation (days; in survivors) | 4 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.46, 0.41] |
| 1.13.1 S‐TC vs INSURE | 4 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.46, 0.41] |
| 1.14 Duration of any respiratory support (days; in survivors) | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.25, 0.03] |
| 1.14.1 S‐TC vs INSURE | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.25, 0.03] |
| 1.15 Duration of oxygen therapy (days; in survivors) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐2.04 [‐4.51, 0.43] |
| 1.15.1 S‐TC vs INSURE | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐2.04 [‐4.51, 0.43] |
| 1.16 Postnatal systemic corticosteroid therapy for BPD mitigation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.21] |
| 1.16.1 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.21] |
| 1.17 BPD (physiological definition) | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 1.17.1 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 1.18 IVH, any grade | 9 | 1353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.30] |
| 1.18.1 S‐TC vs INSURE | 9 | 1353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.30] |
| 1.19 Cystic PVL | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.15, 1.11] |
| 1.19.1 S‐TC vs INSURE | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.18] |
| 1.19.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.12, 1.07] |
| 1.20 PDA requiring medical therapy | 4 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.33] |
| 1.20.1 S‐TC vs INSURE | 4 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.33] |
| 1.21 NEC, modified Bell stage ≥2 | 3 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.81] |
| 1.21.1 S‐TC vs INSURE | 3 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.81] |
| 1.22 ROP stage ≥ 3 | 5 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.29] |
| 1.22.1 S‐TC vs INSURE | 4 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 1.98] |
| 1.22.2 S‐TC vs surfactant via ETT with delayed extubation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.06, 1.31] |
| 1.23 Duration of hospitalisation (days; in survivors) | 5 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐3.26, 1.06] |
| 1.23.1 S‐TC vs INSURE | 5 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐3.26, 1.06] |
| 1.24 Discharged home with oxygen | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
| 1.24.1 S‐TC vs INSURE | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.27] |
Comparison 2. Trials comparing S‐TC with S‐ETT ‐ sub‐group analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death or BPD | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Death or BPD: ≤ 28 weeks' gestation | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.13] |
| 2.1.2 Death or BPD: 29 to 32 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.3 Death or BPD: 33 to 36 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.4 Death or BPD: surfactant prophylaxis trials | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.5 Death or BPD: surfactant rescue trials | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.6 Death or BPD: trials using sedation for S‐TC | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.7 Death or BPD: trials using no sedation for S‐TC | 6 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.46, 0.73] |
Comparison 3. Trials comparing S‐TC with S‐ETT ‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death or BPD | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.78] |
| 3.2 Need for intubation within the first 72 hours | 6 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.51, 0.72] |
| 3.3 Air leak requiring drainage | 4 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.02] |
| 3.4 Severe IVH | 4 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.34, 0.89] |
| 3.5 Death during first hospitalisation | 5 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.09] |
| 3.6 BPD (clinical definition); in survivors to 36 weeks' PMA | 5 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.31, 0.64] |
Comparison 4. Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Death or BPD | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.74] |
| 4.2 Incidence of air leak requiring drainage | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.67] |
| 4.3 Severe IVH | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.50, 3.85] |
| 4.4 Death during first hospitalisation | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.48, 4.44] |
| 4.5 BPD (clinical definition); in survivors to 36 weeks' PMA | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.41] |
| 4.6 Catheter/ETT placement unsuccessful at first attempt | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.80 [0.46, 167.44] |
| 4.7 Bradycardia (heart rate < 100 bpm) during the intervention | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.33 [0.51, 171.25] |
| 4.8 Need for intubation within the first 72 hours or not intubated but reached failure criteria | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.88] |
| 4.9 Need for intubation at any time | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.61] |
| 4.10 Postnatal systemic corticosteroid therapy for BPD mitigation | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.27] |
| 4.11 Cystic PVL | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.51, 13.08] |
| 4.12 ROP ≥ stage 3 | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.33, 29.45] |
| 4.13 Discharged home with oxygen | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.37] |
| 4.14 Cerebral palsy by clinical examination or other means | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.60, 6.52] |
4.14. Analysis.

Comparison 4: Trials comparing S‐TC with continuation of non‐invasive support ‐ overall analysis, Outcome 14: Cerebral palsy by clinical examination or other means
Comparison 5. Trials comparing different methods of surfactant delivery via thin catheter ‐ overall analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Air leak | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.28, 23.65] |
| 5.2 Severe IVH | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.21, 86.79] |
| 5.3 Need for intubation during the procedure | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.83] |
| 5.4 Need for intubation within the first 24 hours | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.51, 4.83] |
| 5.5 Death during first hospitalisation | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.06, 13.22] |
| 5.6 Need for positive‐pressure ventilation during the intervention | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.53 [3.79, 55.73] |
| 5.7 Duration of the procedure (seconds) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐78.68, 78.68] |
| 5.8 Pain score using a validated instrument for measuring discomfort/pain during the procedure (e.g. COMFORTneo score) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐6.59, ‐3.41] |
| 5.9 Hypotension requiring treatment | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.47 [0.32, 129.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bao 2015.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial conducted at a tertiary NICU in China from January 2012 to December 2012 | |
| Participants | 90 preterm infants with gestational age between 28 and 32 weeks who were stabilised with nasal continuous positive airway pressure (CPAP) and were eligible for surfactant administration within 2 hours after birth. Infants who were intubated in the delivery room were excluded Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | The following data were recorded during surfactant instillation
|
|
| Notes | This study was conducted in an upper‐middle‐income country (China) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by a computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered with Chinese Current Controlled Trials ChiCTR‐ICR‐15006001, on 20 February 2015, after patient recruitment was completed |
| Other bias | Low risk | Nil noted |
Boskabadi 2019.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial conducted at tertiary NICUs at Ghaem Hospital of Mashhad, Iran, from 2012 to 2015 | |
| Participants | 40 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary and secondary outcomes not specified separately Outcomes
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Iran). It included relatively mature infants (mean gestational age 30 (SD 2) to 31 (SD 2) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. Study authors mentioned that (quote) "the infants who were included in the study were divided into two groups using the random block method" |
| Allocation concealment (selection bias) | Unclear risk | It is not clear whether or not allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear whether or not personnel and outcome assessors were blinded. Study authors described the study as (quote) "single‐blind" |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Unclear risk | It is not clear whether or not personnel and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not available for review. The trial does not seems to be prospectively registered with any trial registry |
| Other bias | Low risk | Intervention and control groups were similar in gestation and birth weight |
Choupani 2018.
| Study characteristics | ||
| Methods | Parallel‐group, single‐centre randomised controlled trial conducted at Hajar Hospital of Shahrekord NICU in Iran, from 2016 to 2017 | |
| Participants | 104 preterm infants with gestational age between 28 and 37 weeks who were stabilised with nasal continuous positive airway pressure (CPAP) and were eligible for surfactant administration within 2 hours after birth. Infants were enrolled when they reached FiO₂> 40%, or if they had moderate to severe respiratory distress Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | The following data were recorded during surfactant instillation
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Iran). It included relatively mature infants (mean (SD) gestational age 33.06 (2.3) to 32.9 (2.6) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "convenience sampling method was used to select participants from the neonates diagnosed with RDS in the neonatal intensive care unit (NICU) of Hajar hospital in Shahrekord. Participants were then randomly allocated into two groups by random allocation software" |
| Allocation concealment (selection bias) | Unclear risk | It is not clear whether or not allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study authors mentioned (quote): "this double blind clinical trial"; however, no details were mentioned |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Unclear risk | Study authors mentioned (quote): "this double blind clinical trial"; however, no details were mentioned |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Protocol of trial was not available for review. The trial was not registered with a trial registry |
| Other bias | High risk | The study was not reported according to CONSORT guidelines; hence it is difficult to judge its quality |
Dekker 2019.
