Abstract
Background.
The diet of most adults is low in fish and, therefore, provides limited quantities of the long-chain, omega-3 fatty acids (LCn-3FAs), eicosapentaenoic and docosahexaenoic acids (EPA, DHA). Since these compounds serve important roles in the brain, we sought to determine if healthy adults with low-LCn-3FA consumption would exhibit improvements in neuropsychological performance and parallel changes in brain morphology following repletion through fish oil supplementation.
Methods.
In a randomized, controlled trial, 271 mid-life adults (30–54 years of age, 118 men, 153 women) consuming ⩽300 mg/day of LCn-3FAs received 18 weeks of supplementation with fish oil capsules (1400 mg/day of EPA and DHA) or matching placebo. All participants completed a neuropsychological test battery examining four cognitive domains: psychomotor speed, executive function, learning/episodic memory, and fluid intelligence. A subset of 122 underwent neuroimaging before and after supplementation to measure whole-brain and subcortical tissue volumes.
Results.
Capsule adherence was over 95%, participant blinding was verified, and red blood cell EPA and DHA levels increased as expected. Supplementation did not affect performance in any of the four cognitive domains. Exploratory analyses revealed that, compared to placebo, fish oil supplementation improved executive function in participants with low-baseline DHA levels. No changes were observed in any indicator of brain morphology.
Conclusions.
In healthy mid-life adults reporting low-dietary intake, supplementation with LCn-3FAs in moderate dose for moderate duration did not affect neuropsychological performance or brain morphology. Whether salutary effects occur in individuals with particularly low-DHA exposure requires further study.
Keywords: Cognitive functioning, magnetic resonance imaging, neuropsychological performance, omega-3 fatty acids, randomized clinical trial
Introduction
During the past 10 years, the use of omega-3 long-chain polyunsaturated fatty acid (LCn-3FA) supplements in the form of fish oil has burgeoned around the world due, in part, to widespread claims related to brain health. The primary LCn-3FAs are eicosapentaenoic (20:5n-3, EPA) and docosahexaenoic (22:6n-3, DHA) acids. Humans cannot synthesize LCn-3FAs de novo and rely primarily on direct ingestion of EPA and DHA by eating fish. Other foods, such as poultry, red meat, and vegetable oils supply polyunsaturated fatty acids mostly in the form of omega-6 fatty acids, particularly linoleic and arachidonic acids.
Fish is consumed infrequently in the Western diet, and the median daily intake among US is adults is 80–100 mg of EPA and DHA combined (Kris-Etherton et al., 2002; Panikolaou et al., 2014). This is in contrast to the diet of early humans in which fish served as a primary protein source and may have represented greater than 60% of the typical diet in a coastal population (Brenna and Carlson, 2014). Among today’s developed countries only the Japanese eat fish almost daily, consuming 500–1000 mg of LCn-3FAs on average each day (Joordens et al., 2014; Zazzo et al., 2014; Fernandes et al., 2015). There is no Food and Drug Administration recommendation on the daily allowance of LCn-3FAs, but the World Health Organization, Academy of Nutrition and Dietetics and the European Food Safety Agency recommend 250–500 mg per day (Flock et al., 2013). Of the LCn-3FAs, DHA is particularly highly concentrated in the brain and these compounds serve several functions in mammalian neurons and glia, comprising gray and white matter tissue, respectively (Luchtman and Song, 2013).
In humans, research has focused on either the role of LCn-3FAs in brain development or in the preservation of cognitive function during aging. The former literature supports the importance of LCn-3FA accrual in the brain pre- and postnatally (Hibbeln et al., 2007; Brenna and Carlson, 2014) whereas the effectiveness of maternal or infant supplementation remains unclear due to methodological limitations in completed clinical trials (Lauritzen et al., 2016). Late in life, low intake of fish and LCn-3FAs predicts a high risk of dementia (Zhang et al., 2016). In large clinical trials in the elderly, modest supplementation with fish oil has not affected age-related cognitive decline (Cukierman-Yaffe et al., 2014; Chew et al., 2015; Andrieu et al., 2017), whereas high doses of DHA may favorably impact memory (Yurko-Mauro et al., 2015).
The role of LCn-3FAs and any consequences of varying dietary consumptions in adults between ages 18 and 60 are comparatively understudied. As noted, customary dietary intake in the US and other western nations is very low and may prevent many individuals from performing cognitively at their optimum. Low intake or blood levels of LCn-3FAs are associated with a relatively poor performance on tests of executive function and memory (Muldoon et al., 2010; Muldoon et al., 2014), yet the existing clinical trial evidence base is inconclusive on whether measures of cognition can be improved following intervention in this age range. The two largest studies each had fewer than 200 subjects with analyzable data and yielded conflicting results (Rogers et al., 2008; Stonehouse et al., 2013). At the level of the brain, prior cross-sectional and dietary self-report evidence suggests that reduced dietary intake of LCn-3FAs relates to a reduced brain tissue volume, particularly in subcortical (basal ganglia and limbic) regions of the forebrain serving diverse neurobehavioral and cognitive functions (Conklin et al., 2007). Intervention evidence bearing on LCn-3FAs and brain outcomes, however, is mixed (Puri et al., 2001; McNamara et al., 2010; Bauer et al., 2014; Bos et al., 2016; Ginty et al., 2017).
Here, we report findings from a placebo-controlled trial that tested whether redressing low consumption of LCn-3FAs favorably impacts cognitive functioning in healthy, mid-life adults. Also examined were indicators of subcortical and whole-brain tissue morphology, with a particular focus on subcortical (basal ganglia and limbic) structures implicated in a broad range of cognitive functions possibly related to LCn-3FAs.
Methods
Data were derived from a single-site, randomized, and double-blinded placebo-controlled trial of fish oil supplementation in healthy mid-life adults who report low intake of LCn-3FAs (ClinicalTrails.gov RCT00663871). Participants were assigned to receive 1400 mg daily of either fish oil (n = 134) or soybean oil (n = 137) for 18 weeks. Of these participants, 125 completed a brain imaging protocol, with complete structural brain imaging data available for 122 (n = 62 placebo; n = 60 control). This exploratory clinical trial was designed to test whether supplemented dietary intake of EPA and DHA in healthy mid-life adults affects mediators of chronic disease, mood or impulsivity (Muldoon et al., 2016), (Ginty et al., 2017). The current report presents secondary findings related to neuropsychological function and brain morphology.
