Abstract
Recent studies have highlighted the dentate gyrus as a region of increased vulnerability in mouse models of Down syndrome (DS). It is unclear to what extent these findings are reflected in the memory profile of people with the condition. We developed a series of novel tasks to probe distinct medial temporal functions in children and young adults with DS, including object, spatial, and temporal order memory. Relative to mental age-matched controls (n=45), individuals with DS (n=28) were unimpaired on subtests involving short-term object or configural recall that was divorced from spatial or temporal contexts. By contrast, the DS group had difficulty recalling spatial locations when contextual information was salient and recalling the order in which objects were serially presented. Results are consistent with dysfunction of spatial and temporal contextual pattern separation abilities in individuals with DS, mediated by the hippocampus, including the dentate gyrus. Amidst increasing calls to bridge human and animal work, the memory profile demonstrated here in humans with DS is strikingly similar to that of the Ts65Dn mouse model of DS. The study highlights the trisynaptic circuit as a potentially fruitful intervention target to mitigate cognitive impairments associated with DS.
Keywords: Down syndrome, hippocampus, object recognition, spatial memory, trisynaptic circuit
1 ∣. INTRODUCTION
The hippocampus plays a central role in binding our memories to distinct spatial and temporal contexts (Cohen et al., 1999; Diana, Yonelinas, & Ranganath, 2007). Human and animal studies suggest that regions within the medial temporal lobe are functionally specialized to facilitate this process (Jones & Mchugh, 2011; Kesner, Morris, & Weeden, 2012). The dentate gyrus (DG) is theorized to act as a competitive network, parsing overlapping neural representations during encoding so that their similarity does not induce interference, a computational process known as pattern separation (Bakker, Kirwan, Miller, & Stark, 2008; Doxey & Kirwan, 2014; Kesner, Taylor, Hoge, & Andy, 2015; Morris, Churchwell, Kesner, & Gilbert, 2012; Reilly, Bhattacharyya, Howard, & Ketz, 2014). The sparse mossy fibers of DG project to CA3, which is posited as an auto-associative network, pairing representations (e.g., an object within a particular context) such that even degraded inputs are able to trigger recall of the complete memory (Neunuebel & Knierim, 2014; Rolls, 2007). Subregion CA1 completes this trisynaptic circuit, with rodent literature suggesting that this region plays a prominent role in organizing events based on their occurrence in time (Kesner et al., 2012). Outside of the hippocampus proper, recognition of visual items in the absence of spatial or temporal contexts has been ascribed to perirhinal cortex, a convergence point for the ventral visual stream (Brown & Aggleton, 2001; Davachi, Mitchell, & Wagner, 2003; although see Kirwan & Stark, 2007; Yassa et al., 2010; Yonelinas, Hopfinger, Buonocore, Kroll, & Baynes, 2001 for findings implicating the hippocampus in pattern separation of object features). Parahippocampal cortex, in contrast, appears particularly important for the holistic processing and recognition of visual scenes (Eichenbaum, 1987; Goodrich-Hunsaker, Hunsaker, & Kesner, 2008; Howard, Umaran, Ólafsdóttir, & Spiers, 2011; Kesner et al., 2010). Although the hippocampus traditionally has been associated with long term memory (Bontempi, Laurent-Demir, Destrade, & Jaffard, 1999; Frankland & Bontempi, 2005; Hammond, Tull, & Stackman, 2004; Mcgaugh, 2000), patients with damage to the hippocampus have difficulty remembering relational mappings, such as objects within particular spatial contexts, even over short intervals, suggesting that the hippocampus may facilitate rapid encoding of multi-modal associations (Hannula, Tranel, & Cohen, 2006; Olson, Page, Moore, Chatterjee, & Verfaellie, 2006; Piekema, Kessels, Rijpkema, & Fernández, 2009; Watson, Voss, Warren, Tranel, & Cohen, 2013).
Down syndrome (DS, trisomy 21), the most common intellectual disability of known genetic origin, is characterized by pervasive deficits in episodic memory and learning that have widespread adverse implications for daily functioning (Carlesimo, Marotta, & Vicari, 1997; Edgin, Pennington, & Mervis, 2010b; Spanò; & Edgin, 2016). Neuroimaging and neuropsychological studies suggest that later-maturing neural regions, including areas of the medial temporal lobe, may be differentially vulnerable in individuals with DS, with structural imaging studies reporting hippocampal volume loss (Pinter, Eliez, Schmitt, Capone, & Reiss, 2001; White, Alkire, & Haier, 2003), autopsy studies showing reduced myelination in the hilar region of the DG (Ábrahám et al., 2012), and several studies showing that DS groups have difficulties on tasks that are sensitive to hippocampal disruption relative to mental age-matched controls (Edgin et al., 2010a; Lavenex et al., 2015; Pennington, Moon, Edgin, Stedron, & Nadel, 2003; Purser et al., 2015; Ribordy, Jabès, Banta Lavenex, & Lavenex, 2013).