| Study characteristics | ||
| Methods | Parallel‐group, single‐centre randomised controlled trial conducted at Leiden University Medical Centre in the Netherlands, from January 2015 and July 2017 | |
| Participants | 88 preterm infants Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in a high‐income country (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated by www.randomization.com. Allocation was stratified by GA (26 to 31 + 6 and 32 to 36 + 6 weeks) using variable block sizes (4 to 8) |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used to determine randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neonatologists performing the procedure were not blinded |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Low risk | Researchers who analysed both primary and secondary outcomes were blinded to treatment allocation: 2 independent NICU nurses, blinded to allocation, reviewed recordings and measured level of comfort using the COMFORTneo scale |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | All infants recruited were accounted for; however, total of 10 of the 88 infants (11%) were randomised and received allocated treatment; they were later excluded from final analysis due to lack of parental consent (n = 7) or lack of availability of video recordings (n = 3) |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Low risk | Trial was registered with the Dutch Trial Registry (NTR5010) on 18 December 2014, and the first patient was recruited on 5 January 2015 (https://www.trialregister.nl/trial/4765) |
| Other bias | Low risk | Nil noted |
Göpel 2011.
| Study characteristics | ||
| Methods |
Göpel 2011 Parallel‐group, multi‐centre randomised controlled trial (the Avoiding Mechanical Ventilation (AMV) trial) involving 12 German NICUs, conducted between October 2007 and January 2010 Herting 2020 2‐year follow‐up for AMV trial |
|
| Participants |
Göpel 2011 220 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
Herting 2020 The follow‐up study reported 2‐year outcomes for 179 infants from the 206 surviving infants (86.9%) among the original study participants (n = 220) Inclusion and exclusion criteria for the primary trial were as above All infants from the primary trial (n = 220) who were alive at 2 years of age (n = 206) were eligible for follow‐up. Of the total cohort of 220 infants:
2‐year follow‐up was completed in 95 infants from the intervention group and in 84 infants from the control group |
|
| Interventions |
|
|
| Outcomes |
Göpel 2011
Herting 2020
|
|
| Notes | This study was conducted in a high‐income country (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomly assigned with Randomization In Treatment Arms (RITA) programme (version 1.2) in a 1:1 ratio with variable block sizes (4 and 6) by an independent statistician |
| Allocation concealment (selection bias) | Low risk | An independent statistician prepared sequentially numbered, sealed, opaque envelopes stratified by centre and multiple birth status |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None of the participants and none of those giving the interventions, assessing outcomes, or analysing the data were masked to treatment |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | None of the participants and none of those giving the interventions, assessing outcomes, or analysing the data were masked to treatment |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | High risk | None of the participants and none of those giving the interventions, assessing outcomes, or analysing the data were masked to treatment |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | All infants recruited were accounted for |
| Selective reporting (reporting bias) | Low risk |
Göpel 2011 (low risk) The trial was prospectively registered with a clinical trial registry (ISRCTN05025922) Herting 2020 (unclear risk) The trial protocol was not available for review and was not prospectively registered with any trial registry |
| Other bias | Low risk |
Göpel 2011 (low risk) Pragmatic trial with no treatment standardisation between centres (e.g. caffeine, surfactant dose) leading to variability between centres. However, multi‐variate logistical regression analysis was implemented and showed no significant centre effect Herting 2020 (high risk) The initial design of the AMV study did not include the planning of a follow‐up study. However, this was planned towards the end of the AMV study. An addendum to the ethical approval for additional follow‐up data collection was sought, and parents were contacted and were asked for additional information. A protocol/case record form for follow‐up data was filled in by respective participating centres Follow‐up assessment was done by different examiners who were not all blinded. Follow‐up examinations were carried out at individual institutes under different conditions, all of which may influence the quality of data. Furthermore, collected data are not complete for all items. Of note is that the AMV trial was powered to assess differences in BPD but not to assess neurodevelopment. Subgroup analyses in each GA stratum, especially the 23‐ and 24‐week GA strata, are based on rather small numbers of patients |
Gupta 2020.
| Study characteristics | ||
| Methods | Parallel‐group, single‐centre randomised controlled trial conducted at a level III NICU in a tertiary care hospital in Kolkata, India, from March 2019 to December 2019 | |
| Participants | 58 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (India). It included relatively mature infants (median gestational age 30.07 weeks (intervention) and 29.90 weeks (control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding in the study, and even outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | There was no blinding in the study, and even outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were analysed |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Low risk | Registered with clinical trial registry of India (registration number CTRI/2019/03/017992) prospectively on 8 March 2019 |
| Other bias | Low risk | Nil noted |
Halim 2019.
| Study characteristics | ||
| Methods | Parallel‐group, single‐centre randomised controlled trial conducted at a NICU in Islamabad, Pakistan, from April till December 2017 | |
| Participants | 100 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary and secondary outcomes are not clearly defined. Study authors studied multiple demographic and clinical data
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Pakistan). It included relatively mature infants (only 10% of infants were at < 28 weeks' GA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Serial numbers from 1 to 100 were randomly divided into 2 groups via a Web‐based randomisation tool (www.randomizer.org) |
| Allocation concealment (selection bias) | Unclear risk | It is not clear whether or not allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear whether or not personnel were blinded |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Unclear risk | It is not clear whether or not outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the trial was not available for review. Trial does not seem to be prospectively registered with any trial registry |
| Other bias | High risk | Control (InSurE) group had slightly less antenatal steroid coverage. This group also was intubated a bit later compared to the intervention (thin catheter) group. No adjustment for these differences was made in the final analysis |
Han 2020.
| Study characteristics | ||
| Methods | Parallel‐group, multi‐centre randomised controlled trial (Minimally Invasive Surfactant Administration (MISA) trial) involving 8 level III Chinese NICUs, conducted between 1 July 2017, and 31 December 2018. It appears that patients were followed up until 30 March 2019; however follow‐up data are not reported in this manuscript | |
| Participants | 344 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (China). It included relatively mature infants (mean gestational age 30.6 (SD 1.6) to 30.8 (SD 1.3) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation for randomisation was unclear. Allocation ratio was 1:1 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelopes were used to complete group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Assigned treatment was not blinded, as the mode of respiratory management was apparent to clinicians and nurses in the NICU |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Assigned treatment was not blinded |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Outcomes for 13.4% of randomised infants not reported |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered with ClinicalTrials.gov (NCT04077333) on 4 September 2019, after patient recruitment was completed |
| Other bias | High risk | Multi‐centre study, with variation in proportion of eligible infants enrolled at each centre (12.8% to 56.5%). Practices were not consistent between centres, particularly in relation to extubation criteria and ventilatory practices. Infants who died or were transferred to other hospitals for surgery and those with incomplete data (n = 34) were excluded after randomisation and after completing/starting the intervention. For the intervention arm (MISA group), infants who were given a second surfactant dose by intubation and/or were ventilated during the first 72 hours (n = 12) were excluded from final analysis, and infants given a second dose of surfactant by MISA were included. Data analyses were performed on a per‐protocol basis rather than on an intention‐to‐treat basis |
Jena 2019.
| Study characteristics | ||
| Methods | Parallel‐group, multi‐centre randomised controlled trial involving 3 Indian NICUs, conducted between 2013 and 2017 | |
| Participants | Total of 350 preterm infants at ≤ 34 weeks were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
No sedation nor pre‐medication was used in both groups. Criteria for subsequent doses of surfactant, intubation, and MV were the same in both groups |
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (India). It included relatively mature infants (median (IQR) gestational age 31 (29 to 33) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer‐generated random sequence number. Allocation ratio was 1:1 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done by using an opaque sealed envelope. Generation of random numbers and assignment was completed by a person not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Blinding of the intervention was not possible due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the trial was not available for review. Trial was not prospectively registered |
| Other bias | Low risk | Data analysis was based on intention‐to‐treat analysis using StataCorp 11.1, Houston, TX Researcher estimated a sample size of 150 for each group and managed to recruit 175 |
Kanmaz 2013.