Participants
The trial participants were drawn from the Adult Health and Behavior Project-Phase 2 (AHAB-II), a volunteer-based, cross-sectional study of psychosocial factors, behavioral and biological risk factors, and subclinical cardiovascular disease (Muldoon et al., 2016). The AHAB-II protocol included medical, demographic, and social histories, biomedical measures, psychosocial questionnaires, ambulatory monitoring of physical activity, mood and social interactions, brain imaging, and neuropsychological testing. AHAB-II participants were recruited through the mass mailing of recruitment letters to individuals selected from voter registration and other public domain lists for the greater Pittsburgh metropolitan area.
To be eligible to participate in AHAB-II, individuals had to be between the ages of 30 and 54 years and work at least 25 h per week outside of the home (because of a sub-study focusing on occupational stress). Individuals were excluded if they: (i) had a history of cardiovascular disease, stage 2 hypertension (blood pressure ⩾160/100 mmHg), schizophrenia or bipolar disorder, chronic hepatitis, renal failure, major neurological disorder, or chronic lung disease; (ii) reported drinking ⩾35 units of alcohol per week; (iii) took fish oil supplements; (iv) were prescribed insulin or glucocorticoid, anti-arrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight-loss medications; (v) were pregnant; (vi) had less than an eighth grade reading level; (vii) were shift workers; or (viii) had any contraindications that would preclude brain imaging.
Between January of 2008 and May of 2011, we mailed 177 415 recruitment letters for the AHAB-II parent study and received 8957 study inquiries (response rate 5%). Of the 3431 individuals who could be contacted for telephone screening, 490 completed the AHAB-II protocol and were considered for enrollment in the current fish oil trial if they met three additional eligibility criteria: (i) dietary EPA + DHA ⩽300 mg/day, based on the modified 2005 Block Food Frequency Questionnaire (NutritionQuest, Berkeley, CA, USA), (ii) lack of fish allergy, and (iii) fasting serum triglyceride concentration <400 mg/dL. A total of 272 individuals met these criteria, agreed to participate. One subject was excluded from neuropsychological outcome analysis based on an intelligence quotient (IQ) of <70. Those AHAB-II participants who underwent brain imaging started the trial a mean of 2 weeks after the initial (pre-supplementation) brain-imaging visit. Post-supplementation, brain imaging was conducted a mean of 16 weeks after the onset of supplementation and 2 weeks prior to trial conclusion. Additional details are depicted in the Consort flow chart (Fig. 1).
Fig. 1.
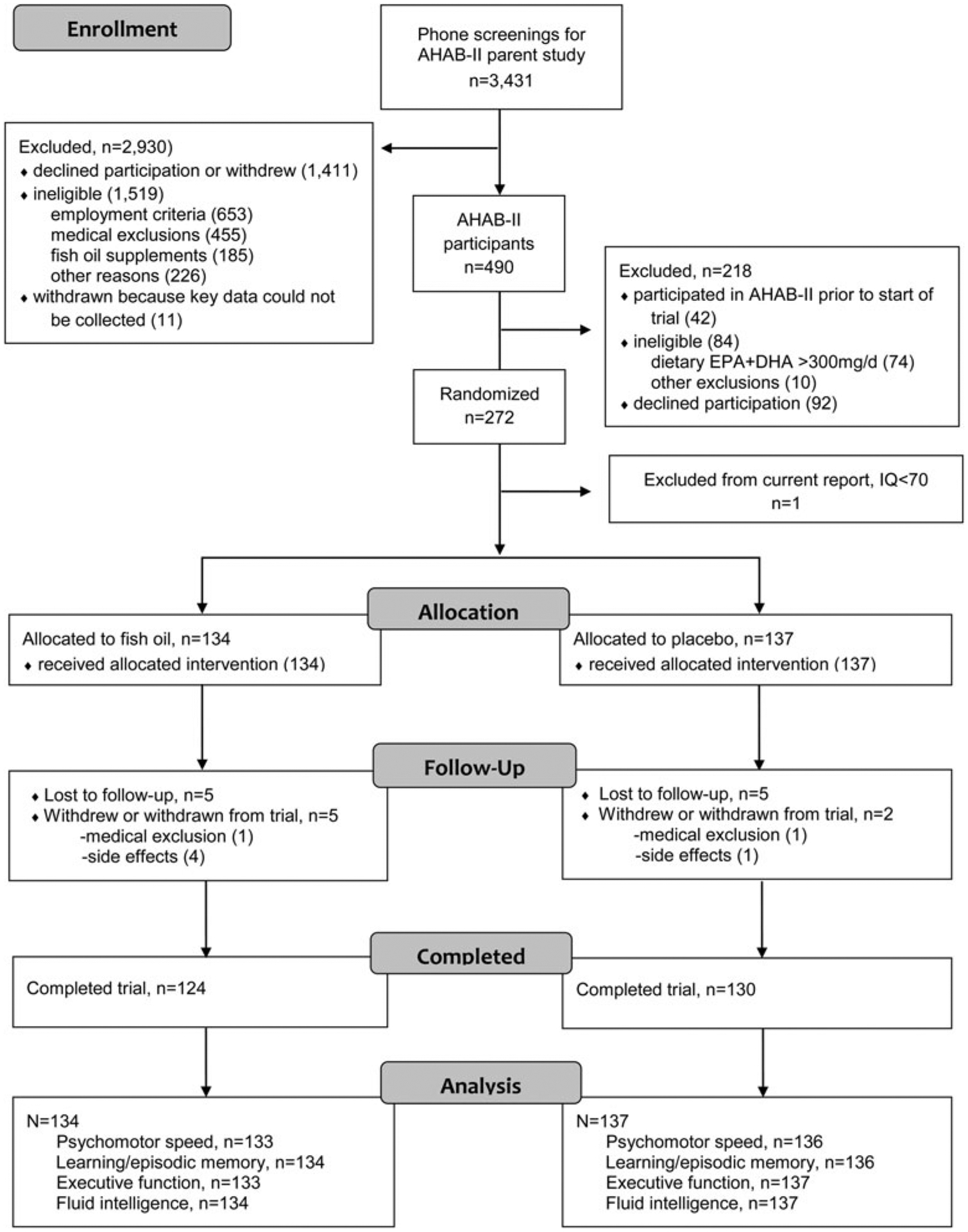
Study Participant Flow Chart [bold] AHAB-II denotes Adult Health and Behavior Project, Phase 2.