Over the past two decades, mouse models of DS, such as Ts65Dn and Tc1, have advanced our understanding of the underlying neurobiology of the disorder (Edgin, Mason, Spanò, Fernández, & Nadel, 2012; Kleschevnikov et al., 2012a). The Ts65Dn mouse is trisomic for large, contiguous segments of chromosome 16, a homologue of human chromosome 21, which is completely or partially triplicated in humans with DS. Ts65Dn mice exhibit widespread changes to the subfield structure and electrophysiology of the hippocampus, including reduced neuronal and synaptic density in DG, CA1 and CA3, reduced connectivity in mossy fiber CA3 cell circuitry, impaired CA3 place cell activity, reduced perforant path and Schaffer collateral long term potentiation, and a disruption of the basic stoichiometry underlying excitatory vs. inhibitory signaling (Ayberk Kurt, Ilker Kafa, Dierssen, & Ceri Davies, 2004; Best, Cramer, Chakrabarti, Haydar, & Galdzicki, 2012; Deidda et al., 2015; Insausti et al., 1998). These abnormalities are accompanied by deficits on rodent “hippocampal” tasks requiring spatial navigation or context discrimination (Hyde, Frisone, & Crnic, 2001; Reeves et al., 1995; Sérégaza, Roubertoux, Jamon, & Soumireu-Mourat, 2006). For instance, a recent study with Tc1 mice showed impaired short-term plasticity (mossy fiber transmission tested via paired-pulse facilitation) in DG-CA3 excitatory synapses, suggesting less developed input into the CA3 auto-associative network over even short-term stimulation intervals (Witton et al., 2015). The authors suggest that these changes are likely to impair disambiguation and integration of temporal and spatial contextual information during pattern completion and separation. Notably, several studies have reported the reversal of memory deficits in mouse models of DS following behavioral or pharmaceutical interventions, sparking clinical trials aimed at mitigating learning impairments in humans with DS (Fernandez et al., 2007; Guidi et al., 2014; Latchney, Jaramillo, Rivera, Eisch,& Powell, 2015; Pons-espinal, Lagran, & Dierssen, 2013). These clinical trials rely on translational work that links animal models with outcome measures in humans.
In a recent study, Smith, Kesner, & Korenberg (2014) tested object, spatial and configural novelty detection in Ts65Dn/TnJ mice. Relative to control littermates, Ts65Dn mice exhibited short-term deficits in object recognition only when objects were presented in an environment rich with spatial cues, suggesting that context cues interfered with memory representations for the familiar object. The mice also showed less discrimination for objects that changed location relative to those that remained stationary in the environment. They were unimpaired when short-term object recognition tasks did not involve contextual cues. Such findings suggest an uneven profile of medial temporal lobe function in the Ts65Dn mice, characterized by relative weaknesses on tasks that are linked to DG and involve the resolution of interference associated with spatial contexts, coupled with strengths on short-term object recognition tasks thought to rely primarily on perirhinal cortex (Fernandez & Garner, 2008).
Few studies have examined medial temporal function with this degree of precision in humans with DS. In those studies that have assessed hippocampal function in humans with DS, the tendency has been to rely on global measures such as the CANTAB Paired Associates Learning Task, which has no direct analogue in the animal literature (Edgin et al., 2010a; Pennington et al., 2003). With a view to moving animal models “from bench to bedside,” we developed a battery of measures to assess different medial temporal subfunctions, including visual and spatial item memory and memory for the associations of items with specific spatial and temporal contexts. Based on Smith et al. (2014), we hypothesized that individuals with DS would experience deficits on context-bound visual, spatial and temporal memory tasks.
2 ∣. METHOD
2.1 ∣. Participants
Participants were recruited via newspaper advertisements, fliers and information booths at community events in Southern Arizona. The 28 participants with DS had a mean age of 18.09 years (SD=5.64; range=6.24–25.42) and 54% were male. Group ethnicity breakdown was 48% White, 37% Hispanic, 4% African American, 4% Native American, and 7% biracial. Median household income was between $50 and $75,000.
The typically developing (TD) control group included 45 (38% male) children with a mean age of 4.39 years (SD=1.24; range=2.25–6.58). Exclusion criteria for this group were language delays, neurological conditions, attention deficit hyperactivity disorder, autism or IQ<70. Ethnic breakdown was 80% White, 16% Hispanic, 2% Asian, and 2% biracial. Median household income was between 50 - $75,000 As is typical when assessing populations with developmental delay, the control group was selected to match the DS group on mental age, rather than chronological age (Jarrold & Brock, 2004). Accordingly, the DS and control groups achieved similar verbal [t(65)=.36, p=.72] and nonverbal [t(65)=−1.08, p=.29] scores on the Kaufman Brief Intelligence Test (Kaufman & Kaufman, 2004) and there were no group differences in gender [χ2(1)=1.75, p=.19], race [χ2(4)=5.11, p=.28], or household income bracket [χ2(4)=2.02, p=.73].
2.2 ∣. Procedures
All procedures were approved by the University of Arizona Institutional Review Board. Parents or legal guardians provided written consent and participants provided assent. Participants completed subtests from the newly developed Arizona Memory Assessment for Preschoolers and Special Populations (A-MAP; Edgin & Clark, 2016 2015.) as part of a laboratory-based neuropsychological assessment. Subtests included here were designed to tap functions theoretically ascribed to distinct medial temporal regions.
2.3 ∣. Measures
The A-MAP uses small, 3-dimensional, concrete objects (e.g., balloon, toy car, plastic star) and an object hiding board with 12 wells randomly positioned on its surface. Objects were chosen carefully as well-known, everyday items that are listed on toddler vocabulary checklists (Fenson et al., 2007). In pilot work, participants with DS and a preschool control group each named an average of 84% of the objects correctly.
Temporal order. After a brief training phase to establish understanding of the concepts of first and last, participants are told that they will see a “parade of objects.” Twelve objects are drawn one at a time from an opaque box. Participants are given 5 s to view and manipulate each object before it is placed back in the box out of view. Immediately thereafter, participants are presented with sets of two items that appear in distinct ordinal positions in the parade and are asked which of the two objects appeared first in the parade sequence. The design of this task is similar to tasks tapping temporal order in older populations (Roberts, Ly, Murray, & Yassa, 2014). The dependent variable is the proportion of correct responses over six total trials.