| Study characteristics | ||
| Methods | Parallel‐group, single‐centre randomised controlled trial (Take Care Trial) conducted at a tertiary NICU in Turkey between December 2010 and December 2011 | |
| Participants | 200 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Turkey) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered with ClinicalTrials.gov registry (NCT01329432) on 6 April 2011, after patient recruitment began |
| Other bias | High risk | Single‐centre study. Some infants who might have been eligible for the study could not be enrolled because of concern for standardisation of the intervention (e.g. unavailability of the study team) |
Kribs 2015.
| Study characteristics | ||
| Methods |
Kribs 2015 Parallel‐group, multi‐centre randomised controlled trial involving 13 German NICUs (Nonintubated Surfactant Application (NINSAPP) trial) between April 2009 and June 2012 Mehler 2020 2‐year follow‐up for NINSAPP trial |
|
| Participants |
Kribs 2015 211 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
Mehler 2020 All infants from the primary trial (n = 211) who were alive at 2 years of age (n = 182) were eligible for follow‐up. Of the total cohort of 211 infants
2‐year follow‐up was completed in 78 infants from the intervention group and in 78 infants from the control group |
|
| Interventions |
|
|
| Outcomes |
Kribs 2015
Mehler 2020
|
|
| Notes | This study was conducted in a high‐income country (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was designed in a 1:1 ratio with variable block sizes by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the procedure was not attempted |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | All infants recruited were accounted for |
| Selective reporting (reporting bias) | Low risk |
Kribs 2015 (low risk) Trial was prospectively registered with a clinical trial registry (NCT00751959) Mehler 2020 (unclear risk) Protocol of trial was not available for review. Trial does not seems to be prospectively registered with any trial registry |
| Other bias | Low risk |
Kribs 2015 (low risk) Pragmatic trial with no treatment standardisation between centres (e.g. caffeine, surfactant dose) leading to variability between centres. However, multi‐variate logistical regression analysis was implemented and showed no significant centre effect Mehler 2020 (unclear risk) The initial design of the NINSAPP study did not include the planning of a follow‐up study. However, this was planned after the end of the NINSAPP study. An addendum to the ethical approval for additional follow‐up data collection was sought, and parents were contacted and were asked for additional information. A protocol/case record form for follow‐up data was filled in by the respective participating centres Follow‐up assessment was done by different examiners who were not all blinded. Follow‐up examinations were carried out at the individual institutes under different conditions, all of which may influence the quality of data. Furthermore, collected data are not complete for all items. Of note is that the NINSAPP trial was powered to assess differences in BPD but not for assessment of neurodevelopment. Subgroup analyses in each GA stratum, especially the 23‐ and 24‐week GA strata, are based on rather small numbers of patients |
Mirnia 2013a.
| Study characteristics | ||
| Methods | Parallel‐group, multi‐centre randomised controlled trial conducted at 3 tertiary NICUs in Iran (Tabriz, Isfahan, and Mashhad) from February 2010 to October 2012 | |
| Participants | 136 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Iran). Atropine was given before intubation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not reported |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Unclear risk | Method not reported |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Protocol of trial was not available for review. Study was not registered with prospective trial registry |
| Other bias | Unclear risk | Study was not reported according to CONSORT guidelines; hence it is difficult to judge its quality |
Mohammadizadeh 2015.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial conducted at 2 tertiary NICUs in Isfahan, Iran, from December 2012 to May 2013 | |
| Participants | 38 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (Iran). It included relatively mature infants (mean gestational age 30 (SD 2) to 31 (SD 2) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the intervention was performed by a medical practitioner (level 2 neonatal trainee) who was not involved in randomisation or outcome assessment" It is not clear whether or not other clinicians were blinded |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Unclear risk | It is not clear whether or not outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Protocol of trial was not available for review. Trial does not seems to be prospectively registered with any trial registry |
| Other bias | Unclear risk | Intervention (thin catheter) group was slightly smaller in birth weight. An adjustment was made for this difference. There was no comment about practices in the 2 collaborating centres (e.g. extubation criteria, adjuvant treatments such as caffeine) |
Mosayebi 2017.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial conducted at a single tertiary NICU in Tehran (Roointan‐Arash Maternity Hospital), Iran, from April 2013 to February 2014 | |
| Participants | 53 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
No sedation nor pre‐medication was used in both groups Treatment was considered a failure if pH < 7.2, FiO₂ > 60%, and PCO₂ > 60 mmHg persisted for longer than 2 hours, or if apnoea occurred, upon which the infant was intubated, and if required, surfactant was administered |
|
| Notes | This study was conducted in an upper‐middle‐income country (Iran). It included relatively mature infants (mean gestational age 31.9 (SD 1.5) to 32.6 (SD 1.1) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details (quote): "simple randomization was used in the allocation of participants" |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not performed at any stage of the study ‐ from intervention to data analysis and interpretation of results |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Blinding was not performed at any stage of the study ‐ from intervention to data analysis and interpretation of results |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Study was retrospectively registered with the Iranian Registry of clinical trials, IRCT2014080716937N4 |
| Other bias | Unclear risk | Study was not reported according to CONSORT guidelines; hence it is difficult to judge its quality |
Olivier 2017.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial conducted at 3 Canadian centres from January 2014 to May 2016 | |
| Participants | 45 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in a high‐income country (Canada). Infants meeting failure criteria were regarded as having reached the primary outcome in the analysis, even if MV was not initiated. Multi‐variate regression was used to control for age at oxygen introduction and age at surfactant administration among other confounders. All patients received atropine and fentanyl before the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was created in a 1:1 ratio in blocks of 4 by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Participants were randomised immediately after inclusion via sealed opaque envelopes (prepared by a nurse not involved in the study) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the procedure was not attempted |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for. A total of 7 eligible patients were not recruited and 4 patients were randomised before inclusion criteria were fulfilled |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered with any trial registry |
| Other bias | Unclear risk | Multi‐centre study. The clinical approach to management of patients in the control group was not standardised after randomisation and was left to the judgement of the clinician (e.g. there was no intubation criteria). Surfactant given after intubation via ETT (S‐ETT) on the judgement of attending physician |
Yang 2020.