Procedure
Randomization
Treatment assignments were computer-generated at randomization using an adaptive randomization approach, which is securely implemented via the internet. Marginal treatment distribution is balanced through minimization within levels of stratification factors of race (white v. non-white), age (<45 v. ⩾45 years), and sex. Participants were randomly assigned at the time that trial eligibility was verified in a 1:1 ratio to receive either fish oil or placebo.
Study intervention & follow-up
To assist with adherence, capsules were distributed in weekly blister packs, each labeled with the week number and a code for the treatment assignment. The assigned supplement was distributed by a blinded study nurse immediately after randomization and through the 18 week supplementation period. Through this standardized procedure, treatment allocation concealment was maintained.
Participants assigned to the fish oil group received a daily dose of two 1000 mg fish oil capsules, providing a total of 1000 mg EPA and 400 mg DHA. Those assigned to the placebo group received a daily dose of two identical-appearing 1000 mg soybean oil capsules. To help maintain participant blinding, the placebo capsules contained 1% fish oil and both supplements contained mint flavor. All supplements also contained 10 IU vitamin E. Pre-trial chemical analysis established that the capsules were devoid of vitamin D. Study staff assessed side effects and adherence by contacting participants by telephone during weeks 2 and 12, and at a brief appointment during week 7 when additional supplement was distributed.
Measures
The neuropsychological performance was assessed by a series of tests administered at baseline and post-intervention by research assistants blinded to treatment condition. All tests were administered using paper and pencil versions. Testers were trained and supervised by the trial neuropsychologist (CMR). IQ was estimated from the Block Design and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997a, 1997b; Homack, 2007). The neuropsychological performance test battery consisted of matrix reasoning, block design, digit span, spatial span, four-word memory, Rey verbal learning, trail-making, and the Stroop task. See online study protocol for descriptions of the neuropsychological tests.
Magnetic resonance structural brain imaging data for volumetric processing were collected with a 3Tesla Trio TIM whole-body magnetic resonance (MR) scanner (Siemens, Erlangen, Germany) and 12-channel head coil. Structural MR images were specifically acquired by a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence, which yielded 192 slices (1 mm thickness, no spacing, field-of-view = 256 × 208 mm, matrix size = 256 × 208 mm, time-to-repetition = 2100 ms, time-to-inversion = 1100 ms, time-to-echo = 3.29 ms, flipangle = 8°).Acquired MPRAGEimages were submitted to an a priori segmentation and volumetric processing pipeline to compute whole-brain tissue volumes, as well as volumes of the hippocampus, amygdala, and basal ganglia (i.e. caudate nucleus, putamen, nucleus accumbens, and globus pallidus). This pipeline was implemented using the Integrated Registration and Segmentation Tool within the Software Library of the Oxford University Centre for Functional MRI of the Brain [v4.0; (Patenaude et al., 2011)]. Segmented volumes of each region-of-interest are in units of mm3. Prior to analyses, volumes were summed over the left and right hemi-spheresto reduce the numberof statistical tests and because no laterality was hypothesized in view of the existing literature.
Fatty acid composition of participant red blood cells (RBCs) was determined at baseline and post-supplementation using methods described previously (Muldoon et al., 2016).
Sample size estimation
This study had a target sample size of 250 subjects (125 per treatment group) in order to achieve at least 0.80 power to detect differences in mean changes in primary outcomes (systemic inflammation, heart rate variability, and affect and hostility) from baseline to post-supplementation in terms of a standardized mean difference (d) as small as d = 0.433 between treatment groups. Sample size determination assumed a test-wise significance level of 0.01 when conducting two-sided hypothesis testing using linear contrasts within a repeated measures framework. At the time this study was designed, improvements in cognitive performance of d > 0.5 had been reported (Fontani et al., 2005).
Data management and statistical analysis
Post-supplementation data were missing for the 17 participants lost to follow-up (10 participants from the fish oil group and seven from the placebo group; Fig. 1). In addition, ~1% of neuropsychological performance data were missing points due to tester error, equipment malfunction, or participant failure to bring reading glasses or correctly follow instructions during neuropsychological testing. Structural imaging was conducted in 125 participants, with complete structural brain imaging data available for 122 (n = 62 placebo; n = 60 control).
Statistical outliers, defined as ⩾3.3 standard deviations s.d.) from the mean, were winsorized as described in Rivest (1994) to 3.3 s.d. from the mean. In cases of more than one outlier, the original rank order of the values was maintained after winsorization. Only neuropsychological performance data were subjected to winsorizing, as no raw volumetric data met outlier criteria. A regression model was used for each neuropsychological performance score, with baseline performance treated as the independent variable and post-supplementation performance as the dependent variable. Residuals were then calculated for each subject for each neuropsychological performance score to identify instances in which a within-subject change fell outside of the population distribution. Residuals > ± 3.3 s.d. were manually examined for degree of deviation and in the context of tester comments and the raw scores. The investigators then made decisions to delete select data points that were deemed spurious (<1%). The same procedure was implemented for structural brain imaging data, with no data being identified as spurious.
Missing neuropsychological performance data were estimated and replaced with single imputation using regression imputation conducted separately by treatment condition (Li et al., 2015). Missing values for baseline test scores were imputed by the predicted values yielded from a fitted regression model with the baseline test score as the dependent variable and the subject’s age, sex, race, verbal IQ, and follow-up score value as predictor variables. Similarly, the missing values for post-supplementation test scores were imputed based on a treatment group-specific regression model using the subject’s age, sex, race, verbal IQ, and baseline score value as predictor variables. We did not impute missing brain imaging data owing to the anatomical implausibility of imputed outcomes based on subject characteristics.
The neuropsychological tests were organized into four cognitive domains of interest: psychomotor speed, executive function, learning/episodic memory, and fluid intelligence. First, all neuropsychological outcome variables were organized conceptually by the research team. Organization was based on common groupings of tasks, as seen in previous literature (Lim et al., 2013; Farrell et al., 2017). To ensure measures within the domains were statistically related, confirmatory factor analysis was performed, and all four domains demonstrated good model fit (RMSEA = 0.078, CFI = 0.94). The psychomotor speed was composed of trail making A time, Stroop word-only time, and color-only time. Executive function was composed of trail making B–A time, digit span forward and digit span backward completions. Learning/episodic memory was composed of d’ from the four word memory task (d’) and the average performance across all trials of the Rey auditory verbal learning test. Fluid intelligence was composed of matrix reasoning task raw score, block design task raw score, and spatial span forward raw score. Tasks were converted into z-scores before averaging across tasks resulting in equal weighting among tasks within each domain (Rodrigue et al., 2012).
