Abstract
Purpose of the Review
The purpose of this review is to highlight the clinical significance of increased renal risk that has its origins in fetal life. This review will also discuss the critical need to identify therapeutic interventions for use in a pregnancy complicated by placental dysfunction and intrauterine growth restriction that can mitigate the developmental origins of kidney disease without inflicting additional harm on the developing fetus.
Recent Findings
A reduction in nephron number is a contributory factor in the pathogenesis of hypertension and kidney disease in low birth weight individuals. Reduced nephron number may heighten susceptibility to a secondary renal insult, and recent studies suggest that perinatal history including birth weight should be considered in the assessment of renal risk in kidney donors.
Summary
This review highlights current findings related to placental dysfunction, intrauterine growth restriction, increased risk for renal injury and disease, and potential therapeutic interventions.
Keywords: Placental dysfunction, Intrauterine growth restriction, Low birth weight, Blood pressure, Renal disease
Introduction
Complications during pregnancy that impair fetal growth contribute to the developmental origins of increased blood pressure and renal disease in the offspring [1]. The sources of adverse exposures during fetal life are varied and include preeclampsia, diabetes, parental smoking, maternal obesity, maternal stress, maternal alcohol consumption, maternal age or poor perinatal care [2]. Some of these factors are preventable. However, the etiology of pregnancy specific conditions such as preeclampsia involves an etiology that is not completely understood. Additionally, effective drug treatments to prevent or treat preeclampsia are unavailable. The only treatment option for preeclampsia involves early delivery or removal of the placenta [3]. Yet, birth before 37 weeks results in preterm or low birth weight which is also associated with increased blood pressure in the offspring [4••, 5].
Placental dysfunction is a critical contributor in the development of preeclampsia [3]. The placenta, the interface between the maternal and fetal circulations, is a vital organ during pregnancy that provides nutrients and oxygen to the developing fetus [6]. In preeclampsia, placental dysfunction can impair maternal-fetal nutrient and oxygen exchange resulting in intrauterine growth restriction (IUGR) [7]. IUGR, defined as the rate of fetal growth below expected growth potential, is the most common pregnancy-associated complication [8]. Maternal undernutrition, the major contributory factor to adverse pregnancy outcomes in third world countries [9], also impairs placental development and function [10]. Maternal undernourishment can result in IUGR when alterations in placental weight, morphology, vascular development, and nutrient transport function are not sufficient to maintain proper fetal growth [10]. When nutrition is insufficient, the fetus undergoes structural and functional changes to ensure survival. Yet, when nutritional insults, regardless of etiology, occur during a critical time of rapid cell growth and differentiation during fetal development, normal physiological, anatomical, and metabolic functions in the offspring are impaired [11, 12]. These changes in organ structure and function persist after birth and predispose offspring to increased risk for chronic disease throughout their lifespan [13].
The concept that adverse influences during fetal life correlate to increased risk of cardiovascular disease in adulthood was first proposed by Dr. David Barker [14]. Origination of the hypothesis developed in the late twentieth century from studies which indicated that infants born low birth weight (5.5 pounds or less) had increased risk for hypertension, renal disease, type 2 diabetes, and death from cardiovascular-related events [14, 15]. Low birth weight is indicated as a crude marker of an adverse intrauterine environment. Although low birth weight does not have the same definition of IUGR [2], numerous studies indicate a strong association between indicators of low birth weight and later chronic disease [16]. The percent of infants born low birth weight within the USA is 8.2%; a rate that is higher within the black population (13.8%) compared to the white (7.0%) [17]. The incidence of IUGR is approximately six times higher in third world countries compared to the USA [2]. Clearly, the prevalence of low birth weight and IUGR indicates the importance of early life events on long-term chronic health in an individual. Thus, the purpose of this review is to highlight changes in placental function and structure that initiate the developmental origins of chronic disease and the post-natal mechanisms that underlie enhanced renal susceptibility to renal injury and disease. In addition, this review will conclude with an overview of studies suggestive of therapeutic approaches and interventions designed to improve maternal health during gestation with added benefit of mitigation of poor fetal growth and increased risk for hypertension and renal disease in the offspring.
The Placenta and Fetal Growth
Preeclampsia and maternal undernutrition both alter placental development with subsequent adverse effects on fetal growth. Although maternal, genetic, and fetal factors can play a causative role in the development of IUGR, the placenta is the primary inter-face between the mother and the fetus and serves as the critical regulator of nutrient supply to the developing fetus [7, 18]. Nutrient transport from the mother to the fetus is greatly dependent upon maternal food intake [10]. Nutrients that are necessary for proper fetal growth include oxygen, glucose, amino acids, and fatty acids, and there are a number of factors that regulate the ability of the placenta to transfer the nutrients to the fetus [7, 18]. The placenta is highly permeable to the rapid diffusion of gases, and changes in utero-placental blood flow can alter the diffusion of gases [19]. Nutrient availability, which is a primary determinant of fetal growth, also involves transfer of larger, less permeable substances via nutrient transport proteins [19]. Nutrient transport proteins are sensitive to utero-placental blood flow [20]. Nutrient concentration gradients in addition to the expression and activity of the nutrient transport systems also alter placental transport mechanisms [20]. Placental weight or size is another factor that directly affects the capacity for nutrient transfer through changes in surface area [21].