| Study characteristics | ||
| Methods | Parallel‐group, quasi‐randomised controlled trial conducted at tertiary NICUs at Children’s Hospital of Shanxi, China, from February 2017 to January 2018 Patients were randomised according to 'numbered list': odd number – LISA group, even number ‐ InSurE group (verbal communication with study authors) |
|
| Participants | 97 preterm infants were included in the trial. Inclusion and exclusion criteria were as follows Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in an upper‐middle‐income country (China). It included relatively mature infants (mean gestational age 33.7 (SD 1.0) to 34.1 (SD 1.3) weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randmised according to 'numbered list': odd number – LISA group, even number ‐ InSurE group (verbal communication with study authors) |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data collector (research assistant) and statistician did not participate in patient management. The medical team who administered surfactant did not participate in patient management thereafter |
| Blinding of outcome assessment (detection bias) Short‐term outcomes | Low risk | Data collector (research assistant) and statistician did not participate in patient management. The medical team who administered surfactant did not participate in patient management thereafter |
| Blinding of outcome assessment (detection bias) Long‐term outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | All infants recruited were accounted for |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered with any trial registry |
| Other bias | Low risk | Intervention and control groups were similar in gestation, birth weight, and other background variables |
AMV: Avoiding Mechanical Ventilation trial; APGAR: appearance, pulse, grimace, activity, and respiration; BPD: bronchopulmonary dysplasia; bpm: beats per minute; CONSORT: Consolidated Standards of Reporting Trials; CPAP: continuous positive airway pressure; EISA: endotracheal intubation surfactant administration; EOS: early‐onset sepsis; ETT: endotracheal tube; GA: gestational age; HR: heart rate; InSurE: Intubate, Surfactant, Extubate; IQR: interquartile range; IVH: intraventricular haemorrhage; LISA: less invasive surfactant administration; MAP: mean airway pressure; MDI: mental development index; MISA: minimally invasive surfactant administration; MIST: minimally invasive surfactant therapy; MV: mechanical ventilation; nCPAP: nasal continuous positive airway pressure; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; NINSAPP: Nonintubated Surfactant Application trials; NIPPV: nasal intermittent positive‐pressure ventilation; PDA: patent ductus arteriosus; PDI: psychomotor development index; PEEP: positive end‐expiratory pressure; RDS: respiratory distress syndrome; RITA: Randomization In Treatment Arms (RITA) programme; ROP: retinopathy of prematurity; SD: standard deviation; SAINT: Surfactant Administration by Insure or Thin Catheter; S‐ETT: surfactant administration via ETT; SurE: Surfactant, Extubate; SWI: surfactant‐without‐intubation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mirnia 2013b | Study participants are reported as part of another Iranian multi‐centre randomised trial (Mirnia 2013a |
| Oncel 2016 | Single‐centre randomised controlled study comparing the effectiveness of nCPAP and NIPPV as initial respiratory support within the MIST approach in preterm infants with respiratory distress syndrome. Both study groups received MIST |
MIST: minimally invasive surfactant therapy; nCPAP: nasal continuous positive airway pressure; NIPPV: nasal intermittent positive‐pressure ventilation.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000916943.
| Study name | OPTIMIST‐A trial: multi‐centre randomised controlled trial of minimally invasive surfactant therapy in preterm infants 25 to 28 weeks' gestation on continuous positive airway pressure |
| Methods | Multi‐centre parallel masked randomised controlled trial of minimally invasive surfactant therapy in preterm infants |
| Participants | Preterm infants at 25 to 29 weeks' gestation on continuous positive airway pressure |
| Interventions |
|
| Outcomes |
|
| Starting date | 1 December 2011 |
| Contact information | Prof Peter Dargaville; Department of Paediatrics, Royal Hobart Hospital, Liverpool St., Hobart, Tasmania 7000; Australia; Tel: +61 3 62228308; Email: peter.dargaville@dhhs.tas.gov.au |
| Notes | Recruitment status of this study is (quote) "active, recruiting" Registered with ANZCTR: ACTRN12611000916943, on 26 August 2011 Registered with ClinicalTrials.gov: NCT02140580, on 16 May 2014 |
ACTRN12611000917932.
| Study name | OPTIMIST‐B trial: multi‐centre randomised controlled trial of minimally invasive surfactant therapy in preterm infants 29 to 32 weeks' gestation on continuous positive airway pressure |
| Methods | Multi‐centre parallel blinded randomised controlled trial of minimally invasive surfactant therapy in preterm infants |
| Participants | Preterm infants at 29 to 32 weeks' gestation on continuous positive airway pressure |
| Interventions |
|
| Outcomes |
|
| Starting date | Recruitment not commenced |
| Contact information | Prof Peter Dargaville; Department of Paediatrics, Royal Hobart Hospital, Liverpool St., Hobart, Tasmania 7000; Australia; Tel: +61 3 62228308; Email:peter.dargaville@ths.tas.gov.au |
| Notes | Recruitment status of this study is (quote) "not recruiting" Registered with ANZCTR: ACTRN12611000917932, on 26 August 2011 |
ChiCTR1900020970.
| Study name | LPPSA: less invasive surfactant administration versus endotracheal surfactant instillation followed by limited peak pressure ventilation in preterm infants with respiratory distress syndrome in China: study protocol for a randomized controlled trial |
| Methods | Multi‐centre randomised prospective trial; will be conducted at 14 tertiary NICUs in China from January 2019 to December 2020 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | To compare surfactant application via 2 techniques
|
| Outcomes |
|
| Starting date | January 2019 |
| Contact information | Jiajun Zhu, Women’s Hospital, Zhejiang University, School of Medicine, Hangzhou 310006, China; Lizhong Du, The Children’s Hospital, Zhejiang University, School of Medicine, Hangzhou 310052, China |
| Notes | Funded by major scientific and technological projects of medicine and health in Zhejiang Province (WKJ‐ZJ‐2032) Recruitment status of this study is (quote) "recruiting" Registered with Chinese Clinical Trial Registry: ChiCTR1900020970, on 23 January 2019 |
NCT01615016.
| Study name | MISurf: MISurF versus InSurE. A comparison of minimally invasive surfactant application techniques in preterm infants |
| Methods | Feasibility of a masked, prospective randomised controlled trial with 2 intervention arms |
| Participants | Eligible are all preterm infants born at ≤ 30 weeks' gestation at McMaster Inclusion criteria
Exclusion criteria
|
| Interventions | To compare surfactant application using 2 techniques
|
| Outcomes |
|
| Starting date | July 2012 |
| Contact information | Salhab el Helou,McMaster University, Children's Hospital/Hamilton Health Sciences |
| Notes | Recruitment status of this study is (quote) "terminated (delay due to infrastructure reasons. Funding withdrawn)" Registered with ClinicalTrials.gov: NCT01615016, on 8 June 2012 |
NCT01848262.
| Study name | ECALMIST: ECALMIST versus InSurE in preterm infant < 32 weeks, multi‐centre, multi‐national RCT |
| Methods | Prospective open‐label randomised clinical trial |
| Participants | Newborn at < 32 weeks' gestation at birth Inclusion criteria
Exclusion criteria
|
| Interventions | Minimally invasive surfactant therapy via a small vascular catheter ‐ ECALMIST (Early CPAP And Large Volume Minimal Invasive Surfactant Therapy) versus InSurE in preterm infants with RDS
|
| Outcomes |
|
| Starting date | June 2013 |
| Contact information | Yahya Al Ethawi, University of Manitoba. |
| Notes | Recruitment status of this study is (quote) "unknown". Completion date has passed and status has not been verified in more than 2 years Registered with ClinicalTrials.gov: NCT01848262, on 7 May 2013 |
NCT02772081.
| Study name | LISPAP: a randomized, controlled study in preterm neonates with RDS to compare two procedures for porcine surfactant (Poractant Alfa, CUROSURF®) administration: a less invasive method (LISA) and the conventional administration |
| Methods | Multi‐centre randomised, open‐label, parallel‐assignment, controlled study ‐ phase 3 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | To compare surfactant application via 2 techniques
|
| Outcomes | Numbers of neonates with surfactant‐ and procedure‐related adverse events (time frame: from application of the laryngoscope up to removal of the CHF 6440 catheter or the endotracheal tube within 1 hour after instillation of poractant alfa, device occlusion, apnoea, neonatal oxygen desaturation, bradycardia, hypotension requiring treatment, cough, sneezing, choking, laryngospasm, surfactant regurgitation, vomiting) |
| Starting date | June 2018 |
| Contact information | Gianluca Lista, Buzzi Hospital, Milan |
| Notes | Sponsored by Chiesi Farmaceutici S.p.A. Recruitment status of this study is (quote) "suspended (sponsor decision)" Registered with ClinicalTrials.gov: NCT02772081, on 12 May 2019 |
NCT03989960.
| Study name | MOLISAN: modified intubation‐surfactant‐extubation (InSurE) technique in preterm neonates with respiratory distress syndrome |
| Methods | Parallel‐assignment, non‐randomised, double‐blind (participant, outcomes assessor) study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | To compare surfactant application via 2 techniques
|
| Outcomes |
|
| Starting date | 1 August 2018 |
| Contact information | Dr. Xiaoqing Chen, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China, 210029 |
| Notes | Recruitment status of this study is (quote) "active, not recruiting" Registered with ClinicalTrials.gov: NCT03989960, on 18 June 2019 |
NCT04016246.
