ABSTRACT
Prescription drug spending in the USA has soared, fueled by rising drug prices. A critical mechanism for restraining drug prices is the formulary tiering system. Although tiering should reflect the cost of a drug—and reward patients who choose less-expensive drugs—something is seriously amiss. Using Medicare claims data from roughly one million patients between 2010 and 2017, this article finds troubling amounts of distorted tiering and wasted cost. Increasingly, generics are shifted to more expensive—and therefore less accessible—tiers. The percentage of generics on the least-expensive tier drops from 73% to 28%; the percentage of drugs on inappropriate tiers rises from 47% to 74%. Considering only costs paid by patients and the federal Low-Income Subsidy Program, tier misplacement cumulatively costs society $13.25 billion over the time period. An unruly problem demands a disruptive solution. This article advances the counterintuitive regulatory reform that tiering should be based on a drug’s list price. Yes, list price—that roundly dismissed figure—should become the touchstone. This would deter incentive-distorting rebate schemes while recognizing that many people already pay list price. It is a remarkably streamlined approach for cutting through a wide swath of perverse incentives and manipulations.
Keywords: drugs, formulary, pharmaceuticals, pricing, regulation, tier
I. INTRODUCTION: FORMULARY GAMES
Prescription drug spending in the USA has soared in the last decade, fueled by rising drug prices.2 Designer drugs, arriving with stunning price tags, are grabbing headlines,3 but day-to-day increases on ordinary medications are causing their fair share of pain. These included medicines for treating arthritis, diabetes, reflux, depression, high blood pressure, and high cholesterol.4
The pain of these rising prices reverberates through many levels of the system, including household consumer budgets. Nearly one in four Americans say that affording their prescription drugs is difficult, whereas three in 10 say they have not taken their medicines as prescribed due to costs.5 Government budgets are strained as well, as taxpayers support the increased costs of Medicare, Medicaid, and other government programs.6
With drug spending and other healthcare costs on the rise, healthcare has become a key focus across the nation. Eighty percent of polled voters consider healthcare an extremely or very important issue, more than any other topic.7 Moreover, the anxiety about healthcare costs cuts across party lines: 87% of Democrats and 72% of Republicans consider it a critical issue, making it the first and third most pressing concern for each voter bloc, respectively.8 In the first four debates of the 2020 election, Democratic presidential hopefuls spent more time discussing healthcare than any other topic—roughly twice as much as other frequently mentioned topics such as foreign policy and climate change.9 In the polarized political climate of this era, one would be hard pressed to think of another issue that so unifies the American public.
A critical mechanism for restraining drug spending is the formulary system, which dictates the drugs for which patients will receive reimbursement. The roots of the system can be traced back to the American Revolution—when the Continental army developed a list of reliable medicines—although modern formulary systems have gradually expanded far from their historic roots. Today’s formularies are divided into tiers that determine just how much a patient will pay. When drugs are on low tiers, such as Tiers 1 and 2, the patient pays less. When drugs are on high tiers, such as Tiers 4 and 5, the patient pays more.
In theory, tiering reflects the cost of a drug—and rewards patients who choose generics over brands.10 The patient’s copay is less, the cost to the healthcare system on the whole is less, and the market for cheaper drugs thrives. In other words, tiering should be part of a virtuous cycle creating the proper market and system incentives. That is the concept, in theory, but something is seriously amiss. In fact, the tiering system has gone off its rails.
Prior to this article, anecdotal evidence had suggested that something odd is happening in the tiering system. Press reports and scattered lawsuits have hinted that at least some health plans are punishing patients for purchasing generics, rather than brand-name drugs, or excluding generics from reimbursement.11 In light of these clues, this study set out to examine, on a systematic basis, whether tiers are doing their jobs. The study follows roughly one million Medicare patients from 2010 to 2017. In the process, the study finds clear evidence of widespread irrational tiering and wasted spending. By placing costly brand-name drugs on preferred tiers while placing cheaper generics on more-expensive tiers, the principal actors involved have cost patients and the government billions of dollars.
To understand how dollars are wasted within the formulary system, the study focuses on whether cheaper drugs are being placed on more-expensive tiers relative to more expensive competitors. In order to define competing drugs, the study applies the novel concept of ‘therapeutic competitors’, a term that has not been previously used in the literature.
An existing term of art, ‘therapeutic equivalent’ is too narrow to fully capture market dynamics. To be therapeutically equivalent under the Food and Drug Administration’s (FDA’s) definition, the main active ingredients must be the same, as well as the dosage form, route of administration, and strength.12 Drug companies compete with drugs far beyond drugs that mirror each of these parameters. Thus, equivalence provides only a limited view of market competition. Other drug classification systems fail to capture the full competitive picture, particularly with drugs that are marketed for off-label uses.13
Drug companies undoubtedly know which drugs compete with their own, even if those drugs only compete off-label or in certain subsections of the market. The concept of ‘therapeutic competitors’ encompasses this broader range of market dynamics. As described below,14 this study examines a subset of therapeutic competitors defined as drugs containing the same active ingredient. Within this framework, the study quantitatively examines certain instances of irrational tiering in the formulary system—specifically, instances in which two or more drugs with the same active ingredient (such as a brand and a generic) are improperly placed on the same tier or when the cheaper drug is placed on a more-expensive tier.
The results are striking, but empirical results are of little use to society if they simply set off rounds of finger-pointing—an activity that has been rampant in Washington DC since the nation has focused its attention on drug pricing. Drug companies blame both health insurers and middle players known as pharmacy benefit managers (PBMs); PBMs blame drug companies; and so on.15 Finger-pointing exercises, however, are rarely productive (except perhaps in piano practice). And as academic literature suggests, there is plenty of blame to go around.16 To varying degrees, many players are profiting on the backs of patients and the government. These players are, after all, profit-making entities, and they are likely to respond to the incentives created by the system. Thus, at the end of the day, finger-pointing merely distracts from the task at hand: creating a better legal framework that more successfully aligns public and private incentives. And in a free-market system, those incentives necessarily include encouraging the competitive forces that erode monopoly positions and bring prices to competitive levels.
Price, of course, is a murky term in the world of prescription drugs. Drug companies point out—and rightfully so—that headline-grabbing list prices do not necessarily reflect the price of an individual drug purchase.17 Rather, the actual price of an individual drug purchase can only be determined after subtracting rebate amounts, which themselves will be determined long after the patient has left the drug counter. Those rebates will be calculated based on complex formulas established in contracts between drug companies and middle players known as PBMs.18 Drug companies and PBMs assert that the pricing information is a trade secret, with the result that health plans, and even plan auditors, are not allowed to know the full terms of the contracts or the net price of drugs.19 In other words, true net price is a slippery term that is disassociated from key buying moments and decisions.20
An unruly problem demands a disruptive solution, and therein lies the startling recommendation that emerges from this work. ‘One actually need not know the true net price.’ Rather, one can restore sanity to drug pricing without the parties actually knowing the true price. To repeat for emphasis, one can fix price without knowing the price, at least in the context described in this article. To accomplish this feat of magic, tiering should be based on list price. Yes, list price—that badly maligned, roundly dismissed figure—should become the touchstone.
Focusing on list price eliminates the need to ferret out and decipher complex deals. It also avoids the practical problem of navigating around the parties’ claims that trade secret law protects net price information.21 Further, focusing on list price is grounded in the reality that many patients payment do reflect the list price, either because (i) they must pay the full list price before reaching a deductible, (ii) they lack complete drug coverage or any drug coverage at all, or 3) their co-sharing payment is determined as a percentage of the list price.22
Using list price also has the happy side effect of disincentivizing the rebate and quasi-kickback games that drug companies use and middle players demand. Those games are like raising the price of a jacket before a sale, so the sale price looks appealing.23 Unfortunately, many people end up paying the pre-sale price for the jacket—and, of course, many people pay the full price for drugs.24 Worse yet, as this study demonstrates, drug prices are rising at a faster pace than rebates, with the result that the rebates only begin to offset the substantial increases.25 Finally, rebate games create bloated spending (because middle players pocket the spread) and harm competition (because drug companies provide rebates so that cheaper competitors are disadvantaged).
Despite widespread recognition of the problem, complex legislative and regulatory attempts to reform this practice have failed.26 In contrast, a simple, Congressional or regulatory mandate that government programs use list prices for tiering would sweep aside the incentives for playing the rebate game. Back-room negotiations and tempting rebate payments would not matter. The price is the price.
Although there is no silver bullet, and all approaches have challenges, basing tiering on list price is a remarkably streamlined approach for cutting through a wide swath of perverse incentives and manipulations. After all, at the end of the day, it is the price that matters.
II. FORMULARIES, DRUGS, AND PRICES: AN OVERVIEW OF THE HEALTHCARE SYSTEM
II.A. A Brief History of Formularies
Formularies, at the most basic level, are a list of medicines. Formularies for hospitals have existed in the USA since the days of the American Revolution when the Lititz Pharmacopeia was published in 1778 for use by the Continental forces.27 It was not until 1816, however, that a formulary for a private civilian hospital was compiled, which was called the Pharmacopeia of the New York Hospital.28 Four years later, the first national pharmacopeia in the USA was published, with the objective of ‘select [ing] from among substances which possess medicinal power, those, the utility of which is most fully established and best understood’.29 It is unclear when the transition of terminology from ‘pharmacopeia’ to ‘formulary’ occurred in the USA.30 Historians document, however, that the idea of a formulary as a simple clinical management tool was established in the USA early on in its history.
Following World War II, formularies expanded beyond the role of merely identifying clinical sufficiency and entered the realm of supply and inventory management. The immense scale and sophistication of the penicillin development effort during the war marked a new era for the pharmaceutical industry’s approach to developing drugs, ushering in unprecedented levels of new development and mass production.31 As many more medications became available to treat a range of diseases, hospitals began to use formularies for managing and controlling their drug inventory and supply needs.32 Formularies edged closer to government regulation in 1965. In that year, the private, nonprofit organization for evaluating hospitals—now, known as The Joint Commission—began requiring that hospitals maintain an active Pharmacy and Therapeutics Committee that would communicate on drug-use issues.33 At this point, formularies were recognized as a useful tool for hospitals, rather than a broad tool for all health insurers, and governmental agencies had yet to apply formal, widespread regulation.
The first, widespread, formal government regulation of formularies—outside of episodes such as military use in the American Revolution—occurred with the passage of the Social Security Amendments of 1965. In addition to having an impact on drug development, the period following World War II saw changing attitudes about federal administration of the Social Security Program.34 Prior to that time, states were viewed as the preferred administrators of health insurance and other forms of social insurance. Following World War II, the view shifted entirely, and states were viewed as unreliable and inefficient administrators of social welfare programs, who, by handling the same social problems in highly disparate ways, created chaos rather than coherence.35
As a practical matter, states were already too embedded in the welfare system to be swept aside.36 Thus, when the Social Security Amendments of 1965 created the Medicare and Medicaid Programs, the Amendments established Medicaid as a joint effort between the federal and state governments to provide basic hospital insurance for the poor.37 The same legislation established Medicare, designed to provide basic hospital insurance for the elderly, as a program administered by the Federal Government.38 Thus, 1965 marked the beginning of formal, federal government regulation of formularies, but only in their capacity as a useful tool for hospitals.
In the 1970s, formularies evolved beyond hospital use to become an essential cost control tool for health insurers in the outpatient realm. Unexpectedly high Medicare expenditures, rapid inflation, expansion of hospital expenses and profits, and changes in medical care contributed to the escalating healthcare costs in the 1970s.39 The increasing healthcare costs drove a paradigm shift in health insurance, away from fee-for-service reimbursement and toward a managed care environment.40 Enrollment in managed care organizations surged, and insurers began to look for ways to control costs, including the prescription drug benefit.41 Most health plans already had experience with hospital formularies, and insurers initially considered using formularies that took a unified approach to medications for inpatient and outpatient care.42 The cost of medication varied tremendously, however, between hospital pharmacies and community pharmacies, even for the same drug.43 Insurers, therefore, needed a separate outpatient formulary.
As formularies entered into widespread use for cost control, however, they had yet to expand into the final, key role for today’s modern formulary: a tool for drug selection and rebate negotiations with drug manufacturers. This final step would fall into place through a combination of the 1980s Hatch–Waxman Act (for the rapid approval of generic drugs)44 and the Medicare Modernization Act of 2003 (offering prescription drug benefits to all Medicare beneficiaries).45 Hatch–Waxman created a wave of generics entering the market, including multiple generic versions of some medications.46 With the influx of competing drugs, brand companies began offering rebates to health insurers, in exchange for preferred placement on formularies.47
In the Medicare Modernization Act, Congress expanded Medicare to include widespread coverage of drugs that patients purchased at a retail pharmacy, as opposed to those related to hospital stays.48 Just as Hatch–Waxman brought a wave of additional competing drugs, the Medicare Modernization Act brought a wave of additional patients who had insurance coverage for day-to-day prescription medications. Thus, throughout the early 21st century, the modern formulary system evolved into a tool that health insurers used, not just for cost control but also for drug selection and as leverage with manufacturers to obtain rebates.
Implementation of the Medicare Modernization Act also transformed the rising PBM industry in healthcare. PBMs originated in the 1970s, emerging largely as claims processors.49 The industry changed in the 1990s, with the advent of electronic claims. That shift, however, was minor in comparison to the tectonic shift that occurred as the Medicare Modernization Act became fully implemented in 2006.50 With the influx of new patients having prescription drug coverage, PBMs took on a new responsibility for their clients, the insurers: negotiating rebates with drug manufacturers, as well as helping design and manage formularies. This final, historic shift led to the modern formulary as a drug choice tool for health insurance plans with the dual goals of clinical effectiveness and cost control. In the words of one PBM executive, tiering is designed to ‘encourage desirable outcomes while saving considerable costs….Treatment—not just prescribing—becomes better and more cost-effective. Ultimately, overall health care improves’.
II.B. The Modern Formulary
The majority of patients in the USA rely on a health insurance plan to cover at least part of their prescription drug cost. As described above, the core of this system is a plan’s drug formulary, which determines both which drugs will be covered by insurance and how much customers will pay through the plan.51 In the modern context, formularies also function as a way of translating complicated pricing information into relatively easily understood, out-of-pocket costs for individual insurance plan subscribers.
Insurance companies create formularies to differentiate preferred and nonpreferred drugs by using a multi-tier system. When a drug is on the lowest tier—tier one—patients who buy the drug have lower cost-sharing burdens; when drugs are on higher tiers, patients pay more. A patient’s payment can come in the form of a flat copay, a percentage of the cost of the drug (which is known as co-insurance), or a combination of both.52 Thus, when drugs are on the same tier, patients will have the same copay amount, regardless of the drug’s wholesale price. Any co-insurance amount, however, could vary.
The following is an example of a five-tier formulary, provided by a BlueShield health plan, which is typical of five-tier formularies:53
| Tier 1 | The prescription drug tier that consists of the lowest-cost tier of prescription drugs: most are generic. |
| Tier 2 | The prescription drug tier that consists of medium-cost prescription drugs: most are generic and some brand-name prescription drugs. |
| Tier 3 | The prescription drug tier that consists of high-cost prescription drugs: most are brand-name prescription drugs. |
| Tier 4 | The prescription drug tier that consists of the higher-cost prescription drugs: most are brand-name prescription drugs and some specialty drugs. |
| Tier 5 | The prescription drug tier that consists of the highest-cost prescription drugs: most are specialty drugs. |
Although specialty drugs are normally placed on nonpreferred tiers, the definition of a specialty drug can vary among plans and within literature. Medicare sets a floor (at least $670 per month in 2019) to define a drug as ‘specialty’.54 Beyond Medicare and in general, high list prices tend to distinguish specialty drugs from other drugs, along with four corollary features: treating rare conditions, requiring special handling, using a limited distribution network, or necessitating ongoing clinical assessment and monitoring.55
An insurance plan will normally cover only the drugs listed on its formulary tiers. For all other (nonlisted) drugs, patients will have to pay the full price out of their own pockets.56
One can think of tiering as loosely analogous to product placement in a grocery store. A company will sell more of its soda cans if the cans are placed on the end-cap display of a grocery aisle than on the top shelf of the drinks section. Thus, to the extent tier placement is intended to incentivize the purchase of drugs that the health plan prefers, the plan is placing the drugs certain drugs on the end cap. The term ‘loosely analogous’ is used because patients cannot decide what prescription medicine to purchase alone. The doctor’s prescription drives the purchase, and both patient and doctor may suffer from insufficient information to make pricing-based choices. Thus, being on the ‘end-cap’ of the prescription drug-pricing tier is not a perfect incentivizing mechanism. Nevertheless, the tiering system is designed to drive choices in the market, however imperfectly that market operates.
In modern formularies, one of the most important factors for tier placement involves deals between PBMs and drug manufacturers. As described above, PBMs are the middle players who negotiate with drug companies on behalf of health plans and help the plans create their formularies.57 PBMs that can promise drug companies a certain amount of revenue (ie sales volume) from a particular health plan can obtain sizable rebates off the list price for a particular drug. In theory, the PBM’s ability to negotiate with drug companies should lead to lower prices for prescription drugs, ultimately improving consumer welfare for individuals.58
In short, the modern formulary theoretically serves not only as a tool for ensuring that drugs are clinically effective, it also helps to control the cost of prescription medication, minimizing health plan spending and patient out-of-pocket costs. This is accomplished through a combination of prioritizing generics over brand drugs and preferred brands over nonpreferred brands. Price, however, is a slippery term in the modern world of pharmaceuticals.
II.C. The Price of a Drug
The strange and shrouded notion of price in the pharmaceutical industry begins in a perfectly ordinary fashion. Drug companies sell their product to wholesalers. The price of a drug, however, will quickly dissolve into a tangle of timing oddities, rebates, and an impressively obscure alphabet soup of terms used in the industry—wholesale acquisition cost (WAC),59 maximum allowable cost (MAC),60 average wholesale price (AWP),61 average sales price (ASP),62 estimated acquisition cost (EAC),63 and others—many of which lack a consistent definition, are unverified, or are not based on actual sales transactions. Despite all of this, drug price begins with drug companies selling their products to wholesalers; that basic price is already reported to the federal government. The figure, which must be based on actual sales, is statutorily defined and reported to the Centers for Medicare and Medicaid Services (CMS).64 For simplicity, this paper will refer to this critical figure as the wholesale price of the drug.
Various other indexes are available as a measure of wholesale price, although each one is flawed. For example, drug companies voluntarily supply reports to third-party commercial services that, in theory, report the price at which the companies sell to wholesalers.65 Drug-makers also voluntarily provide the average of that cost to the third-party commercial services.66 Both sets of figures are unverified, and the Health and Human Services (HHS) Office of Inspector General has reported that those numbers are not necessarily based on actual sales.67
In theory, the patient would pay the wholesale price with a small markup for the wholesaler’s profit—in other words, the ‘list price’. In many circumstances, however, the list price is only the beginning. Drug companies offer substantial rebates to health plans, generally in recognition of the volume of product that the health plan’s patients will purchase.
This critical juncture is where the system begins to go off the rails. As noted above, middle players, known as PBMs negotiate prices on behalf of their health plan clients. The health plans pay PBMs based on the size of the discount that the PBM can wrestle from the drug company, sometimes even allowing the PBMs to pocket part of the spread.68 This method—called spread pricing69— should lead PBMs to negotiate more substantial discounts, which would, in turn, lower net prices. After all, one would negotiate hard for discounts if one’s pay were determined by the size of that discount.
Perverse incentives and strategic behaviors, however, have derailed the process. To increase the spread and profitability for the PBMs, drug companies can raise the list prices of their drugs and then offer steeper rebates. As a result, PBMs can report a greater spread, thereby increasing their pay, even if net price remains the same or increases.70 This creates upward pressure on drug prices, as drug companies offer—and PBMs demand—greater and greater spreads.
