Abstract
Background:
Guidelines recommend targeting systolic blood pressure (SBP) <130 mm Hg in heart failure with preserved ejection fraction (HFpEF) with limited data.
Objectives:
This study sought to determine the optimal achieved SBP and whether the treatment effects of sacubitril/valsartan on outcomes are related to BP lowering, particularly among women who derive greater benefit from sacubitril/valsartan.
Methods:
Using 4,795 trial participants, this study related baseline and time-updated mean achieved SBP quartiles (<120, 120 to 129, 130 to 139, ≥140 mm Hg) to the primary outcome (cardiovascular death and total heart failure hospitalizations), its components, myocardial infarction or stroke, and a renal composite outcome. At the 16-week visit, the study assessed the relationship between SBP change and Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS) and N-terminal pro-B-type natriuretic peptide (NT-proBNP). The study analyzed whether the BP-lowering effects of sacubitril/valsartan accounted for its treatment effects.
Results:
Average age was 73 ± 8 years, and 52% of participants were women. After multivariable adjustment, baseline and mean achieved SBP of 120 to 129 mm Hg demonstrated the lowest risk for all outcomes. Sacubitril/valsartan reduced SBP by 5.2 mm Hg (95% confidence interval: 4.4 to 6.0) compared with valsartan at 4 weeks, which was not modified by baseline SBP. However, sacubitril/valsartan reduced SBP more in women (6.3 mm Hg) than men (4.0 mm Hg) (interaction p = 0.005). Change in SBP was directly associated with change in NT-proBNP (p < 0.001) but not KCCQ-OSS (p = 0.40). The association between sacubitril/valsartan and the primary outcome was not modified by baseline SBP (interaction p = 0.50) and was similar when adjusting for time-updated SBP, regardless of sex.
Conclusions:
Baseline and mean achieved SBP of 120 to 129 mm Hg identified the lowest risk patients with HFpEF. Baseline SBP did not modify the treatment effect of sacubitril/valsartan, and the BP-lowering effects of sacubitril/valsartan did not account for its effects on outcomes, regardless of sex.
Keywords: heart failure with preserved ejection fraction, heart failure hospitalization, sacubitril/valsartan, blood pressure
CONDENSED ABSTRACT.
Guidelines recommend targeting SBP<130mmHg in HFpEF, but the clinical benefits of, and influence of sacubitril/valsartan on, BP lowering are unknown. In PARAGON-HF, baseline and mean achieved SBP 120–129mmHg was associated with the lowest risk for adverse outcomes. Sacubitril/valsartan reduced SBP by 5.2mmHg compared with valsartan. The associations between sacubitril/valsartan and outcomes were not modified by baseline SBP and was similar when adjusting for time-updated SBP. In sum, SBP 120–129 mmHg identified the lowest risk patients, supporting current guidelines. Baseline SBP did not modify the treatment effect of sacubitril/valsartan and the BP lowering effects of sacubitril/valsartan did not account for its effects on outcomes.
INTRODUCTION
Few therapeutic options exist in treating patients with heart failure with preserved ejection fraction (HFpEF), and accordingly, treatment has generally focused on optimizing management of comorbidities (1–4). Hypertension is very common in HFpEF, is thought to play an etiologic role, and because it leads to left ventricular hypertrophy, diastolic dysfunction, abnormal ventricular arterial coupling, and end organ damage, it has been conjectured that blood pressure control could relate to improvement in outcomes (5–7). In fact, professional society guidelines have recommended targeting a systolic blood pressure (SBP) of less than 130 mmHg in HFpEF (8,9). However, there is limited evidence to support this recommendation, particularly since the Systolic Blood Pressure Intervention Trial (SPRINT), which demonstrated that intensive versus standard BP control improved cardiovascular outcomes, excluded patients with symptomatic HF (10). In addition, while several agents that lower BP have been studied in clinical trials of HFpEF (1–3,11,12), a dedicated trial using BP targets has not been performed in this population. Further, a substudy of Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Americas did not identify a significant relationship between SBP quartiles and cardiovascular outcomes, and blood pressure reduction did not explain the potential beneficial effects of spironolactone (13).
Thus, the extent to which SBP control influences clinical outcomes remains unclear. In addition, whether BP reduction is associated with clinical benefit (reflected by quality of life and cardiovascular outcomes) is of significant interest. Moreover, the relationship between BP reduction and biomarkers in HFpEF is not well established. The Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ARB [angiotensin-receptor blockers] Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial is the largest randomized study in HFpEF to date (12), and whether the anti-hypertensive effects of sacubitril/valsartan mediate its effects on outcomes, particularly among women and those with lower EF given significant effect modification observed in these subgroups, is unknown (14,15).