The primary hypotheses were tested according to the principal of intention to treat. We conducted a repeated measures MANOVA for each of the four cognitive domains. The between-subjects factor was supplementation assignment, and the within-subjects factor was time. Coefficients for the effect of time, supplement, and the interaction of time × supplement were calculated. The test-wise level of significance was 0.01 to mitigate inflation of type 1 error.
A repeated measures MANOVA was also applied to structural brain imaging data, with all subcortical volumes included in the same MANOVA to reduce statistical testing and type 1 error inflation. Likewise, total (whole-brain) gray and white matter tissue volumes were tested in a single MANOVA, separate from the subcortical MANOVA. The Pillai–Bartlett trace was used as the omnibus multivariate F statistic. Follow-up repeated measures ANOVAs were planned for each dependent measure if the multivariate omnibus test reached statistical significance at 0.01. Finally, MANOVAs were executed with and without covariate control for intra-cranial volume (ICV) to test for the influence of overall head size on any observed effects.
In exploratory analyses, Pearson correlations between a change in LCn-3FAs in RBCs and a change in neuropsychological performance and brain tissue volumes were examined in both groups combined. Here, performance scores were standardized and combined within performance domains in order to calculate domain change scores. Similar change scores were also computed for brain tissue volumes. Finally, we sought to test whether any effects of supplementation varied as a function of LCn-3FA exposure at baseline. Statistical analysis similar to that conducted in primary hypothesis testing was performed for each cognitive domain and volumetric measure with the additional between-subjects factor of low v. high-baseline RBC LCn-3FA content based on median split using the entire sample. Separate analyses were performed for EPA, DHA, and EPA + DHA.
Results
Participants were mostly Caucasian, employed and relatively well-educated Pittsburgh-area adult volunteers with a mean age of 43 years and slight female predominance (Table 1). The sample generally had above average intelligence, and the median intake of LCn-3FAs was less than 100 mg per day. Of the 271 participants, 17 dropped-out (6.3%); missing data were retained for analysis through multiple imputations (Fig. 1). Adherence to supplement capsules was evaluated by self-report and fatty acid analysis of RBCs. Pill count was available on >90% of participants in whom adherence averaged >95% in both groups. In the fish oil group, EPA and DHA in RBCs increased consistent with expectations, and did not change in the placebo group (Table 2). There were no demographic or other differences between those with and without brain imaging data.
Table 1.
Participant characteristics
| Fish oil (n = 134) | Placebo (n = 137) | |
|---|---|---|
| Age (years) | 43.1 ± 7.5 | 42.4 ± 7.0 |
| Sex (% female) | 56.7 | 56.2 |
| Race (% white) | 81.3 | 84.7 |
| Employment (% employed) | 100 | 100 |
| Family income (% <$ 50 000/year) | 37.3% | 39.9% |
| Schooling (years) | 16.5 ±2.8 | 16.8 ±2.7 |
| Intelligence quotient | 113 ±12.6 | 113 ±12.2 |
| Alcohol consumption (drinks/week) | 2(4)a | 1(5)a |
| Dietary EPA+ DHA (mg/day)b | 84.2 (11)a | 96.4 (10)a |
Median (interquartile range).
Dietary consumption calculated from food questionnaire
Table 2.
RBC fatty acid compositiona at baseline and following supplementation
| Fish oil group | Placebo group | |||||
|---|---|---|---|---|---|---|
| Week 0 Mean (s.d.) | Week 18 Mean (s.d.) | Absolute Change (95% CI) | Week 0 Mean ± s.d. | Week 18 Mean ± s.d. | Absolute Change (95% CI) | |
| EPA | 0.46% (0.26) | 1.45% (0.75) | 1.00% (0.86–1.14) | 0.45% (0.23) | 0.41% (0.89) | −0.03% (−0.76 to 0.06) |
| DHA | 2.53% (0.92) | 3.50% (1.12) | 1.02% (0.80–1.24) | 2.48% (0.89) | 2.44% (0.97) | 0.01% (−0.14 to 0.17) |
Expressed as percentage of total fatty acids.
The neuropsychological performance outcomes and related statistics are displayed in Table 3. At baseline, there were not ceiling effects in the performance scores, and the composite scores were within limits of a normal distribution (executive function skewness is 0.238, learning/episodic memory skewness is −0.371, fluid intelligence skewness is −0.687, and psychomotor speed skewness is 0.024 where standard error is 0.148 for all). Both the fish oil and placebo groups exhibited small and non-significant improvements over the 18-week supplementation period. There was no effect of fish oil on neuropsychological performance in any of the four cognitive domains. Specifically, the time × treatment interaction F statistics for psychomotor speed, learning/episodic memory, executive function, and fluid intelligence were: F(1,267) = 0.665, p = 0.42; F(1,268) = 0.243, p = 0.62; F(1,268) = 0.091, p = 0.76; and F(1,268) = 0.381, p = 0.54.
Table 3.
Effects of fish oil supplementation on four domains of neuropsychological performancea
| Week 0 | Week 18 | Change | |||
|---|---|---|---|---|---|
| Psychomotor speedb | |||||
| Fish oil | 0.040 (0.634)c | 0.125 (0.536) | 0.085 (0.439) | 0.145 (−0.096 to 0.386) | 0.025 (−0.214 to 0.264) |
| Placebo | −0.038 (0.603) | 0.007 (0.570) | 0.044 (0.449) | 0.075 (−0.162 to 0.312) | |
| Executive function | |||||
| Fish oil | −0.019 (0.566) | 0.072 (0.618) | 0.090 (0.518) | 0.152 (−0.089 to 0.393) | 0.147 (−0.092 to 0.386) |
| Placebo | 0.018 (0.470) | 0.034 (0.626) | 0.018 (0.470) | 0.028 (−0.208 to 0.265) | |
| Learning/memory | |||||
| Fish oil | 0.014 (0.866) | 0.177 (0.854) | 0.163 (0.532) | 0.190 (−0.051 to 0.431) | 0.064 (−0.175 to 0.303) |
| Placebo | −0.012 (0.860) | 0.117 (0.869) | 0.129 (0.523) | 0.149 (−0.088 to 0.387) | |
| Fluid intelligence | |||||
| Fish oil | −0.017 (.847) | −0.005 (0.820) | 0.012 (0.376) | 0.014 (−0.226 to 0.255) | −0.062 (−0.301 to 0.177) |
| Placebo | 0.017 (.895) | 0.054 (0.829) | 0.037 (0.429) | 0.043 (−0.194 to 0.280) |
No comparison between groups over time reached statistical significance.