Utero-placental insufficiency is common in pregnancies complicated by preeclampsia and IUGR [7, 18]. Several studies indicate that nutrient transport is altered in experimental models of preeclampsia and IUGR. Placental insufficiency-induced via bilateral uterine ligation at day 19 of gestation in the rat is associated with a decrease in expression of the placental glucose transporter 1 (GLUT1) and the placental glucose diffusion channel (Cx26) [22]. Placental GLUT1 is also reduced in a mouse model of IUGR-induced via bilateral uterine ligation [23]. Overexpression of human adenoviral insulin growth factor, an important regulator of placental and fetal growth, corrects IUGR and restores reduced placental GLUT1 expression [23] suggesting that correction of placental transport can also restore proper fetal growth. Placental expression of Cx26 is also reduced in the model of IUGR-induced via chronic overexpression of sFlt-1 in the pregnant mouse [24]. Therefore, these studies indicate that different models of preeclampsia and IUGR demonstrate similar pathways of impaired placental transport. Furthermore, the use of experimental models of utero-placental insufficiency [25, 26] and maternal undernutrition [27] provides the ability to investigate direct cause and effect between an adverse fetal environment and increased blood pressure and renal risk in the offspring.
Nephron Number and Renal Risk
Barker and Osmond first recognized the link between undernutrition during fetal life and increased risk for coronary heart disease [28]. Barker and Osmond expanded their observation by demonstrating that birth weight was inversely associated with blood pressure suggesting that hypertension may be the link between undernutrition during fetal life and increased risk for cardiovascular disease in later life [15]. Based on these geographical studies, Barker hypothesized that fetal undernutrition leads to disproportionate fetal growth and increased risk for hypertension [14]. Brenner advanced this theory to incorporate a role for the kidney suggesting that a reduction in nephron number at birth favors an increased risk of hypertension in later life [28]. In 1992, Hinchliffe and colleagues reported that nephron number was below control values in IUGR stillborn compared to control stillborn [29]. Using kidneys collected in autopsy, Hughson et al. later reported lower nephron number in low birth weight individuals [30], confirming that slow fetal growth is associated with improper renal development. Collectively, these studies linking a reduction in nephron number with IUGR and low birth weight, respectively, provide credence that events that impair fetal growth are associated with changes in organ structure. In 1994, using an experimental model of maternal low protein during gestation to mimic maternal undernutrition, Evans and Jackson reported that blood pressure was increased in offspring of low protein dams [31]. This was the first experimental study to substantiate the Barker hypothesis. A later study showed that a reduction in nephron number is associated with increased blood pressure in offspring from low protein dams [27] providing correlative evidence linking exposure to undernutrition during fetal life with reduced nephron number and increased blood pressure. In 2000, Lackland and colleagues reported that low birth weight is associated with a greater prevalence of end-stage renal disease (ESRD) [32]. Eriksson et al. reported that low birth weight is associated with greater risk for chronic kidney disease (CKD) [33] and more recently, a review by Starr and Hingorani highlighted the association between preterm birth increased risk for CKD [34••]. Taken together, these studies suggest that insults during fetal life that alter kidney structure and function are not just linked to hypertension. To date, numerous experimental and clinical studies report that a reduction in nephron number at birth increases risk for renal dysfunction and disease [34••, 35]. In 2017, the Low Birth Weight and Nephron Number Working Group published a consensus document highlighting the association between low birth weight, IUGR, and preterm birth with low nephron number [36••]. This consensus developed a number of recommendations and action items aimed at reducing low birth weight and preterm delivery in addition to improvements in detection and management of health birth outcomes. A list of action items was developed to combat the growing evidence that low birth weight and preterm birth are associated with increased risk for hypertension and chronic kidney disease. However, concerns related to the effect of low birth weight and nephron number on adverse health outcomes were recently expanded to include the effect of low birth weight on renal donation.
Birth Weight and Kidney Donation
Numerous studies indicate that kidney donation does not heighten renal or cardiovascular risk within the general population [37, 38]. Yet, recent studies suggest that low birth weight or low nephron number may compromise long-term health following renal donation. Nephron number is reduced in Australian Aborigines, a population that has a high incidence of renal failure [39]. In 2009, a study by Rogers et al. reported that renal risk is significantly higher in Aborigines donors [40]. In 2019, Issa et al. reported that albuminuria is increased in low birth weight kidney donors [41•], a finding also reported by Berglund et al. In 2014, Berglund et al. did not observe a greater prevalence of hypertension in low birth weight renal donors [42•]. Yet, a greater prevalence of hypertension in low birth weight kidney donors was observed in a study by Jedrzejko et al. published in 2018 [43•]. Differences in male and female predominance and time since donation within each study cohort could contribute to differences in study outcomes in these two studies. However, these studies highlight the need for more in-depth investigation into the effect of renal donation in individuals with a congenital reduction in nephron number. Renal risk may also be increased in those that receive a kidney from a low birth weight donor. In 2017, Schachtner and Reinke reported that recipients of kidneys from low birth weight donors require greater anti-hypertensive therapy [44•]. Collectively, these studies suggest that in addition to other exclusion criteria for kidney donors, low birth weight as a surrogate marker of nephron number should be considered in the selection process for kidney donation.
In humans, nephrons are formed between 28 and 34 weeks’ gestation, after which no additional nephrons are formed [30, 45]. In the rat, nephrogenesis continues after birth up to postnatal day 10 [46]. In 1999, to address the importance of a significant loss in nephron number during development on later chronic health, Woods reported that uninephrectomy at birth in the rat was associated with hypertension in the adult rat [47]. Using a genetic model of reduced nephron number, Ruta and colleagues demonstrated that a 25% reduction in nephron endowment did not alter blood pressure on a regular salt diet but was associated with a significant increase in blood pressure on a high salt diet [48] suggesting exposure to adverse environmental factors after birth heightens renal susceptibility. A reduction in nephron number is associated with a loss in glomerular surface area, a determinant of glomerular filtration rate (GFR). Based on Brenner’s theory of hyperfiltration, compensatory glomerular hypertrophy occurs in response to nephron loss leading to glomerular damage, proteinuria, and over time renal failure [49]. Exposure to an insult that provides an additional challenge to the functional components of a kidney or kidneys that are already compromised by low nephron number may be a contributory factor to increased risk for renal injury and CKD in low birth weight individuals.