| Study name | PROLISA: propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double‐blind, placebo controlled trial |
| Methods | Multi‐centre, parallel‐blinded randomised controlled trial of minimally invasive surfactant therapy in preterm infants with vs without sedation with LISA |
| Participants | Newborn < 32 weeks' gestation at birth Inclusion criteria
Exclusion criteria
|
| Interventions |
In both groups, when the team is ready, a solution of atropine, caffeine, and oral sugar solution 30% or 24% is given orally via a syringe before the LISA procedure LISA procedure involves surfactant treatment during spontaneous breathing via an aspiration probe (CH6) or the LISAcath® (catheter for oral endotracheal instillation, CHIESI SAS, Bois Colombes, France). The type of probe is left to the choice of each investigator |
| Outcomes |
|
| Starting date | 7 October 2019 |
| Contact information | Marie Chevallier, UMR 5525 ThEMAS, CNRS, TIMC‐IMAG, Grenoble Alps University, Grenoble, France, and Neonatal Intensive Care Unit, Grenoble Alps University Hospital, Grenoble, France. Email: mchevallier3@chu-grenoble.fr |
| Notes | Recruitment status of this study is (quote) "recruiting" Registered with ClinicalTrials.gov: NCT04016246, on 06 June 2019, N°EUDRACT: 2018–002876‐41 |
NCT04073173.
| Study name | StrAAS: stress assessment in preterm infants with respiratory distress syndrome treated or not with an analgesic drug during traditional or less invasive method of surfactant therapy |
| Methods | Single‐centre parallel open‐label randomised controlled trial. Planning to recruit 80 participants |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 November 2020. |
| Contact information | Virgilio Carnielli; Azienda Ospedaliero‐Universitaria Ospedali Riuniti di Ancona; Tel: +390715962045; Email: v.carnielli@staff.univpm.it Clementina Rondina; Azienda Ospedaliero‐Universitaria Ospedali Riuniti di Ancona; Tel +390715962014; Email clementina.rondina@ospedaliriuniti.marche.it |
| Notes | Recruitment status of this study is (quote) "active, not recruiting" Registered with ClinicalTrials.gov: NCT04073173, on 29 August 2019 |
NCT04445571.
| Study name | SAINT: Surfactant Administration by Insure or Thin Catheter |
| Methods | Single‐centre parallel open‐label randomised controlled trial. Planning to recruit 160 participants |
| Participants |
Inclusion criteria
Exclusion criteria
Infants will be excluded from the final analysis if they have a congenital abnormality or condition that might have an adverse effect on breathing or ventilation, including congenital diaphragmatic hernia; tracheo‐oesophageal fistula; or cyanotic heart disease |
| Interventions |
|
| Outcomes |
|
| Starting date | 15 October 2020 |
| Contact information | Kajsa Bohlin, Karolinska University Hospital, Stockholm, Sweden, 14186, Tel 0858580000 ext 81356; Email: kajsa.bohlin@ki.se Mats Blennow,Karolinska University Hospital, Stockholm, Sweden, 14186, Tel 0858580000 ext 81428; Email: mats.blennow@ki.se |
| Notes | Recruitment status of this study is (quote) "recruiting" Registered with ClinicalTrials.gov: NCT04445571, on 24 June 2020 |
UMIN000021785.
| Study name | Effectiveness of MIST (minimally invasive surfactant therapy) under bronchoscopy in treating neonatal respiratory distress syndrome |
| Methods | Single‐arm, non‐randomised, open‐label study |
| Participants |
Inclusion criteria: patients fulfilling all of the following criteria will be included
Exclusion criteria: patients fulfilling any of the following criteria will be excluded
|
| Interventions | To compare surfactant application using 2 techniques
|
| Outcomes |
|
| Starting date | January 2016 |
| Contact information | Masanori Wasa, Tokyo Women's Medical University Medical Centre East, 2‐1‐10 Nishiogu, Arakawaku, Tokyo |
| Notes | Funded by Tokyo Women's Medical University Medical Centre East Recruitment status of this study is (quote) "recruiting" Registered with UMIN‐CTR Clinical Trial (Japan): UMIN000021785, on 5 April 2016 |
ASQ: age and stage questionnaire; BPD: bronchopulmonary dysplasia; bpm: beats per minute; CLD: chronic lung disease; CPAP: continuous positive airway pressure; CRF: case report form; CSF: cerebrospinal fluid; ECALMIST: Early CPAP And Large Volume Minimal Invasive Surfactant Therapy;ETT: endotracheal tube; FANS: Faceless Acute Neonatal Pain Scale; GA: gestational age; hsPDA: haemodynamically significant patent ductus arteriosus;InSurE: intubate, surfactant, extubate; IVH: intraventricular haemorrhage; LISA: less invasive surfactant administration; LPPSA: A Randomized, Controlled Study in Preterm Neonates With RDS to Compare Two Procedures for PorcineSurfactant (Poractant Alfa, CUROSURF®) Administration: A Less Invasive Method (LISA) and ConventionalAdministration; MIST: minimally invasive surfactant therapy; MISurF: minimally invasive surfactant; MV: mechanical ventilation; nCPAP: nasal continuous positive airway pressure;NEC: necrotising enterocolitis; nHFOV: nasal high‐frequency oscillatory ventilation; NICU: neonatal intensive care unit; NIPPV: nasal intermittent positive‐pressure ventilation; NIRS: near‐infrared spectroscopy; NIV: non‐invasive ventilation; PEEP: positive end‐expiratory pressure; PSC: pulmonary severity score; SAE: serious adverse event; SNIPPV: synchronised nasal intermittent positive‐pressure ventilation technique; StrAAS: Stress Assessment in Preterm Infants With Respiratory Distress Syndrome Treated or Not With an Analgesic Drug During the Traditional or the Less Invasive Method of Surfactant Therapy.
Differences between protocol and review
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see www.cochranelibrary.com/central/central-creation). Cochrane Neonatal has validated its searches to ensure that relevant Embase records are found while CENTRAL is searched.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs from ClinicalTrials.gov nor from the International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see www.cochranelibrary.com/central/central-creation). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (at http://www.isrctn.com/, formerly Controlled‐trials.com) is searched separately.
We modified the title of the Review from ‘Surfactant therapy via brief tracheal catheterisation in preterm infants with or at risk of respiratory distress syndrome’ to ‘Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome’, and changed ‘surfactant via brief tracheal catheterisation’ to ‘surfactant via thin catheter’ in the text.
We added the methods and the plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol (Wheeler 2015).
-
We also added the following outcomes, which were not included in the original protocol.
Catheter/ETT placement unsuccessful at first attempt (during trial‐related intervention).
Incidence of the need for mechanical ventilation within first 72 hours, or not ventilated but reached failure criteria.
Incidence of spontaneous intestinal perforation..
We added a definition for neurosensory disability.
-
We added six new outcomes to the primary outcome section (now a total of seven primary outcomes). These added outcomes were listed as secondary outcomes in the protocol (Wheeler 2015).
Need for intubation within the first 72 hours of life.
Air leak requiring drainage (during first hospitalisation).
Severe intraventricular haemorrhage (IVH), including grades III and IV (Papile 1978).
Death during first hospitalisation (all causes).
BPD (clinical definition) among survivors to 36 weeks' PMA.
Death or survival with neurosensory disability, with the latter measured beyond one year PMA and defined as any of (i) cerebral palsy by clinical examination or other means; (ii) developmental delay more than two standard deviations below the population mean on standardised testing; (iii) blindness (visual acuity less than 6/60); or (iv) deafness (hearing impairment requiring amplification).