The spread pricing and rebate system might be less of a problem if no one actually paid the higher price but many people do. Some health plans require that patients pay the full amount for a drug until reaching a deductible level.71 The full amount is the list price and ignores all rebates. Other plans require that patients pay a co-share amount based on a percentage of the full list price.72 Some patients lack health insurance or lack plans that cover medications.73 Even when full Medicare coverage is in place, gaps can occur that leave patients paying the full cost of the drug.74 Most importantly, prices are rising faster than rebates.75 Thus, for those who do not pay the full list price, the cost of drugs is increasing at an alarming rate, even with rebates.
The timing of rebates completely obscures the actual price of any individual drug transaction. When a patient goes to the pharmacy to purchase a prescription drug, the price includes the list price—that is, the wholesale price with markup for the wholesaler’s profit—plus a small markup for the pharmacists. To cover that cost, the pharmacist collects the patient’s copay or co-insurance amount, along with the insurance plan’s contribution (generally processed by the PBM).76 Rebates arrive long after the patient has left the pharmacy counter. A health plan’s PBM will provide rebates to the health plan that cover a large number of drug transactions and likely a large number of drugs. Thus, for example, a health plan knows what it paid for a particular patient’s heart medication at the point of the sale—and what it paid overall for all transactions. Nevertheless, the plan never knows the true net price, because the rebate on that purchase will be lumped with rebates for many other transactions and delivered long after the patient has left the pharmacy counter.
Timing and aggregation are not the only issues obscuring net prices. After all, a plan could simply disaggregate those prices to obtain net prices, at least some time down the road. The rebate amounts, however, normally flow from long and complex calculations that are set out in contracts between the PBM and the drug company. Drug companies and PBMs claim that net prices, and the calculations that produce those net prices, are trade secrets. Although courts have not squarely addressed whether that information constitutes a trade secret77 and academics have cast doubt on the claim,78 the information is fiercely guarded, even from the PBMs’ own client, the health plan. The health plan’s auditors are not even allowed full access to the terms.79 As a result, actual net prices are hidden—from the patient, from the health plan, from government regulators, and from pesky academics. Lack of information makes it difficult for government regulators to ferret out inappropriate behavior—or reform the process—and for patients to make fully informed decisions about drug purchases and health plan purchases.
Moreover, the PBM industry is highly concentrated, with three PBMs controlling 85% of the market.80 Given the market structure, PBMs reportedly engage in lockstep demands, making it difficult for health plans to bargain for different terms.81
The system benefits drug manufacturers as well. In exchange for the lucrative rebates that drive PBM profitability, drug manufacturers can demand that the PBM guarantee a certain volume flow from the health plan’s patients by giving their drugs exclusive or preferred formulary placement. These volume rebates allow drug companies that hold a substantial position in the market to prevent new competitors from gaining ground.
In competition terms, one can conceptualize this as a form of raising rivals’ costs, that is, engaging in a behavior that will impose costs on your competitor without imposing similar costs on you.82 Imagine if Budweiser approached bar owners in a state offering $1 off each bottle of Bud sold, if the owners agree not to put any craft beers on the menu.83 If the bar owners normally sell two million bottles of Bud in a year, that offer is worth $2 million. Now imagine a small craft beer company trying to break into the market—an entrant that might start off by selling 10,000 bottles at $3 each. Even if the new entrant discounted the price down to a single penny per bottle in comparison to the normal $3 price, the bar owners would save only about $30,000. The new entrant could never match Budweiser’s $2 million offer to the PBM. And remember, the PBM will be paid based on the amount of the discount spread, and it may even be able to pocket the spread.
The danger of volume rebates can be more pronounced in the context of large drug manufacturers offering a variety of drugs. A drug company offering multiple drugs can use its market dominance in one drug to protect its less competitive drug. And of course, brand drugs whose patents are expiring may hold monopoly positions that allow for this type of volume rebate behavior.84
Anecdotal evidence has hinted at abuses in the formulary system, driven by the incentive structure in place and the type of strategic behaviors described above. One lawsuit alleged that health insurance plans essentially excluded a lower-priced version of the arthritis drug Remicade, following bundled rebate deals from the brand.85 Another alleged that a vaccine company significantly raised its prices for any consumers that did not buy a certain number of its bundled drugs, in order to prevent customers from jumping ship to a recently introduced competitor.86 Another alleged that a company used bundled rebates and exclusive formulary contracts to disadvantage competitors of the blockbuster dry eye medication, Restasis.87
Outside the lawsuit setting, press and individual reports have described patients paradoxically paying a ‘higher’ co-share for filling their prescriptions with the generic and a lower co-share for buying the brand.88 The press piece reported of pharmacists being told in 2017 that some Medicare plans, with formularies designed by the same PBM, would cover only the brand version of 12 drugs, despite the fact that some of the drugs had generic competitors on the market.89 In the same vein, generics and consulting industry sources have asserted that patients are being overcharged for generics and that generics are being placed on irrational tiers, harming the generics industry and inflating patient costs.90 These sources provide few details on the assertions, however.
Although academic research on tier placement is sparse, the literature suggests that over the past decade, a number of players in the pharmaceutical industry have engaged in strategic behavior related to tiering. One paper, for instance, found that insurers used tiers as a way around the Affordable Care Act’s laws barring discrimination based upon preexisting conditions.91 Patients who are human immunodeficiency virus (HIV) positive may have greater medical needs and higher-cost medications. By placing all HIV drugs on the highest-cost tier, insurance companies discourage high-cost, HIV-positive patients from enrolling.92
Researchers also have identified tiering behaviors designed to evade other aspects of the Affordable Care Act, such as those related to the Essential Health Benefits Provision.93 This provision requires that health insurers must cover at least one drug in each class and that all drugs must be covered in six protected classes. The provision does not regulate related dimensions of formulary design, such as cost-sharing and tier placement.94 As a result, insurers may comply with the Affordable Care Act while also undermining the Act’s goals of expanding prescription drug access for patients by manipulating tier placement. One econometric study, for example, found that while insurers complied with the Affordable Care Act’s provisions to cover additional drugs, compliance increased the probability that a newly covered drug was assigned to a more-costly formulary tier.95
Along the same lines, protests alleging discrimination in tiering have been lodged with state and federal regulatory agencies. For example, two groups filed an administrative complaint with the federal Department of Health and Human Services’ Office for Civil Rights arguing that four insurers violated the Affordable Care Act’s nondiscrimination provisions through adverse tiering on their plans in the federal health exchange in Florida.96 The complaint settled, with the insurers agreeing to improve the tier placement of HIV medications, which would, in turn, make those medications more affordable.97 In Georgia, letters from a state senator and a coalition of groups successfully convinced the Georgia Department of Community Health to maintain certain HIV medications on the preferred tier of the state’s Medicaid formulary, rather than downgrading them.98
Studies outside the academic realm report other types of concerns with formularies and tiering. One investigative journalism piece, for example, found that when pharmaceutical companies gave perks to doctors voting on Medicaid systems, those doctors were more likely to recommend placement of the pharmaceuticals on state formularies.99
In light of the many allegations, the study sets out to examine empirically whether evidence exists of widespread irrational tiering—along with problems created by that irrationality—and if so, how to address the problems. To do so, the study follows roughly one million Medicare patients from 2006 to 2017, examining all of the cohort’s drug purchase claims filed during this period. These data are available for purchase from the federal government through the CMS.100
Medicare provides a particularly useful pool of information for studying health insurance tiering. The availability of detailed, government-verified claims data provides an excellent basis for empirical research, particularly given that similar information on private insurance plan patients is not available to researchers.101 Moreover, Medicare has significant market presence and purchasing muscle, accounting for 29% of the money spent on prescription drugs in the USA. Compared with an analysis of all insurance plans with prescription drug coverage, however, there is presumably less variation in a number of factors, including: the drugs the cohort buys, how the drugs are covered by their insurance, the prices that they pay, the kind of rebates one expects the plans to obtain, etc.102 Nevertheless, certain problems within the Medicare system are likely to apply to the US health insurance system as a whole, making Medicare a fertile research venue. Moreover, for reasons of political expediency discussed below, it may be easier to craft initial reforms within the Medicare system.103
Developing a methodology to test the hypothesis was no easy task, given that legislators, regulators, and researchers are caught in a bind. As noted above, drug companies assert that drug prices constitute trade secrets. Thus, true price is shielded even from health plans themselves—wrapped in layers of aggregated rebates, which are paid long after any individual drug purchase takes place.104 Worse yet, there is no consistent definition or list of brand versus generic drugs.105
The study’s analysis breaks through these barriers to reveal what is happening behind the tiering curtain. A detailed methodology, including statistical significance testing, will be made available to guide future researchers who wish to expand on or confirm the results.106
The results of the study confirm that the manner in which drugs are currently being placed on formulary tiers is adversely affecting patients and costing society. The sections below will describe the results and analyses in detail, but key conclusions include the following:
From 2010 to 2017, the percentage of generics on the least-expensive tier drops from 73% to 28%. The shift creates considerable burden for patients, given that the average copay triples when a drug moves from the first tier even up to just the second tier.
During the same period, the percentage of drugs placed on inappropriate tiers in relation to drugs with the same active ingredient increases from 47% to 74%.
Considering only patient out-of-pocket costs and payments from the federal Low-Income Subsidy program, abuses of the formulary system conservatively costs $13.25 billion over the eight-year period, with the costs rising significantly from the beginning to the end of the period.
After factoring in rebates, the average dosage-unit price for brand drugs increases by 313%, whereas the average dosage-unit price for generics remains stable.
III. STUDY DETAILS AND RESULTS
A few notes may be helpful at the outset. First, CMS began recording complete formulary data in 2010.107 Thus, certain analyses cover only the eight-year period from 2010 to 2017, rather than the entire 12-year study period. In addition, although the number of tiers in a health plan’s formulary can range from three to seven, the study finds that plans with five tiers were the most common configuration within the data. This finding is consistent with other reports.108 Thus, for the calculations related to tiering, the study focuses on five-tier plans. Finally, although data on the rebates for individual drug purchases are never available in any form, the Medicare Trustees Report provides average rebate data across all drugs for a particular year. That figure could be used to derive the average rebates for brand drug manufacturers, a calculation that is set out in Appendix A2.
Before delving into an analysis of formulary tiering, the study begins by examining the availability of drugs and the amounts that patients paid for their prescriptions, to verify the commonly held assumption that the rising availability of generic drugs leads to lower patient expenditures. This assumption lies behind modern initiatives to increase the number of available generics as an antidote to concerns about rising pharmaceutical prices and patient costs.109
An examination of the claims data finds that over the time period, an increasing percentage of the drugs on formularies were, indeed, generics rather than brands.110 If the formulary tier system works as hypothesized, patient expenditure should, therefore, decrease. However, the study shows an entirely different picture. Patients, on average, pay more for both brand and generic drugs between 2006 and 2017. In this 12-year period, the average amount patients pay for brand drugs increases drastically, rising from $18 to $47. Thus, a patient’s out-of-pocket payment for each brand drug more than doubles during the 12 years of the study—and this is only for prescriptions that were covered. Although the increase in average price paid is less stark, generic prices still rise 75%, from $4 to $7. Given these results, the study finds that the growing prevalence of generics has not prevented rising costs for consumers. In other words, from the perspective of what a patient specifically pays out-of-pocket, more generic drugs alone do not solve the problem of rapidly increasing drug prices.111
It is important to emphasize that these figures are for patient out-of-pocket costs. During the same period, the net price of generic drugs stays roughly stable.112 One would not expect the patient’s payment burden to rise 75% for generics when the insurance plan’s cost of acquiring those drugs remains the same.
III.A. Broadening the Lens: Dosage-Unit Prices for Brands and Generics
The analyses above examine average patient out-of-pocket costs for brand and generic drugs. To broaden the lens on drug prices, the study examines other aspects of drug prices—not just those paid by the patient. Drugs can be dispensed in different dosages, creating the need for a method of normalizing dosages and prices across different drugs. To solve this problem, the study uses a novel metric: the average dosage-unit price.
Consider an analogy from the beer industry. Imagine a 12-pack of Bud Light cans standing next to a single bottle of Heineken. To compare pricing between the two, one would need to consider the price of one ounce of Bud Light in comparison to one ounce of Heineken.
As described below, this novel metric also allows the study to examine the ever-elusive category of prices after rebates. Specifically, the study finds that rebates are not fully eliminating price increases. Rather, the price of brand drugs continues to rise at an astonishing pace, even after factoring in rebates. Moreover, the cost of a brand drug far exceeds the cost of a generic. After factoring in rebates,113 the average dosage-unit price of brand drugs overwhelmingly exceeds that of generic drugs. In the discussion below, the article uses the term, ‘net price’, to refer to the price after factoring in rebates.
As described above,114 net prices are important for understanding the true price trajectory of a drug. If the list price of a drug rises, but the drug company discounts the drug by the amount of the rise or more in the form of a rebate, it would be misleading to suggest that the price has risen at all. With drug companies asserting that net price information constitutes a trade secret—a secret so delicate that it cannot be revealed even to the health plan itself—one cannot fully analyze drug prices.
This conundrum has stymied researchers and policymakers for years. Drug companies can defend against criticisms of rising drug prices by noting that prices are lower than anyone realizes due to rebates—and then refuse to reveal any information about the rebates. Any discussion of drug prices becomes a game of shadow boxing.
The study breaks this impasse. Using actual claims data and applying the methodology of dosage-unit price, the study shows that net prices climb dramatically between 2006 and 2017. As Figure 1 demonstrates, ‘after rebates’, the average dosage-unit prices for brand drugs experiences a shocking 313% increase across the decade. Specifically, the average dosage-unit price of brand drugs after rebate increases from $38 to $157, whereas generic drugs remain at a relatively stable $3–4.
Figure 1.
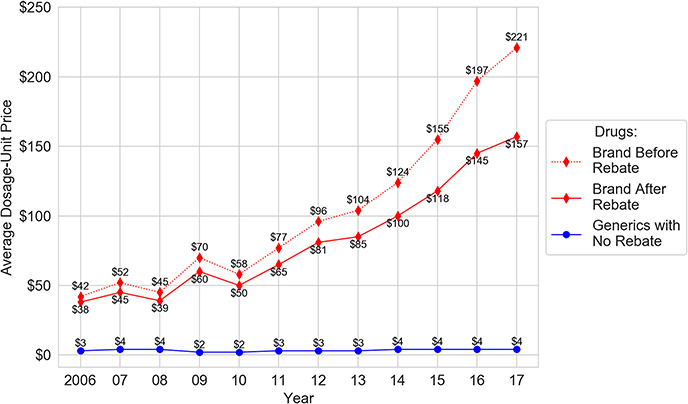
Average dosage-unit price.
One should note the following: The previous section found that the out-of-pocket costs patients pay for generics rises 75% to roughly $7 per prescription across the study period. This section finds that the average dosage-unit price ‘health insurers’ pay remains roughly stable. Thus, patient expenditures for generics are rising, whereas insurers pay roughly the same amount for generics over time.
In short, although rebates rise over the decade, prices rise faster, outstripping the effects of the rebates. Most important, the prices for brand drugs, both before and after rebates, soar far above the price of generic drugs.
The net price, however, is not the only relevant figure; understanding the list price is important for a different aspect of price trajectory. Quite simply, many people do pay the full list price—at least at certain times—and others have payments that are tied to the list price. Specifically, some patients pay full price until reaching a deductible; some pay co-insurance as a percentage of the list price, rather than a flat copay. Thus, the study also examines list price information.
Unsurprisingly, the trajectory for the list prices paid by health insurance plans is far worse than for net prices. Between 2006 and 2017, the average dosage-unit list price for brand drugs rises dramatically from $42 to $221, a 426% increase. In contrast, the average dosage-unit price that health plans pay for generics remains relatively stable at a low $3 to $4.115
III.B. The Shrinking Access to Generics
No matter how broad the lens, prices alone do not explain the full story of drug accessibility in the USA. As discussed in previous sections, insurers organize their drugs within tiered formularies. Tiering ought to reflect the cost of a drug, with cheaper generics placed on lower, less-expensive tiers and expensive brand drugs placed on higher, more-expensive tiers. Insurers reward patients who choose cheaper drugs by requiring smaller copays; more expensive drugs on higher tiers, in contrast, command progressively large copays.116 The formulary system, in theory, drives down patients’ copays, reduces overall healthcare costs, and promotes the market for cheaper drugs.
The primary goal of the study involved empirically assessing whether the formulary system is working as it should. Strikingly, the analysis reveals strong evidence of distorted tier placement for generic drugs. First, generics are increasingly placed on tiers that have higher costs for patients. For example, in 2010, 96% of generics are placed on the two least-expensive tiers combined, formulary Tiers 1 and 2; by 2017, this number shrinks to 66%. In particular, there is a serious decrease in the percentage share of generics on Tier 1—the tier with the lowest cost for consumers—compared with all other tiers. On that golden tier, the percentage of generics drops from 73% to 28% between 2010 and 2017.117 This occurs even though the cost that health plans pay for generics remains stable.118
One might speculate that the reduction of the percentage of generics on the first tier occurs because health plans are choosing one generic to favor over another generic. However, given that tiering should reflect price119 and generics do not compete on price,120 there should be no reason to preference certain generics over others in the widespread manner that the study reveals, absent other economic distortions. To the extent additional economic distortions are occurring, it may reflect other market dynamics that are beyond the scope of this paper.
The data also show an increase in the percentage of generics on Tier 2, rising from 23% to 38%. Similarly, the percentage of generic drugs on Tiers 3 and 4 grows from a negligible number to a combined 33% in 2017. Together, these represent a significant shift toward more-expensive tiers for generics. In general, generics are noticeably shifted from Tier 1 to higher tiers, with Tier 2 now containing a plurality of the generics. Nevertheless, although the percentage of generic drugs on Tiers 1 and 2 significantly decreases during 2010–2017, nearly three in five generic drugs are still assigned to the first two tiers. Thus, the major trend in generics is a shift from Tier 1 to Tier 2.
Pushing generics toward more-expensive tiers—even from Tier 1 to Tier 2—has significant cost implications for consumers. According to one study, the average copay increases three-fold when a drug moves from Tier 1 to Tier 2, increasing from $11 to $33 as shown in Figure 3.122 Such copay amounts can add up quickly for a patient using multiple medications across the entire year. Moreover, this increase in patient out-of-pocket payments occurs despite the fact that the price health plans pay for generics on Tier 2 is roughly the same as on Tier 1, as shown in the section below.123
Figure 3.
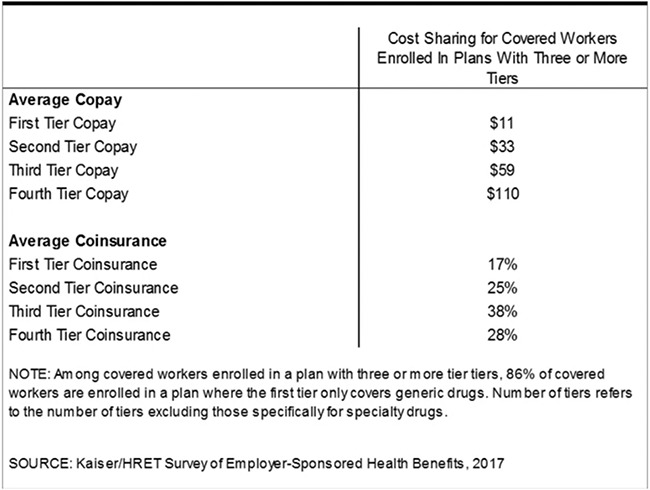
Average copayment and co-insurance for the different tiers among workers with insurance plans with three or more tiers.124
As a general matter, formularies are intended to be designed so that drugs are separated onto different tiers according to their price, with the exception of higher-priced specialty generics and specialty brands grouped together on Tier 5.125 Thus, an initial consideration concerns whether it makes sense from a price perspective to have brands and generics on the same tier—that is, to what extent are brands and generics priced similarly.