Visual object recognition. Participants are shown an A4 booklet with three, horizontally-aligned black and white photographs on each page (Figure 1). One of the photographs shows an object presented during the temporal order task, while the other two pictures show novel objects with overlapping features. The dependent variable is the proportion of correct selections of the previously seen object over 12 trials.
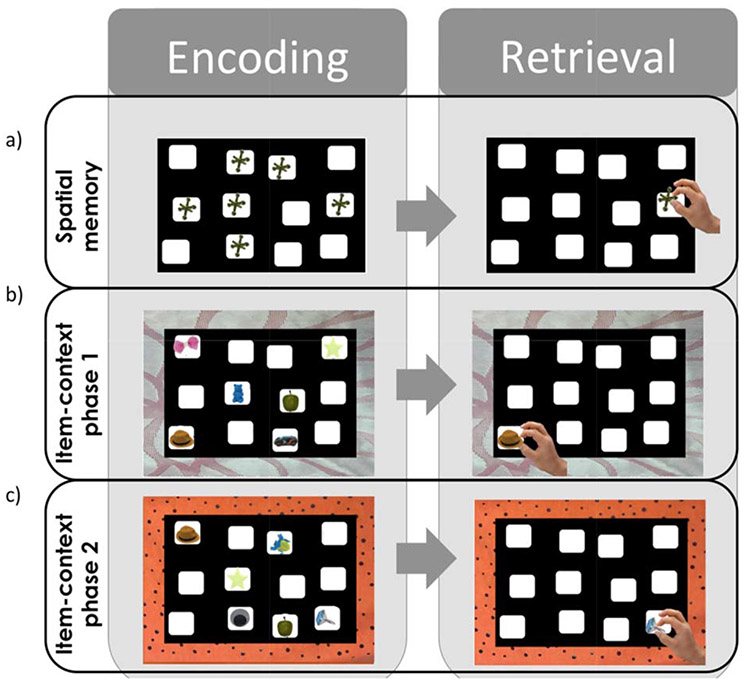
Spatial position recall. The examiner places six identical toy jacks in wells on the hiding board in a standardized configuration and invites the participant to look carefully at the board for 30 s (Figure 2a). The examiner then removes the jacks from their wells and invites the participant to replicate the spatial configuration. Up to six trials are administered, with corrective feedback provided after each trial. Based on pilot analyses, the subtest is discontinued and the participant is allocated full credit for remaining trials after successfully replicating the complete board configuration on two consecutive trials. The dependent variable is the proportion of correct placements over the six trials of the task. Data were not available for two TD 2-year-old participants who did not complete the task due to attention difficulties.
- Item in context
- Phase 1 (IIC 1): Six everyday objects are placed in specific wells on the hiding board (see Figure 2b). The board is placed on a distinctive orange or pink mat (counterbalanced across IIC 1 and 2) and the participant is told, “This is where the objects belong when the board is on the orange/pink mat.” After a 30 s study phase, the examiner places the six target objects and three lure objects in a tray in front of the participant and the participant is asked to place the objects in their respective wells, with the mat operating as a cue or context for recall. After participants complete two consecutive trials with no errors, full credit is granted for remaining trials.
- Phase 2 (IIC 2): A new, distinctive orange/pink mat is placed below the hiding board to provide a novel context and the participant is told, “this is where the objects belong when the board is on the pink/orange mat” (Figure 2c). Three objects from IIC 1 and three objects from the original object parade are placed in new locations on the board. Administration is similar to that for IIC1. Four TD participants did not complete this phase due to attention difficulties.
FIGURE 1.
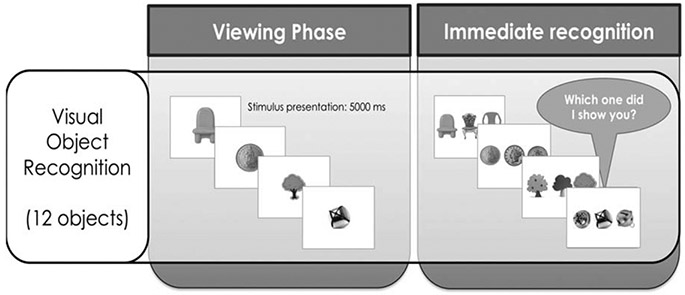
Visual object recognition task. Participants see objects during the object parade and then must identify them from an array of similar objects.
FIGURE 2.
Board configurations used to assess spatial position memory and item in context recall. The participant is required to place the objects in their demonstrated spatial locations across six consecutive trials. For all phases, all nine objects are presented to the participant during all trials, with the three remaining objects acting as lures for each board.
The two IIC phases are designed to assess memory in relation to specific contextual cues (i.e., the colored mats). Unlike the spatial position recall task, distinct items are used, pressing for an encoding strategy that relies on allocentric spatial processing of the relative positions of the objects (Blue, Kazama, & Bachevalier, 2013). Note that there is also an overlap in the objects employed across these phases, making the contextual information particularly relevant and potentially inducing interference. The primary dependent variable for each phase was the number of correct placements of non-lure objects. Several additional metrics were calculated to provide further insight into the nature of participant errors. Intrusion errors included the total number of placements of lure items that should not have been placed on the board. Intrusion errors during IIC phase 1 suggest difficulties remembering the items themselves, whereas intrusions during phase 2 suggest difficulties with contextual bindings of specific items to the separate mat contexts. The total number of pairwise swap errors included instances where participants transposed the locations of two items, indicating a difficulty with relational binding of items to specific locations (Watson et al., 2013). Finally, deformation errors included the ratio of placements into wells that should have been left empty relative to the total number of placements for each trial. These errors suggest difficulties representing the overall shape of the board configuration as opposed to difficulties representing the relational configuration of objects within that shape.