-
A number of outcomes in the protocol ‐ Wheeler 2015 ‐ were not included in the review, largely based on anticipated infrequency of reporting. These were:
incidence of discontinuation of the intervention;
number of surfactant doses post intervention (need for intratracheal surfactant therapy post intervention remains);
incidence of dosing failure;
incidence of the need for early intubation (within one hour of surfactant administration);
use of diuretic therapy as prophylactic or rescue treatment;
incidence of death in the first 28 days (in this case, a single death outcome was opted for);
incidence of PDA requiring surgical therapy;
major morbidity and death, or major morbidity (here, the concern was the variability of definitions for this outcome);
time to regain birth weight; and
number of hospital re‐admissions in the first year (all).
-
One of the sub‐group analyses proposed in the protocol ‐ Wheeler 2015 ‐ was omitted.
Surfactant type (animal‐derived, synthetic).
Contributions of authors
The protocol ‐ Wheeler 2015 ‐ was developed by KIW and PAD. All review authors provided feedback on the content of the protocol (Wheeler 2015).
The review manuscript was developed in RevMan 5 by MEA. MEA and PAD independently performed electronic database searches, assessed the certainty of evidence, and synthesised the evidence. PGD also provided methodological input. All review authors provided feedback on the content of the draft and the final manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
MEA has no interests to declare.
KIW has no interests to declare.
PGD has no interests to declare.
AGDP has no interests to declare.
PAD is the Chief Investigator of the OPTIMIST‐A trial, a multi‐centre RCT of surfactant via tracheal catheterisation in preterm infants on CPAP (ACTRN12611000916943). Chiesi Farmaceutici (Parma, Italy) is providing in‐kind support for this trial by providing surfactant at reduced cost for the OPTIMIST‐A trial. Dr. Dargaville has served as a consultant for Chiesi Farmaceutici and AbbVie Inc. Neither company is involved with the protocol, analysis, manuscript preparation, or publication processes of this review. The Australian National Health and Medical Research Council (NHMRC) has awarded a project grant (#1049114) for conduct of an RCT of minimally invasive surfactant therapy in preterm infants on CPAP, for which PAD is the Chief Investigator.
Edited (no change to conclusions)
References
References to studies included in this review
Bao 2015 {published and unpublished data}
- Bao Y, Zhang G, Wu M, Ma L, Zhu J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatrics 2015;15(21):1-6. [DOI: 10.1186/s12887-015-0342-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boskabadi 2019 {published data only}
- Boskabadi H, Maamouri G, Gharaei Jomeh R, Zakerihamidi M. Comparative study of the effect of the administration of surfactant through a thin endotracheal catheter into trachea during spontaneous breathing with intubation (intubation-surfactant-extubation method). Journal of Clinical Neonatology 2019;8(4):227-31. [DOI: 10.4103/jcn.JCN_32_19] [DOI] [Google Scholar]
Choupani 2018 {published data only}
- Choupani R, Mashayekhy G, Hmidi M, Kheiri S, Khalili Dehkordi M. A comparative study of the efficacy of surfactant administration through a thin intratracheal catheter and its administration via an endotracheal tube in neonatal respiratory distress syndrome. Iranian Journal of Neonatology 2018;9(4):33-40. [DOI: 10.22038/ijn.2018.30057.1408] [DOI] [Google Scholar]
Dekker 2019 {published and unpublished data}
- Dekker J, Lopriore E, Zanten HA, Tan RN, Hooper SB, te Pas AB. Sedation during minimal invasive surfactant therapy: a randomised controlled trial (PROMISES). Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(4):F378–83. [DOI: 10.1136/archdischild-2018-315015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Göpel 2011 {published data only}
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al, German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants: an open-label, randomised, controlled trial (AMV Trial). Lancet 2011;378(9803):1727-34. [DOI: 10.1016/S0140-6736(11)60986-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Herting E, Kribs A, Härtel C, Wense A, Weller U, Hoehn T, et al, German Neonatal Network (GNN). Two-year outcome data suggest that less invasive surfactant administration (LISA) is safe. Results from the follow-up of the randomized controlled AMV (avoid mechanical ventilation) study. European Journal of Pediatrics 2020;179(8):1309-13. [DOI: 10.1007/s00431-020-03572-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2020 {published data only}
- Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation - a randomized controlled trial. European Journal of Pediatrics 2020;179(8):1287–93. [DOI: 10.1007/s00431-020-03682-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halim 2019 {published data only}
- Halim A, Shirazi H, Riaz S, Gul SS, Ali W. Less Invasive surfactant administration in preterm infants with respiratory distress syndrome. Journal of the College of Physicians and Surgeons Pakistan 2019;29(3):226-30. [DOI: 10.29271/jcpsp.2019.03.226] [PMID: ] [DOI] [PubMed] [Google Scholar]
Han 2020 {published and unpublished data}
- Han T, Liu H, Zhang H, Guo M, Zhang X, Duan Y, et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Frontiers in Pediatrics 2020;8(182):1-12. [DOI: 10.3389/fped.2020.00182] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jena 2019 {published and unpublished data}
- Jena SR, Bains HS, Pandita A, Verma A, Gupta V, Kallem VR, et al, On Behalf of Sure Group. Surfactant therapy in premature babies: SurE or InSurE. Pediatric Pulmonology 2019;54(11):1747-52. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanmaz 2013 {published and unpublished data}
- Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 2013;131(2):e502-9. [DOI: 10.1542/peds.2012-0603] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kribs 2015 {published data only}
- Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R, Teig N, et al, NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants a randomized clinical trial. JAMA Pediatrics 2015;169(8):723-30. [DOI: 10.1001/jamapediatrics.2015.0504] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mehler K, Broer A, Roll C, Göpel W, Wieg C, Norbert PJ, et al. Developmental outcome of extremely preterm infants is improved after less invasive surfactant application (LISA). Acta Paediatrica 2020;00:1-8. [DOI: 10.1111/apa.15565] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mirnia 2013a {published data only}
- Mirnia K, Heidarzadeh M, Hosseini MB, Sadeghnia A, Balila M, Ghojazadeh M. Comparison outcome of surfactant administration via tracheal catheterization during spontaneous breathing with INSURE. Medical Journal of Islamic World Academy of Sciences 2013;21(4):143-8. [DOI: 10.12816/0002647] [DOI] [Google Scholar]
Mohammadizadeh 2015 {published data only}
- Mohammadizadeh M, Ardestani AG, Sadeghnia AR. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. Journal of Research in Pharmacy Practice 2015;4(1):31-6. [DOI: 10.4103/2279-042X.150053] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosayebi 2017 {published data only}
- Mosayebi Z, Kadivar M, Taheri-Derakhsh N, Nariman S, Mahdi Marash Si, Farsi Z. A randomized trial comparing surfactant administration using InSurE technique and the minimally invasive surfactant therapy in preterm infants (28 to 34 weeks of gestation) with respiratory distress syndrome. Journal of Comprehensive Pediatrics 2017;8(4):e60724. [DOI: 10.5812/compreped.60724] [DOI] [Google Scholar]
Olivier 2017 {published and unpublished data}
- Olivier F, Nadeau S, Bélanger S, Julien AS, Massé E, Ali N, et al. Efficacy of minimally invasive surfactant therapy in moderate and late preterm infants: a multicentre randomized control trial. Paediatrics & Child Health 2017;22(3):120-4. [DOI: 10.1093/pch/pxx033] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2020 {published and unpublished data}
- Yang G, Hei M, Xue Z, Zhao Y, Zhang X, Wang C. Effects of less invasive surfactant administration (LISA) via a gastric tube on the treatment of respiratory distress syndrome in premature infants aged 32 to 36 weeks. Medicine (Baltimore) 2020;99(9):e19216. [DOI: 10.1097/MD.0000000000019216] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Mirnia 2013b {published data only}
- Heidarzadeh M, Mirnia K, Hoseini MB, Sadeghnia A, Akrami F, Balila M, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra Hospital. Iranian Journal of Neonatology 2013;4(2):5-9. [DOI: 10.22038/IJN.2013.1075] [ijn.mums.ac.ir/article_1075.html] [DOI] [Google Scholar]
- Mirnia K, Heidarzadeh M, Hoseini MB, Sadeghnia A, Akrami F, Balila M, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra Hospital. Iranian Journal of Neonatology 2013;4(2):5-9. [DOI: 10.22038/IJN.2013.1075] [jn.mums.ac.ir/article_1075.html] [DOI] [Google Scholar]
Oncel 2016 {published data only}
- Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Archives of Disease in Childhood. Fetal Neonatal Edition 2016;101(4):F323-8. [DOI: 10.1136/archdischild-2015-308204] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12611000916943 {published data only}
- ACTRN12611000916943. OPTIMIST-A trial: multicentre randomised controlled trial in preterm infants 25-28 weeks gestation on continuous positive airway pressure of the effect of minimally-invasive surfactant therapy in comparison to standard care (continuation of CPAP) on the incidence of the composite outcome of death or physiological BPD. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336668 (first received 25 August 2011). [https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336668&isReview=true (Registered on 26 Aug 2011)]
- Dargaville PA, Kamlin CO, De Paoli AG, Carlin JB, Orsini F, Soll RF, et al. The OPTIMIST-A trial: evaluation of minimally-invasive surfactant therapy in preterm infants 25–28 weeks gestation. BMC Pediatrics 2014;14:213. [DOI: 10.1186/1471-2431-14-213] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02140580. OPTIMIST-A trial: minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation on CPAP (OPTIMIST-A) [Multicentre randomised controlled trial of minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation on continuous positive airways pressure]. clinicaltrials.gov/ct2/show/NCT02140580 (first received 16 May 2014).