The study answers this question using rigorous and conservative statistical comparison testing of brands and generics placed on the same tier.126 Subsequent analysis confirms that in the case of all tiers except for Tier 1, brand and generic drugs should not be placed on the same tier: the dosage-unit price for brands is simply far greater than the dosage-unit price for generics. Only for Tier 1 do brands and generics sometimes belong together, given that some brand prices are as low as the price range for generics.
III.C. Irrational Tiering
Academic literature has explained that the prize of avoiding generic substitution drives many strategic games in modern pharmaceutical markets.127 For example, in what is euphemistically known as ‘life cycle management’, brand drug companies alter various aspects of a drug—such as a drug’s dosage, formulation, or delivery system—shifting the market away from the version of their drug that is facing generic competition.128 As a result, simply comparing drugs with their generic counterparts may not fully capture the competitive dynamics of the market. Thus, this section of the study looks beyond brand drugs and their direct generic substitutes, expanding the comparison to consider drugs with the same active ingredient. For example, a brand drug in tablet form may be compared to a generic with the same active ingredient in capsule form, with a different dosage, etc.
Of course, an approach that truly considers the entire competitive landscape would also encompass competitors that have drugs with different active ingredients. Drug companies undoubtedly know who their competitors are, even if those drugs only compete off-label or in certain subsections of the market. One could think of such as approach as comparing therapeutic competitors, a term that has not been used previously in the literature.129 Identifying all such therapeutic competitors in order to compare their formulary placement would be a truly daunting task. Nevertheless, the study successfully expands the comparison to a degree by examining a subset of therapeutic competitors—specifically, drugs containing the same active ingredient.130
Comparing active ingredients, of course, is not a perfect measurement of substitutability. Certain routes of administration, for example, may be particularly expensive, although they may be better suited for a patient’s condition. Nevertheless, the dosage-unit price calculations and the comparisons of brand costs with costs of generics demonstrate that brands and generics, on the whole, have vastly different prices.131 Thus, although there may be outlying examples, brands and generics, as a general matter, should not be on the same tier—which means that brands and generics of the same active ingredient certainly should not be on the same tier.
Thus, this section of the study undertakes an empirically original analysis of ‘irrational tiering’ in the formulary system—instances in which a brand and a generic with the same active ingredient are placed on the same tier or when the generic is placed on a more-expensive tier.132 Given that the CMS began recording complete formulary data in 2010, the analyses in this section focus only on claims from 2010 to 2017, rather than the 12-year study period for pricing data.133
To ensure a valid comparison among drugs that have the potential for direct competition with each other, the study examines only the subgroup of generics and brand drugs that have the same active ingredient within the same, single health plan formulary. Generics with such brand competitors represent a relatively small portion of the drugs on five-tier formularies, with the average percentage of these drugs dropping from 32% to 29% between 2010 and 2017. Thus, it is important to note that in the section below, the study demonstrates that a minor change to tier placement for a limited percentage of drugs could lead to massive cost savings for patients and the federal government.
Even looking at this limited group, the study finds troubling evidence of irrational tiering. In 2015, for example, 69% of generics experience at least one abnormal placing relative to more expensive drugs with the same active ingredient. Moreover, the trend is worsening over time. Specifically, the study finds that the percentage of all generics that are irrationally tiered rises from 47% in 2010 to 74% in 2017.
The problem is not just that generics are being placed on the same tiers as brands with the same active ingredient; increasingly, the generic is being placed on a ‘worse’ tier. Within the cohort of irrational placements, the distortion of same-tier placement drops from 98% to 93%, whereas worse-tier placement more than triples from 2% to 7%. Thus, the combination of all results demonstrates that irrational tiering of generics is increasing, with an increasing share of misplacements shifting toward placing generics on a ‘more-expensive’ tier than a brand competitor with the same active ingredient. Both of these trends indicate increasing irrationality in the tier-placement system.
III.D. Wasted Spending Due to Irrational Tier Placement
Clearly, the formulary system is not working as intended. The question now is: how much does improper drug placement affect spending? Addressing the question requires a definition of spending.
III.D.i. Defining Spending
As described above, although average rebate information can be calculated for brand drugs, the rebate for any particular drug is not available. Thus, calculating true wasted costs when looking at a subset of drugs would be difficult to accomplish with precision. This particular challenge has confounded pharmaceutical researchers in other circumstances, leaving uncertainty in its wake.
The study, however, is able to overcome this perennial problem. Although net price remains out of reach, one can measure certain types of spending for which detailed data are available. Thus, the study examines spending by calculating what patients actually paid out of their own pockets for an individual drug purchase, combined with any amount that the federal government paid for that purchase in the form of its Low-Income Subsidy Program.134 These amounts provide a concrete and reliable method for measuring true dollars out the door.
Details on the calculation of the cost to patients and the federal government can be found at Appendix A3, but a few points are worth noting here. First, the calculation is based on the assumption that abnormally placed generics should have been placed a single tier below their actual tier placement. This is a highly conservative estimate; there are likely circumstances in which the difference in tier placement should be greater, based on the cost differential.
Second, Medicare enrollments135 play an important role in driving up wasted costs. In 2010, irrationally tiered generics leads to more than $50 million of wasted spending. By 2017, the amount of wasted spending increases by nearly a factor of 83, reaching $4.17 billion. This dramatic increase flows both from the continuous increase in Medicare drug prescription plan enrollees and the increase in the cost of irrationally tiered generics per beneficiary. In other words, when the number of plan enrollees increases, the amount of spending increases, as does the amount of waste from that spending.
Third, the results are likely to seriously understate the costs of irrational tiering from another perspective, as well. Specifically, the study examines only brand drugs with a generic competitor having the same active ingredient. A drug may have other types of therapeutic competitors, such as another drug with a different active ingredient that could treat the same disease state or a subsection of the population with the disease state, even if it might not treat all patients. To the extent that other forms of therapeutic competitors are cheaper and there is irrational tiering, those would be added costs to the patient and to the government. Even with these conservative approaches, the total wasted spending across the 2010–2017 study period amounts to $13.25 billion.
III.E. Implications and Limitations
As noted above,136 examining Medicare claims data provides a useful approach for understanding the inner workings of the health insurance system and the pharmaceutical industry. Four elements make the Medicare system a particularly attractive venue for researchers: (i) Medicare accounts for 29% of prescription drug spending in the USA; (ii) the detailed, government-verified claims data available from the CMS are unavailable from private insurers; (iii) certain problems in Medicare are likely to appear in the health insurance system in general; and (iv) reforms may be easier to craft for the Medicare system as an initial matter, before venturing into reforms of the private insurance market.
Nevertheless, there would be limitations in generalizing from the Medicare system to the whole. These may include less variation in the drugs the cohort buys, how the drugs are covered by their insurance, the prices that they pay, the kind of rebates one expects the plans to obtain, and so on. For example, an industry analyst report concludes that rebates amount to a far larger percentage of point-of-service spending for drug spending in Medicare Part D than in private health insurance planes.137 In Medicare, rebates amount to 22% of spending; in private plans, the percentage is 12%, almost half of that amount.138 The analyst report hypothesizes that patients may have different preferences in shopping for private plans than they do in shopping for Medicare plans. Prescription coverage is purchased separately with Medicare plans, in contrast to private plans, which may lead patients to evaluate the plans, and the interactions between elements of the plans, differently.
It is possible that given differences in the private insurance and Medicare markets, brand drug companies are relying less on rebates to drive consumers to their drugs and more on coupons. With coupons and similar systems, drug companies reimburse a patient for all or part of the patient’s copayment or co-insurance.139 Patients pay less at the pharmacy counter. They may pay more; however, given that the insurance plan’s cost for the higher-priced drug will filter through to higher premiums. For this reason, Medicare does not allow the use of coupons, and some private plans discourage coupons by refusing to count the amount covered by a coupon in a patient’s deductible.140 And in the long run, of course, patients may pay more as lower-priced substitutes are unable to gain much of a foothold in the market or are discouraged from entering the market at all.
The point is simply that differences between the private insurance market and Medicare insurance markets counsel caution in comparing the two. Comparisons between the two may be instructive but not necessarily conclusive.
One should also note that the study does not examine how rebate dollars that are returned to the health plan (as opposed to those retained by the PBMs) might flow back into overall costs in the system. There is some evidence that rebates help to defray ‘premium’ costs—that is, the cost paid to enroll in a health plan—to patients.141 One would have to determine how much flows back into premium reduction (as opposed to executive pay or other expenditures), how those flowbacks affect the payment allocations among different types of patients. For example, some have suggested that to the extent rebates reduce premiums, they have the effect of shifting burdens away from healthy patients and onto sick patients, the opposite of the manner in which an insurance system is supposed to operate. 142
One would also have to calculate how much of the premium reduction is inadvertently funded by increased government subsidies through the Low-Income Subsidy Program and potentially other increased reimbursements from Medicare. For example, the increased prices that fuel rebates also push patients more quickly into the portion of Medicare in which the government picks up 80% of a patient’s costs.143 Thus, rebates can have the effect of shifting expenditures from the health plan to the federal government.144 All of these variables transform any calculation of how rebate dollars flow into overall system expenditures into an exercise fraught with uncertainty and potential inaccuracies. And of course, much of the necessary information is claimed as a trade secret and deeply hidden.145 Thus, calculating the increased amounts patients must pay out of pocket, along with the portion of that payment subsidized by the government, provides a useful window into a tangible and immediate impact on those parties. Moreover, given the complexities and timing shifts of the healthcare reimbursement one would expect significant leakage to occur, even if every penny of the rebate dollars—both the dollars retained by the PBMs and the dollars returned to the health plans—were to flow back into premium reduction, which is an unlikely scenario itself.
Finally, interactions between drug costs and other elements of an insurance plan, both for the patient and for the plan, raise tantalizing questions for future research. Medication purchasing is only one element of the overall risk profile of a patient, and it creates a form of signaling effect for both the patient and the plan. In other words, plans want more people with a more profitable risk profile, and patients want plans that cover their needs. The issue is more complicated than simply trying to attract healthy patients and discourage those who are less healthy—something that could be accomplished by placing medications for HIV, for example, on a less-favored tier, thereby discouraging patients who might impose a range of nonmedication costs on a plan. On a more complex level, suppose that certain conditions are more profitable for a plan given reimbursement levels or other factors. In that case, if the plan believes that patients may prefer brand drugs for that disease state, the plan might choose to place both the brand and generic on a favorable tier to attract patients with that disease state—or do the reverse if it wished to discourage such patients. Comparisons between stand-alone Medicare Part D drug plans and certain types of Medicare plans that also offer Part D coverage146 could provide the opportunity to tease out some of these effects.
The results of the study, nevertheless, point to a clear, unmistakable conclusion: we do not nearly live in an ideal world. The current formulary tier system for drug coverage, which is intended to reduce both patient and government expenditures for prescription drugs, is being manipulated to the advantage of insurers, drug manufacturers, and PBMs seeking greater profits at the expense of patients. Based purely on cost, the standard for drug placement should be that cheaper generic alternatives are placed on lower, less-expensive tiers with lower copays, whereas more-expensive brand drugs should be placed on higher, more-expensive tiers requiring higher copays.147
Although insurers maintain that their formularies are structured according to this ideal,148 the study’s examination paints a different picture. Brand and generic drugs are increasingly placed together on the same tiers, despite significant cost differences between the two. Within the nebulous haze that is the formulary system, few things are clearer than the fact that the current system is being gamed and costing society dearly. Observations such as these are most useful, however, if they illuminate potential pathways for reform.
IV. THE PROBLEM AND ITS SOLUTIONS149
Only a few solutions have been proposed to correct abuses of the formulary system, in part, because formulary abuses seem to be a recently identified phenomenon.150 One Canadian academic suggested designing formularies based on evidence of head-to-head comparisons of drug efficacy and cost-effectiveness analyses with competing medications, including real-world, postmarketing evaluations of effectiveness. 151 The approach would establish a set of predetermined criteria in the formulary placement process, restricting how certain drugs could be added or removed from formularies. Such an approach, however, would be far easier to implement in a nation with a single-payor system, such as Canada, than in the USA.
Generics industry actors, naturally, wish to restrict the ability of insurance plans to place brand drugs on lower, less-expensive tiers.152 Similarly, the CMS proposed—and subsequently scrapped—a rule that the lower tiers should be exclusively reserved for generic drugs, with higher tiers reserved for brand drugs.153 Although these approaches would be an improvement over the current system, they leave much room for mischief. There is no single, accepted definition of what is a generic, and brand drug companies engaged in strategic behavior regarding the term. For example, anecdotal evidence has revealed brand companies withdrawing their product from the market so that the generic version, as the only one on the market, would be treated as a brand under reimbursement procedures.154 Moreover, the term ‘generic’ itself has become muddied by the entrance of so-called authorized generics. Authorized generics are versions made or licensed by the brand company using the generic name of the drug, normally at a lower price than the brand. Any system that relies on a name with no agreed definition of the naming convention is likely to encounter serious problems.155 At the end of the day, one can call something a brand, a generic, or an elephant. It is the price that matters.
IV.A. Focusing on List Price
To solve the game playing and waste, one must return to the core rationale of the tiering system. Specifically, what is the tiering system intended to accomplish, and how might society direct the system back to its goals, despite the complexities and competing incentives. As a touchstone for the inquiry, modern tiering systems are supposed to be based on the price of the drug, a rationale that is explicitly set out both in the academic literature and in insurance plan materials.156 But what price? As described above, price is a slippery and murky concept.157
One might argue that tiering should be based on the net price. After all, that is the bottom-line cost to the health insurer. Basing tiering on net price, however, presents a host of problems. The first is that true net price is hidden—from the health insurance plan, auditors, and regulators. This disappearing act makes it nearly impossible to track and monitor strategic or even downright illegal behavior. The convoluted nature of net price also blunts its ability to operate as a signaling mechanism—for patients or even for health plans.158
Most important, volume rebates allow companies with market power to provide deals that cheaper, new entrants cannot meet. This is particularly problematic in the context of new generic entering the market in the face of brand drugs coming off patent, when those patents may have created a monopoly position in the market. Thus, net price in the context of volume rebates may create insurmountable barriers to competition, undermining the goal of having generic drugs bring down the price of prescription medication. The long-term benefits of generic competition may be left in the dust. In short, net price as a solution to tiering leaves much to be desired.
A problem of this magnitude and degree of challenge requires an innovative solution. The sweeping solution that this paper offers is that tiering should be based on ‘list’ price. Yes, list price, a term that is roundly dismissed because it does not embody the hidden rebate deals. Nevertheless, list price should be the key component in determining the tier placement for all drugs. And as the discussion below will explain, list price is the perfect regulatory solution for bringing some measure of sanity to the tiering system.
First, basing tiering on list price brings an end to the rebate and quasi-kickback games that can harm competition and contribute to rising prices.159 One cannot overestimate how difficult the rebate problem has been to address. Despite widespread recognition of the problem involving rebates, regulatory attempts to reform this practice have failed. Most recently, in February 2019, the Trump administration proposed a rule to alter safe harbor protections under the federal Anti-Kickback statute so that drug companies would be prohibited in federal healthcare programs from paying rebates tied to a percentage of a drug’s list price to PBMs under federal health programs.160 The proposal was limited in some aspects, offering two new safe harbor provisions: one that would have allowed PBMs to negotiate rebates with drug manufacturers as long as those rebates were shared directly with patients at the point of sale and another that would have allowed PBMs to receive fixed fees for services provided on behalf of insurers.161 In a report released in May, however, the Congressional Budget Office (CBO) projected total prescription drug costs in the USA would not decrease if the proposed rule were finalized.162 The CBO predicted that under the rule, drugmakers would ‘offer the renegotiated discounts in the form of chargebacks’ instead of lowering drug list prices.163 As a result, the CBO estimated that the rule would have increased government spending by a total of $177 billion from 2020 to 2029.164 The administration eventually pulled the proposal to eliminate drug rebates in July.165
In addition to the risk of developing new games, efforts to reform rebates have been stymied by the concern that drug companies could simply pocket the amounts previously offered as rebates without lowering prices. In the CBO’s assessment of the Trump administration’s proposal to eliminate rebates, for example, the agency predicted that drug companies would ‘withhold about 15% of the amounts they currently rebate to PBMs in Part D and would negotiate discounts approximately equal to the remaining 85%’.166 Rather than lowering list prices, the agency expected that manufacturers would pocket most of the savings themselves rather than pass them on to consumers.167 Without the capacity to negotiate drug discounts, Medicare would have to bear the brunt of the costs, leading to increases in premiums and government spending.168
Focusing on list price, however, has the potential to directly address high pharmaceutical prices, while avoiding the unintended consequences of directly eliminating rebates. If tiers were based on list price, a drug company that raised its price to give space for rebates and other payments to PBMs would find that the strategy backfires. The high list price would drive the company’s product to a less-advantageous tier, in comparison to cheaper substitutes. This would flip the perverse incentives of the current system—in which ‘raising’ prices provides a competitive advantage—on its head.169 And as one would expect in a free-market system, lower price would make a company more competitive.170 Hidden negotiations and tempting rebate deals would not matter, regardless of whether payments were designed as rebates, chargebacks, or in some other manner; they simply would not factor into competitive placement.
Basing tiering on list prices provides additional advantages. The approach sidesteps the need for greater transparency regarding negotiations between PBMs and drugmakers by avoiding the practical problem of navigating around claims to trade secret protection.171 No one claims that ‘list’ price is a trade secret. Moreover, Medicare regulations already requires drug companies to report the list price, including providing penalties for failure to report.
Basing tiering on list price potentially could be accomplished with regulatory changes through the Department of Health and Human Services that could be mandated by Congress. The CMS, the part of HHS that oversees Medicare,172 already exercises authority over formularies and formulary design.173 Medicare’s Prescription Drug Manual, as updated in 2018, specifies that a plan must include ‘Provision of an Adequate Formulary’, and that the agency will ‘consider specific drugs, tiering and utilization management strategies employed in each formulary’.174 At present, the Manual suggests that CMS’ exercise of authority will revolve around ensuring that plans follow common practice in the industry. For example, the Manual notes that plans are encouraged to submit formularies similar to those in widespread use today, and that the Agency will identity outliers for further evaluation.175
As of 2018, CMS’ Manual identifies its authority to regulate formularies as flowing from the Medicare Modernization Act of 2003, with its requirement that a plan’s categorization system does not ‘substantially discourage enrollment by any group of beneficiaries’.176 Earlier CMS guidelines on formularies, however, describe the goal of the Medicare Modernization Act (and the Agency’s guidelines, themselves) in terms of cost, explaining that the CMS will review plans to ‘assure that beneficiaries receive clinically appropriate medications at the lowest possible costs’ and that ‘the goal is for plans to provide high-quality cost-effective drug benefits by negotiating the best possible prices and using effective drug utilization management techniques’.177
To the extent CMS’ earlier view of its authority is accurate, the Agency could reach beyond simply requiring that plans look like other plans on the market and specify tiering according to list prices, in the interests of providing high-quality cost-effective drug benefits. Even if courts were to conclude that CMS’ authority under the Medicare Modernization Act is narrower, the basic pathway exists. With CMS already reviewing and providing guidance on formularies, Congress could provide any additional authority necessary to further regulate formularies.
Given that CMS already requires that drug companies submit their list prices, 178 the Agency has the necessary information. Expansion of the regulations to provide for verification of the information, along with a willingness to enforce the regulation, would be important.179 Although companies that provide inaccurate reports can be subject to civil monetary penalties or terminated from the drug rebate program, compliance problems have existed in the past. For example, in 2008, over half of the manufacturers did not fully comply with the quarterly submission requirements and three-quarters did not fully comply with monthly requirements.180 Over time, the Office of Inspector General has indicated a willingness to step up enforcement efforts.181 The Inspector General’s Office even suggested that CMS use its authority to impose $10,000 per day fines for late reporting.182 Nevertheless, the existence of open list pricing creates an easy avenue for developing a modicum of accountability.
Ideally, tiering reforms would be applied to all insurance plans. The political difficulty of instituting such a broad change for the entire healthcare insurance industry, however, could be challenging, and the prospect of wading into the treacherous waters of the private healthcare market would be daunting. Instead, moving tiering to list prices would be more easily adopted through the Medicare system, which has the potential to create ripple effects in the private insurance market.