Secondary Renal Insult and Renal Risk
Using a model of reduced nephron number induced by fetal exposure to maternal protein restriction, Zimanyi et al. tested the hypothesis that exposure to a secondary renal insult would result in greater renal injury in low-protein offspring [50]. Low-protein offspring did not exhibit overt renal injury prior to a secondary insult. However, exposure to exogenous advanced glycation end-products (AGEs), a natural byproduct that accumulates in the kidney in response to injury, resulted in additional accumulation of AGEs and a greater increase in markers of renal injury in low-protein offspring compared to their control counterpart [50]. Plank et al. reported that IUGR low-protein offspring exhibit greater renal susceptibility in young adulthood to acute mesangioproliferative glomerulonephritis (GN) [51], a disorder that results in a progressive loss of renal function. Plank et al. reported similar findings in IUGR offspring from dams exposed to placental ischemia [52], and Ojeda reported that IUGR is also associated with heightened renal susceptibility to an acute secondary insult such renal ischemia/reperfusion [53]. Based on recent studies reporting low-birth weight heightens the risk for adverse outcome after renal donation [41•, 42•, 43•, 44•], our laboratory used a model of IUGR-induced via placental insufficiency in the pregnant rat to test the hypothesis that uninephrectomy in adult IUGR rat offspring programs enhanced renal injury relative to control or normal birth weight counterparts. We reported that uninephrectomy in male IUGR offspring is associated with an increase in proteinuria and urinary excretion of neutrophil gelatinase-associated lipocalin (NGAL), a marker of renal injury, compared to control counterparts [54]. Unlike previous studies exploring enhanced renal susceptibility to a secondary insult in young adulthood, uninephrectomy was induced in rat offspring at 18 months of age, or the age equivalent to the average age of kidney donors [55]. We also reported that an increase in NGAL but not proteinuria is observed in uninephrectomized female IUGR offspring [54]. It is well established that women differ in their susceptibility to renal disease relative to age-matched men [56]. Thus, in our study, female IUGR offspring demonstrated less susceptibility to uninephrectomy compared to male IGUR counterparts. Black et al. reported that female low-protein IUGR offspring lose their protection against a decrease in renal function with aging compared to male low-protein IUGR offspring [57]. GFR was not altered in female or male IUGR offspring in our study regardless of intact or uninephrectomy [54]. The degree of protein restriction used in the study by Black et al. was severe, 8.7% versus 20% fed to controls [57]. Therefore, differences in the severity of fetal insult could affect whether GFR under baseline conditions is reduced or not. Whether aging including a longer exposure to uninephrectomy would result in a similar degree of renal injury in female uninephrectomized offspring in our study compared to their male counterparts is not known. Clearly, additional studies are needed to clarify how sex and age alter enhanced susceptibility to a renal insult in individuals compromised by IUGR and low birth weight. Investigations into the mechanisms that contribute to enhanced renal risk following a developmental insult are also warranted in order to aid in the development of preventative strategies and treatments. Yet, enhanced renal susceptibly induced by an adverse fetal environment is not limited to a secondary renal insult. Dietary challenges can also serve as a second hit on renal health following a fetal insult.
Pre- and Postnatal Diet and Renal Risk
Chronic intake of a high protein diet does not adversely affect renal function or GFR in healthy individuals [58]; yet, Shen et al. and Chen et al. showed that postnatal exposure to a diet high in protein is associated with a greater increase in blood pressure and proteinuria in IUGR induced by maternal low protein compared to IUGR rat offspring placed on a standard protein diet [59, 60]. These studies indicated that a postnatal diet high in protein content enhances the adverse effects of IUGR, and that IUGR programs susceptibility to dietary challenges that would not alter renal health in normal birth weight counterparts.
Obesity is an emerging risk factor for renal disease [61]. Obesity was a recent topic at World Kidney Day highlighting the association of obesity as an independent risk factor for CKD [61]. It is well established that maternal obesity programs metabolic risk in the offspring [62]. However, human studies addressing the effect of maternal obesity on renal health in the child are very limited [63]. Studies exploring whether fetal exposure to overnutrition programs impaired renal health and increased risk for kidney disease are limited. In 2005, Armitage et al. reported that offspring of lard-fed rats does not exhibit a change in glomerular number or volume [64]. In 2017, Glastras et al. demonstrated that proteinuria and serum creatinine levels are increased in offspring of high fat-fed mothers [65]. Whether these changes are due to direct exposure to maternal obesity or a result of metabolic disturbances is unknown. Thus, maternal dietary excess, like maternal dietary undernutrition, can have long-term effects on renal well-being in the offspring. Moreover, these studies indicate that extensive additional investigation is needed to determine the mechanism(s) that are involved the programming of adverse health following exposure to overnutrition during development, and to determine the long-term effect of prenatal exposure to maternal obesity or maternal overnutrition on renal health and susceptibility to renal injury.
Yet, another gap exists in our understanding of overnutrition and its adverse consequences on renal function. The effect of a postnatal diet rich in fat following undernutrition during gestation has only recently been explored. In 2017, Rizzi et al. reported that low protein rat offspring exhibit a significant increase in markers of renal injury associated with altered renal structure when exposed to a postnatal diet high in fat [66]. Our laboratory reported in 2019 that IUGR induced by placental ischemia in the pregnant rat results in a reduction in GFR in male IUGR offspring by 6 months of age [67], an observation not seen at 4 months of age in this model [25]. We also showed that chronic exposure to a postnatal diet enriched in fat and sucrose results in a further decrease in GFR in male IUGR offspring with no effect in male control; GFR is not reduced in female IUGR offspring regardless of diet [67]. Boubred et al. explored a more subtle effect of overfeeding on renal function, a reduction in litter size during lactation that results in early accelerated growth in IUGR low-protein rat offspring [68]. This study demonstrated that GFR is not reduced in low-protein IUGR even up to 22 months of age [68]. However, exposure to immediate postnatal accelerated growth by lactation results in a decrease in GFR in IUGR low-protein rat offspring not observed in normal birth weight rat offspring [68]. Taken together, these studies emphasize the importance of the postnatal diet following low birth weight or IUGR on renal function.