ACTRN12611000917932 {published data only}
- ACTRN12611000917932. The OPTIMIST-B trial: multicentre randomised controlled trial in preterm infants 29-32 weeks gestation on continuous positive airway pressure of the effect of minimally-invasive surfactant therapy in comparison to standard care (continuation of CPAP) on the duration of respiratory support (all hours of intubation, nasal CPAP and high flow nasal cannula). anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343305 (first received 26 August 2011).
ChiCTR1900020970 {published data only}
- ChiCTR1900020970. A multicenter clinical randomized controlled study comparing the application of modified minimally invasive pulmonary surfactant and low inspiratory peak pressure supporting pulmonary surfactant instillation technology in the treatment of respiratory distress syndrome in very premature infants. chictr.org.cn/searchproj.aspx?ishtml=sponsorproj&type=cn&institution=浙江大学医学院附属妇产科医院&country=中国&province=广东&city=&createyear=0 (first received 23 January 2019). [DOI: 10.1186/s13063-020-04390-3] [DOI]
NCT01615016 {published data only}
- NCT01615016. MISurf versus InSurE. A comparison of minimally invasive surfactant application techniques in preterm infants (MIsurf) [Feasibility study of a comparison of minimally invasive surfactant application techniques in preterm infants]. clinicaltrials.gov/ct2/show/NCT01615016 (first received 8 June 2012).
NCT01848262 {published data only}
- NCT01848262. ECALMIST versus InSurE in preterm infant < 32 weeks, multicenter, multinational RCT (ECALMIST) [ECALMIST (Early CPAP And Large Volume Minimal Invasive Surfactant Therapy) versus InSurE (Intubate, Surfactant, Extubate) in preterm infants with respiratory distress syndrome (RDS): prospective randomised control clinical trial]. clinicaltrials.gov/ct2/show/NCT01848262 (first received 7 May 2013).
NCT02772081 {published data only}
- NCT02772081. An open-label, multicenter, randomized, controlled study in spontaneously breathing preterm neonates with respiratory distress syndrome to compare two procedures for porcine surfactant (poractant alfa, CUROSURF®) administration: a less invasive method (LISA) during non-invasive ventilation (NIV) and the conventional administration during brief invasive ventilation (LISPAP). ClinicalTrials.gov/show/NCT02772081 (first received 13 May 2019).
NCT03989960 {published data only}
- NCT03989960. Application of modified intubation-surfactant-extubation (InSurE) technique in preterm neonates with respiratory distress syndrome (MOLISAN). ClinicalTrials.gov/show/NCT03989960 (first received 18 June 2019).
NCT04016246 {published data only}
- NCT04016246 Chevallier M, Durrmeyer X, Ego A, Debillon T, the PROLISA Study Group. Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double-blind, placebo controlled trial (PROLISA). BMC Pediatrics 2020;20(100):1-9. [DOI: 10.1186/s12887-020-02112-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04016246. Respiratory effect of the LISA (less invasive surfactant administration) method with sedation by propofol versus absence of sedation: double-blind comparative randomized clinical trial (PROLISA). ClinicalTrials.gov/show/NCT04016246 (first received 11 July 2019).
NCT04073173 {published data only}
- NCT04073173. Stress assessment in preterm infants with respiratory distress syndrome treated or not with an analgesic drug during the traditional or the less invasive method of surfactant therapy (StrAAS). clinicaltrials.gov/ct2/show/NCT04073173 (first received 29 August 2020).
NCT04445571 {published data only}
- NCT04445571. Surfactant Administration by Insure or Thin Catheter (SAINT). clinicaltrials.gov/ct2/show/NCT0444557 (first received 24 June 2020).
UMIN000021785 {published data only}
- UMIN000021785. Effectiveness of MIST (minimally invasive surfactant therapy) under bronchoscopy in treating neonatal respiratory distress syndrome. rctportal.niph.go.jp/en/detail?trial_id=UMIN000021785 (first received 5 April 2016).