IV.B. Staying Ahead of the Game
Legislative and regulatory changes happen slowly and infrequently. In contrast, strategic behavior by the players in the system constantly shifts and evolves. As any chess player can attest, blocking the last move will never be enough to succeed. One has to play forward, anticipating reactions and setting pathways for managing likely responses. With this in mind, two additional sets of reforms would be important within the CMS regulatory approach: transparency and rational tiering rules.
IV.B.i. Transparency
Creating tiers based on list price is a sensible departure from the current system. Nevertheless, without transparency regulations and the capacity for enforcement, the formulary system remains ripe for abuse. Strategic behaviors can simply shift their forms—from gaming the tiering system, to gaming net prices, to gaming list prices, to new games.
Information symmetry is one of the prerequisites for functioning markets,183 and, as many scholars have observed,184 the healthcare and insurance markets are plagued by asymmetric information—with sophisticated institutions having vastly more knowledgeable than individual consumers. Although formulary tiers are supposed to serve as a way of partially addressing this information asymmetry, the murkiness permeating the formulary system can only enhance the likelihood that insurers construct irrational tiers. To ensure that the door guarding formulary tiers is closed to further abuse, as well as to prevent arbitrary construction of tiers, it is imperative to accompany tiering based on list price with regulations heightening transparency.185
For example, data regarding the tiering system should be fully available to the public in an easily understood format so that any interested party, including patients, government regulators, academics, competitors, and the press can easily access information regarding how tiers are structured and how drugs are placed on those tiers. By making this information publicly available, various stakeholders can use the data as a check to ensure that the formulary system remains free from abuse. Publicly available information also helps to ensure that driving patient behavior—which is the goal of the tiering system in the first place—both occurs and occurs in an appropriate manner. Moreover, the capacity to more easily investigate PBMs and health insurance companies fosters greater accountability and lessens the incentive for these players to game the tier system for short-term profits. These checks—regulation by interested parties and self-regulation—facilitate the construction of sensible tiers and reinforce the storied principle that a free press, an informed citizenry, and a free market go hand in hand.
To be clear, transparency measures should go beyond information about list prices and tiering. Pharmaceutical market functioning and competition suffer dramatically from a shadowy lack of information.186 As I have noted in the past, ‘[M] arkets, like gardens, grow best in the sun’.187
Different pathways are available for transparency. These could include resolving the issue through trade secret law itself, either by court decisions clarifying that pharmaceutical prices and pricing terms are not trade secrets188 or by Congressional clarification of the point. Taking a page from the requirement that pharmaceutical companies share safety and efficacy data for the benefit of prospective generics,189 legislative changes could mandate that to obtain marketing approval of a drug, the company must agree to share pricing information with the market. Regardless of the pathway, games will continue to flourish in dark corners unless we shine a little light.
IV.B.ii. Rational Tiering Rules
In addition to the need for greater transparency, regulatory authorities would need to implement both a rules-based and a standards-based approach. Even after adopting a tiering system based on list price, one could imagine a plethora of avenues to game the formulary system and circumvent the spirit of this proposal. Suppose pharmaceutical companies induce health insurers to construct tiers such that copayment patterns reflect little about a drug’s actual price. One could accomplish that, for example, by having patients pay roughly the same for different tiers or by grouping large swaths of drugs on the same tier.
To make the example easier to understand, imagine a system with 10 or even 20 tiers and minimal differences in copays between the tiers. For example, suppose patients pay a $20 copay for drugs on Tier 8 (a less-expensive tier) and a $20.50 copay for drugs on Tier 9 (a slightly more-expensive tier) and that the generic version of a drug is placed on the less-expensive Tier 8, whereas the brand version of the drug is placed on the slightly more-expensive Tier 9. At first glance, that might appear perfectly acceptable: the generic is on a tier that is preferred over the brand tier. The patient, however, would be paying a tiny amount more for the brand than for the generic. The brand drug name may be more familiar to the patient—particularly if the brand’s patent protection has recently ended—or the patient may believe that the ‘brand’ is better. Advertisers already know that these issues can affect patient buying patterns, with advertisers in all fields urging consumers to buy the original and pharmaceutical advertisers urging patients ‘tell your doctor to write the prescription for the brand only’.190 In those circumstances, patients could easily choose the brand over the generic when the copays are roughly the same.
Similarly, imagine tiering organized with most drugs grouped onto one tier. Suppose the least-expensive tier—Tier 1—is designated for drugs with a list price of $0–$0.01, Tier 2 for drugs with a list price of $0.02–$0.03, and Tier 3 for drugs with a list price of $0.04–$10,000. Health insurers could charge copays on Tier 3 drugs that do not correspond to the real price of the drugs; a Tier-3 drug could have a list price of $4 or $4000. These examples are drawn in the extreme, but the point is that if tiers are constructed so that placement is meaningless, the tiering system loses its power.
Complexity breeds opportunity,191 and the pharmaceutical payment and delivery is a marvel of complexity. In these circumstances, a rules and standards approach is important for avoiding a whack-a-mole approach to the problem of pharmaceutical pricing, particularly considering that the mole can move faster and more frequently than the mallet. As this author has noted, ‘[t]he goal with a standards-based approach would be look at the overall effect of a behavior in an effort to thwart those who follow the letter of the law, but manage to arrive at a destination that the law intends to forbid’.192
In pursuing a rules-based and standards-based approach, tax law’s step-transaction doctrine provides a helpful analogy. As with healthcare, tax law is a heavily rules-based area with precise regulations. Nevertheless, tax law’s step-transaction doctrine states that even if firms may have managed to follow all the rules, tax authorities have the requisite authority to collapse all steps in a transaction if the steps are part of an overall plan to avoid taxation.
The same principle would apply to drug price tiering. A standards-based approach, in tandem with a rules-based approach, is necessary to ensure that formularies properly implement tiering based on list price, without taking advantage of loopholes that might ‘follow all the rules’ but circumvent the spirit of the proposal. Specifically, a rational-tiering standard would head off future problems by requiring that formularies cannot be set in a way that conflicts with the rule’s intended end, that is greater transparency, greater competition, and lower prices. Once again, a rational-tiering standard could be implemented by regulation or legislation in concert with implementation of the rule to base tiering on list price.
CMS regulation, of course, is itself subject to the limitations of any agency action. Scholars have spilled oceans of ink on the topic of agency capture and the challenges of creating efficient and effective expert agencies that are sufficiently independent of influence by industry or politics.193 Nevertheless, regulatory agencies remain the nation’s best method for the type of detailed and nimble supervision that would be necessary to impose a measure of rationality in insurance plan reimbursement systems.
IV.C. What Basing Tiering on List Price Cannot Solve
No single solution can possibly solve all problems within the formulary system, let alone with pharmaceutical pricing. The study and its recommendations cannot address the staggering price of new drugs coming on the market, particularly in the realm of cancer treatment. In those realms, it will be years, or more likely decades, before generic alternatives may enter the market.194
One should also note this study and its resulting recommendations focus on competition between brand and generic drugs. As such, the recommendations presuppose a functioning generics market. If other strategic behaviors block or hinder that market,195 all the formulary reform in the world will not help. This is the case regardless of the extent to which the problems stem from behaviors by brand companies, behaviors within the generic market itself,196 or structural problems such as tiny markets with low demands.197 If generic markets are not competitive and thriving, little ground can be gained.
In addition, the generics industry must be able to deliver medications of reliable quality. Recent concerns have arisen regarding the quality of ingredients in medications. For example, in 2020, the FDA recommended that five generic companies recall their versions of the diabetes medication, metformin, in light of tests showing the presence of the carcinogen NDMA.198 The potential concerns are not limited to generic drugs, of course. For example, the FDA in 2020 recalled the brand-name drug Zantac in light of the same carcinogen.199
Beyond contamination issues, there can be slight variations between brand and generic drugs. Although a generic must have the same active ingredient as the brand, the FDA allows certain variations in inactive ingredients.200 Similarly, the FDA sets the parameters for the extent to which there may be small variations in purity, size, and strength—not just between the brand version of a drug and the generic version but even among different batches of the same drug from the same brand company.201 The generics industry, as well as the entire industry, must be able to deliver medications of consistent quality and potency, for consumers and health plans to be willing to trust them.
Of course, if a generic drug is of poor quality, the solution is not to charge patients more to purchase it. That would be a strange approach given that drugs of poor quality simply should not be on a formulary list. Moreover, in that scenario, the plan would save money by buying a cheap drug of low quality and then would be able to line its pockets by charging patients a premium for that low-quality product. That would be truly perverse. The solution for quality concerns should lie in testing, both at the time of approval and across the production period. Charging patients more for low quality should never be a solution.
In short, soaring pharmaceutical pricing is a difficult and complex problem that deeply affects the lives of patients throughout the USA. There is no magic bullet. Nevertheless, the off-kilter tiering system makes a substantial contribution to the problem. As the study demonstrates, abuses of the formulary system conservatively cost patients and the government over $50 million in 2010 and over $4.17 billion in 2017 alone. And the problem is growing across time. The number of drugs placed on irrational tiers increases from 47% to 74% across the eight-year study period. And the percentage of generics on the least-expensive tier drops from 73% to 28%. Society cannot hope for a thriving generic market—one that can inject competition and bring down pricing—when generics are increasingly losing out in the battle for valuable tiering space.
Tiering lies at the heart of what drives patient behavior, but the devil is in the tiers. By reforming legislative or regulatory rules to require that tiers are based on list price, government officials can restore proper incentives in the drug coverage system, with the happy side effect of discouraging anticompetitive rebate and kickback schemes.
ACKNOWLEDGEMENTS
I wish to thank Ehrik Aldana, Colin Burke, John Gray, Christopher Kim, Nicholas Massoni, Prianka Misra, Sophia Tao, and David Toppelberg for their incomparable research assistance. I am grateful beyond measure to Ramy Alsaffar, Chief Data Scientist at C4i, for his invaluable role in the design and implementation of this and other empirical work at the Center. I am also immensely grateful to Gerard Anderson, Jeromie Ballreich, Juliette Cubanski, Leemore Dafney, Ben Depoorter, Scott Dodson, Jane Horvath, Elizabeth Jex, Cheryl Johnson, Michael Kades, Myresha Lewis, Joshua Sarnoff, Fiona Scott-Morton, David Simon, Kara Stein, and Mariana.
The research was supported in part by a grant from the Laura and John Arnold Foundation.
Appendix A
Figure 6.
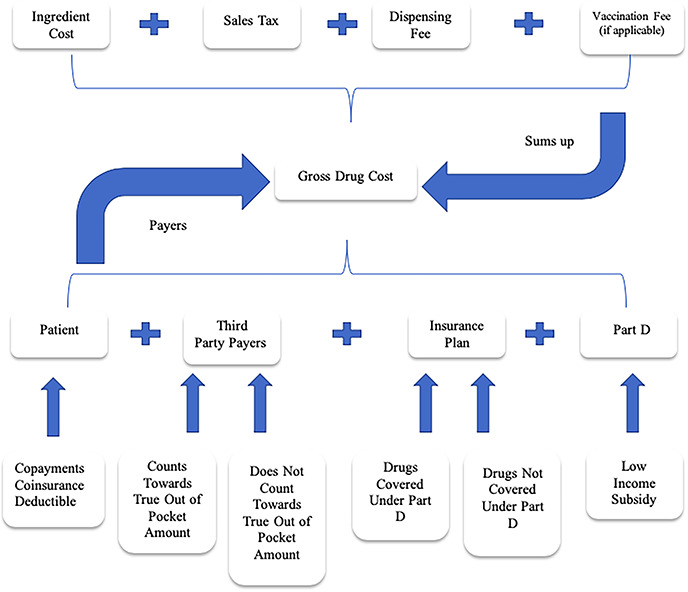
Breakdown of PDE gross drug cost & payers.
Appendix B
Rebate Calculations
The average rebate percentages provided in the Medicare Trustees Reports202 are calculated across all prescription drugs (brands and generics). Therefore, the study needed an approach to modify these rebate percentages to reflect brand drugs only. The methodology of deriving average rebates of brand drugs from the average rebates of all drugs is according to the following formulas:
Rebate % = (Rebate Amount/Total Spending) * 100
Total Spending = Brand Spending + Generics Spending
Rebate % = [Rebate Amount/(Brand Spending + Generics Spending)] * 100
Assuming generics did not receive any rebates, then:
Rebate % for Brand = (Rebate Amount/Brand Spending) * 100
Brand Spending percentages are derived from the CMS data.
The calculations and findings are as follows:
Step 1: Rebates across all prescription drugs from Medicare Trustees reports.
| From Medicare Trustees reports | ||
|---|---|---|
| Year | Rebate % for total spending | Total spending |
| 2006 | 8.6 | 100 |
| 2007 | 9.6 | 100 |
| 2008 | 10.4 | 100 |
| 2009 | 11.1 | 100 |
| 2010 | 11.3 | 100 |
| 2011 | 11.5 | 100 |
| 2012 | 11.7 | 100 |
| 2013 | 12.9 | 100 |
| 2014 | 14.3 | 100 |
| 2015 | 18.2 | 100 |
| 2016 | 19.9 | 100 |
| 2017 | 21.8 | 100 |
Step 2: Brand and generics spending from CMS sample data.
| From the CMS data | |||
|---|---|---|---|
| Rebate amount | Brand spending | Generics spending | Total spending |
| 8.6 | 81 | 19 | 100 |
| 9.6 | 80 | 20 | 100 |
| 10.4 | 78 | 22 | 100 |
| 11.1 | 77 | 23 | 100 |
| 11.3 | 77 | 23 | 100 |
| 11.5 | 78 | 22 | 100 |
| 11.7 | 73 | 27 | 100 |
| 12.9 | 72 | 28 | 100 |
| 14.3 | 74 | 26 | 100 |
| 18.2 | 76 | 24 | 100 |
| 19.9 | 75 | 25 | 100 |
| 21.8 | 75 | 25 | 100 |
Step 3: Rebate % for brand.
| Year | Rebate amount | Brand spending | Rebate % for brand |
|---|---|---|---|
| 2006 | 8.6 | 81 | 10.6 |
| 2007 | 9.6 | 80 | 12.0 |
| 2008 | 10.4 | 78 | 13.3 |
| 2009 | 11.1 | 77 | 14.4 |
| 2010 | 11.3 | 77 | 14.7 |
| 2011 | 11.5 | 78 | 14.7 |
| 2012 | 11.7 | 73 | 16.0 |
| 2013 | 12.9 | 72 | 17.9 |
| 2014 | 14.3 | 74 | 19.3 |
| 2015 | 18.2 | 76 | 23.9 |
| 2016 | 19.9 | 75 | 26.5 |
| 2017 | 21.8 | 75 | 29.1 |
Appendix C
Estimated Cost to Patients and the Federal Government Calculations
The main formula used to calculate the cost estimate to society of the abnormal placement of generic drugs on formulary tiers is as follows:
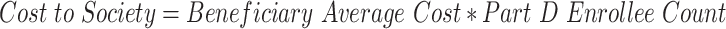 |
Where the Beneficiary Average Cost is calculated based on five-tier formularies, and Part D Enrollee Count is weighted to reflect only the count of five-tier enrollees.
Part D Enrollee Count is provided by the Chronic Conditions Data Warehouse website.203 The average cost of abnormally placed generics per beneficiary was determined by calculating the cost per formulary and dividing it by its beneficiary count:
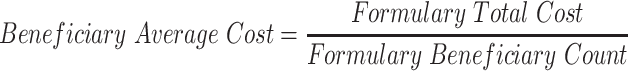 |
The calculation of the Formulary Total Cost (of abnormal generics placement) is based on the assumption that the proper placement of a generic drug is one tier lower than its actual tier placement. The formula used to calculate the Formulary Total Cost is as follows:
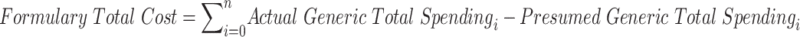 |
Where n is the number of generics with abnormal placement in a formulary.
To compute the actual total spending of a generic, the study adds the total amount patients paid to what Part D subsidized for the specified drug, as per the following formula:
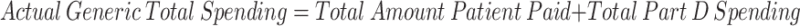 |
To compute the presumed generic total spending, the study calculates the generic volume, or the number of times a generic was filled, and multiplies it by its formulary generics average spending of one tier lower than its actual tier ID. Formulary generics average spending is the average of patient paid amounts and Part D spending calculated separately for each formulary. The formula used to calculate the presumed generic total spending is as follows:
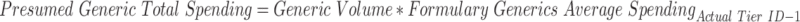 |
The study calculates the Formulary Generics Average Spending for each tier in the five-tier formulary. The average generic spending of each formulary tier is the result of the sum of total patient paid amounts and the total Part D spending, divided by generics count, or the number of generic drugs on this specific tier.
 |
Where i is between [1, 5]
Appendix D
Figure 7.
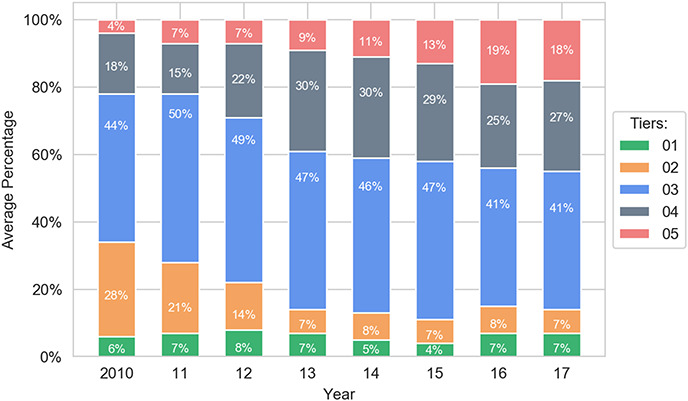
Average distribution of brand drugs on five-tier formularies.
Figure 8.
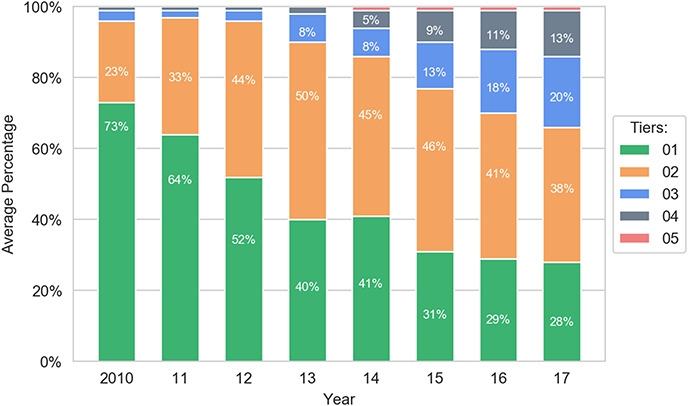
Percentage of generics on all tiers.
Appendix E
V. METHODOLOGY
V.A. Overview
The study seeks to identify and measure game playing within the formulary tiering system using Medicare Part D data collected by the CMS. The study hypothesizes that brand drugs are being placed in advantageous tiers, whereas generic drugs are being placed in disadvantaged tiers and that this behavior is increasing across time.
V.B. Methodology Details
V.B.i. The Data
The study period covers a 12-year period from 2006 to 2017 following a cohort of just under one million patients. The cohort request was designed with the following parameters: all patients in the cohort were alive during the entire study period, and each patient had at least one prescription drug event during the study period. A prescription drug event represents a patient filling a prescription drug at a pharmacy, and this article will refer to it as a drug purchase. All the patients in the cohort were enrolled in Medicare Part A, B, and D coverage only—excluding Part C. In other words, all the patients in the cohort were only enrolled in a prescription drug plan and not a private insurance plan with prescription drug coverage.
This study defines a ‘drug’ by its unique National Drug Code (NDC), which serves as a universal product identifier for a drug. An NDC is a numerical code with three segments representing: the drug’s labeler204 (eg Pfizer Consumer Healthcare), product and dosage (e.g. Advil), and package (eg 24 tablets in one bottle).205 As is required by the Drug Listing Act of 1972, drugs are identified and reported to the FDA using NDCs.