Recent studies suggest that the effect of maternal diet on renal function and disease is not limited to just over- or undernutrition. The Dahl salt-sensitive (Dahl SS) rat is a genetic model of salt-sensitive hypertension outbred from the Sprague Dawley rat by Lewis K. Dahl. This model has been used extensively to study mechanisms related to salt-sensitive hypertension. In 2004, Mattson and colleagues reported that renal outcomes in the Dahl SS rat differed between a casein-based diet versus a corn and wheat protein-based grain diet, though both diets had similar protein content, 18–20% [69]. Blood pressure, albuminuria, urinary protein excretion, and histological evidence of renal injury were higher in the Dahl SS exposed to a casein diet versus the grain diet [69]. To determine the specificity of the diet versus potential genetic drift, in 2015, this group readdressed the effect of maternal dietary source of protein during gestation on offspring chronic health [70•]. Using colonies of Dahl SS rats maintained for many generations on the two respective diets, embryo-transfer experiments demonstrated that hypertension and renal injury were exacerbated in offspring derived from grain-fed dams and transferred to dams maintained on a casein-based diet [70•]. Transfer from a dam maintained on a casein-based diet to a grain-fed dam attenuated the increase in blood pressure and degree of renal injury in the offspring [70•]. Genetic differences were ruled out [70•] confirming the importance of the maternal diet on susceptibility to renal disease in later life.
Therapeutic Approaches, Fetal Growth, and Benefit Without Harm
The prevention of increased blood pressure induced by an adverse fetal environment is a growing area of study. Yet, a caveat in regard to administration of therapeutic approaches during pregnancy involves consideration of benefit without harm to the developing fetus in the prevention of programmed susceptibility to chronic disease. Torrens et al. were one of the first groups to demonstrate the beneficial effect of a maternal intervention on offspring chronic health. They demonstrated that folate supplementation, a factor critical for proper fetal development, during pregnancy prevents the increase in blood pressure in male offspring programmed by fetal exposure to maternal protein restriction [71]. Franco et al. expanded this finding to report that maternal administration of antioxidant nutrients including folate, selenium, and vitamins C and E during pregnancy also reduced blood pressure in low-protein offspring [72], demonstrating the importance of micronutrient supplementation on long-term benefit in offspring. However, in this study by Franco et al., a reduction in nephron number was not prevented indicating that long-term susceptibility to a secondary renal insult including enhanced aging-related injury may remain. Roghair et al. showed that administration of the antioxidant, tempol, abolishes stress-induced blood pressure reactivity in male offspring exposed to excess fetal glucocorticoid exposure [73]. Yet, aortic dysfunction in female offspring was not improved [73]. Sex differences in blood pressure and renal injury are observed in many models of developmental origins [1]. Thus, an important outcome of this study by Roghair et al. involved the sex-specific programming response to a gestational intervention [73]. Interventions using manipulation of the nitric oxide (NO) pathway, another vasoactive factor, also prevent increased blood pressure that has its origins in fetal life. L-citrulline, a precursor for NO, abolishes increased blood pressure in rats exposed to maternal nutrient restriction [74], maternal dexamethasone [75], or type 1 diabetes induced by streptozotocin in the rat [76]. Therefore, these studies are important as they highlight that a single intervention provides benefit in offspring exposed to multiple different fetal insults.
Preeclampsia, a leading cause of IUGR and maternal morbidity and mortality, is associated with a reduction in NO-cyclic guanosine monophosphate (cGMP) signaling [77]. However, the long-term benefit for prevention/treatment of preeclampsia using therapeutic approaches that target the balance of NO and reactive oxygen species via chronic L-arginine, L-citrulline, tempol, or other antioxidants is not clear [78, 79] indicating a need to identify new therapeutic targets that improve maternal health and provide benefit to the offspring. In 2009, Samangaya et al. reported the sildenafil citrate, a phosphodiesterase type 5 inhibitor that prevents hydrolysis of the NO secondary messenger cGMP, was reported to be well-tolerated during pregnancy and women with preeclampsia [80]. In 2019, Groom et al. reported that although maternal sildenafil use in pregnancies complicated by early-onset fetal growth restriction had no beneficial effect on fetal growth, there was no indication of fetal harm [81]. A systematic review of the literature by Paauw et al. published 2017 that evaluated experimental studies. Reported sildenafil is associated with improved fetal growth, but at higher doses used clinically [82]. In 2013, George et al. reported that administration of sildenafil abolishes hypertension in the well-established and clinically relevant rat model of reduced uterine perfusion pressure (RUPP) of preeclampsia, suggesting benefit to the mother [83]. In 2016, Gillis et al. showed that sildenafil administered during gestation in the Dahl SS rat lowered blood pressure in the offspring although this perinatal intervention had no effect on GFR [84]. Collectively, these studies provided support for a potential therapeutic that might not only provide benefit to the mother with no harm in the offspring, but also mitigate increased blood pressure in the offspring. Yet, based on adverse outcomes including infant death after birth, a clinical trial addressing sildenafil therapy during pregnancy on fetal growth was halted [85] bringing into question the safety and efficacy of this particular therapeutic intervention. Clearly new therapeutic approaches are needed to mitigate the developmental origins of chronic disease without inducing additional harm in the offspring.