Psychomotor speed is reverse scored in order that higher values indicate better performance.
Performance data constitutes the averaged, standardized scores of the tests in each domain.
Structural brain imaging data are provided in Table 4. There were no statistical changes over time in subcortical brain tissue volumes over the 18-week supplementation period across the groups, main effect of time F statistic (1,115) = 0.871, p = 0.52. In parallel to the neuropsychological performance, there was no effect of fish oil on any indicator of the subcortical tissue volume, treatment × time interaction F statistic (1,115) = 0.197, p = 0.97. These findings were unchanged with covariate control for ICV.
Table 4.
Effects of fish oil supplementation on subcortical and whole-brain tissue volumesa
| Week 0 | Week 18 | Change | Between groups | ||
|---|---|---|---|---|---|
| Amygdala | |||||
| Fish oil | 2150.71 (433.06) | 2162.68 (418.64) | 11.97 (267.24) | 0.03 (−0.33 to 0.39) | −0.01 (−0.26 to 0.24) |
| Placebo | 2174.94 (450.88) | 2189.54 (466.41) | 14.60 (263.34) | 0.03 (−0.32 to 0.38) | |
| Caudate | |||||
| Fish oil | 6779.88 (731.30) | 6806.83 (782.98) | 26.95 (297.59) | 0.04 (−0.32 to 0.39) | 0.04 (−0.21 to 0.29) |
| Placebo | 6844.56 (820.19) | 6861.15 (804.40) | 16.59 (209.17) | 0.02 (−0.33 to 0.37) | |
| Globus pallidus | |||||
| Fish oil | 3262.02 (335.64) | 3259.96 (362.09) | −2.07 (117.06) | −0.01 (−0.36 to 0.35) | 0.02 (−0.23 to 0.27) |
| Placebo | 3240.98 (289.25) | 3236.89 (324.39) | −4.09 (111.67) | −0.01 (−0.37 to 0.34) | |
| Hippocampus | |||||
| Fish oil | 7381.25 (859.94) | 7398.37 (847.26) | 17.12 (269.55) | 0.02 (−0.34 to 0.38) | −0.03 (−0.28 to 0.23) |
| Placebo | 7484.92 (879.23) | 7508.81 (784.38) | 23.88 (251.32) | 0.03 (−0.32 to 0.38) | |
| Nucleus accumbens | |||||
| Fish oil | 873.48 (209.40) | 870.70 (221.20) | −2.78 (120.58) | −0.01 (−0.37 to 0.34) | 0.14 (−0.11 to 0.39) |
| Placebo | 870.87 (179.13) | 851.41 (162.38) | −19.46 (112.53) | −0.11 (−0.47 to 0.24) | |
| Total gay matter | |||||
| Fish oil | 594 081.84 (54561.47) | 591311.60 (55633.34) | −2770.25 (9491.80) | −0.05 (−0.41 to 0.31) | −0.02 (−0.27 to 0.23) |
| Placebo | 607 242.96 (51775.85) | 604 666.41 (50 650.98) | −2576.54 (9626.18) | −0.05 (−0.40 to 0.30) | |
| Total white matter | |||||
| Fish oil | 540 831.42 (60158.67) | 542 851.52 (59 887.33) | 2020.10 (12 350.40) | 0.03 (−0.32 to 0.39) | 0.10 (−0.16 to 0.35) |
| Placebo | 544 596.68 (49 800.44) | 545 547.12 (49 323.68) | 950.45 (9854.20) | 0.02 (−0.33 to 0.37) |
All tissue volumes (mm3) are presented in raw units, unadjusted for intracranial volume. No comparisons between groups over time reached statistical significance in repeated measures MANOVAs with and without covariate control for intracranial volume. Fish oil n = 60, placebo n = 62, total N = 122.
There was a statistical change over time in total (gray and white matter) brain tissue volume over the 18-week supplementation period across the groups, main effect of time F statistic (1,119) = 4.729, p = 0.011. This multivariate effect was explained by a small decline in whole-brain gray matter volume (−2671.808 mm3, 95% confidence interval (CI) −4378.391 mm3 to −965.224 mm3). The latter decline, however, was not observed after covariate control for individual differences in ICV, F(1,118) = 0.656, p = 0.521. In parallel to subcortical brain tissue volumes, there was no effect of fish oil on total gray or white matter, treatment × time interaction F statistic (1,119) = 0.153, p = 0.859. The latter findings were unchanged with covariate control for ICV.
Exploratory analyses
In supplementary analyses, changes in neuropsychological performance and brain tissue volumes did not correlate with changes in RBC LCn-3FA composition (online Supplementary Table S1). We also tested whether fish oil supplementation affects neuropsychological performance or brain tissue volumes in individuals with especially low-LCn-3FA exposure. Low exposure was based on a median split of RBC LCn-3FA composition at enrollment. A significant treatment × baseline DHA effect was observed for executive function (F(1,266) = 7.384, p = 0.007). In comparison with placebo, fish oil supplementation improved executive function (Cohen’s d 0.497, CI 0.157–0.838) in participants with low-baseline DHA (<2.45% of total fatty acids). No treatment effect occurred in participants with high-baseline RBC DHA (⩾2.46% of total fatty acids). Of note, there were no significant differences in baseline performance of any domain between high and low-baseline DHA groups (online Supplementary Tables S2a and S2b). No treatment × baseline DHA interactions were observed in the other three cognitive domains (psychomotor speed, episodic memory and learning, and fluid intelligence), and none were observed with low v. high-baseline RBC EPA and EPA + DHA (data not shown). Finally, we observed no moderating effects of LCn-3FA exposure at enrollment on brain tissue volume outcomes.
Participant blinding and adverse events
Participants in the two groups were similarly accurate in guessing their treatment assignments (p = 0.58), verifying that participant blinding was maintained. No major adverse events occurred, whereas subjective side effects varied by treatment condition. Detailed findings regarding participant blinding and side effects were reported previously (Muldoon et al., 2016).