Each data entry represents one drug purchase in a larger file called the Part D Event file. Each drug purchase entry corresponds to one NDC—in other words, there is never more than one drug associated with one drug purchase entry. From each drug purchase entry, there were corresponding data about the drug itself, including the formulary the drug purchased was in, and how much the drug cost for the various payers. Payers include the patient, Medicare Part D, and the health insurance plan. The drug purchase files reflect the payments at the point of sale and do not include amounts that may be given as rebates to the health insurance plan later on.206
Before starting the data analysis, the data were filtered multiple times in order to analyze and draw accurate conclusions based on relevant data. The first layer of elimination is removing all drug event data where the drug purchased was covered under an ‘enhanced’ benefit package. Some plan sponsors offer an ‘enhanced’ benefit package that covers non-Part D drugs, such as over-the-counter (OTC) medications. In other words, the study eliminates all drug event data where the drug purchased was covered under the ‘enhanced’ benefit package that covers non-Part D drugs or OTC medications.207 Non-Part D drugs are not relevant to the analysis, which draws conclusions based on the Medicare Part D program. The study takes more steps to filter and organize the data, which is detailed further in this section.
V.B.ii. Categorizing Drug Data as Brand or Generic
Comparing brand and generic drug placement on formulary tiers required categorizing all the drugs in the data, represented by NDCs, as either brand or generic. One would assume that categorizing drugs as brand or generic would be simple enough, but there are no hard and fast rules for classifying a particular drug as brand or generic.
One possible categorization method is to map each drug to their associated New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) codes. Respectively, NDAs and ANDAs represent brand and generic drugs at the FDA approval level.208 However, there is no existing mapping system between NDCs and NDAs or ANDAs.209 The FDA only publishes records of active NDCs,210 and a significant number of NDCs have been removed during the observed time period (2006–2017).211 The study attempted to find historical records through internet archiving but was unsuccessful. Commercial databases (such as Cerner Multum, First Databank, Medi-Span) also had limited to no data on historical NDCs codes.
Thus, the drug data were categorized as brand or generic using three sources in the following order: the original CMS data, National Library of Medicine’s RxNorm database, and Cerner Multum’s commercial drug database.
The CMS dataset includes a variable that indicated whether the insurance plan processed the drug purchased as a brand or generic drug.212 However, CMS only began collecting data under this variable in 2012, which would have limited the years of analysis from 2006 to 2011.
The National Library of Medicine’s RxNorm database categorized the drugs that CMS could not determine.213 The study uses a web service developed at the National Library of Medicine, called RESTful Web API, that allows a user to access, make queries to, and customize RxNorm data.214
Cerner Multum’s commercial database provided final categorization for the remaining drugs which were left uncategorized by CMS and RxNorm.215
Using the three databases mentioned above, the study is able to categorize 95.5% of all drugs in the CMS data as brand name or generic (60,317 out of a total of 63,141 NDCs). The remaining 4.5%, or 2824 drugs, did not have a clear pattern as to why they defied categorization.216 As a result, the study eliminates the uncategorized pool of drugs from analysis. The results and conclusions detailed below are based on the 95.5% that could be defined as either brand or generic drug.
V.B.iii. Formulary-Specific Analysis
CMS began recording complete formulary data beginning in 2010. Thus, the analyses regarding formularies only cover the eight-year period from 2010 to 2017.217 In contrast, the analyses of brand versus generic drug availability, average drug-dosage unit price, and average amount patient paid cover the entire 12-year period.
As noted above, formularies can have different numbers of tiers. Because formularies with five tiers were the most common, the study chooses to focus its analyses across the eight-year period on five-tier formularies. This choice imposes additional limitations on the study: drug costs could have been markedly different in other formulary types.
Abridged sample Blue Cross Blue Shield five-tier formulary 218 .
| Tier ID | Drug type | Description |
|---|---|---|
| 01 | Preferred generic | Lowest-cost drugs, mostly generics |
| 02 | Nonpreferred generic | Medium-cost drugs, mostly generics and some brand-name drugs |
| 03 | Preferred brand | High-cost drugs, mostly brand-name drugs |
| 04 | Nonpreferred brand | Higher-cost drugs, mostly brand-name drugs |
| 05 | Specialty | Highest-cost brand-name and generic drugs |
V.C. Establishing Key Metrics
The study seeks to identify and measure game playing within the formulary tiering system. The methodology hypothesized that brand drugs are being placed on preferred tiers, whereas generic drugs are being placed in nonpreferred tiers, and that this behavior is increasing across time. In order to accomplish the qualitative analysis, the study examines:
Generic and brand drug availability across 2006–17
Patient expenditure for generic and brand drugs across 2006–17
Significance in cost difference of generic drugs on different tiers, 2010–17
Significance in cost difference of brand drugs on different tiers, 2010–17
Significance in cost difference of generic and brand drugs on the same tiers, 2010–17
Estimated cost to Medicare society of irrational formulary tier placement, 2010–17
V.C.i. Drug Availability
Drug availability in Figure 1 represents the number of unique drugs in all drug purchase events. All drug purchase events are dated by a prescription service date variable split into corresponding years over the study period of 2006–17.219 Within each year, the analysis counts the number of unique NDCs (y-axis on Figure 1).
V.C.ii. Patient Expenditure—Average Amount Patient Paid
Each drug purchase event has a corresponding ‘Gross Drug Cost’ number that represents the price paid for the drug at point of sale (ie the pharmacy counter).220 The Gross Drug Cost number does not include any rebates or discounts. Appendix A1 includes a detailed explanation of costs, fees, and taxes that determine the Gross Drug Cost.221
Patients pay a part of each drug purchase event’s Gross Drug Cost. All drug purchase events have a corresponding variable indicating how much the patient paid (without being reimbursed by a third party).221 The Average Amount Patient Paid in Figure 2 (see Methodology section 5.2.2.) represents the average payment made by patients for a drug in all drug purchase events.
Figure 2.
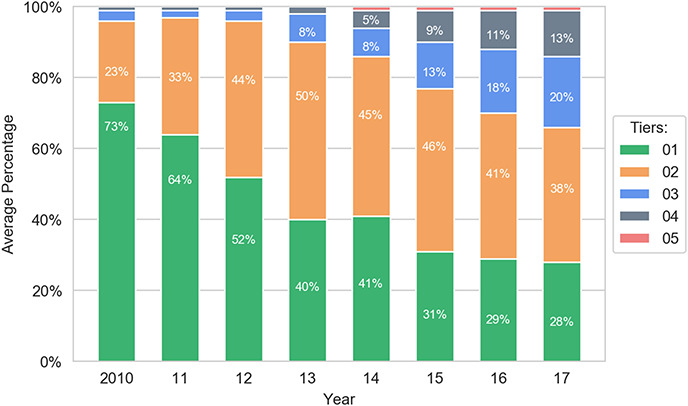
Average percentage of generic drugs across tiers on five-tier formularies.121
To calculate Average Amount Patient Paid, the study first splits up all the drug purchase events into corresponding years. The study then splits all drug purchase events within their year into brand or generic drug groups based on their associated NDC (see Problems and Solutions section 4.2.2.). Within each annual brand or generic category, the study then adds up all the patient payment amounts and divided them by the number of drug purchase events in each. The calculation is as follows:
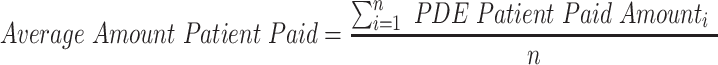 |
where n is the number of drug purchase events in either the brand or generic drug category in a given year.
V.C.iii. Patient Expenditure—Average Dosage-Unit Price
After splitting up all the drug purchase events into their annual brand or generic groups, the study calculates every drug purchase event’s Drug Dosage-Unit Price by dividing the Gross Drug Cost over the drug’s quantity dispensed.223 The average dosage-unit price was then calculated by taking the arithmetic mean of all the Dosage-Unit Prices within the annual brand or generic group.
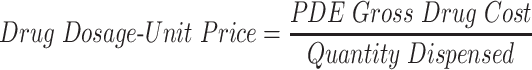 |
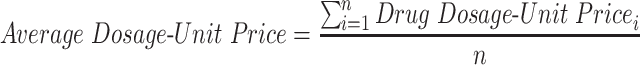 |
where n is the number of drug purchase events in either the brand or generic drug category in a given year.
V.C.iii.a. Including Rebates into Average Dosage-Unit Price
As previously mentioned, the Gross Drug Cost variable provided by CMS does not include any drug manufacturer rebates or discounts. Therefore, the average dosage-unit price calculated above, derived from the Gross Drug Cost, also does not factor in rebates. Rebates are provided by brand companies, not by generics.224 CMS provides aggregate rebate figures for each year.225 In order to establish a fair comparison between the cost of brand versus generic drugs, the analysis applies average percentage rebates on the average dosage-unit prices for brand drugs.226 This new calculation is represented in Figure 1 as the ‘Brand After Rebate’ variable.
V.C.iv. Formulary Analysis—Average Percentage Generic/Brand Drugs Per Tier
The study first splits all drug purchase events into their annualized brand or generic groups. For Figure 4 (see Methodology section 5.3.1.), it started with a pool of all drug purchase events associated with a generic drug in a given year, then calculated what percentage of all those drugs were on Tier 1, what percentage of all those drugs were on Tier 2, and so on, until Tier 5.227 For Figure 5 (see Methodology section 5.3.1.), the exact same calculations were done, except with brand drugs.
Figure 4.
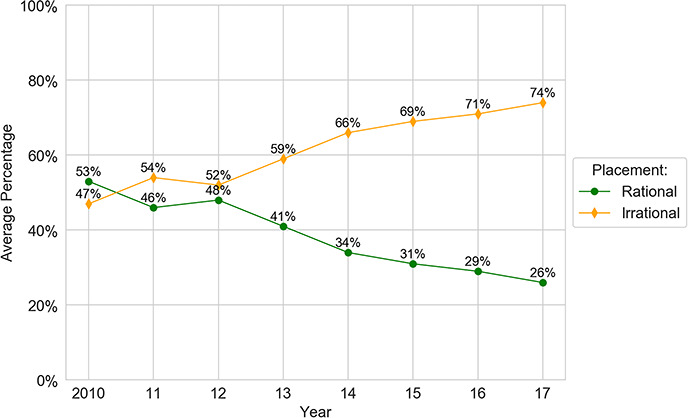
Irrationally placed generics.
Figure 5.
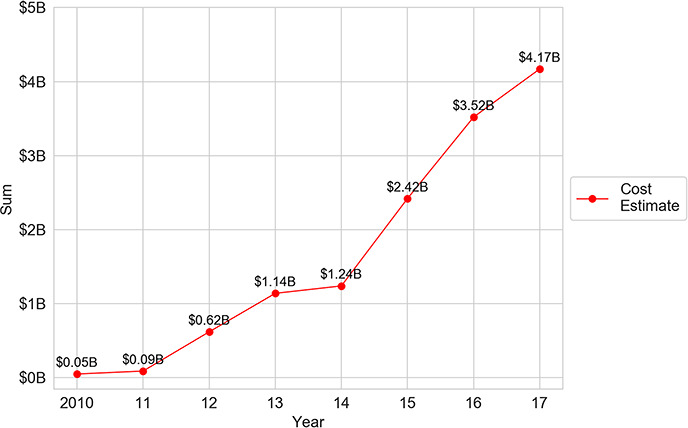
Wasted spending from irrationally placed generics.
V.C.v. Formulary Analysis—Significance in Cost Differences
Several factors determine drug tier placement, the most critical of which is cost-effectiveness. The analysis tests the logic of brand and generic spread (based on drug cost) across formulary tiers. For example, did the price of generics or brand drugs vary across the five tiers such that placing the drugs on different tiers make sense? To answer this question, statistical analyses checked whether there were statistically significant differences in the average dosage-unit price across formulary tiers. If the answer to the question is yes, then one would expect to see statistically significant differences in the average dosage-unit prices across the tiers; specifically, one would expect to see a significant increase in average dosage-unit prices as the tier number increases—that is, moving from preferred to nonpreferred tiers.
Three tests were performed: (i) a two-sided Kruskal–Wallis H test among generics only, (ii) the same test among brand drugs only, and (iii) a two-sided Mann–Whitney U test among generic and brand drugs placed on the same tier.228 The data that are graphed onto Figure 6 (Methodology section 5.3.2.) is shown below on Tables 1 and 2.
Table 1.
Average dosage-unit price for generic drugs per tier
| Generic average dosage-unit price ($) | |||||
|---|---|---|---|---|---|
| Tier ID (row) Year (column) | 01 | 02 | 03 | 04 | 05 |
| 2010 | 1 | 2 | 3 | 10 | 29 |
| 2011 | 1 | 2 | 3 | 13 | 39 |
| 2012 | 1 | 2 | 5 | 13 | 59 |
| 2013 | 1 | 2 | 3 | 10 | 44 |
| 2014 | 1 | 2 | 3 | 7 | 15 |
| 2015 | 1 | 1 | 2 | 6 | 33 |
| 2016 | 1 | 1 | 2 | 6 | 34 |
| 2017 | 1 | 1 | 2 | 6 | 41 |
Table 2.
Net average dosage-unit price for brand drugs per tier
| Brand drug average dosage-unit price ($) | |||||
|---|---|---|---|---|---|
| Tier ID (row) Year (column) | 01 | 02 | 03 | 04 | 05 |
| 2010 | 1 | 12 | 11 | 26 | 365 |
| 2011 | 1 | 12 | 13 | 41 | 368 |
| 2012 | 2 | 11 | 16 | 31 | 459 |
| 2013 | 1 | 5 | 17 | 27 | 562 |
| 2014 | 1 | 10 | 16 | 35 | 471 |
| 2015 | 1 | 3 | 20 | 36 | 439 |
| 2016 | 1 | 4 | 20 | 47 | 481 |
| 2017 | 1 | 5 | 21 | 50 | 524 |
V.C.v.a. Significance in Cost Difference of Generic Drugs on Different Tiers
In this section, the study examines whether generic drugs, based on their average dosage-unit price, should be assigned to five different formulary tiers or to a smaller number of tiers. The hypothesis was that generic drugs placed on different formulary tiers have significantly different average dosage-unit prices.229 The Kruskal–Wallis H test, which determines if there are statistically significant differences between groups of an independent variable on a continuous dependent variable, was applied.
The resultant P-value was 2.3E−07, far less than the standard significance level of 0.05. Therefore, this gives one reason to accept the hypothesis, namely that generic drugs placed on different formulary tiers have significantly different average dosage-unit prices. However, the test itself does not indicate which tiers have significant average dosage-unit price differences compared with the other tiers. Therefore, the study opted to perform pairwise comparisons between the formulary tiers to find the tier pairs that feature significant differences in average dosage-unit price using the Tukey test, a statistical test that is used to find means that are significantly different from each other.230 The results are shown in Table 3.
Table 3.
Pairwise comparisons of average dosage-unit price for generic drugs—Tukey (honestly significant difference (HSD), FWER = 0.05
| Tier ID | Average difference | Lower | Upper | HypothesisNull | |
|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||
| 01 | 02 | 0.625 | −7.7892 | 9.0392 | Not Rejected |
| 01 | 03 | 1.875 | −6.5392 | 10.2892 | Not rejected |
| 01 | 04 | 7.875 | −0.5392 | 16.2892 | Not rejected |
| 01 | 05 | 35.75 | 27.3358 | 44.1642 | Rejected |
| 02 | 03 | 1.25 | −7.1642 | 9.6642 | Not rejected |
| 02 | 04 | 7.25 | −1.1642 | 15.6642 | Not rejected |
| 02 | 05 | 35.125 | 26.7108 | 43.5392 | Rejected |
| 03 | 04 | 6 | −2.4142 | 14.4142 | Not rejected |
| 03 | 05 | 33.875 | 25.4608 | 42.2892 | Rejected |
| 04 | 05 | 27.875 | 19.4608 | 36.2892 | Rejected |
From Table 3’s results, the following conclusions can be drawn:
There is evidence to reject the null hypothesis when performing the pairwise comparisons of generics placed on Tier 5 with the other tiers. The Tier 5 pairwise comparisons have P-values that are <0.05 significance level. More simply, generic drugs placed on Tier 5 have significant differences in average dosage-unit price from generic drugs placed on the other formulary tiers (1, 2, 3, and 4).
There are no significant differences in the average dosage-unit price among Tiers 1, 2, 3, and 4. In other words, generics placed on Tiers 1, 2, 3, and 4 have similar average dosage-unit prices and could theoretically be placed on a single formulary tier.
Therefore, all generics could theoretically be divided and placed between only two formulary tiers instead of spreading them across five tiers.231
V.C.v.b. Significance in Cost Difference of Brand Drugs on Different Tiers
In this section, the study examines whether brand drugs, based on their average dosage-unit price, should be assigned to five different formulary tiers or to a smaller number of tiers. The hypothesis was that brand drugs placed on different formulary tiers have significantly different average dosage-unit prices using the same Kruskal–Wallis H test that was applied in the previous analysis of generic drugs.232
The resultant P-value was 2.3E−06, which is less than the standard significance level of 0.05. Therefore, this gives us reason to accept the hypothesis, namely that brand drugs placed on different formulary tiers have significantly different average dosage-unit prices. However, since the test itself does not indicate which pairs of tiers have significant average dosage-unit price differences, another examination tested pairwise comparisons between formulary tiers on brand drugs using the Tukey test in a similar manner to the previous analysis on generics.
From Table 4’s results, the following conclusion can be drawn:
There is evidence to reject the null hypothesis when performing the pairwise comparisons of the average dosage-unit prices between Tiers 1, 2, 3, and 4, except between Tiers 1 and 2. In other words, there is no significant difference in average dosage-unit prices between brand drugs on Tiers 1 and 2; therefore the brand drugs on these tiers can theoretically be placed on the same formulary tier.
Table 4.
Pairwise comparisons of average dosage-unit price for brand drugs—Tukey HSD, FWER = 0.05
| Tier ID | Average difference | Lower | Upper | HypothesisNull | |
|---|---|---|---|---|---|
| Group1 | Group2 | ||||
| 01 | 02 | 6.625 | −0.3824 | 13.6324 | Not rejected |
| 01 | 03 | 15.625 | 8.6176 | 22.6324 | Rejected |
| 01 | 04 | 35.5 | 28.4926 | 42.5074 | Rejected |
| 02 | 03 | 9 | 1.9926 | 16.0074 | Rejected |
| 02 | 04 | 28.875 | 21.8676 | 35.8824 | Rejected |
| 03 | 04 | 19.875 | 12.8676 | 26.8824 | Rejected |
V.C.v.c. Significance in Cost Difference of Generic and Brand drugs on the Same Tiers
Formularies are supposed to be designed so that brand and generic drugs are generally separated onto different tiers, with the exception that specialty generics and specialty brand drugs are supposed to be placed together on Tier 5. Even though Tiers 1 and 2 are primarily designated for generics, and Tiers 3 and 4 are primarily designated for brand drugs,233 the results indicate that a combination of brand drugs and generic drugs appear on all tiers. Thus, the study seeks to examine the cost criterion further to determine whether brand drugs and generic drugs were appropriately placed together when on the same formulary tiers.
The study tests the average dosage-unit price of brand and generic drugs that appear on the same formulary tier in the data. If both the brand and generic categories of drugs have equal or similar average dosage-unit prices, then the observed placement of brand and generic drugs on the same tier is justified. If both categories have differences in average dosage-unit prices that are statistically significant, then they should not be placed together on the same formulary tier. To test these hypotheses, the methodology utilized a two-sided Mann–Whitney U test.234 The results of applying the test in a pairwise manner between brand drugs and generic drugs for each formulary tier are shown below, in Table 5.
Table 5.