Preeclampsia is associated with an increase in production of several factors including sFlt-1 and an agonistic angiotensin II type 1 receptor autoantibody (AT1-AA). Cunningham and colleagues recently reported that administration of a 7-amino acid inhibitory peptide against the AT1-AA abolishes the increase in blood pressure in RUPP dam [86]. LaMarca et al. also reported that chronic administration of a TNF-alpha antagonist attenuates hypertension in the RUPP dam [87]. For both interventions, pup weight was not reduce in RUPP-treated dams suggestive of benefit not harm [86, 87]. Although the use of many therapeutics are contraindicated during pregnancy, anti-TNF compounds are commonly utilized in women with immune-mediated inflammatory diseases such as Crohn’s disease, rheumatoid arthritis, and psoriasis and others prior to and throughout gestation [88–91]. The benefit of the 7-amino acid inhibitory peptide against the AT1-AA in human preeclampsia is not yet known. However, these preclinical studies indicate potential new therapeutics for the treatment of preeclampsia. Whether they induce harm to the baby or mitigate the adverse consequence of placental ischemia on IUGR and later chronic health of the offspring is not yet known. Thus, additional studies are needed to clarify benefit versus harm for these approaches in the offspring.
Conclusion
The developmental response to an adverse fetal environment provides a mechanism for the fetus to adapt to conditions in the intrauterine environment to ensure survival to birth. A consequence of these adaptations is the developmental programming of pathophysiological changes that underlie the progression to increased blood pressure and renal risk later in life (Fig. 1). This review provides an overview of the pathogenesis of IUGR and the renal changes that contribute to the development of increased blood pressure and renal risk in IUGR offspring. This review also highlights the critical need to identify potential maternal interventions that will provide benefit without harm to fetus and mitigate the increased risk for renal disease that originates during fetal life.
Fig. 1.
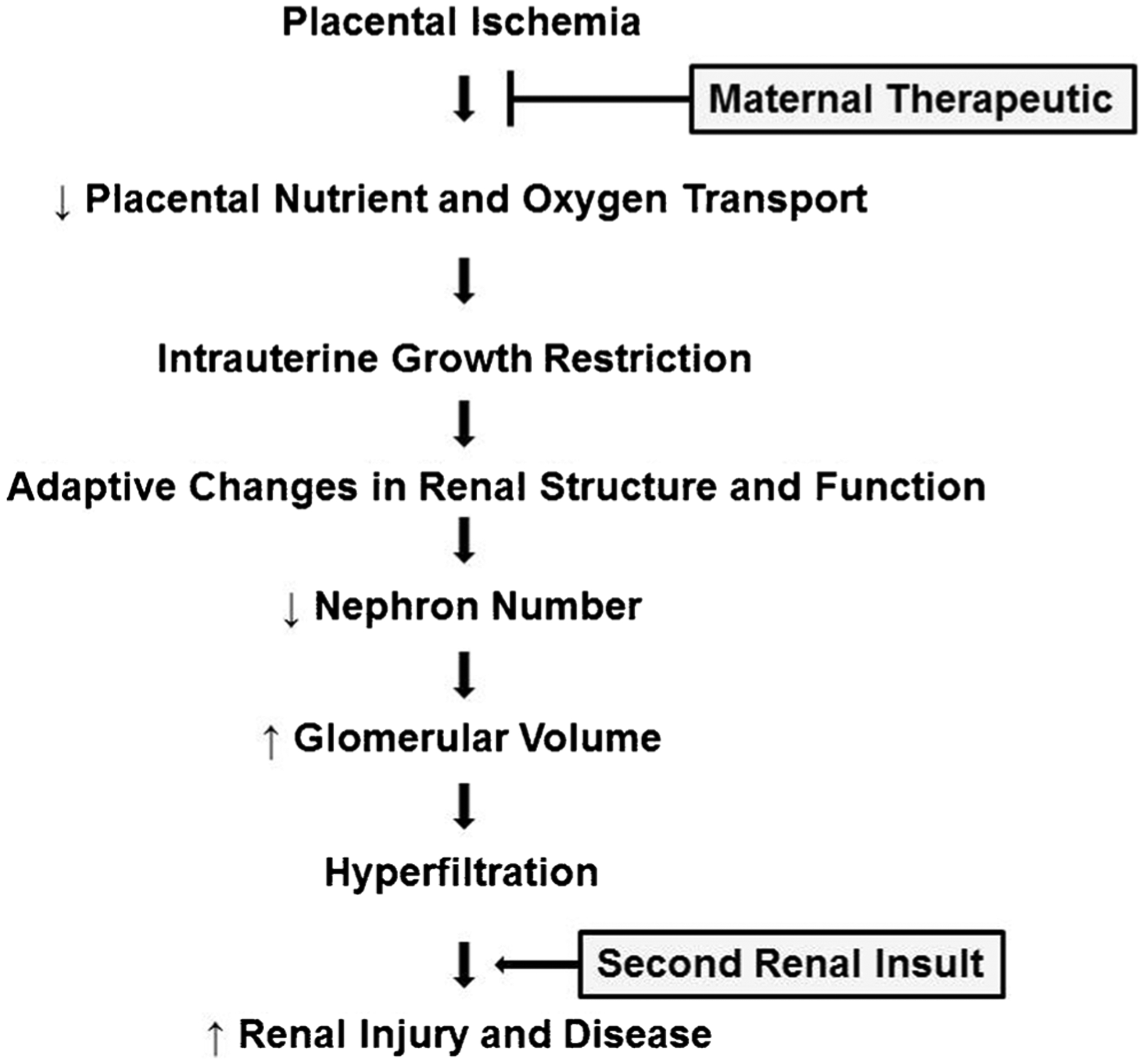
Summary of the effect of placental ischemia on fetal growth and the adaptive changes in renal structure and function that program an enhanced renal susceptibility to a secondary renal insult leading to increased risk for renal injury and disease in intrauterine growth-restricted offspring
Sources of Funding
This work was supported by the National Institutes of Health Grants R56HL143459 with additional funding provided by HL51971, P20GM104357, and P20GM121334. Ms. Coats, Dr. Davis, and Dr. Newsome received funding from NIH T32HL105324, and AHA PRE34060010 (Davis) and NIH F30DK112718 (Newsome).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Dasinger JH, Davis GK, Newsome AD, Alexander BT. Developmental programming of hypertension: physiological mechanisms. Hypertension. 2016;68(4):826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma D, Shastri S, Sharma S. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016;10:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2(2):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.••.Andraweera PH, Lassi ZS. Cardiovascular risk factors in offspring of preeclamptic pregnancies—systematic review and meta-analysis. J Pediatr. 2019;208:104–13. [DOI] [PubMed] [Google Scholar]; This is a concise summary of all studies that indicate increased cardiovascular risk in offspring of women with preeclampsia.