3 ∣. RESULTS
Table 1 describes DS and TD group performance on each A-MAP subtest. To minimize the risk of type 2 error, we first performed a multivariate analysis of group differences across all A-MAP subtests, which revealed a group difference in overall performance, F(6,62)=4.03, p=.002. Follow-up t tests showed that groups performed equivalently on the object recognition subtest and on the subtest assessing memory for spatial positions in the absence of contextual cues. However, participants with DS were, on average, less able to remember the temporal order of presented objects. Furthermore, the DS group scored lower in the second, but not the first, IIC phase.
TABLE 1.
Mean A-MAP subtest performance of DS and typically developing groups
| A-MAP subtest M (SD) % Correct |
TD (n=45) | DS (n=28) | p | Cohen’s d |
|---|---|---|---|---|
| Temporal order memory | 70.74 (20.46) | 57.14 (21.96) | <.01 | .64 |
| Visual recognition | 71.48 (19.50) | 67.26 (15.86) | .34 | .24 |
| Spatial position memory | 74.48 (15.73) | 73.41 (15.63) | .78 | .07 |
| Item in context phase 1 | 73.21 (28.27) | 65.77 (27.98) | .28 | .26 |
| Item in context phase 2 | 69.31 (30.07) | 51.19 (25.43) | .01 | .65 |
Note: Scores reflect the proportion of correct responses over all administered trials.
Figure 3 provides a more detailed description of trajectories of group performance over the six trials administered for the spatial position task. A repeated measures ANOVA showed no significant difference in group performance on any trial, with both groups demonstrating increasing accuracy over the course of the task, Fgroup(1,69)=.10, p=.752, Ftrial(5,345)=12.23, p<.001, Fgroup × trial(5,345)=.50, p=.776.
FIGURE 3.
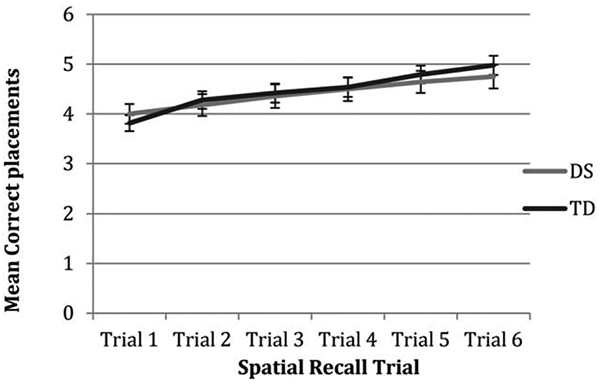
Average correct placements for each trial of the spatial position recall task in DS and TD groups.
Figure 4 describes group performance across the six IIC phase 1 trials. Again, there were no group differences in performance for any trial, Fgroup(1,71)=1.20, p=.28, Ftrial(5,355)=32.24, p <. 001, Fgroup × trial(5,355)=.57, p=.721. Additionally, groups made a similar number of incorrect placements of lure items over the course of all IIC phase 1 trials, M(SD)DS=5.79 (6.15) vs. M(SD)TD=3.58 (4.93); t(71)=1.69, p=.095. Participants made very few pairwise swap errors (maximum=2 per group) and the proportion of swap errors was equivalent across the two groups, 14 vs. 20%, χ2(1)=.38, p=.535. There were no group differences in the rate of deformation errors over the six trials, F(70,1)=3.40, p=.069.
FIGURE 4.
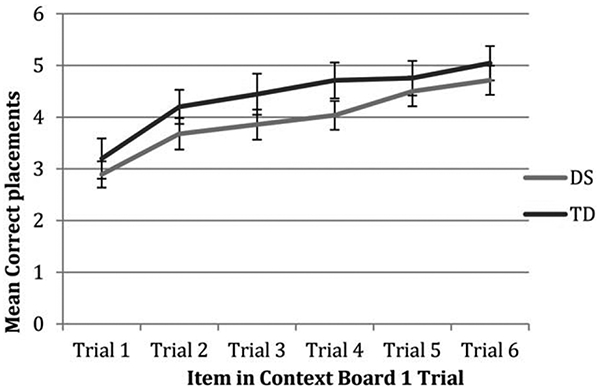
Average correct placements for each trial of the IIC phase 1 in DS and TD groups.
By contrast, the mean performance of the DS group was approximately 1 point below the TD group throughout the course of IIC phase 2, Fgroup(1,67)=6.32, p=.014, Ftrial(5,335)=39.72, p <.001, Fgroup × trial(5,335)=.35, p=.883 (see Figure 5). The DS group also placed more lure items on the board during these IIC 2 trials, M (SD)DS=6.29 (6.42) vs. M(SD)TD=3.41 (45.35); t(67)=2.12, p=.048. Pairwise swap errors occurred infrequently, with equivalent proportions of participants making such errors in each group, 29 vs. 16%, χ2(1)=1.79, p=.181. There were no group differences in terms of deformation errors, F(69,1)=.13, p=.718.
FIGURE 5.
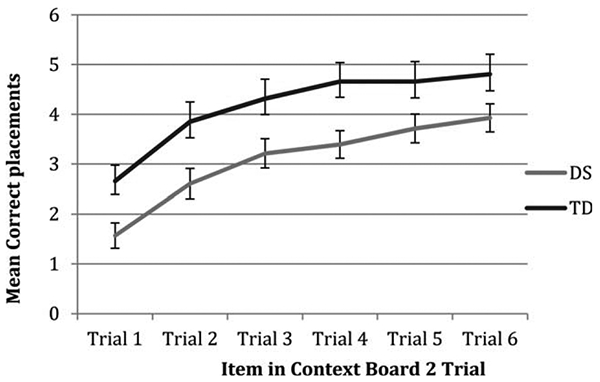
Average correct placements for each trial of IIC phase 2 in DS and TD groups.