Mann–Whitney P-Values between average dosage-unit prices of brand and generic drugs per formulary tier
| Tier ID | P-value | HypothesisNull | |
|---|---|---|---|
| Brand drugs | Generics | ||
| 01 | 01 | 0.19078695 | Not rejected |
| 02 | 02 | 0.00037139 | Rejected |
| 03 | 03 | 0.00040731 | Rejected |
| 04 | 04 | 0.00044551 | Rejected |
| 05 | 05 | 0.00046955 | Rejected |
From Table 5’s results, the following conclusions can be drawn:
There is evidence to reject the null hypotheses for Tiers 2, 3, 4, and 5. In other words, brand drugs and generic drugs should not be placed together on these formulary tiers based on the cost criterion. Brand drugs and generic drugs placed on Tier 1, however, have similar average dosage unit prices and are justified in being placed together.
Although Tier 5 allows the placement of both brand and generic drugs by default, the difference between their average dosage-unit prices is large enough to be noted without doing any inferential statistical tests.
Footnotes
In accordance with the protocols outlined in the Harvard Journal of Law & Technology Open Letter on Ethical Norms, detailed data and methodology information will be made available at Center for Innovation, UC Hastings Law, https://www.uchastings.edu/academics/centers/center-for-innovation/ (accessed Sept. 21, 2020). See Robin Feldman, Mark A. Lemley, Jonathan S. Masur & Arti K. Rai, Open Letter on Ethical Norms in Intellectual Property Scholarship, 29 Harv. J.L. & Tech. 339, 350–2 (2016) (with list of signatories).
See Medicare Payment Advisory Comm’n, Report to the Congress: Medicare Payment Policy, 408–9 (2017) (hereinafter MedPAC Report) (concluding that price increases in brand drugs are overwhelming the effects of patients using lower-cost generics in Medicare); see also Cal. Office of Statewide Health Planning and Development, Prescription Drug Wholesale Acquisition Cost (WAC) Increases (2019) (detailing wholesale price increases of more than 16% for hundreds of drugs between 2017 and Q2 of 2019) (hereinafter State of California Report); see also U.S. Dep’t. of Health & Hum. Serv., Office of Inspector General, OEI-03-15-0080, Increases in Reimbursement for Brand-Name Drugs in Part D, 4 (2018) (hereinafter HHS Inspector General Report) (reporting that even after accounting for rebates, Medicare spending for brand-name drugs increased 62% between 2011 and 2015 (Exhibit 3), despite a decrease in the number of prescriptions (Exhibit 2)).
See, eg, Katie Thomas & Reed Abelson, The $6 Million Drug Claim, NY Times (Aug. 25, 2019) (describing the drug Strensiq, which treats a debilitating genetic disorder with an annual treatment cost of $2 million), https://www.nytimes.com/2019/08/25/health/drug-prices-rare-diseases.html (accessed Oct. 30, 2020); MegTirrell, A U.S. Drugmaker Offers to Cure Rare Blindness for $850,000, CNBC (Jan. 3, 2018) (detailing $850,000 cost for Spark Therapeutics’ cure for a rare form of blindness), www.cnbc.com/2018/01/03/spark-therapeutics-luxturna-gene-therapy-will-cost-about-850000.html (accessed Oct. 30, 2020). Trends in specialty drugs that are widely used by elderly Americans—generally, the most expensive drugs in the healthcare system—are even more stark. The average annual retail price of specialty drugs nearly tripled between 2006 and 2017, approaching $79,000 in 2017. See Stephen W. Schondelmeyer & Leigh Purvis, Trends in Retail Prices of Specialty Prescription Drugs Widely Used by Older Americans: 2017 Year-End Update, AARP Pub. Pol’y Inst. (2019).
See Ctr. for Medicare & Medicaid Serv., Fact sheet, Drug Spending Information Products (2018) [listing the ten drugs with the highest annual price increases from 2012 to 2016 covered by Medicare, including Embrel (arthritis), Humira (arthritis), Januvia (diabetes), Lyrica (nerve pain associated with diabetes), Xarelto (blood clots), and Crestor (high cholesterol)], https://www.cms.gov/newsroom/fact-sheets/drug-spending-information-products-fact-sheet (accessed Oct. 30, 2020); State of California Report, supra note 2 [showing increases for the period of 2017 through mid-2019 of 31% for Lumigan (glaucoma eye drops), 31% for Lexapro (antidepressant), 18% for Wellbutrin (depression), 26% for Diovan HCT (high blood pressure), 45% for nonextended release Effexor (antidepressant), and 43% for Protonix (reflux)].
Kaiser Family Foundation, Public Opinion on Prescription Drugs and their Prices (Oct. 15, 2019), https://www.kff.org/slideshow/public-opinion-on-prescription-drugs-and-their-prices/ (accessed Oct. 30, 2020). Cf. Sara R. Collins, David C. Radley & Jesse C. Baumgartner, Trends in Employer Health Care Coverage, 2008-2018: Higher Costs for Workers and Their Families, The Commonwealth Fund (2019).
See Robin Feldman, Drugs, Money, and Secret Handshakes: The Unstoppable Growth of Prescription Drug Prices 6-7 (2019); see also MedPac Report, supra note 2, at 408–9 (describing increased costs of Medicare); HHS Inspector General Report, supra note 2.
Frank Newport, Top Issues for Voters: Healthcare, Economy, Immigration, Gallup (Nov. 2, 2018), https://news.gallup.com/poll/244367/top-issues-voters-healthcare-economy-immigration.aspx.
Id.
Hannah Brown & Dylan Scott, The Democratic Debates Have Spent 93 Minutes on Health Care, Vox (Oct. 15, 2019), https://www.vox.com/policy-and-politics/2019/10/15/20914415/democratic-debates-health-care-issues; Emmarie Huetteman, Healthcare Stayed Front and Center at the Democratic Debate, Kaiser Health News (Oct. 16, 2019) https://khn.org/news/health-care-stayed-front-and-center-at-democratic-debate/.
See, eg, Copayment Tier Definitions, BlueCross BlueShield of North Carolina, https://www.bluecrossnc.com/understanding-insurance/how-drug-benefits-work/copayment-tier-definitions (accessed Nov. 15, 2019) (chart explaining this health plan’s tiering system, asserting that on the two least-expensive tiers, ‘most are generic’, on the next two tiers ‘most are brand’, and on the most-expensive tier, most are highest-cost specialty drugs); see also Haiden A. Huskamp et al., The Impact of a Three-Tier Formulary on Demand Response for Prescription Drugs, 14 J. Econ. & Mgmt. Strategy 729, 731 (2005) (discussing how formulary-tier structures incentivize preferred drugs by placing preferred drugs on lower tiers, which corresponds to lower copays for the individual consumer); John Jones, The Pros and Cons of Formularies, 6 J. Managed Care Pharm., 203, 203, n. 3 (2000) (article authored by PBM executive explaining that tiering is designed to ‘encourage desirable outcomes while saving considerable costs…. Treatment—not just prescribing—becomes better and more cost-effective. Ultimately, overall healthcare improves’.).
See Charles Ornstein & Katie Thomas, Take the Generic, Patients Are Told. Until They Are Not, NY Times (Aug. 6, 2017) (interviewing one patient whose health plan required an additional $50 per month for using the generic version of the attention-deficit/hyperactivity disorder (ADHD) drug, Adderall, rather than the brand, and another whose plan did not cover the generic at all, only the brand), https://www.nytimes.com/2017/08/06/health/prescription-drugs-brand-name-generic.html (accessed Oct. 30, 2020); see also id. (describing communication to pharmacists at the end of 2016 that some Medicare prescription drug plans with formularies designed by a major PBM would cover only the brand-name version of 12 drugs, some of which had generic competitors); see also Complaint at 2, Grabstald v Walgreens Boots Alliance, Inc., No. 17–05789 (N.D.Ill. Aug. 9, 2017) (alleging that pharmacies charged patients more for certain generics that the patients would have paid without insurance or if they had paid cash); see also Complaint at 1, Pfizer Inc. v Johnson & Johnson and Janssen Biotech, Inc., U.S. Dist. LEXIS 31690, No. 17–4180 (E.D. Pa. 2018) (alleging that hospitals and health plans essentially excluded the lower-priced biosimilar version of the rheumatoid arthritis drug, Remicade); see also Complaint at 6, 21–23, Shire U.S. Inc. v Allergan, Inc., No. 17–7716 (D.N.J. 2017) (alleging bundled-rebate scheme by a pharmaceutical company to induce health plans to exclude or disadvantage the dry eye medication, Restasis).
See Drugs@FDA Glossary of Terms, U. S Food and Drug Administration, https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms (accessed Dec. 16, 2019).
For example, in the World Health Organization’s complex, five-level Anatomical-Therapeutic-Chemical (ATC) classification system, drugs are characterized according to the main therapeutic use or pharmacological class according to the principle of only one ATC code for each route of administration. See Structure and Principles, WHO Collaborating Ctr. for Drug Stats. Methodology, https://www.whocc.no/atc/structure_and_principles/#Therapeu (accessed Sep. 21, 2020).
See infra text accompanying notes 123–127.
See, eg, Eric Sagonowsky, Bring it on, Pharma: Finger-Pointing at Insurers Provokes AHIP Tweetstorm, FiercePharma (Sept. 28, 2017), www.fiercepharma.com/pharma/insurance-lobby-phrma-duke-it-out-over-healthcare-costs. See, eg, Berkeley Lovelace Jr. & Ashley Turner, CVS, Cigna, Humana Blame Big Pharma at Senate Hearing for Skyrocketing US Drug Prices, CNBC (Apr. 9, 2019). (‘A major factor contributing to the increase in drug spending is the list price of prescription drugs. Drug manufacturers alone set the list price of prescription drugs’, said William Fleming, President of Health-Care Services at Humana). See also Philip Moeller, Drug Companies Try Shifting Blame for High Prices Under Lawmakers’ Scrutiny, Public Broadcasting Service, (Apr. 11, 2019), https://www.pbs.org/newshour/economy/making-sense/column-drug-companies-try-shifting-blame-for-high-prices-under-lawmakers-scrutiny (showing drug companies directing blame at PBMs for their role in increasing drug prices); see also Shira Stein, Insurers to Disclose Drug Copays, Treatment Terms Under Proposal, Bloomberg Law (Nov. 15, 2019) https://news.bloomberglaw.com/health-law-and-business/insurers-to-disclose-drug-copays-treatment-terms-under-proposal.
Feldman, supra note 6. See generally Steven Brill, America’s Bitter Pill: Money, Politics, Backroom Deals, and the Fight to Fix Our Healthcare System (2015) (explaining how hospitals can play a role in increasing prices for consumers even for standard care).
See Neeraj Sood, Understanding Competition in Prescription Drug Markets: Entry and Supply Chain Dynamics, Fed. Trade Comm’n Workshop Slides 105, 107 (2017), https://www.ftc.gov/system/files/documents/public_events/1255653/understanding_competition_in_prescription_drug_markets_workshop_slides_11-8-17.pdf (accessed Oct. 30, 2020) (noting that Jenny Bryan, Senior Vice President at PhRMA, says brand drug price increased only 3.5% when accounting for rebates).
See Michael Hiltzik, How ‘Price-Cutting’ Middlemen are Making Crucial Drugs Vastly More Expensive, LA Times (2017), https://www.latimes.com/business/hiltzik/la-fi-hiltzik-pbm-drugs-20170611-story.html (accessed Oct. 30, 2020) (noting that size of rebates are guarded as trade secrets in contracts).
Robin Feldman & Charles Tait Graves, Naked Price and Pharmaceutical Trade Secret Overreach, 22 Yale J.L. & Tech. 61, 74 (2020) (noting that industry claims that naked price constitutes protectable trade secrets de secret law).
Id. at 72.
Id. at 74.
See Feldman, supra note 6.
See Mark Meador, Squeezing the Middleman: Ending the Underhanded Dealing in the Pharmaceutical Benefit Management Industry through Regulation, 20 Ann. Health L. 77, 82 (2011) (noting that PBMs take advantage of the price range in various price lists for generic drugs, negotiating with manufacturers for a lower price and setting reimbursement rates with plan sponsors using a higher list price, to maximize the spread); cf. Fiona Scott Morton & Lysle T. Boller, Enabling Competition in the Pharmaceutical Markets, Working Paper 30, Hutchings Ctr. on Fiscal & Monetary Pol’y (2017), https://www.brookings.edu/wp-content/uploads/2017/05/wp30_scottmorton_competitioninpharma1.pdf (accessed Oct. 30, 2020).
See MedPAC Report, supra note 2; Norman Augustine, Guru Madhavan & Sharyl Nass, Making Medicines Affordable: A National Imperative, Nat’l Academies of Sci., Eng’g, and Med. 76 (2018) (hereinafter NAS Report).
See infra Figure 1 and text accompanying notes 113–114.
Eg 84 FR 2340 (Proposed Jan. 31, 2019; rejected July 10, 2019) (to be codified at 42 C.F.R 1001); See Robin Feldman & Evan Frondorf, Drug Wars: How Big Pharma Raises Prices and Keeps Generics Off the Market (2017); see also David Brady & Daniel Kessler, Why is Health Reform so Difficult?, 35 J. Health Pol., Pol’y & Law (2010); See also Anders Asland, Opinion: US Health care is an Ongoing Miserable Failure, The Hill (Jan. 5, 2019), https://thehill.com/opinion/healthcare/423865-us-health-care-is-an-ongoing-miserable-failure.
Donald E. Francke, Clifton J. Latiolais, Gloria N. Francke & Norman Ho, The Formulary System: Brief History and 1960s Perspective: Excerpted from Mirror to Hospital Pharmacy, 43 Am. J. Hosp. Pharmacy 2838, 2838 (1986).
Id.
Malcolm J. Pearce & Evan J. Begg, A Review of Limited Lists and Formularies, 1 PharmacoEconomics 191, 192 (1992) [citing United States Pharmacopoeia Convention Inc., History of the Pharmacopeia of the United States xli-xliii (21st ed. 1984)].
Id. The transition may have occurred in 1936, when ‘pharmacy and therapeutic committees (P&T) were first established in American Hospitals to consider all matters related to the use of drugs in hospitals. Such committees became an integral part of the formation and management of hospital formularies’. P&T are responsible for managing the formulary system and are composed of active healthcare providers including nurses, doctors, and pharmacists. They manage formularies with the intent to improve safety of drug therapy and therapeutic outcomes, among other clinical concerns. Linda S. Tyler et al., ASHP Guidelines on the Pharmacy and Therapeutics Committee and the Formulary System, 65 Am. J. Health-Syst. Pharm. 1272, 1273–4, 223 (2008).
Robin Walsh, A History of the Pharmaceutical Industry, Pharmaphorum (Oct. 1, 2010), https://pharmaphorum.com/articles/a_history_of_the_pharmaceutical_industry/.
See Robert B. Goldberg, Managing the Pharmacy Benefit: The Formulary System, 3 J. Managed Care Pharmacy & Spec. Pharmacy 565, 565 (1997).
See Tyler et al., supra note 30, at 1275. The Joint Commission is a nonprofit organization that certifies more than 22,000 US healthcare organizations and programs. About Us, The Joint Commission, https://www.jointcommission.org/about_us/about_the_joint_commission_main.aspx (accessed Nov. 15, 2019).
Edward Berkowitz, Medicare and Medicaid: The Past as Prologue, 27 Health Care Fin. Rev. 11, 14 (2005).
Id. [citing Arthur J. Altmeyer, The Formative Years of Social Security (1966)].
Berkowitz, supra note 34, at 14.
See Gary Smith et al., Office of the Assistant Sec’y for Planning & Evaluation, U.S. Dep’t. of Health & Human Servs., Using Medicaid to Support Working Age Adults with Serious Mental Illnesses in the Community: A Handbook 19 (2005). See also Social Security Amendments of 1965, Pub. L. No. 89-97, 79 Stat. 286 (1965).
See Smith et al., supra note 37.
See Healthcare Crisis: Healthcare Timeline, Public Broadcasting Service, https://www.pbs.org/healthcarecrisis/history.htm (accessed Nov. 15, 2019).
Goldberg, supra note 32, at 565. Fee-for-service health insurance allows patients to choose whatever provider and health service they would like. Patients pay for everything out of pocket and then file the paperwork for reimbursement with their insurers; this makes fee-for-service generally the most expensive (and least common) insurance offered today. In contrast, managed-care health insurance limits coverage to providers and hospitals in the insurer’s network (‘in-network’). In doing so, costs are decreased in general, and the savings are passed to the patient in the form of lower deductibles, premiums, and copays. See Jeremy Vohwinkle, Types of Individual Health Insurance Policies: HMOs, PPOs, and FFS, The Balance (Aug. 28, 2019), https://www.thebalance.com/health-insurance-ppo-s-hmo-s-1289671.
Goldberg, supra note 32, at 565–66.
Goldberg, supra note 32, at 566.
Id.
See Drug Price Competition and Patent Term Restoration Act, Pub. L. No. 98–417, 98 Stat. 1585 (1984).
See Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Pub. L. No. 108–173, 117 Stat. 2067 (2003).
See Cole Werble, Health Policy Brief: Formularies, Health Affairs (Sept. 14, 2017), https://www.healthaffairs.org/do/10.1377/hpb20171409.000177/full/ (stating that ‘the emergence of formularies in the late 1980s was driven by a structural change in the drug industry—the development of several multibrand categories in which up to a half-dozen related, but not interchangeable, brands existed, with each commanding a similar price’.)
See id. (stating ‘formularies gained prominence as a tool for purchasers to use in selecting among these treatment options, with purchasers often obtaining rebates from drug manufacturers in exchange for preferred formulary placement’.).
See Thomas R. Oliver, Philip R. Lee & Helene L. Lipton, A Political History of Medicare and Prescription Drug Coverage, 82 Milbank Q. 283, 305 (2004) [citing Mary A. Laschober, Michelle Kitchman, Patricia Neuman & Allison A. Strabic, Trends in Medicare Supplemental Insurance and Prescription Drug Coverage, 1996-1999, Health Aff. (Feb. 27, 2002)] (stating that ‘in 1999, … millions of [Medicare] beneficiaries [were left] to shop elsewhere for prescription drug benefits—if they could afford them’). The Medicare Modernization Act also led to increased government control of formularies in the context of health plans that provide prescription drug coverage within the Medicare system. Following full implementation of the Act in 2006, the federal agency charged with managing Medicare, the CMS, developed additional rules for prescription drug plans within the Medicare system regarding the creation and management of formularies, including that a P&T ‘will approve inclusion or exclusion of the therapeutic classes in the formulary on an annual basis’. See Ctrs. for Medicare & Medicaid Servs., Medicare Modernization Act 2007 Final Guidelines—Formularies 2 (2007) (hereinafter MMA Final Guidelines on Formularies); 42 C.F.R.§ 423.120(b)(1). Together, the legislation and the regulations laid the groundwork for the modern formulary in the Medicare system, also providing pathways for reforms.
Allison Dabbs Garrett & Robert Garis, Leveling the Playing Field in the Pharmacy Benefit Management Industry, 42 Val. U. L. Rev. 33, 34 (2007).
Robin Feldman, Why Prescription Drug Prices Have Skyrocketed, Wash. Post (Nov. 26, 2018), https://www.washingtonpost.com/outlook/2018/11/26/why-prescription-drug-prices-have-skyrocketed/.
Werble, supra note 46.
Kaiser Family Found., 2017 Employer Health Benefits Survey 98 (2017) (hereinafter 2017 Employer Health Benefits Survey) (explaining the difference between various employee cost-sharing structures including coinsurance and copayment), http://files.kff.org/attachment/Report-Employer-Health-Benefits-Annual-Survey-2017 (accessed Oct. 30, 2020).
See Copayment Tier Definitions, supra note 10.
Juliette Cubanski, Wyatt Koma & Tricia Neuman, The Out of Pocket Cost Burden for Specialty Drugs in Medicare Part D in 2019, Kaiser Family Foundation (Feb. 1, 2019) https://www.kff.org/medicare/issue-brief/the-out-of-pocket-cost-burden-for-specialty-drugs-in-medicare-part-d-in-2019/.