Additional references
Abdel‐Latif 2011a
- Abdel-Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD008311. [DOI: 10.1002/14651858.CD008311.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2011b
- Abdel-Latif ME, Osborn D. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008309. [DOI: 10.1002/14651858.CD008309.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2012
- Abdel-Latif ME, Osborn DA. Nebulised surfactant in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD008310. [DOI: 10.1002/14651858.CD008310.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aguar 2014
- Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy (MIST) with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 2014;103(6):e229-33 2014;103(6):e229-33. [DOI: 10.1111/apa.12611] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aldana‐Aguirre 2016
- Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Archieves of Disease in Childhood. Fetal and Neonatalal Edition 2016;102(1):F17-23. [DOI: 10.1136/archdischild-2015-310299] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ammari 2005
- Ammari A, Suri M, Milisavljelic V, Sahni R, Bateman D, Sanocka U, et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. Journal of Pediatrics 2005;147(3):341-7. [DOI: 10.1016/j.jpeds.2005.04.062] [PMID: ] [DOI] [PubMed] [Google Scholar]
Avery 1959
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA Journal of Diseases of Children 1959;97(5 Part 1):517-23. [DOI: 10.1001/archpedi.1959.02070010519001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2013
- Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology 2013;104(4):265-74. [DOI: 10.1159/000354419] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhayat 2020
- Bhayat S, Kaur A, Premadeva I, Reynolds P, Gowda H. Survey of less invasive surfactant administration in England, slow adoption and variable practice. Acta Paediatrica 2020;109:505-10. [DOI: 10.1111/apa.14995] [PMID: ] [DOI] [PubMed] [Google Scholar]
Björklund 1997
- Björklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatric Research 1997;42(3):348-55. [DOI: 10.1203/00006450-199709000-00016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dargaville 2011
- Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Archives of Disease in Childhood. Fetal & Neonatal Edition 2011;96(4):F243-8. [DOI: 10.1136/adc.2010.192518] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dargaville 2013a
- Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Archives of Disease in Childhood. Fetal Neonatal Edition 2013;98(2):F122-6. [DOI: 10.1136/archdischild-2011-301314] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dargaville 2013b
- Dargaville PA, Aiyappan A, De Paoli AG, Dalton RG, Kuschel CA, Kamlin CO, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 2013;104(1):8-14. [DOI: 10.1159/000346460] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dargaville 2016
- Dargaville PA, Gerber A, Johansson S, De Paoli AG, Kamlin CO, Orsini F, et al, Australian and New Zealand Neonatal Network. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 2016;138(1):e20153985. [DOI: 10.1542/peds.2015-3985] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dekker 2016
- Dekker J, Lopriore E, Rijken M, Rijntjes-Jacobs E, Smits-Wintjens V, Te Pas A. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 2016;109(4):308-13. [DOI: 10.1159/000443823] [DOI: ] [DOI] [PubMed] [Google Scholar]
Dunn 2011
- Dunn MS, Kaempf J, Klerk A, Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128(5):e1069–76. [DOI: 10.1542/peds.2010-3848] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finer 2010
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. [DOI: 10.1056/NEJMoa0911783] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 2 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gupta 2012
- Gupta S, Donn SM. Novel approaches to surfactant administration. Critical Care Research and Practice 2012;2012(278483):1-5. [DOI: 10.1155/2012/278483] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haberman 2002
- Haberman B, Shankaran S, Stevenson DK, Papile LA, Stark A, Korones S, et al. Does surfactant and immediate extubation to nasal continuous positive airway pressure (CPAP) reduce use of mechanical ventilation? Pediatric Research 2002;51(4 Suppl):349A. [Google Scholar]
Heiring 2017
- Heiring C, Jonsson B, Andersson S, Björklund LJ. Survey shows large differences between the Nordic countries in the use of less invasive surfactant administration. Acta Pædiatrica 2017;106(3):382–6. [DOI: 10.1111/apa.13694] [PMID: 27992064 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Isayama 2016
- Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 2016;316(6):611-24. [DOI: 10.1001/jama.2016.10708] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jeffreys 2019
- Jeffreys E, Hunt K, Dassios T, Greenough A. UK survey of less invasive surfactant administration [Letter]. Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(5):F567. [DOI: 10.1136/archdischild-2018-316466] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 1993
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328(12):861-8. [DOI: 10.1056/NEJM199303253281208] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klotz 2017
- Klotz D, Porcaro U, Fleck T, Fuchs H. European perspective on less invasive surfactant administration - a survey. European Journal of Pediatrics 2017;176(2):147-54. [DOI: 10.1007/s00431-016-2812-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kribs 2007
- Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤ 27 weeks). Paediatric Anaesthesia 2007;17(4):264-9. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kribs 2008
- Kribs A, Vierzig A, Hunseler C, Eifinger F, Welzing L, Stutzer H, Roth B. Early surfactant in spontaneously breathing with nCPAP in ELBW infants – a single centre four year experience. Acta Pædiatrica 2008;97(3):293-8. [DOI: 10.1111/j.1651-2227.2007.00617.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kribs 2009
- Kribs A. Early administration of surfactant in spontaneous breathing with nCPAP through a thin endotracheal catheter - an option in the treatment of RDS in ELBW infants? Journal of Perinatology 2009;29(3):256. [DOI: 10.1038/jp.2008.24] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kribs 2010
- Kribs A, Härtel C, Kattner E, Vochem M, Küster H, Möller J, Müller D, et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klinische Pädiatrie 2010;222(1):13-7. [DOI: 10.1055/s-0029-1241867] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lau 2017
- Lau CS, Chamberlain RS, Sun S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants: a meta-analysis. Global Pediatric Health 2017;4:1-9. [DOI: 10.1177/2333794X17696683] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehler 2013
- Mehler K, Oberthuer A, Haertel C, Herting E, Roth B, Goepel W, German Neonatal Network (GNN). Use of analgesic and sedative drugs in VLBW infants in German NICUs from 2003–2010. European Journal of Pediatrics 2013;172(12):1633–9. [DOI: 10.1007/s00431-013-2095-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
More 2014
- More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatrics 2014;168(10):901-8. [DOI: 10.1001/jamapediatrics.2014.1148] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morley 2008
- Morley CJ, David PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, et al, COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700-8. [DOI: 10.1056/NEJMoa072788] [PMID: ] [DOI] [PubMed] [Google Scholar]
Panza 2020
- Panza R, Laforgia N, Bellos I, Pandita A. Systematic review found that using thin catheters to deliver surfactant to preterm neonates was associated with reduced bronchopulmonary dysplasia and mechanical ventilation. Acta Paediatrica 2020;109(11):2219-2225. [DOI: 10.1111/apa.15374] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of the subependymal intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Porth 2011
- Porath M, Korp L, Wendrich D, Dlugay V, Roth B, Kribs A. Surfactant in spontaneous breathing with nCPAP: neurodevelopmental outcome at early school age of infants ≤ 27 weeks. Acta Paediatrica 2011;100(3):352–9 2011;100(3):352-9. [DOI: 10.1111/j.1651-2227.2010.02068.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reininger 2005
- Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks' gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. Journal of Perinatology 2005;25(11):703-8. [DOI: 10.1038/sj.jp.7211381] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roberts 2020
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] [PubMed] [Google Scholar]
Sinclair 2009
- Sinclair SE, Chi E, Lin HI, Altemeier WA. Positive end-expiratory pressure alters the severity and spatial heterogeneity of ventilator-induced lung injury: an argument for cyclical airway collapse. Journal of Critical Care 2009;24(2):206-11. [DOI: 10.1016/j.jcrc.2008.04.005] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2013
- Soll RF, Edwards EM, Badger GJ, Kenny MJ, Morrow KA, Buzas JS, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 2013;132(2):222-8. [DOI: 10.1542/peds.2013-0501] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevens 2007
- Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD003063. [DOI: 10.1002/14651858.CD003063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suresh 2005
- Suresh GK, Soll RF. Overview of surfactant replacement trials. Journal of Perinatology 2005;25 Suppl 2:S40-4. [DOI: 10.1038/sj.jp.7211320] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sweet 2016
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2016 update. Neonatology 2017;111(2):107–25. [DOI: 10.1159/000448985] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verder 1994
- Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. New England Journal of Medicine 1994;331(16):1051-5. [DOI: 10.1056/NEJM199410203311603] [PMID: ] [DOI] [PubMed] [Google Scholar]
Victorin 1990
- Victorin LH, Deverajan LV, Curstedt T, Robertson B. Surfactant replacement in spontaneously breathing babies with hyaline membrane disease – a pilot study. Biology of the Neonate 1990;58(3):121-6. [DOI: 10.1159/000243250] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1988
- Walsh MC, Kliegman RM, Fanaroff AA. Necrotizing enterocolitis: a practitioner's perspective. Pediatrics in Review 1988;9(7):219-26. [DOI: 10.1542/pir.9-7-219] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2017
- Wu W, Shi Y, Li F, Wen Z, Liu H. Surfactant administration via a thin endotracheal catheter during spontaneous breathing in preterm infants. Pediatric Pulmonology 2017;52(6):844-54. [DOI: 10.1002/ppul.23651] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zwicker 2016
- Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. Journal of Pediatrics 2016;172:81-7.e2. [DOI: 10.1016/j.jpeds.2015.12.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wheeler 2015
- Wheeler KI, Abdel‐Latif ME, Davis PG, De Paoli AG, Dargaville PA. Surfactant therapy via brief tracheal catheterization in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011672. [DOI: 10.1002/14651858.CD011672] [DOI] [PMC free article] [PubMed] [Google Scholar]