- 5.Alsnes IV, Vatten LJ, Fraser A, Bjorngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (Nord-Trøndelag Health Study) in Norway. Hypertension. 2017;69(4):591–8. [DOI] [PubMed] [Google Scholar]
- 6.Salmani D, Purushothaman S, Somashekara S, Gnanagurudasan E, Sumangaladevi K, et al. Study of structural changes in placenta in pregnancy-induced hypertension. J Nat Sci Biol Med. 2014;5(2):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol. 2014;38(3):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. In seminars in fetal and neonatal medicine. Semin Fetal Neonatal Med. 2018;23(2):119–25. [DOI] [PubMed] [Google Scholar]
- 9.Morrison J, Regnault T. Nutrition in pregnancy: optimising maternal diet and fetal adaptations to altered nutrient supply. Nutrients. 2016;8(6):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010;83(3):325–31. [DOI] [PubMed] [Google Scholar]
- 11.Mandy M, Nyirenda M. Developmental origins of health and disease: the relevance to developing nations. Int Health. 2018;10(2):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longtine M, Nelson D. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29(03):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon E, Kim K. What is fetal programming?: a lifetime health is under the control of utero health. Obstet Gynecol. 2017;49(10): 506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker D, Bull A, Osmond C, Simmonds S. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301(6746):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker D, Osmond C. Low birth weight and hypertension. BMJ. 1988;297(6641):134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7646267 participants from 135 studies. J Am Heart Assoc. 2018;7(23):e008870. 10.1161/JAHA.118.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton B, Martin J, Osterman MJK, Driscoll A, Rossen M. Births: provisional data for 2017. NVVS. 2018;67(8):1–50. [PubMed] [Google Scholar]
- 18.Zhang S, Regnault TR, Barker PL, Botting KJ, McMillan CM, et al. Placental adaptations in growth restriction. Nutrients. 2015;7(1): 360–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gude NM, Roberts CT, Kakionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407. [DOI] [PubMed] [Google Scholar]
- 20.Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport- a review. Placenta. 2007;28(8–9):763–4. [DOI] [PubMed] [Google Scholar]
- 21.Herrera E, Camm E, Cross C, Mullender J, Wooding F, Giussani D. Morphological and functional alterations in the aorta of the chronically hypoxic fetal rat. J Vasc Res. 2012;49(1):50–8. [DOI] [PubMed] [Google Scholar]
- 22.Nüsken E, Gellhaus A, Kuhnel E, Swoboda I, Wohlfarth M, et al. Increased rat placental fatty acid, but decreased amino acid and glucose transporters potentially modify intrauterine programming. J Cell Biochem. 2016;117(7):1594–603. [DOI] [PubMed] [Google Scholar]
- 23.Jones HN, Crombleholme T, Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS One. 2013;8(9):e74632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kühnel E, Kleff V, Stojanovaska V, Kaiser S, Waldschutz R, Herse F, et al. Placental-specific overexpression of sFlt-1 alters trophoblast differentiation and nutrient transporter expression in an IUGR mouse model. J Cell Biochem. 2017;118(6):1316–29. [DOI] [PubMed] [Google Scholar]
- 25.Alexander B Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–62. [DOI] [PubMed] [Google Scholar]
- 26.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, et al. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587(11):2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64(11):965–74. [DOI] [PubMed] [Google Scholar]
- 28.Brenner BM, Garcia D, Anderson S. Glomeruli and blood pressure: less of one, more the other? Am J Hypertens. 1988;1(4.1):335–47. [DOI] [PubMed] [Google Scholar]
- 29.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. BJOG Int J Obstet Gynaecol. 1992;99(4):296–301. [DOI] [PubMed] [Google Scholar]
- 30.Hughson M, Farris A III, Douglas-Denton R, Hoy W, Bertram J. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–22. [DOI] [PubMed] [Google Scholar]
- 31.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci. 1994;86(2):217–22. [DOI] [PubMed] [Google Scholar]
- 32.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med. 2000;160(10):1472–6. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson JG, Salonen K, Kajantie E, Osmond C. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–4. [DOI] [PubMed] [Google Scholar]
- 34.••.Starr M, Hingorani S. Prematurity and future kidney health: the growing risk of chronic kidney disease. Curr Opin Pediatr. 2018;30(2):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive review of the role of preterm or low birth weight on increased risk of renal dysfunction.