See Werble, supra note 46.
Id.
See supra notes 49–51 and accompanying text.
See generally Feldman, supra note 6 (describing perverse incentives in the PBM rebate system).
Office of Inspector Gen., U.S. Dep’t. of Health & HUM. Servs., Medicaid Drug Price Comparisons: Average Manufacturer Price to Published Prices I (2005) (hereinafter HHS Report) (noting that WAC is an estimate of the pharmacy’s acquisition cost through commercially available reports).
Id. at 2 (defining MAC as the upper limit that state Medicaid programs will reimburse pharmacies for selected drugs).
Id. at 2 (explaining that AWP ‘is often considered a price for wholesalers to charge retailers’ and is based off of commercially available reports).
Id. at 3 (explaining that ASP is used by Medicare to determine reimbursement for drugs covered under Medicare Part B).
Id. at 2 (noting EAC is the ‘best’ estimate of the cost providers paid for the drug). EAC is the benchmark determining how much state Medicaid programs will reimburse pharmacies for their drugs (in addition to a dispensing fee or a pharmacy’s general charge to the consumer).
HHS Report, supra note 59, at 4 [defining average manufacturer price (AMP) as the ‘average price paid by wholesalers for drugs distributed to the retail class of trade, net of customary prompt pay discounts’]. The definition of AMP has changed under the ACA (and implemented by a CMS final rule in 2012, effective on Apr. 1, 2016). The previous definition defined it as the average price paid to a manufacturer by a pharmacy class of trade, which included mail order pharmacies and specialty pharmacies. Nontraditional pharmacies generally purchase drugs at higher costs, which means there is a lower rebate for Medicaid (when Medicaid reimburses the pharmacies and the rebates go to Medicaid). The change excluded mail order and specialty pharmacies from the definition of ‘pharmacy class of trade’ increasing rebates to Medicaid. See Office of Inspector Gen., Dep’t. of U.S. Health & Human Servs., Average Manufacturer Price Determinations by Selected Drug Manufacturers Generally Were Consistent With Federal Requirements i–ii (2014).
See 42 U.S.C. § 1395w-3aI(6)(B) (defining WAC as ‘the manufacturer’s list price for the drug or biological to wholesalers or direct purchasers in the USA, not including prompt pay or other discounts, rebates, or reductions in price, for the most recent month for which the information is available, as reported in wholesale price guides or other publications of drug or biological pricing data’). Within the alphabet soup of listings, this figure is charmingly named WAC for ‘Wholesale Acquisition Cost’.
Commercial publishers of AWP data include Truven Health Analytics, Red Book, Medi-Span, and First Databank. AWP technically stands for ‘Average Wholesale Price’ and is colloquially referred to as ‘Ain’t What’s Paid’.
See HHS Report, supra note 59.
For an extensive examination of the rebate system and its effects on competition, see Feldman, supra note 6.
See generally Christy A. Rentmeester & Robert I. Garis, Rebates and Spreads: Pharmacy Benefit Management Practices and Corporate Citizenship, 33 J. Health Pol. Pol’y & L. 943, 948–50, (2008).
See Feldman, supra note 6, at 18.
For example, 30% of all employer-sponsored health insurance plans are structured in this manner. See NAS Report, supra note 24.
See MedPAC Report, supra note 2, at 408–9.
See Stacie B. Dusetzina & Peter B. Bach, Prescription Drugs—List Price, Net Price, and the Rebate Caught in the Middle, 321 JAMA 1563, 1563 (2019) (noting that those without insurance pay the full list price).
See MedPac Report, supra note 2, at 404.
See infra Figure 1.
For a description of a practice known as clawbacks, in which PBMs collect more from the pharmacist than the health plan’s contribution, creating the potential for the pharmacist to lose money on the sale, see Feldman, supra note 6, at 49–50.
See Feldman & Graves, supra note 19.
See Feldman & Graves, supra note 19; see also Annemarie Bridy, Trade Secret Prices and High-Tech Devices: How Medical Device Manufacturers are Seeking to Sustain Profits by Propertizing Prices, 17 Tex. Intell. Prop. L.J. 187 (2009).
See Feldman, supra note 6, at 14.
See Feldman, supra note 6, at 14.
For an extended discussion of the PBM market and its effect on prices, see Feldman, supra note 6, at 13–45.
See Thomas G. Krattenmaker & Steven C. Salop, Anticompetitive Exclusion: Raising Rivals’ Costs To Achieve Power over Price, 96 Yale L.J. 209 (1986); see also Feldman, supra note 6, at 38 (comparing the strategy of raising prices at the tail end of a monopoly period to ‘raising rivals’ costs’).
The beer example, along with a full discussion of the implications of volume rebating in the pharmaceutical industry, was first presented by the author in Feldman, supra note 6, at 22. See also Robin Feldman, Defensive Leveraging in Antitrust, 87 Geo. L.J. 2079 (1999) (hereinafter Defensive Leveraging) (discussing volume rebates in the context of the cephalosporin market).
See Defensive Leveraging, supra note 83. For a discussion of the fact that holding a patent does not guarantee a monopoly, see Robin Feldman, Rethinking Patent Law 65–67 (2012). For a discussion of how increased costs from improper tiering might interact with the cost of health insurance premiums, see infra notes 134–139 and accompanying text.
Complaint at 1, Pfizer, Inc. v. Johnson & Johnson and Janssen Biotech, Inc., 2018 U.S. Dist. LEXIS 31690 (E.D. Pa. 2018) (No. 17–4180).
Class Action Complaint at 3, Castro, M.D., P.A. v. Sanofi Pasteur Inc., LEXIS 96001 (D.N.J. 2012) (No. 11–7178).
See Complaint at 6, 21–23, Shire U.S., Inc. v. Allergan, Inc., No. 17–7716 (D.N.J. 2017) (complaint alleging that according to one Medicare plan administrator, the new competitor could give its drug away for free and the numbers still would not work).
See Ornstein & Thomas, supra note 11 (reporting on insurance plans that punished patients for filling their prescriptions with the generic version of attention-deficit, stroke prevention, pain-relieving, and cholesterol drugs); see also Posting of Dr Rosemary Jane Jolly, rjj14@psu.edu, to l-new-faculty-net@lists.psu.edu (Jan. 7, 2020) (faculty member at Pennsylvania State University, speaking from her experience on the Faculty Senate and Faculty Advisory Committee to the President, explaining in an email to faculty that the university’s employer-provided insurance plan was forcing faculty members to use brand drugs when cheaper generics were available) (on file with author).
See Ornstein & Thomas, supra note 11.
See, eg, Letter from Marcie McClintic Coates, Head of Global Policy, Mylan, to Aaron Zajic, Office of Inspector General, U.S. Dep’t of Health & Human Servs. (Apr. 8, 2019), https://www.regulations.gov/document?D=HHSIG-2019-0001-19845 (hereinafter Letter from Mylan); see also Press Release, Avalere Health, Seniors Pay More for Medicare Part D Generics Despite Stable Prices (May 22, 2018), https://avalere.com/press-releases/seniors-pay-more-for-generics-in-medicare-prescription-drug-plans-despite-stable-prices) (healthcare consulting firm press release asserting that due to improper formulary placement, seniors in Medicare Part D overpay for generic drugs and that the 53% decrease in the number of generic drugs placed on the lowest tier between 2011 and 2015 cost patients an additional $6.2 billion in 2015—a 93% increase relative to 2011). https://avalere.com/press-releases/seniors-pay-more-for-generics-in-medicare-prescription-drug-plans-despite-stable-prices (accessed Oct. 30, 2020).
Douglas Jacobs & Benjamin Sommers, Using Drugs to Discriminate—Adverse Selection in the Insurance Marketplace, 372 New Eng. J. Med. 399 (2015).
Although not related to inappropriate tier placement, other literature documents issues related to challenges in providing mental health drugs for patients intrinsic to formulary design as well as concerns with formularies in general. See, eg, Dominic Hodgkin, Cindy Parks Thomas, Linda Simoni-Wastila, Grant A. Ritter & Sue Lee, The Effect of a Three-Tier Formulary on Antidepressant Utilization and Expenditures, 11 J. Mental Health Pol’y & Econ. 67 (2008).
See, eg, Martin Andersen, Constraints on Formulary Design Under the Affordable Care Act, 26 Health Econ. 160 (2017).
45 C.F.R. § 156.122. See Govind Persad, Priority-Setting, Cost-Effectiveness, and the Affordable Care Act, 41 Am. J.L. & Med. 119 (2015).
See Andersen, supra note 93.
Although administrative complaints are treated as confidential by the HHS, one of the complaining groups posted a copy on its website. See Jane Perkins and Wayne Turner, NHELP and The AIDS Institute Complaint to HHS re HIV/AIDS Discrimination by FL, The Nat’l Health Law Program (May 28, 2014), https://healthlaw.org/resource/nhelp-and-the-aids-institute-complaint-to-hhs-re-hiv-aids-discrimination-by-fl/; see also Katie Thomas, Bias Claims for Insurers in Coverage of HIV, N.Y. Times, (May 29, 2014), https://www.nytimes.com/2014/05/30/business/four-insurers-accused-of-discriminating-against-people-with-hiv.html.
Natalie Kean, Fighting Against Discriminatory Health Insurance Practices: Successful Advocacy in Florida, in U.S. Conference on AIDS (Sept. 16, 2016), http://theaidsinstitute.org/sites/default/files/attachments/Kean%20-%20USCA%202016%20ACA%20Disc.pdf.
Patrick Saunders, UPDATE: HIV meds to remain on preferred status with Medicaid, Georgia Voice (Aug. 25, 2015), https://thegavoice.com/news/atlanta/georgia-senator-lgbt-groups-criticize-health-department-on-hiv-meds-change/.
Liz Whyte, Joe Yerardi & Alison Kodjak, Investigation: Patients’ Drug Options Under Medicaid Heavily Influenced By Drugmakers, NPR, (July 18, 2018), https://www.npr.org/sections/health-shots/2018/07/18/629575118/medicaid-under-the-influence-how-drugmakers-sway-medication-options-for-patients.
The study used Research Identifiable Files, meaning that files contain beneficiary level protected health information. See Lori Siedelman, Differences between RIF, LDS, and PUF Data Files, Research Data Assistance Ctr. (Aug. 10, 2016), https://www.resdac.org/articles/differences-between-rif-lds-and-puf-data-files. The work was reviewed and approved by the Institutional Review Board in compliance with the requirements of the Common Rule and the Health Insurance Portability and Accountability Act. Health Insurance Portability and Accountability Act, Pub. L. No. 104–191, 110 Stat. 1936 (1996).
Feldman, supra note 6, at 92.
See infra Appendix A5 for more detailed information about the requested data and cohort specifications. For a discussion of differences between Medicare and private plans, see infra text accompanying notes 132–140.
See infra Section 4 for a detailed discussion of suggested reforms.
See supra notes 68–79 and accompanying text.
Comparing brand and generic drug placement on formulary tiers required categorizing all the drugs in the data, represented with NDC, as either brand or generic. One would assume that categorizing drugs as brand or generic would be simple enough, but there are no hard and fast rules. There is no consistent, universal method of brand or generic drug classification (or even within a single drug information database). Thus, the methodology categorized drugs as brand or generic using three different sources in the following order: the original CMS data, the National Library of Medicine’s RxNorm database, and finally, Cerner Multum’s commercial drug database. The National Library of Medicine is a medical library and an institute within the National Institutes of Health, operated by the federal government. RxNorm, NIH Nat’l Library of Med., https://www.nlm.nih.gov/research/umls/rxnorm/index.html (accessed Feb. 3, 2020). The study also purchased access to a drug database managed by Cerner Multum, a medical information company. The study used Cerner Multum’s database last to categorize drugs because it was not a governmental source. Drug Database, Cerner, https://www.cerner.com/solutions/drug-database (accessed Nov. 15, 2019).
Center for Innovation, UC Hastings Law, https://www.uchastings.edu/academics/centers/center-for-innovati on/ (accessed Oct. 30, 2020). See Robin Feldman, Mark A. Lemley, Jonathan S. Masur, & Arti K. Rai, Open Letter on Ethical Norms in Intellectual Property Scholarship, 29 Harv. J.L.&Tech. 339, 350–2 (2016) (with list of signatories).
See Data Dictionaries infra note 204; Part D Formulary File, Research Data Assistance Center (accessed Aug. 17, 2020), https://www.resdac.org/cms-data/files/part-d-formulary-file.
See, eg, 2017 Employer Health Benefits Survey, supra note 52.
See, eg, Drug Price Competition and Patent Term Restoration Act, Pub. L. No. 98–417, 98 Stat. 1585 (1984). Informally known as the Hatch–Waxman Act, this Act incentivizes the rapid development and entry of generic drugs once brand drug patents expire.
See infra Figure 6.
Expenditures for patients also include upfront premiums. See infra text accompanying notes 136–137 for a discussion of the argument that rising drug costs are not harming patient premium payments.
See infra Figure 1 (showing that average dosage-unit price for generic drugs stays roughly stable). Generic companies generally do not engage in rebating; the cost to the insurance company is the list price. See Ctrs. for Medicare & Medicaid Servs., 2011 Medicare Trustees Report, at 183 (hereinafter 2011 Medicare Trustees Report).
See 2011 Medicare Trustees Report, supra note 112, at 183 (noting that generic drugs generally do not provide rebates). For a discussion of the rebate methodology and calculations, see infra Appendix A5.
See supra text accompanying notes 69–79.
See 2011 Medicare Trustees Report, supra note 112, at 183 (noting that generic prescriptions generally do not receive rebates).
See, eg, Copayment Tier Definitions, supra note 10; see also Huskamp et al., supra note 10; Jones, supra note 10.
See infra Figure 8. In contrast, brand drugs on Tier 1 remained relatively constant throughout this time period. See Appendix A4 (showing distribution of brand drugs on five-tier formularies). More broadly, the percentage of brand drugs on formulary Tiers 3, 4, and 5 increased, particularly on Tiers 4 and 5, but there was not a noticeable change shift away from Tier 1, as there was with generics. There was also a noticeable decrease in the Tier 2 percentage share of brand drugs, from 28% to 7%. Taken together, the findings show that while both brand and generic drugs are increasing placed on higher tiers, generics specifically are being shifted away from the least-expensive tier, whereas brand drugs are remaining relatively stable on that tier.
See supra Figure 1.
See supra note 10 and accompanying text.
See Ctrs. for Medicare & Medicaid Servs, supra note 112, at 183.
Tier 1 drugs offer the lowest copayment and are often generic versions of brand-name drugs; copayments rise with higher tiers.
2017 Employer Health Benefits Survey, supra note 52. One should note that the Employer Health Benefit Study reflects only co-pays, not co-insurance, although one would expect any co-insurance portions of the total payment to be low for generic drugs, given that generic prices are relatively low, ranging from $3 to $4. See supra Figure 1.
See supra Figure 1 (comparing average dosage-unit prices for generics on different tiers).
2017 Employer Health Benefits Survey, supra note 52.
See supra note 10 and accompanying text.
Note: the study used a Mann–Whitney U Test to compare net brand and generic average dosage-unit prices.
See, eg, Feldman & Frondorf, supra note 26, at 34 and sources cited within (discussing the tactics and strategies brand companies use to delay or prevent generic entry into the market); see Feldman, supra note 6, at 18–26 and sources cited within (discussing the use of volume games by brand companies in collaboration with PBMs to block generic entry into the market); Michael Carrier & Carl Minniti, Citizen Petitions: Long, Late-Filed, and At-Last Denied, 66 Am. L. Rev 305 (Aug. 2016).
See Feldman, supra note 6.
Therapeutic competitor is distinct from phrases such as ‘therapeutically equivalent’ or ‘directly substitutable’. To be therapeutically equivalent under the FDA’s definition, the main active ingredients must be the same, as well as the dosage form, route of administration, and strength. See Drugs@FDA Glossary of Terms, U. S Food and Drug Administration (accessed Dec.16, 2019), https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms. The same general characteristics must be true for pharmacists in a particular state to substitute a generic drug for a brand.
The use of active ingredients provided practical advantages, as well. FDA data are organized by ANDA and NDA, whereas the CMS data are organized by NDC code. ANDA refers to Abbreviated New Drug Application, which is the application for a generic to receive product approval from the FDA, placing it in the Orange Book once approved. Abbreviated New Drug Application (ANDA), U. S Food and Drug Administration (accessed Dec. 16, 2019), https://www.fda.gov/drugs/types-applications/abbreviated-new-drug-application-anda. NDC code refers to National Drug Code Directory, which is a national directory of all drugs ‘manufactured, prepared, propagated, compounded, or processed’, published by the FDA. National Drug Code Directory, U. S Food and Drug Administration (accessed Dec. 16, 2019), https://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory. Without a viable way of mapping NDC codes to ANDA or NDA codes, it is difficult to pair up brand and generic drugs, which would make the analysis far more challenging.
See supra text accompanying notes 69–79 and Figure 1.
See supra text accompanying notes 13–14. One should note that in the relevant years, between 2010 and 2017, the majority of irrational tiering involved brand drugs with an active ingredient competitor placed on the same formulary tier as their generic counterparts; only a small percentage of generic drugs fell on a more-expensive tier.
See Data Dictionaries infra note 205; Part D Formulary File, Research Data Assistance Center (accessed Aug. 17, 2020), https://www.resdac.org/cms-data/files/part-d-formulary-file.
The amount that the government subsidizes is the Low-Income Subsidy; the government also subsidizes Medicare premiums through a complex calculation. See The Ctrs. For Medicare & Medicaid Servs., Guidance to States on the Low-Income Subsidy (2009).
In order to keep this estimate conserve, the study weighs the number of Medicare enrollments to reflect only the count of five-tier enrollees. See infra Appendix A3.
See infra text accompanying notes 123–127
Charles Roehrig, The Impact of Prescription Drug Rebates on Health Plans and Consumers, Altarum 10 (Apr. 2018), https://altarum.org/sites/default/files/Altarum-Prescription-Drug-Rebate-Report_April-2018.pdf.
See id.
See Feldman, supra note 6, at 53–55.
Michelle Andrews, Why Cannot Medicare Patients Use Drugmakers’ Discount Coupons? NPR (May 9, 2018), https://www.npr.org/sections/health-shots/2018/05/09/609150868/why-cant-medicare-patients-use-drugmakers-discount-coupons; Emma Ryan & Emily Fitts, The Hidden Costs of Discount Cards: Understanding Copay Accumulator Adjustment, diaTribe (Mar. 22, 2019) https://diatribe.org/hidden-costs-discount-cards-understanding-copay-accumulator-adjustment.
Cf. Ctrs. for Medicaid & Medicare Servs., Fact sheet, Medicare Part D: Direct and Indirect Remuneration (Jan. 19, 2017), https://www.cms.gov/newsroom/fact-sheets/medicare-part-d-direct-and-indirect-remuneration-dir (stating that ‘Part D premiums … have grown only modestly in comparison to gross drug costs’).
Cf. MedPac Report, supra note 2 (stating that ‘Part D plan sponsors generally use rebates to offset benefit costs [ie lower premiums for all plan enrollees) rather than to lower (point of sale) prices and cost sharing’]; Caitlin Owens, White House Kills Major Drug Pricing Proposal, Axios (Jul. 10, 2019), https://www.axios.com/trump-drug-prices-plan-pharma-ec527a14-0287-492b-937d-a7144c47b734.html [stating that ‘critics of the (Trump Administration anti-kickback rule) proposal argued it did nothing to require drug makers to lower their prices and would’ve cost taxpayers hundreds of billions of dollars’); Bob Herman, All Eyes Are on the Drug Pricing Middlemen, Axios (Apr. 9, 2019), https://www.axios.com/all-eyes-drug-pricing-middlemen-pbms-rebate-rule-senate-79f57f2e-ad63-43e1-bead-e68f48472020.html (stating that ‘experts at the Medicare Payment Advisory Committee and the Pew Charitable Trusts both wrote … the proposed regulation “is unlikely to reduce drug prices” and would create new windfalls for drugmakers’).