An important question that arises from these findings is whether the decrement in IIC 2 performance in the DS group is due to a difficulty in overcoming interference from the board configuration learned in IIC 1. To address this question, we calculated the number of times during the IIC 2 trials that participants placed objects into locations that would previously have been correct for those same items in IIC 1. Controlling for accuracy in IIC 1, the DS group were more likely than the TD group to place items in locations that would have been correct for those items in IIC 1 when completing IIC 2 trials, M(SD)DS=4.28 (4.71) vs. M(SD)TD=2.07 (3.47); F(1,70)=4.60, p=.035.
4 ∣. DISCUSSION
Mouse models highlight the DG as a region of vulnerability in DS (Smith et al., 2014). A key step in translating these findings to clinical work involves conducting similarly precise assessments of medial temporal functioning in people with DS. Consistent with our hypothesis, the profile of strengths and weaknesses reported here in people with DS is strikingly similar to that reported by Smith et al. (2014) in the Ts65Dn model. In our study, the DS group performed poorly on tasks that involved associating items with distinct spatial and temporal contexts. Overall, findings point to disruptions in the trisynaptic circuit as an important contributor to cognitive difficulties in DS.
Similar to Smith et al. (2014), participants with DS in this study did not show deficits relative to what would be expected for their mental age on an object recognition task, which required them to identify the object they had previously seen from an array of objects with similar visual features. These findings are consistent with findings from patients with medial temporal lesions, which show that short term memory for single items generally is intact (Cave & Squire, 1992; Warrington & Baddeley, 1974). Disambiguation of overlapping visual features in the absence of associative spatial contexts has been attributed to perirhinal cortex (Brown & Aggleton, 2001; Davachi, 2006; Staresina & Davachi, 2006), although other studies have linked fine-grained pattern separation of visual items to the hippocampus (Kirwan & Stark, 2007). The relative sparing of performance on the object recognition task raises the possibility that some medial temporal regions, including perhaps perirhinal cortex, may be relatively intact in children and young adults with DS.
The DS group also performed relatively well on the spatial position recall task, which involved remembering the locations of non-distinct items (i.e., toy jacks) when there were no associated contextual cues. This finding is consistent with studies suggesting that immediate spatial memory may be commensurate with general cognitive ability or perhaps even an area of strength in individuals with DS (Edgin et al., 2010b; Yang et al., 2014). In addition, the DS group learned the IIC board 1 configuration to an equivalent degree as the TD group, with both groups showing high levels of accuracy by the sixth learning trial. Of course, these findings are somewhat distinct from Smith et al., who reported deficits in spatial location memory in their Ts65Dn mice. They also are dissimilar to studies indicating that hippocampal compromise in humans disrupts the ability to bind objects to specific locations (Braun et al., 2011; Olson et al., 2006). One possibility is that spatial configurations in these AMAP phases could be encoded imprecisely as an integrated “whole” (not unlike a visual scene) without having to encode exact, fine-grained, metric representations of angles or relative distance (Kesner & Goodrich-Hunsaker, 2010; Kesner et al., 2012). Such a strategy would be consistent with an attentional bias toward global rather than local information in those with DS (Porter & Coltheart, 2006) and with a relative sparing of parahippocampal function (Howard et al., 2011). Relatedly, hippocampal deficits are most pronounced for complex tasks that involve fine-grained, high-resolution encoding of item-context pairings (Yonelinas, 2013). Unlike previous studies with amnesic patients (Watson et al., 2013), our board included distinct wells for item placements, limiting our ability to detect potentially subtle object displacements along a continuous metric.
The DS group showed a decrement in performance compared to the control group when they were required to learn a new configuration associated with a different mat context in the second set of IIC trials. During this second IIC phase, individuals with DS were more likely than TD children to place objects in locations that had previously been, but were no longer, correct for those objects. They were no more likely than controls to distort the overall configuration of objects, suggesting that item displacements occurred within the confines of preserved overall spatial configuration. Overall, findings point to difficulties resolving interference from competing spatial contexts. While it is difficult, of course, to parse contextual pattern separation and pattern completion processes based on behavioral performance (Hunsaker & Kesner, 2013), the individuals with DS were as able as the control group to recall the first board configuration when they were presented with the mat cue, making it difficult to attribute their difficulties to pattern completion alone. Moreover, there were no delays between encoding and testing for any of these tasks, biasing them to capture encoding processes, a distinguishing feature of pattern separation relative to pattern completion tasks (Hunsaker & Kesner, 2013; Liu, Gould, Coulson, Ward, & Howard, 2015). Difficulties emerged when high levels of interference from multi-modal representations were involved (i.e., when a new board configuration with overlapping spatial and contextual representations had to be learned). A deficit in contextual pattern separation is consistent with data from mouse models of DS, which show that microstructural changes to hippocampal dendritic spine morphology, lower rates of neurogenesis and reduced long term potentiation in the DG, as well as alterations in DG-CA3 excitatory connectivity, are among the most pronounced disruptions to neural development (Kleschevnikov et al., 2012b; Witton et al., 2015). Granule cells are critical computational units in pattern separation (Leutgeb et al., 2007) and dysfunction of these cells represents a plausible neurobiological mechanism for memory disruption in DS.