Discussion
Observational studies link fish or LCn-3FA intake to cognitive function throughout the lifespan (de Groot et al., 2007; Muldoon et al., 2010; Leckie et al., 2014). However, few studies have tested the causal relationship between variation in dietary intake of LCn-3FAs and cognitive functioning or its putative neural correlates in non-elderly adults. In the current placebo-controlled trial, we enrolled healthy midlife adults who consumed 300 mg/d of LCn-3FAs or less at baseline, and had them take supplements containing either 1400 mg/d of EPA and DHA, or matching placebo, for 18 weeks. Neuropsychological testing administered at baseline and trial completion revealed no intervention effects on psychomotor speed, executive function, learning/episodic memory, or fluid intelligence. Subgroup analyses detected an improvement in executive functioning with supplementation compared to placebo in participants below the median in baseline DHA exposure. Finally, we observed no intervention effects on any indicator of brain tissue morphology.
Preclinical research has shown that LCn-3FAs play consequential roles in the brain (Luchtman and Song, 2013). Diets deficient in LCn-3FAs reduce neuronal growth and synaptic proliferation, reduce neuron size in brain regions, such as the hippocampus, change gene expression related to synaptic plasticity and learning, alter neurotransmitter function, and cause functional deficits, especially in learning and memory processes putatively subserved by medial temporal lobe and basal ganglia regions (Moriguchi et al., 2000; Ahmad et al., 2002; Berger et al., 2002; Kitajka et al., 2002; Kitajak et al., 2004; Brenna, 2011; Luchtman and Song, 2013). Likewise, small studies in humans have found that short-term supplementation may affect brain morphology as well as activation patterns (Jackson et al., 2012b; Bauer et al., 2014; Boespflug et al., 2016; Bos et al., 2016). Ours is the first clinical trial examining brain morphology in mid-life healthy adults. In a substudy of the current investigation, we used functional brain imaging to examine corticolimbic (e.g. medial temporal lobe) and corticostriatal (e.g. basal ganglia) brain systems, which are thought to support affective and impulsive processes, as well as neurocognitive functions, such as memory. Complementing the present findings on brain tissue morphology in these systems, no effects of supplementation on activation patterns were observed (Ginty et al., 2017).
With respect to cognitive function, we found seven previous randomized clinical trials that enrolled non-elderly adults (Fontani et al., 2005; Rogers et al., 2008; Antypa et al., 2009; Jackson et al., 2012a; Karr et al., 2012; Stonehouse et al., 2013; Dretsch et al., 2014). These trials ranged in size from 22 to 190 participants with analyzable data and provided 540 to 2800 mg/day of LCn-3FAs for 4 to 26 weeks. Several excluded persons who ate fish frequently. Two trials reported that fish oil supplementation improved scores on select neuropsychological tests spanning several cognitive domains. Specifically, Fontani and colleagues report improvement in two out of three tasks of executive function, and not in a task psychomotor speed, while Stonehouse and colleagues report improvements in composite domains of working and episodic memory (in women only) and not in attention or processing speed. Ours and the next largest trial (Rogers et al., 2008) did not find any effect on the major cognitive dimensions of neuropsychological performance.
In exploratory analyses, we found evidence of improved executive function selectively in participants who fell below the median in baseline DHA content in RBCs. DHA constitutes 30% of fatty acids in brain phospholipids, compared to less than 4% in most other tissues (O’Brien et al., 1964; Arterburn et al., 2006). Among LCn-3FAs, DHA is most directly implicated in human brain development and cognitive functioning (Muldoon et al., 2010; Brenna and Carlson, 2014; Yurko-Mauro et al., 2015; Lauritzen et al., 2016). The presented subgroup analyses are exploratory and require further, a priori testing, but suggest that there may be some non-uniformity in the cognitive domains affected by DHA such that some cognitive domains are affected more than others.
The presented trial has some notable strengths. All participants had chronically low-dietary intake of LCn-3FAs, calculated from a modified-food frequency questionnaire and confirmed with RBC fatty acid analyses. Follow-up was complete on 94% of randomized participants and multiple imputation permitted retention of all participants in analyses of neuropsychological performance. The organization of neuropsychological test scores into statistically validated domains and a test-wise level of significance of 0.01 mitigates risk type 1 error and allows for conceptual clarity regarding the domains of cognitive function under investigation. Finally, hypothesis testing by repeated measures MANOVA across treatment conditions examines supplement-related change independent of any placebo effects, as well as practice effects in the case of neuropsychological performance.
Several study limitations warrant consideration as well. Most study participants were Caucasian, well-educated adults with an above average IQ. In addition, participants were mid-life adults in whom cognitive functioning tends to be stable. Cognitive effects may be different in adolescents, older adults, and individuals with behavioral health disorders that include some degree of cognitive dysfunction. The treatment period was 18 weeks, whereas turnover of brain membrane lipids is slow and there is uncertainty regarding the duration necessary to reach a new steady state or impact brain function or structure. Along these lines, the behavioral consequences of improvements in brain physiology might take a more prolonged exposure. Furthermore, the optimal intake and ratio of LCn-3FAs for human brain are unknown and, in particular, supplementation with 400 mg/day of DHA may be insufficient. However, a recent trial in older adults found that 1720 mg of DHA and 600 mg of EPA for 18 months did not affect cognitive performance (Danthiir et al., 2018).
Response to supplementation with fish oil may differ from what might be observed following increased fish consumption. Differences in overall nutrient composition (e.g. concurrent vitamin D intake) or ability of the body to utilize LCn-3FAs obtained from food as opposed to supplements may explain the discord between observational and interventional investigations (Muldoon et al., 2010; Muldoon et al., 2014).
While this new evidence does not support the general efficacy of 18-weeks of supplementation for improving cognitive functioning in mid-life healthy adults whose dietary habits include infrequent fish consumption, other topics merit exploration. As alluded to above, the optimal dose and duration of treatment remain unknown. Our exploratory finding may warrant targeted trials of DHA supplementation in persons with particularly low-DHA intake during midlife or at-risk elderly populations. Another avenue of investigation concerns effects on the brain of supplementation in persons at risk for dementia by virtue of carrying an APOE*E4 allele (Singh et al., 2006; Conway et al., 2014; van de Rest et al., 2016).
Overall, the clinical implications of this study are mixed. Fish continues to constitute only a minor component of the US diet whereas pre-clinical and epidemiological evidence suggests that adequate levels of omega-3 fatty acid consumption have many benefits, particularly for secondary prevention in persons with heart disease (Del Gobbo et al., 2016; Sala-Vila et al., 2016; Siscovick et al., 2017). The use of fish oil supplements has exploded exponentially in the general public over the past decade and, paradoxically, may be unwarranted (Cohen, 2016). Aside from that in populations with heart disease or depression, evidence of benefit based on randomized clinical trials remains sparse, and the trial reported here fails to show improvement in suspected aspects of brain health; namely, optimal cognitive performance and tissue volume during mid-life. Therefore, the current state of the evidence should not cause a clinician to recommend strongly for, or against, increased LCn-3FAs intake among his or her patients. No doubt, ongoing and future studies of other doses or diet modification, longer duration, and other endpoints conducted in specific populations will further illuminate the role of these nutrients in disease prevention.