In this study, we assessed the prognostic role of baseline SBP and mean achieved SBP in patients with HFpEF enrolled in PARAGON-HF, the relationship between SBP lowering with biomarker and clinical outcomes, and whether the SBP lowering effect of sacubitril-valsartan related to its treatment effects. We hypothesized that the relationship between SBP and outcomes would be J-shaped (13), and the SBP lowering effect of sacubitril-valsartan would not be responsible for its treatment effects.
METHODS
PARAGON-HF study design
The design of the PARAGON-HF study has been described in detail previously (5). Briefly, PARAGON-HF was an international, randomized, double blind, parallel group, actively-controlled, 2-arm event-driven trial comparing the efficacy and safety of sacubitril/valsartan versus valsartan in patients with HFpEF. PARAGON-HF enrolled patients with signs and symptoms of heart failure (New York Heart Association class II–IV), left ventricular EF ≥45%, increased plasma concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) (degree of elevation depending on history of HF hospitalization within 9 months and presence or absence of atrial fibrillation on screening electrocardiogram), evidence of structural heart disease (increased left atrial size or left ventricular hypertrophy), and diuretic therapy within 30 days. Before randomization, patients entered sequential single-blind run-in periods ensuring that both treatments were tolerated at half the target doses. The primary endpoint for the trial was cardiovascular death and total (first and recurrent) HF hospitalizations. The study was approved by institutional review boards at individual study sites, and all patients signed written informed consent.
Key exclusion criteria included prior left ventricular EF <40%, and SBP <110 or ≥180 mm Hg. Patients with SBP >150 mm Hg were excluded unless they were receiving at least 3 antihypertensive medications at screening. In addition, participants were excluded with estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 as calculated by the Modification in Diet in Renal Disease formula at visit 1. Detailed exclusion criteria are listed elsewhere (5). For the present study, we excluded 1 participant with missing SBP at baseline and 26 participants enrolled from a site closed for violations of Good Clinical Practice.
Study Outcomes
Endpoints studied in this analysis include the primary composite outcome of total (first and recurrent) hospitalizations for HF and death from cardiovascular causes, total HF hospitalizations, cardiovascular death, myocardial infarction or stroke, all-cause mortality, and a renal composite outcome (decrease in the eGFR of ≥50%, development of end-stage renal disease, or death due to renal failure). For safety assessment, we analyzed dose reduction or discontinuation. Among a subgroup of participants with available data, we also assessed the relationship of the change in SBP to several endpoints included at the 16-week visit. These included quality of life assessed using the overall summary score on the Kansas City Cardiomyopathy Questionnaire (KCCQ-OSS) (scores range from 0 to 100, with higher scores indicating better health status) (16), NT-proBNP, and high-sensitivity troponin T.
Statistical analysis
Baseline characteristics grouped by quartiles (<120, 120–129, 130–139, ≥140 mmHg) of baseline SBP were described using means±SD and medians and interquartile ranges or percentages as appropriate for the levels of measurement and distributions of the variables. These quartiles also approximated clinical guideline thresholds for classification and treatment of hypertension (9). The SBP quartiles were compared using ANOVA for continuous variables and chi-squared tests for categorical variables.
The association between baseline SBP quartiles and the efficacy and safety outcomes were assessed using crude and multivariable-adjusted Cox regression, using the 2nd quartile (120–129mmHg) as the referent group (the quartile that had the lowest event rate), given a J-shaped relationship observed. In a complementary analysis using restricted cubic splines, we examined the continuous association between SBP and all outcomes. Four knots placed at the 5th, 35th, 65th, and 95th percentiles were used for all outcomes except the renal composite outcome, which was analyzed linearly. Multivariable models adjusted for covariates used in a previous analysis of SBP in HFpEF, including region, atrial fibrillation, creatinine, diabetes mellitus, New York Heart Association class, heart rate, sex, age, race, current smoking, peripheral vascular disease, number of anti-hypertensive medications, and treatment group (model 1) (13). We additionally adjusted for diastolic blood pressure in model 2. We repeated these analyses using mean SBP as a time-updated covariate, which was updated at each BP ascertainment to represent the average observed blood pressure up to that time point (17).