Findings suggest that memory for temporal order may be another area of weakness for individuals with DS. In a recent study using an elicited imitation procedure, Milojevich & Lukowski (2016) found that preschoolers with DS showed similar recall of individual actions relative to mental age-matched control children both during an immediate posttest and after a 1-month delay. At the 1-month delay, however, they were less likely than the control group to recall the learned sequences in the correct order. Difficulties in temporal order memory may therefore be present in children with DS even younger than those tested in the current study.
Theories of typical memory development have focused on long-term consolidation and strategy use as the primary mechanisms driving developmental shifts in typical children’s memory through infancy and the transition out of childhood amnesia (Bauer, 2005; Flavell, 1970). However, the current data from individuals with DS provide a model of dysfunction in the hippocampus that may help inform what key processes could be impaired. While some work has begun to address differences in encoding of overlapping spatial and temporal patterns across early childhood, few studies have employed tasks that directly compare functions likely to be mediated by distinct medial temporal regions. Our findings suggest that developing individuals with DS, who do show disruptions in the trisynaptic circuit (Contestabile et al., 2007), appear to have the most difficulty on tasks that require the encoding of overlapping patterns in space and time. It is unlikely that difficulties remembering overlapping patterns on the IIC are due to working memory load, because of the control for the number of items to be remembered in each of the previous hiding boards (i.e., spatial position and the first IIC board). It is also unlikely that our results are attributable to attention difficulties, as the temporal order test was administered very early in the sequence and attentional difficulties would have been manifested across a number of the other measures. In total, these data add to previous investigations using similar measures in the Ts65dn mouse model (Smith et al., 2014), allowing for the first corroboration of this profile of deficits in humans with the condition. Down syndrome may provide a model for understanding trisynaptic circuit function in more detail. While DS does not provide a perfect model, it represents one of the most well characterized nonlesion conditions affecting the development of this region.
From a practical standpoint, there have been calls for valid and reliable measures of cognitive performance for use in clinical trials with DS (de Sola et al., 2015). With its links to basic neuroscience, the A-MAP promises to address this need. Interference between competing associative memories may lead to “representational inflexibility” in individuals with DS (Deng, Aimone, & Gage, 2010; Yassa & Stark, 2011). Examples of tasks that may become challenging as a result of these impairments include learning the locations of rooms in two buildings, remembering where one has placed one toy relative to another, or discriminating between two conversations held in close succession. Effective remediation of these difficulties in individuals with DS would have clear implications for quality of life.
Future studies using the A-MAP would benefit from inclusion of a wider age range of participants with DS, particularly given the varying developmental courses of specific hippocampal subfields in typical development and their long-range connectivity with the prefrontal cortex (Lavenex & Banta Lavenex, 2013; Simons & Spiers, 2003). Deficits in prefrontal function, for instance, are likely to become more pronounced over time, potentially sabotaging the ability of adults with DS to use alternative strategies to compensate for poor hippocampal binding processes. Although our findings are consistent with a deficit in memory for object-context associations, neuroimaging studies will be necessary to shed light on whether the difficulties reported here are indeed related to hippocampal activation or whether they are driven by interference control mechanisms typically modulated by anterior cortical regions. Unfortunately, functional imaging studies are extremely challenging in preschoolers and special populations. Tying behavioral studies to information gained from tightly controlled animal models reflects a “best effort” attempt to overcome these challenges.
Although past studies of individuals with DS have suggested deficits in medial temporal processes, the tasks used in these studies often have been too complex to allow for the delineation of precise areas of weakness in the memory profile. The current study draws upon animal models of DS to reveal specific weakness in effectively encoding and reducing interference between item-context pairings in this population. Therapies targeting the trisynaptic circuit may have promise for alleviating these impairments and improving quality of life for those in the DS community.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Child Health and Human Development RO1 HD088409 R1 (to J.O.E.) and by the LuMind Research Down Syndrome Foundation. We are grateful to Danielle Abel for her work on the study and for the generous support of families who gave up their time to participate in the research.
REFERENCES
- Ábrahám H, Vincze A, Veszprémi B, Kravják A, Gömöri É, Kovács GG, & Seress L (2012). Impaired myelination of the human hippocampal formation in Down syndrome. International Journal of Developmental Neuroscience, 30, 147–158. [DOI] [PubMed] [Google Scholar]
- Ayberk Kurt M, Ilker Kafa M, Dierssen M, & Ceri Davies D (2004). Deficits of neuronal density in CA1 and synaptic density in the dentate gyrus, CA3 and CA1, in a mouse model of Down syndrome. Brain Research, 1022, 101–109. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, & Stark CEL (2008). Pattern separation in the human. Science, 319, 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ (2005). Developments in declarative memory. Psychological Sciences, 16, 41–47. [DOI] [PubMed] [Google Scholar]
- Best TK, Cramer NP, Chakrabarti L, Haydar TF, & Galdzicki Z (2012). Dysfunctional hippocampal inhibition in the Ts65Dn mouse model of Down syndrome. Experimental Neurology, 233, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, & Bachevalier J (2013). Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of the International Neuropsychological Society, 19, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, & Jaffard R (1999). Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature, 400, 671–675. [DOI] [PubMed] [Google Scholar]
- Braun M, Weinrich C, Finke C, Ostendorf F, Lehmann TN, & Ploner CJ (2011). Lesions affecting the right hippocampal formation differentially impair short-term memory of spatial and nonspatial associations. Hippocampus, 21, 309–318. [DOI] [PubMed] [Google Scholar]
- Brown MW, & Aggleton JP (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Marotta L, & Vicari S (1997). Long-term memory in mental retardation: Evidence for a specific impairment in subjects with Down’s syndrome. Neuropsychologia, 35, 71–79. [DOI] [PubMed] [Google Scholar]
- Cave CB, & Squire LR (1992). Intact verbal and nonverbal short-term memory following damage to the human. Hippocampus, 2(2), 151–164. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, & Nash C (1999). Hippocampal system and declarative (Relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus, 9, 83–98. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, … Ciani E (2007). Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down Syndrome and in Ts65Dn mice. Hippocampus 678, 665–678. [DOI] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, & Wagner AD (2003). Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Science USA, 100, 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deidda G, Parrini M, Naskar S, Bozarth IF, Contestabile A, & Cancedda L (2015). Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nature Medicine, 21, 318–326. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, & Gage FH (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience, 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Science, 11, 379–386. [DOI] [PubMed] [Google Scholar]
- Doxey CR, & Kirwan CB (2014). Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus, 10, 1–10. [DOI] [PubMed] [Google Scholar]
- Edgin JO & Clark CAC (2015). U.S. Patent Application No. 20150379877 A1.