- 35.Das SK, Mannan M, Faruque AS, Ahmed T, McIntyre HD, et al. Effect of birthweight on adulthood renal function: a biased-adjusted met-analytic approach. Nephrology (Carlton). 2016;21(7):547–65. [DOI] [PubMed] [Google Scholar]
- 36.••.The Low Birth Weight Nephron Number Working Group. The impact of kidney development on the life course: a consensus document for action. Nephron. 2017;136(1). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a consensus statement that emphasizes the recommendations for action based on clinical experience and research studies highlighting the need for early action to prevent chronic kidney disease assocatied with low birth weight.
- 37.Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166(6):2043–7. [PubMed] [Google Scholar]
- 38.Lam NN, Lentine KL, Levey AS, Kasiske SA, Berry H, et al. Long-term medical risks to the living kidney donor. Nat Rev Nephrol. 2015;11(7):411–9. [DOI] [PubMed] [Google Scholar]
- 39.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70(1):104–10. [DOI] [PubMed] [Google Scholar]
- 40.Rogers N, Lawton P, Jose M. Indigenous Australians and living kidney donation. N Engl J Med. 2009;361(15):1513–6. [DOI] [PubMed] [Google Scholar]
- 41.•.Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, et al. Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant. 2019;00:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Low birth weight is associated with albuminuria after renal donation.
- 42.•.Berglund D, MacDonald D, Jackson S, Richard S, Naim I, et al. Low birthweight and risk of albuminuria in living kidney donors. Clin Transpl. 2014;28(3):361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Low birth weight is associated with albuminuria after renal donation.
- 43.•.Jędrzejko K, Kieszek R, Dor FJMF, Kwapisz M, Nita M, et al. Does low birthweight have an impact on living kidney donor outcomes? Transplant Proc. 2018;50(6):1710–4. [DOI] [PubMed] [Google Scholar]; Low birth weight is associated with higher blood pressure after renal donation.
- 44.•.Schachtner T, Reinke P. Estimated nephron number of the donor kidney: impact on allograft kidney outcomes. Transplant Proc. 2017;49(6):1237–43. [DOI] [PubMed] [Google Scholar]; Donor birth weight is an indicator or renal function in kidney transplant recipients.
- 45.Simeoni U, Ligi I, Buffat C, Boubred F. Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatr Nephrol. 2011;26(4):493–508. [DOI] [PubMed] [Google Scholar]
- 46.Guron G, Friberg P. An intact renin–angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18(2):123–37. [DOI] [PubMed] [Google Scholar]
- 47.Woods L Neonatal uninephrectomy causes hypertension in rats. Am J Phys. 1999;276(4):R974–8. [DOI] [PubMed] [Google Scholar]
- 48.Ruta LA, Dickinson H, Thomas MC, Denton KM, Anderson WP, et al. High-salt diet reveals the hypertensive and renal effects of reduced nephron endowment. Am J Physiol Ren Physiol. 2010;298(6):F1384–92. [DOI] [PubMed] [Google Scholar]
- 49.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49(6):1774–7. [DOI] [PubMed] [Google Scholar]
- 50.Zimanyi MA, Denton KM, Forbes JM, Thallas-Bonke V, Thomas MC, Poon F, et al. A developmental nephron deficit in rats is associated with increased susceptibility to a secondary renal injury due to advanced glycation end-products. Diabetologia. 2006;49(4): 801–10. [DOI] [PubMed] [Google Scholar]
- 51.Plank C, Ostreicher I, Hartner A, Marek I, Struwer FG, et al. Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int. 2006;70(11):1974–82. [DOI] [PubMed] [Google Scholar]
- 52.Plank C, Nusken KD, Menendez-Castro C, Hartner A, Ostreicher I, et al. Intrauterine growth restriction following ligation of the uterine arteries leads to more severe glomerulosclerosis after mesangioproliferative glomerulonephritis in the offspring. Am J Nephrol. 2010;32(4):287–95. [DOI] [PubMed] [Google Scholar]
- 53.Ojeda N Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Ren Physiol. 2011;301(2):F420–6. [DOI] [PubMed] [Google Scholar]
- 54.Newsome AD, Davis GK, Adah ON, Ojeda NB, Alexander BT. Renal injury after uninephrectomy in male and female intrauterine growth-restricted aged rats. PLoS One. 2019;14(3):e0213404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis CL, Cooper M. The state of US living kidney donors. Clin J Am Soc Nephrol. 2010;5(10):1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulroney SE, Woda C, Johnson M, Pesce C. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 1999;56(3):944–53. [DOI] [PubMed] [Google Scholar]
- 57.Black MJ, Lim K, Zimanyi MA, Sampson AK, Bubb KJ, Flower RL, et al. Accelerated age-related decline in renal and vascular function in female rats following early-life growth restriction. Am J Phys Regul Integr Comp Phys. 2015;309(9):R1153–61. [DOI] [PubMed] [Google Scholar]
- 58.Devries MC, Sithamparapillai A, Brimble KS, Banfield L, Morton RW, Phillips SM. Changes in kidney function do not differ between healthy adults consuming higher-compared with lower-or normal-protein diets: a systematic review and meta-analysis. J Nutr. 2018;148(11):1760–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen Q, Xu H, Wei LM, Chen J, Liu HM. Intrauterine growth restriction and postnatal high-protein diet affect on kidneys in adult rats. Nutrition. 2011;27(3):364–71. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Xu H, Shen Q, Guo W, Sun L. Effect of postnatal high protein diet on kideny function of rats exposed to intrauterine protein restriction. Pediatr Res. 2010;68(2):100–4. [DOI] [PubMed] [Google Scholar]
- 61.Kovesdy C, Furth S, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Am J Hypertens. 2017;30(3):328–36. [DOI] [PubMed] [Google Scholar]