Ctrs. for Medicaid & Medicare Serv., Fact sheet, Medicare Part D: Direct and Indirect Remuneration (Jan. 19, 2017), www.cms.gov/newsroom/fact-sheets/medicare-part-d-direct-and-indirect-remuneration-dir (explaining the relationship between lower plan liability and higher government costs and noting in particular that the substantial rise experienced in the government’s costs results in part because ‘gross drug cost growth is concentrated in the catastrophic phase … where Medicare covers 80% of drug costs’).
See Feldman, supra note 6.
See supra text accompanying notes 77–79.
These are known as Medicare Advantage plans. Medicare Advantage Plans, Medicare (accessed Aug. 18 2020), https://www.medicare.gov/sign-up-change-plans/types-of-medicare-health-plans/medicare-advantage-plans
Even if there are factors other than cost such as clinical effectiveness that determine drug placement, the cumulative effect of these factors is unable to account for the disparity that is seen between insurers’ claims that cost is the primary criterion for placement and the contradicting reality that features generic and brand drugs with significantly different costs occupying the same tiers.
See supra Section II.B.
For other proposed solutions to issues with the formulary tier system, see Gerard Anderson, Thomas Cordeiro, Mariana Socal & Lauren Vela, Removing Waste from Drug Formularies, John Hopkins Drug Access and Affordability Initiative, the Pacific Business Group on Health and Integrity Pharmaceutical Advisors (Oct. 2019) (proposing an approach to formulary management whereby the plan sponsors remove drugs that are more expensive and do not provide greater clinical value compared to other similar drugs). For prior literature on the formulary tier system, see eg Gordon D. Schiff et al., A Prescription for Improving Drug Formulary Decision Making, 9 PLOS Med. 1 (2012); Bryan L. Walser et al., Do Open Formularies Increase Access to Clinically Useful Drugs?, 15 Health Affairs 95 (1996); Raymond J. Seigfried et al., Deciding Which Drugs Get Onto the Formulary: A Value-Based Approach, 16 Value in Health 901 (2013).
See Avalere Health, supra note 90. For a discussion of entirely different approaches to cost-sharing in health insurance, see Christopher T. Robertson, Exposed: Why Our Health Insurance is Incomplete and What Can Be Done About it (Harv. 2019).
Andreas Laupacis, Geoffrey Anderson & Bernie O’Brien, Drug Policy: Making Effective Drugs Available Without Bankrupting the Healthcare System, 3 Healthcare Papers 12 (Feb. 2002).
Eg Letter from Mylan, supra note 90.
See Ctrs. for Medicare and Medicaid Servs., Advance Notice of Methodological Changes for Calendar Year (CY) 2020 for Medicare Advantage (MA) Capitation Rates, Part C and Part D Payment Policies and 2020 Draft Call Letter 180 (2019).
See Feldman & Frondorf, supra note 26. Along the same lines, one medical information company explained in a call with the author that a brand manufacturer had requested to have its drug not designated as a brand so that health insurance plans would cover it as generic.
Cf. Feldman, supra note 84 (presenting the bargain theory of patents and arguing that lack of a shared conception for things that are new, limitations in language, and the problem of fixation in time make it impossible for a patent to definitively identify the rights that have been granted, no matter what claiming approach society chooses).
See supra notes 36–40 and accompanying text; see also Copayment Tier Definitions, supra note 10 (citing BlueShield materials describing tiers in terms of drug cost).
See discussion supra Section 2.3.
See discussion supra Section 2.3.
See supra notes 23–24 and accompanying text.
Fraud and Abuse; Removal of Safe Harbor Protection for Rebates Involving Prescription Pharmaceuticals and Creation of New Safe Harbor Protection for Certain Point-of-Sale Reductions in Price on Prescription Pharmaceuticals and Certain Pharmacy Benefit Manager Service Fees, 84 Fed. Reg. 2340 (Feb. 6, 2019).
Id.
Congressional Budget Office, Incorporating the Effects of the Proposed Rule on Safe Harbors for Pharmaceutical Rebates in CBO’s Budget Projections—Supplemental Material for Updated Budget Projections: 2019 to 2029 1 (2019).
Id.
Id.
See Caitlin Owens, White House kills major drug pricing proposal, Axios (July 10, 2019), https://www.axios.com/trump-drug-prices-plan-pharma-ec527a14-0287-492b-937d-a7144c47b734.html.
Congressional Budget Office, supra note 162, at 2.
See Congressional Budget Office, supra note 162.
See Yasmeen Abutaleb, Amy Goldstein & Ashley Parker, Trump Kills Key Drug Price Proposal He Once Embraced, Washington Post (July 11, 2019), https://www.washingtonpost.com/business/economy/white-house-kills-key-drug-pricing-rule-to-eliminate-hidden-rebates/2019/07/11/ff595192-a3de-11e9-bd56-eac6bb02d01d_story.html.
See generally, Robin Feldman, Perverse Incentives: Why Everyone Prefers High Drug Prices—Except for Those Who Pay the Bills, 57 Harv. J. on. Legis. 303 (describing perverse incentives of the rebate system in which raising prices allows room for rebates and other payments that disadvantage competitors) https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3162432 (accessed Oct. 30, 2020).
Implementation of this solution may require a transition period for the market. While the market readjusts, prices may be higher for a period of time while market actors acclimate to the new incentive structure. However, one would anticipate that list prices will slowly and steadily be reduced to secure drug placements on more preferable tiers.
See Feldman & Graves, supra note 19.
See Ctrs. For Medicare & Medicaid Servs., About CMS, https://www.cms.gov/About-CMS/About-CMS (accessed Aug. 19, 2020).
See Ctrs. for Medicare & Medicaid Servs., Medicare Prescription Drug Benefit Manual: Chapter 6—Part D Drugs and Formulary Requirements § 30.2 (2018). (hereinafter Medicare PDBM Chapter 6 § 30.2); MMA Final Guidelines on Formularies, supra note 48. See also HPMS Guidance History, Ctrs. for Medicare & Medicaid Servs., https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/HPMS-Guidance-History (accessed Oct. 24, 2012) (listing past Medicare prescription drug guidance materials distributed to plans by CMS’ Health Plan Management System).
Medicare PDBM Chapter 6 § 30.2, supra note 173.
Medicare PDBM Chapter 6 § 30.2, supra note 173.
Medicare PDBM Chapter 6 § 30.2, supra note 173.
MMA Final Guidelines on Formularies, supra note 48.
HHS Report, supra note 59, at 4.
See supra text accompanying notes 62–64 (describing some failure of drug companies to report and efforts to increase enforcement under the regulations).
See Office of Inspector General, Drug Manufactures’ Noncompliance with Average Manufacturers Price Reporting Requirements, Dep’t Health & Human Servs. i–iii (Sept. 2010) https://oig.hhs.gov/oei/reports/oei-03-09-00060.pdf.
See id.
See id. at 4.
What is Information Asymmetry, The Economist, Sept. 5, 2016, https://www.economist.com/the-economist-explains/2016/09/04/what-is-information-asymmetry.
See, eg, Martin J. D’Cruz & Ranjan B. Kini, The Effect of Information Asymmetry on Consumer Driven Health Plans, 1 Integration and Innovation Orient to E-Society 353; James H. Cardon & Igal Hendel, Asymmetric Information in Health Insurance: Evidence from the National Medical Expenditure Survey, 32 The RAND J. of Econ. 408; Gerald Bloom et al., Markets, Information Asymmetry and Health Care: Towards New Social Contracts, Elsevier Soc. Sci. & Med.
On the importance of transparency legislation, see, eg, Feldman, supra note 6 at 95–97; Feldman & Graves, supra note 19, at 11; Feldman & Frondorf, supra note 23, at 135–136.
See Feldman, supra note 6, at 95–97; Robin Feldman, Transparency, 19 Virginia J. L. & Tech. 272, 278–83 (2014) (discussing in depth the need for approaches to ensuring transparency in pharmaceutical markets, as well as the arguments in opposition to transparency).
Feldman, supra note 6, at 95 (discussing in depth the need for approaches to ensuring transparency in pharmaceutical markets, as well as the arguments in opposition to transparency).
See Feldman & Graves, supra note 19.
Known colloquially as the Hatch–Waxman Act, the formal name is the Drug Price Competition and Patent Term Restoration Act, Pub. L. No. 98–417, 98 Stat. 1585 (1984). See also Robin Feldman, Regulatory Property: The New IP, 40 Colum. J. L. & Arts 53, 59–68 (2016) (discussing the requirement for data sharing).
See, eg, Feldman & Frondorf, supra note 26, at 71 (‘As of mid-2016, for example, Genentech provided a CellCept copay card to consumers on the same website pushing doctors to prescribe only the branded medication’.).
See Feldman, supra note 6, at 108–9.
See Feldman, supra note 6, at 109.
See generally Michael E. Levine & Jennifer L. Forrence, Regulatory Capture, Public Interest, and the Public Agenda: Toward a Synthesis, 6 J. L. Econ., & Org. 167 (1990); Jean-Jacques Laffont & Jean Tirole, The Politics of Government Decision-Making: A Theory of Regulatory Capture, 106 The Q. J. of Econ. 1089 (1991); William J. Novak et al., Preventing Regulatory Capture: Special Interest Influence and How to Limit It (Daniel Carpenter & David A. Moss eds., 2013).
Patent protection lasts for twenty years from the date of the patent application, and industry estimates suggest that companies average 12 years of remaining patent protection when a drug reaches market. See Robin Feldman, May Your Drug Price Be Evergreen, 5 Oxford J. L. & Biosci. 590, 598–9 (2018) (‘Estimates suggest that the average remaining patent period for a new drug is 12 years. Although far less than a term of 20 years from the time of a patent application, 12 years of exclusivity is a considerable reward, particularly for a blockbuster drug that will garner many billions of dollars a year in revenue’.). Companies have become adept at extending that protection by piling on additional patents and exclusivities. See id. (finding that 78% of the drugs associated with new patents between 2005 and 2015 were not new drugs but existing ones).
See Feldman, supra note 194, (examining the extent of evergreening or artificially extending the patent protection cliff and its anticompetitive effects); Robin Feldman et al., Empirical Evidence of Drug Pricing Games—A Citizen’s Pathway Gone Astray, 20 Stan. Tech. L. Rev. 39 (2017) (exploring the abuse of the citizen petition process by drug companies to delay the approval of generic competitors); Robin Feldman & Prianka Misra, The Fatal Attraction of Pay-for-Delay, 18 Chicago-Kent J. Intell. Prop. 101 (investigating the use of pay-for-delay tactics to stifle competition).
Jonathan D. Alpern, William M. Stauffer & Aaron S. Kesselheim, High-Cost Generic Drugs—Implications for Patients and Policymakers, New Engl. J. Med. (Nov. 13, 2014) (examining the phenomenon of exorbitant price increases of generic drugs not protected by patents or market exclusivity). See Diane Bartz & Doina Chiacu, U.S. States Accuse Teva, Other Drugmakers, of Price-Fixing: lawsuit, Reuters (May 11, 2019), https://www.reuters.com/article/us-usa-drugs-lawsuit/u-s-states-accuse-teva-other-drugmakers-of-price-fixing-lawsuit-idUSKCN1SH0DP (reporting a lawsuit filed by a coalition of 44 states alleging 20 major drug manufacturers artificially inflated the prices of over 100 generic drugs).
See Nadia Whitehead, Why a Pill That’s 4 Cents in Tanzania Costs Up to $400 in the U.S., NPR (Dec. 11, 2017), https://www.npr.org/sections/goatsandsoda/2017/12/11/567753423/why-a-pill-thats-4-cents-in-tanzania-costs-up-to-400-in-the-u-s.
Press Release, U.S. Food & Drug Admin., FDA Alerts Patients and Health Care Professionals to Nitrosamine Impurity Findings in Certain Metformin Extended-Release Products (May 28, 2020), https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin.
Press Release, U.S. Food & Drug Admin., FDA Requests Removal of All Ranitidine Products (Zantac) from the Market (Apr. 1, 2020), https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market.
U.S. Food & Drug Admin., Generic Drugs: Questions & Answers, https://www.fda.gov/drugs/questions-answers/generic-drugs-questions-answers (accessed Aug. 19, 2020).
See id.
Trustees Reports (current and prior), Ctrs. for Medicare & Medicaid Servs., https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/TrusteesReports (accessed Dec. 6, 2019).
Medicare Part D Charts, Chronic Conditions Data Warehouse, https://www2.ccwdata.org/web/guest/medicare-charts/medicare-part-d-charts (accessed Nov. 15, 2019).
The labeler can be the manufacturer, marketer, repackager, or distributor of the product. What is NDC?, National Drug Codes List, https://ndclist.com/what-is-ndc.
Id. The study regards a ‘drug’ and ‘NDC’ as equivalent terms and use them interchangeably. The study chooses to define a ‘drug’ at the NDC level because it was the drug identifier variable included in the data. Variables included in the data can be found under the Medicare Part D section of the Data Dictionaries provided by the Chronic Conditions Data Warehouse, the research database created by CMS. Data Dictionaries, Ctrs. for Medicare & Medicaid Servs. (CMS), https://www2.ccwdata.org/web/guest/data-dictionaries.
See supra text accompanying notes 68–76 (describing the rebate system and its timing).
Drug Coverage Status Code, Research Data Assistance Center, https://www.resdac.org/cms-data/variables/drug-coverage-status-code.
New Drug Application (NDA), U.S. Food & Drug Administration, https://www.fda.gov/drugs/types-applications/new-drug-application-nda; Abbreviated New Drug Application (ANDA), U.S. Food & Drug Administration, https://www.fda.gov/drugs/types-applications/abbreviated-new-drug-application-anda.
See Himali Saitwal et al., Cross-Terminology Mapping Challenges: A Demonstration Using Medication Terminological Systems, 45 J. Biomed. Inform. 613 (2012) (describing the difficulties of mapping between numerous and nonequivalent medication terminological systems).
See National Drug Code Directory, U.S. Food & Drug Administration, https://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory. The directory is updated daily, meaning that NDCs that are no longer on market disappear from the directory on a daily basis.
NDCs can become obsolete for many reasons, including (but not limited to): discontinuation for safety reasons, a change in drug packaging, or discontinuation for economic reasons. The RxNorm database, which the study uses as part of its methodology in categorizing drugs as brand or generic, does maintain history information for its own identifiers, but does not keep track of obsolete NDCs. Lee B. Peters & Olivier Bodenreider, Approaches to Supporting the Analysis of Historical Medication Datasets with RxNorm, AMIA Ann. Symp. Proc. Arch. 1034 (2015). It is important to note that NDCs also hold potential for game playing, as the FDA does not assign NDCs to approved drugs—the pharmaceutical manufacturers do. Specifically, although manufacturers are supposed to permanently retire NDCs for products that have been removed from the market, but manufacturers have been known to reuse NDCs after a period of inactivity.
Brand-Generic Code Reported by Submitting Plan, Research Data Assistance Center (accessed Aug. 19, 2020), https://www.resdac.org/cms-data/variables/brand-generic-code-reported-submitting-plan.
The National Library of Medicine is a medical library and an institute within the National Institutes of Health, operated by the federal government. The National Library of Medicine’s RxNorm database includes an ‘NDC’ variable and a ‘BNvsGNN’ variable that allowed us to categorize the drugs, represented by NDCs, as brand or generic. RxNorm, NIH U.S. National Library of Medicine (Sept. 25, 2019), https://www.nlm.nih.gov/research/umls/rxnorm/index.html.
RxNorm RESTful Web API, NIH National Library of Medicine Lister Hill National Center for Biomedical Communications, https://rxnav.nlm.nih.gov/RxNormAPIREST.html
The study uses Cerner Multum’s database last to categorize the drugs because their methodology was not exact and did not align exactly with the study’s methodology and purposes. Drug Database, Cerner Corporation, https://www.cerner.com/solutions/drug-database.
These drugs usually had no formulary tier status and were not purchased often (which translates to a relatively low number of drug purchase entries in the data). Some of these drugs were strips, syringes, and OTC drugs.
Data Dictionaries, supra note 205; Part D Formulary File, Research Data Assistance Center (accessed 19 Aug. 2020), https://www.resdac.org/cms-data/files/part-d-formulary-file.
Copayment Tier Definitions, supra note 10.
RX Service Date, Research Data Assistance Center, https://www.resdac.org/cms-data/variables/rx-service-date.
Total Drug Cost, Research Data Assistance Center, https://www.resdac.org/cms-data/variables/total-drug-cost-part-d.
See Appendix A1. Note that CMS does not provide the data for ingredient cost, sales tax, dispensing fee, or vaccination fee (if applicable) that make up the Gross Drug Cost. CMS does not provide the data to this level of detail because the data are considered commercially sensitive information. Even if the data are commercially sensitive information, it is easy to see how this holds potential for game playing to any of the players in the drug supply chain that can benefit from avoiding transparency. This supports the idea that increased transparency is a possible solution to the problem identified in this paper. See Section 4.2.1. Transparency.
Amound Paid by Patient, Research Data Assistance Center, https://www.resdac.org/cms-data/variables/Patient-Pay-Amount.
Quantity Dispensed, Research Data Assistance Center, https://www.resdac.org/cms-data/variables/quantity-dispensed.
Ctrs. for Medicare & Medicaid Servs., 2018 Medicare Trustees Report.
Eg id, at 147 n.4 (explaining that the numbers provided on Table IV.B8. for annual manufacturer rebates are ‘expressed as a percentage of total drug costs’).
See supra Appendix A2 for detailed calculations of rebates. These average rebate percentages were obtained from annual Medicare Trustees Reports. Trustees Reports (current and prior), https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/TrusteesReports.html. The average rebate percentages in these reports are calculated across all Part D prescription drugs (both brand and generic drugs). Therefore, the analysis had to modify these rebates in order to make them reflect rebates for brand drugs only. The analysis does so by assuming that generic drugs do not have rebates. See also 2011 Medicare Trustees Report, supra note 112, at 183 (stating that ‘generic drugs… typically do not carry manufacturer rebates. Many brand-name prescription drugs carry substantial rebates’) (emphasis added).
Each drug in a drug purchase event has an associated formulary tier number. Tier Number (Plan Characteristics), Research Data Assistance Center, https://www.resdac.org/cms-data/variables/tier-number-plan-characteristics.
These statistical tests check if there is a significant difference between two groups. The Kruskal–Wallis test is a nonparametric test that can be applied on non-normal distributions; the Tukey test performs pairwise comparisons; and the Mann–Whitney test tests whether two samples come from the same population.
Null Hypothesis: All generic drugs that are placed on different formulary tiers (1, 2, 3, 4, and 5) have equal average dosage-unit prices or no significant differences among them. The cost criterion for the placement of generic drugs is not met. Alternative Hypothesis: Generic drugs placed on different formulary tiers have significantly different average dosage-unit prices. Hence, the cost criterion for the placement of generic drugs is met.
Tukey’s test is also known as the Tukey’s range test, Tukey method, Tukey’s honest significance test, or Tukey’s HSD test.
Operating on the assumption that drug cost is the critical determinant for drug placement on formulary tiers.
Null Hypothesis: All brand drugs that are placed on different formulary tiers (1, 2, 3, and 4.) have equal dosage-unit price averages or no significant differences among them. The cost criterion for the placement of brand drugs is not met. Alternative Hypothesis: Brand drugs placed on different formulary tiers have significantly different dosage-unit price averages. Hence, the cost criterion for the placement of brand drugs is met.
Copayment Tier Definitions, supra note 10.
Null Hypothesis: Brand drugs and generic drugs placed on the same formulary tier have equal or similar average dosage-unit prices. Therefore, they can be placed together on the same formulary tier and the cost criterion for drug placement is met. Alternative Hypothesis: Brand drugs and generic drugs placed on the same formulary tier have significantly different average dosage unit prices and should not be placed together on the same formulary tier. The cost criterion for drug placement is not met.