- Edgin JO, Mason GM, Allman MJ, Capone GT, DeLeon I, Maslen C, … Nadel L (2010a). Development and validation of the Arizona Cognitive Test Battery for Down syndrome. Journal of Neurodevelopmental Disorders, 2, 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Mason GM, Spanò G, Fernández A, & Nadel L (2012). Human and mouse model cognitive phenotypes in Down syndrome: Implications for assessment. Prog Brain Research, 197, 123–151. [DOI] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF, & Mervis CB (2010b). Neuropsychological components of intellectual disability: The contributions of immediate, working, and associative memory. Journal of Intellectual Disability Research, 54, 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum HB (1987). The hippocampal system and decbtive memory in animals. Journal of Cognitive Neuroscience 4(3), 217–231. [DOI] [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal D, Dale PS, Reznick JS, & Bates E (2007). The MacArthur-Bates communicative development inventories. Baltimore, MD: Paul H. Brooks. [Google Scholar]
- Fernandez F, & Garner CC (2008). Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behavioral Brain Research, 188, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, & Garner CC (2007). Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nature Neuroscience, 10, 411–413. [DOI] [PubMed] [Google Scholar]
- Flavell JH (1970). Developmental studies of mediated memory. In: Reese HW & Lipsitt LP (Eds.), Advances in child development and behavior (Vol. 5, pp. 181–211). New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Frankland PW, & Bontempi B (2005). The organization of recent and remote memories. Nature Reviews Neuroscience, 6, 119–130. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, & Kesner RP (2008). The interactions and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience, 122, 16–26. [DOI] [PubMed] [Google Scholar]
- Guidi S, Stagni F, Bianchi P, Ciani E, Giacomini A, De Franceschi M, … Bartesaghi R (2014). Prenatal pharmacotherapy rescues brain development in a Down’s syndrome mouse model. Brain, 137, 380–401. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, & Stackman RW (2004). On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory, 82, 26–34. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, & Cohen NJ (2006). The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience [Internet], 26, 8352–8359. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.5222-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Ólafsdóttir HF, & Spiers HJ (2011). Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. Journal of Neuroscience, 31, 5253–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, & Kesner RP (2013). The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews, 37, 36–58. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Frisone DF, & Crnic LS (2001). Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behavioral Brain Research, 118, 53–60. [DOI] [PubMed] [Google Scholar]
- Insausti AM, Megías M, Crespo D, Cruz-Orive LM, Dierssen M, Vallina TF, … Flórez J (1998). Hippocampal volume and neuronal number in Ts65Dn mice: A murine model of Down syndrome. Neuroscience Letters, 253, 175–178. [DOI] [PubMed] [Google Scholar]
- Jarrold C, & Brock J (2004). To match or not to match ? Methodological issues in autism related research. Journal of Autism and Developmental Disorders, 34, 81–86. [DOI] [PubMed] [Google Scholar]
- Jones MW, & Mchugh TJ (2011). Updating hippocampal representations : CA2 joins the circuit. Trends in Neuroscience, 34(10), 536–535. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). The Kaufman Brief Intelligence Test (2nd ed). San Antonio, TX: Pearson. [Google Scholar]
- Kesner RP, & Goodrich-Hunsaker NJ (2010). Developing an animal model of human amnesia: The role of the hippocampus. Neuropsychologia, 48, 2290–2302. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, & Ziegler W (2010). The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiology of Learning and Memory, 93, 111–116. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Morris AM, & Weeden CSS (2012). Spatial, temporal, and associative behavioral functions associated with different subregions of the hippocampus. Oxford Handbook of Comparative Cognition, 1–28. [Google Scholar]
- Kesner RP, Taylor JO, Hoge J, & Andy F (2015). Role of the dentate gyrus in mediating object-spatial configuration recognition. Neurobiology of Learning and Memory, 118, 42–48. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, & Stark CEL (2007). Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory, 14, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Salehi A, & Wu C (2012a). Discoveries in Down syndrome: Moving basic science to clinical care (1st ed.). Elsevier B.V. New York, NY. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Gall J, George L, Nosheny R, Maloney MT, … Mobley WC (2012b). Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome. Neurobiology of Diseases, 45, 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchney SE, Jaramillo TC, Rivera PD, Eisch AJ, & Powell CM (2015). Chronic P7C3 treatment restores hippocampal neurogenesis. Neuroscience Letters, 591, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, & Banta Lavenex P (2013). Building hippocampal circuits to learn and remember: insights into the development of human memory. Behavioral Brain Research, 254, 8–21. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Bostelmann M, Brandner C, Costanzo F, Fragnière E, Klencklen G, … Vicari S (2015). Allocentric spatial learning and memory deficits in Down syndrome. Frontiers in Psychology, 6, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M, Moser EI, & Moser I (2007). Pattern separation in the dentate gyrus separation and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Liu KY, Gould RL, Coulson MC, Ward EV, & Howard RJ (2015). Tests of pattern separation and pattern completion in humans – A systematic review. Hippocampus, 0, 2–31. [DOI] [PubMed] [Google Scholar]