Supplementary Material
Acknowledgements.
We acknowledge the efforts of Dr François Lespérance, MD, who served as the trial data safety monitor.
Financial support.
The study was funded by US Public Health Service Awards R01 AT004699, R01 HL101421, P01 HL40962, and T32 HL07560. The US Public Health Service had no role in the study design or implementation, data collection, statistical analysis, interpretation or manuscript composition.
Footnotes
Conflict of interest.
The authors received at no charge identical appearing fish oil and soybean oil (placebo) capsules from PharmaOmega-Life, NourishLife, LLC (Lake Forest, IL). The authors have no other potential conflicts of interest to disclose.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002617.
References
- Ahmad A, Moriguchi T and Salem N (2002) Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatric Neurology 26, 210–218. [DOI] [PubMed] [Google Scholar]
- Andrieu S, Guyonnet S, Coley N, Canete C, Bonnefoy M, Bordes S, Bories L, Cufi MN, Dantoine T, Dartigues JF, Desclaux F, Gabelle A, Gasnier Y, Pesce A, Sudres K, Touchon J, Robert P, Rouaud O, Legard P, Payoux P, Caubere JP, Weiner M, Carrie I, Ousset PJ, Vellas B and Group MS (2017) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurolology 16, 377–389. [DOI] [PubMed] [Google Scholar]
- Antypa N, Van Der Does AJ, Smelt AH and Rogers RD (2009) Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. Journal of Psychopharmacology 23, 831–840. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Hall EB and Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. American Journal of Clinical Nutrition 83, 1467S–1476S. [DOI] [PubMed] [Google Scholar]
- Bauer I, Hughes M, Rowsell R, Cockerell R, Pipingas A, Crewther S and Crewther D (2014) Omega-3 supplementation improves cognition and modifies brain activation in young adults. Human Psychopharmacology 29, 133–144. [DOI] [PubMed] [Google Scholar]
- Berger A, Mutch DM, German JB and Roberts MA (2002) Unraveling lipid metabolism with microarrays: effects of arachidonate and docosahexaenoate acid on murine hepatic and hippocampal gene expression. Genome Biology 3, REPRINT0004. [DOI] [PubMed] [Google Scholar]
- Boespflug EL, McNamara RK, Eliassen JC, Schidler MD and Krikorian R (2016) Fish oil supplementation increases event-related posterior cingulate activation in older adults with subjective memory impairment. J Nutr Health Aging, 20, 161–169. [DOI] [PubMed] [Google Scholar]
- Bos DJ, Van Montfort SJ, Oranje B, Durston S and Smeets PA (2016) Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: what is the evidence? European Neuropsychopharmacology 26, 546–561. [DOI] [PubMed] [Google Scholar]
- Brenna JT (2011) Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life. Maternal & Child Nutrition 7(suppl. 2), 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna JT and Carlson SE (2014) Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. Journal of Human Evolution 77, 99–106. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein R,Bernstein PS and Age-related Eye Disease Study 2 Research Group (2015) Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. Journal of the American Medical Associaton 314, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PA (2016) The supplement paradox: negligible benefits, robust consumption. Journal of the American Medical Associaton 316, 1453–1454. [DOI] [PubMed] [Google Scholar]
- Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB and Muldoon MF (2007) Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neuroscience Letters 421, 209–212. [DOI] [PubMed] [Google Scholar]
- Conway V, Allard MJ, Minihane AM, Jackson KG, Lovegrove JA and Plourde M (2014) Postprandial enrichment of triacylglycerol-rich lipoproteins with omega-3 fatty acids: lack of an interaction with apolipoprotein E genotype? Lipids in Health Disease 13, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Bosch J, Diaz R, Dyal L, Hancu N, Hildebrandt P, Lanas F, Lewis BS, Marre M, Yale JF, Yusuf S, Gerstein HC and ORIGIN Investigators (2014) Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: a substudy of the ORIGIN trial. Lancet Diabetes & Endocrinology 2, 562–572. [DOI] [PubMed] [Google Scholar]
- Danthiir V, Hosking DE, Nettelbeck T, Vincent AD, Wilson C, O’Callaghan N, Calvarsei E, Clifton P and Wittert GA (2018) An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. American Journal of Clinical Nutrition 107, 754–762. [DOI] [PubMed] [Google Scholar]
- De Groot RH, Hornstra G and Jolles J (2007) Exploratory study into the relation between plasma phospholipid fatty acid status and cognitive performance. Prostaglandins Leukotrienes & Essential Fatty Acids 76, 165–172. [DOI] [PubMed] [Google Scholar]
- Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateua P, Campos H, Chaves PH, Djousse L, Giles GG, Gomez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Riserus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, Van Dam RM, Voutilainen S, Willett WC, Woodward M, Mozaffarian D and Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) (2016) Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Internal Medicine 176, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretsch MN, Johnston D, Bradley RS, Macrae H, Deuster PA and Harris WS (2014) Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. Soldiers: a pilot study. Military Medicine 179, 396–403. [DOI] [PubMed] [Google Scholar]
- Farrell ME, Kennedy KM, Rodrigue KM, Wig G, Bischof GN, Rieck JR, Chen X, Festini SB, Devous MD and Park DC (2017) Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose-response relationship. JAMA Neurology 74, 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R, Grootes P, Nadeau MJ and Nehlich O (2015) Quantitative diet reconstruction of a Neolithic population using a Bayesian mixing model (FRUITS): the case study of Ostorf (Germany). American Journal of Physical Anthropology 158, 325–340. [DOI] [PubMed] [Google Scholar]
- Flock MR, Harris WS and Kris-Etherton PM (2013) Long-chain omega-3 fatty acids: time to establish a dietary reference intake. Nutrition Reviews 71, 692–707. [DOI] [PubMed] [Google Scholar]
- Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S and Lodi L (2005) Cognitive and physiological effects of omega-3 polyunsaturated fatty acid supplementation in healthy subjects. European Journal of Clinical Investigation 35, 691–699. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Muldoon MF, Kuan DCH, Schirda B, Kamark TW, Jennings JR, Manuck SB and Gianaros PJ (2017) Omega-3 supplementation and the neural correlates of negative affect and impulsivity: a double-blind, randomized, placebo-controlled trial in midlife adults. Psychosomatic Medicine 79, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williasm C and Golding J (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369, 578–585. [DOI] [PubMed] [Google Scholar]
- Homack SR (2007) Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Brace & Company. [Google Scholar]