- 62.Glastras SJ, Chen H, Pollock CA, Saad S. Maternal obesity increases the risk of metabolic disease and impacts renal health in offspring. Biosci Rep. 2018;38(2):BSR20180050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT. Prenatal risk factors for childhood CKD. J Am Soc Nephrol. 2014;25(9):2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armitage JA, Lakasing L, Taylor PD, Balachandra AA, Jensen RI, et al. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565(1):171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glastras SJ, Chen H, Tsang M, Teh R, McGrath RT, et al. The renal consequences of maternal obesity in offspring are overwhelmed by postnatal high fat diet. PLoS One. 2017;12(2):e0172644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzi VH, Sene LD, Fernandez CD, Gontijo JA, Boer PA. Impact of long-term high-fat diet intake gestational protein-restricted offspring on kidney morphology and function. J Dev Orig Health Dis. 2017;8(1):89–100. [DOI] [PubMed] [Google Scholar]
- 67.Intapad S, Dasinger JH, Johnson JM, Brown AD, Ojeda NB, Alexander BT. Male and female intrauterine growth-restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension. 2019;73(3):620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boubred F, Daniel L, Buffat C, Tsimaratos M, Oliver C, Lelièvre-Pégorier M, et al. The magnitude of nephron number reduction mediates intrauterine growth-restriction-induced long term chronic renal disease in the rat. A comparative study in two experimental models. J Transl Med. 2016;14(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, et al. Influence of diet and genetics on hypertension and renal disease in dahl salt-sensitive rats. Physiol Genomics. 2004;16(2):194–203. [DOI] [PubMed] [Google Scholar]
- 70.•.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in dahl salt-sensitive rats. Hypertension. 2015;65(2):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; Exposure to dietary components during gestation and lactation in the rat has a significant effect on disease outcomes in the offspring.
- 71.Torrens C, Bawley L, Anthony FW, Dance CS, Dunn R, et al. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47(5):982–7. [DOI] [PubMed] [Google Scholar]
- 72.do Carmo Franco M, Ponziio BF, Gomes GN, Gil FZ, Tostes R, et al. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85(7–8):327–33. [DOI] [PubMed] [Google Scholar]
- 73.Roghair RD, Wemmie JA, Volk KA, Scholz TD, Lamb FS, Segar JL. Maternal antioxidant blocks programmed cardiovascular and behavioural stress responses in adult mice. Clin Sci. 2011;121(10):427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tain YL, Hseieh CS, Lin IC, Chen CC, Sheen JM, Huang LT. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: the impact of nitric oxide pathway. Nitric Oxide. 2010;23(1): 34–41. [DOI] [PubMed] [Google Scholar]
- 75.Tain YL, Seen JM, Chen CC, Yu HR, Tiao MM, et al. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic Res. 2014;48(5): 580–6. [DOI] [PubMed] [Google Scholar]
- 76.Tain YL, Lee WC, Hsu CN, Lee WC, Huang LT, et al. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS One. 2013;8(2):e55420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasser J, Murphy S, Granger J. Emerging drugs for preeclampsia– the endothelium as a target. Expert Opin Emerg Drugs. 2015;20(4): 527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hegde C The use of l-arginine in the management of pre-eclampsia and intrauterine growth restriction. J Obstet Gynecol India. 2012;62:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salles A, Galvao TF, Silva MT, Motta LC, Pereira MG. Antioxidants for preventing preeclampsia: a systematic review. Sci World J. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28(4):369–82. [DOI] [PubMed] [Google Scholar]
- 81.Groom KM, McCowan LM, Mackay LK, Lee AC, Gardener G, Unterscheider J, et al. STRIDER NZA us: a multicentre randomised controlled trial of sildenafil therapy in early-onset fetal growth restriction. BJOG Int J Obstet Gynaecol. 2019;126(8):997–1006. [DOI] [PubMed] [Google Scholar]
- 82.Paauw ND, Terstappen F, Ganzevoort W, Joles JA, Gremmels H, Lely AT. Sildenafil during pregnancy: a preclinical meta-analysis on fetal growth and maternal blood pressure. Hypertension. 2017;70(5):998–1006. [DOI] [PubMed] [Google Scholar]
- 83.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Phys Regul Integr Comp Phys. 2013;305(4):R397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil treatment ameliorates the maternal syndrome of preeclampsia and rescues fetal growth in the dahl salt–sensitive rat. Hypertension. 2016;67(3):647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pels A, Jakobsen JC, Ganzevoort W, Naaktgeboren CA, Onland W, van Wassenaer-Leemhuis A, et al. Detailed statistical analysis plan for the Dutch STRIDER (sildenafil therapy in dismal prognosis early-onset fetal growth restriction) randomised clinical trial on sildenafil versus placebo for pregnant women with severe early onset fetal growth restriction. Trials. 2019;20(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cunningham M Jr, Castillo J, Ibrahim T, Cornelius DC, Campbell N, et al. AT1-AA (angiotensin II type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. Hypertension. 2018;71(5):886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-α blockade. Hypertension. 2008;52(6):1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ, et al. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut. 2016;65(8):1261–8. [DOI] [PubMed] [Google Scholar]
- 89.Hazes JM, Coulie PG, Geenen V, Vermeire S, Carbonnel F, et al. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology. 2011;50(11):1955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kammerlander H, Nielsen J, Knudsen T, Kjeldsen J, Friedman S, Nørgård BM. Anti–TNF-α use during the third trimester of pregnancy in women with moderate–severe inflammatory bowel disease and the risk of preterm birth and low birth weight. Inflamm Bowel Dis. 2017;23(11):1916–23. [DOI] [PubMed] [Google Scholar]
- 91.Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220(1):71–6. [DOI] [PubMed] [Google Scholar]