- Mcgaugh JL (2000). Memory — A century of consolidation. Science, 287, 248–251. [DOI] [PubMed] [Google Scholar]
- Milojevich H, & Lukowski A (2016). Recall memory in children with Down syndrome and typically developing peers matched on developmental age 60 (1), 89–100. [DOI] [PubMed] [Google Scholar]
- Morris AM, Churchwell JC, Kesner RP, & Gilbert PE (2012). Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiology of Learning and Memory, 97, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, & Knierim JJ (2014). CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron [Internet], 81, 416–427. Available from: 10.1016/j.neuron.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, & Verfaellie M (2006). Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience, 26, 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, & Nadel L (2003). The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Development, 74, 75–93. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RPC, Rijpkema M, & Fernández G (2009). The hippocampus supports encoding of between-domain associations within working memory. Learning and Memory, 16, 231–234. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Eliez S, Schmitt JE, Capone GT, & Reiss AL (2001). Neuroanatomy of Down’s syndrome: A high-resolution MRI study. American Journal of Psychiatry, 158, 1659–1665. [DOI] [PubMed] [Google Scholar]
- Pons-Espinal M, Lagran MMD, & Dierssen M (2013). Neurobiology of Disease Environmental enrichment rescues DYRK1A activity and hippocampal adult neurogenesis in TgDyrk1A. Neurobiology of Diseases, 60, 18–31. [DOI] [PubMed] [Google Scholar]
- Porter M, & Coltheart M (2006). Global and local processing in William syndrome, autism, and Down syndrome: Percetion, attention, and construction. Developmental Neuropsychology, 30, 771–789. [DOI] [PubMed] [Google Scholar]
- Purser HRM, Farran EK, Courbois Y, Lemahieu A, Sockeel P, Mellier D, & Blades M (2015). The development of route learning in Down syndrome, Williams syndrome and typical development: investigations with virtual environments. Developmental Science, 18, 599–613. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, – Davisson M (1995). A mouse model for Down syndrome exhibits learning and behavior deficits. Nature Genetics, 10, 196–201. [DOI] [PubMed] [Google Scholar]
- Reilly RCO, Bhattacharyya R, Howard MD, & Ketz N (2014). Complementary learning systems. Cognitive Science, 38(2014), 1229–1248. [DOI] [PubMed] [Google Scholar]
- Ribordy F, Jabès A, Banta Lavenex P, & Lavenex P (2013). Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cognitive Psychology, 66, 1–29. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Ly M, Murray E, & Yassa MA (2014). Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus, 24, 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2007). An attractor network in the hippocampus: theory and neurophysiology. Learning and Memory, 14, 714–731. [DOI] [PubMed] [Google Scholar]
- Sérégaza Z, Roubertoux PL, Jamon M, & Soumireu-Mourat B (2006). Mouse models of cognitive disorders in trisomy 21: A review. Behavioral Genetics, 36, 387–404. [DOI] [PubMed] [Google Scholar]
- Simons JS, & Spiers HJ (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience, 4, 637–648. [DOI] [PubMed] [Google Scholar]
- Smith GK, Kesner RP, & Korenberg JR (2014). Dentate gyrus mediates cognitive function in the Ts65Dn/DnJ mouse model of down syndrome. Hippocampus, 24, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sola S, de la Torre R, Sanchez-Benavides G, Benejam B, Cuenca-Royo A, del Hoyo L, … Legout V (2015). A new cognitive evaluation battery for Down syndrome and its relevance for clinical trials. Frontiers in Psychology, 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanò G, & Edgin JO (2016). Everyday memory in individuals with Down syndrome: Validation of the Observer Memory Questionnaire - Parent Form. Child Neuropsychology, 7049, 1–13. [DOI] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2006). Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience, 26, 9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, & Baddeley AD (1974). Amnesia and memory for visual location. Neuropsychologia, 12, 257–263. [DOI] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, & Cohen NJ (2013). Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus, 23, doi: 10.1002/hipo.22115.570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Alkire MT, & Haier RJ (2003). A voxel-based morphometric study of nondemented adults with Down Syndrome. Neuroimage, 20, 393–403. [DOI] [PubMed] [Google Scholar]
- Witton J, Padmashri R, Zinyuk LE, Popov VI, Kraev I, Line SJ, Jensen TP, Tedoldi A, Cummings DM, Tybulewicz VLJ, Fisher EMC, Bannerman DM, Randall AD, Brown JT, Edwards F,A, Rusakov D,A, Stewart MG, & Jones MW (2015). Hippocampal circuit dysfunction in the Tc1 mouse model of Down syndrome. Nature Neuroscience, 18(9), 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Conners FA, & Merrill EC (2014). Visuo-spatial ability in individuals with Down syndrome: Is it really a strength? Research in Developmental Disabilities, 35, 1473–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, & Stark CEL (2011). Pattern separation in the hippocampus. Trends in Neuroscience, 34, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, & Stark CEL (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage, 51, 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioral Brain Research [Internet], 254, 34–44. Available from: 10.1016/j.bbr.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, & Baynes K (2001). Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport, 12, 359–363. [DOI] [PubMed] [Google Scholar]