- Jackson PA, Deary ME, Reay JL, Scholey AB and Kennedy DO (2012a) No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. British Journal of Nutrition 107, 1232–1243. [DOI] [PubMed] [Google Scholar]
- Jackson PA, Reay JL, Scholey AB and Kennedy DO (2012b) DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. British Journal of Nutrition 107, 1093–1098. [DOI] [PubMed] [Google Scholar]
- Joordens JC, Kuipers RS, Wanink JH and Muskiet FA (2014) A fish is not a fish: patterns in fatty acid composition of aquatic food may have had implications for hominin evolution. Journal of Human Evolution 77, 107–116. [DOI] [PubMed] [Google Scholar]
- Karr JE, Grindstaff TR and Alexander JE (2012) Omega-3 polyunsaturated fatty acids and cognition in a college-aged population. Experimental and Clinical Psychopharmacology 20, 236–242. [DOI] [PubMed] [Google Scholar]
- Kitajka K, Puskas LG, Zvara A, Hackler L, Barcelo-Coblijn G, Yeo YK and Farkas T (2002) The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proceedings of the National Academy of Sciences of the United States of America 99, 2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajak K, Sinclair AJ, Weisinger RS, Weisinger HS, Mathai M, Jayasooriya AP, Halver JE and Puskas LG (2004) Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proceedings of the National Academy of Sciences of the United States of America 101, 10931–10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS and Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106, 2747–2757. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Brambilla P, Mazzocchi A, Harslof LB, Ciappolino V and Agostonie C (2016) DHA effects in brain development and function. Nutrients 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie RL, Manuck SB, Bhattacharjee N, Muldoon MF, Flory JM and Erickson KI (2014) Omega-3 fatty acids moderate effects of physical activity on cognitive function. Neuropsychologia 59, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Stuart EA and Allison DB (2015) Multiple imputation: a flexible tool for handling missing data. Journal of the American Medical Associaton 314, 1966–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Oh IK, Han C, Huh YJ, Jung IK, Patkar AA, Steffens DC and Janb BH (2013) Sensitivity of cognitive tests in four cognitive domains in discriminating MDD patients from healthy controls: a meta-analysis. International Psychogeriatrics 25, 1543–1557. [DOI] [PubMed] [Google Scholar]
- Luchtman DW and Song C (2013) Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology 64, 550–565. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliasen JC, Alfieri D, Weber W, Jarvis K, Delbello MP, Strakowski SM and Adler CM (2010) Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. American Journal of Clinical Nutrition 91, 1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS and Salem N (2000) Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. Journal of Neurochemistry 75, 2563–2573. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Ryan CM, Sheu L, Yao JK, Conklin SM and Manuck SB (2010) Serum phospholipid docosahexaenoic acid is associated with cognitive functioning during middle adulthood. Journal of Nutrition 140, 848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Ryan CM, Yao JK, Conklin SM and Manuck SB (2014) Long-chain omega-3 fatty acids and optimization of cognitive performance. Military Medicine 179, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Laderian B, Kuan DC, Sereika SM, Marsland AL and Manuck SB (2016) Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. Journal of Internal Medicine 279, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JS, Fillerup DL and Mead JF (1964) Quantification and fatty acid and fatty aldehyde composition of ethanolamine, choline, and serine glycerophosphatides in human cerebral grey and white matter. Journal of Lipid Research 5, 329–338. [PubMed] [Google Scholar]
- Panikolaou Y, Brooks J, Reider C and Fulgoni VL (2014) U.S. Adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutrition Journal 13, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kenndy DN and Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri BK, Counsell SJ, Hamilton G, Richardson AJ and Horrobin DF (2001) Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. International Journal of Clinical Practice 55, 560–563. [PubMed] [Google Scholar]
- Rivest L (1994) Statistical properties of Winsorized means for skewed distributions. Biometrika 81, 373–383. [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D and Park DC (2012) Beta-amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology 78, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA and Ness AR (2008) No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. British Journal of Nutrition 99, 421–431. [DOI] [PubMed] [Google Scholar]
- Sala-Vila A, Guasch-Ferre M, Hu FB, Sanchez-Tainta A, Bullo M, Serra-Mir M, Lopez-Sabater C, Sorli JV, Aros F, Fiol M, Munoz MA, Serra-Majem L, Martinez JA, Corella D, Fita M, Salas-Salvado J, Martinez-Gonzalez MA, Estruch R, Ros E and PREDIMED Investigators (2016) Dietary alpha-linolenic acid, marine omega-3 fatty acids, and mortality in a population with high fish consumption: findings from the PREvencion con DIeta MEDiterranea (PREDIMED) study. Journal of the American Heart Association 5, e002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Singh M and Mastana SS (2006) APOE distribution in world populations with new data from India and the UK. Annals of Human Biology 33, 279–308. [DOI] [PubMed] [Google Scholar]
- Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Moxaffarian D and American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology (2017) Omega-3 polyunsaturated fattyacid (Fish Oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation 135, e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C and Kennedy D (2013) DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. American Journal of Clinical Nutrition 97, 1134–1143. [DOI] [PubMed] [Google Scholar]
- Van De Rest O, Wang Y, Barnes LL, Tangney C, Bennett DA and Morris MC (2016) APOE epsilon4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology 86, 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechseler D (1997a) Wechsler Adult Intelligence Scale – Third Edition: Administration and Scoring Manual. San Antonio, TX: Harcourt Brace & Company. [Google Scholar]
- Wechsler DB (1997b) THE WMS-III Administration and Scoring Manual. San Antonio, TX: Harcourt Brace & Company. [Google Scholar]
- Yurko-Mauro K, Alexander DD and Van Elswyk ME (2015) Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One 10, e0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazzo A, Munoz O and Saliege JF (2014) Diet and mobility in a late Neolithic population of coastal Oman inferred from radiocarbon dating and stable isotope analysis. American Journal of Physical Anthropology 153, 353–364. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Qiu J, Li Y, Wang J and Jiao J (2016) Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. American Journal of Clinical Nutrition 103, 330–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