We next determined the valsartan-adjusted change in SBP from baseline to the 4-week visit overall and by SBP quartile. Four weeks was selected as this was the time at which maximal SBP change occurred in the trial (12). Interaction terms between treatment and both gender and EF (modeled continuously) were tested (14,15). We subsequently assessed the relationships between change in SBP (expressed per 10 mmHg reduction) from baseline to the 16 and 48-week visits with KCCQ-OSS score, log transformed NT-proBNP, and log transformed high-sensitivity troponin T. An interaction term between treatment and continuous SBP was tested.
To understand whether SBP reduction related to the treatment effect of sacubitril/valsartan, we generated Cox models assessing the relationship between treatment assignment and outcomes adjusting for baseline SBP and time-updated SBP (which was updated at each study visit). Interaction analyses were performed to determine whether sex or EF (modeled continuously) modified these relationships. We assessed the primary outcome, total HF hospitalization, and the renal composite outcome since these outcomes were most strongly associated with a treatment effect (12). Analyses were performed using STATA version 14, and a two-sided p-value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The baseline characteristics of the 4,795 participants meeting study inclusion criteria stratified by quartiles of SBP are shown in Table 1. The mean baseline BP was 131±15 / 74±11 mmHg. The average age was 73±8 years, 52% were women, and 82% were white. Higher SBP quartile was associated with higher diastolic BP, proportionately more people of white race, higher body mass index, lower heart rate, less frequent atrial fibrillation, more frequent diabetes mellitus, higher eGFR, and lower NT-proBNP (p<0.05 for all comparisons).
TABLE 1.
Baseline Clinical Characteristics by Systolic Blood Pressure Quartile
| SBP<120 mmHg N=1146 | 120≤SBP<129 mmHg N=1105 | 130≤SBP<139 mmHg N=1155 | SBP≥140 mmHg N=1389 | P-value for trend | |
|---|---|---|---|---|---|
| SBP (mmHg) | 112 ± 5 | 124 ± 3 | 133 ± 3 | 149 ± 10 | |
| DBP (mmHg) | 67 ± 9 | 73 ± 9 | 76 ± 9 | 79 ± 11 | <0.001 |
| Pulse pressure (mmHg) | 44 ± 9 | 50 ± 10 | 57 ± 9 | 70 ± 14 | <0.001 |
| Randomization to sacubitril/valsartan, n (%) | 584 (51.0%) | 537 (48.6%) | 582 (50.4%) | 704 (50.7%) | 0.86 |
| Age, years | 73 ± 9 | 73 ± 8 | 72 ± 8 | 73 ± 8 | 0.52 |
| Female sex, n (%) | 574 (50.1%) | 554 (50.1%) | 610 (52.8%) | 741 (53.3%) | 0.05 |
| White race, n (%) | 879 (76.7%) | 913 (82.6%) | 942 (81.6%) | 1173 (84.4%) | <0.001 |
| NYHA, n (%) | 0.18 | ||||
| I | 33 (2.9 %) | 33 (3.0 %) | 23 (2.0 %) | 48 (3.5 %) | |
| II | 877 (76.6%) | 851 (77.0%) | 889 (77.0%) | 1088 (78.3%) | |
| III | 229 (20.0%) | 219 (19.8%) | 235 (20.4%) | 249 (17.9%) | |
| IV | 6 (0.5 %) | 2 (0.2 %) | 7 (0.6 %) | 4 (0.3 %) | |
| Geographic region, n (%) | 0.33 | ||||
| Asia-Pacific or other | 238 (20.8%) | 163 (14.8%) | 173 (15.0%) | 188 (13.5%) | |
| Central Europe | 274 (23.9%) | 392 (35.5%) | 502 (43.5%) | 547 (39.4%) | |
| Latin America | 91 (7.9 %) | 92 (8.3 %) | 94 (8.1 %) | 93 (6.7 %) | |
| North America | 209 (18.2%) | 124 (11.2%) | 100 (8.7 %) | 126 (9.1 %) | |
| Western Europe | 334 (29.1%) | 334 (30.2%) | 286 (24.8%) | 435 (31.3%) | |
| KCCQ-OSS | 70.6 ± 19.5 | 71.8 ± 18.6 | 71.6 ± 19.1 | 71.6 ± 18.7 | 0.26 |
| Physical Characteristics | |||||
| Body mass index (kg/m2) | 29.9 ± 5.2 | 30.1 ± 4.9 | 30.3 ± 5.0 | 30.5 ± 4.9 | 0.001 |
| Heart rate (beats/min) | 71.4 ± 12.8 | 70.8 ± 12.3 | 70.4 ± 11.7 | 69.5 ± 12.2 | <0.001 |
| Comorbidities, n (%) | |||||
| Hypertension | 1038 (90.6%) | 1053 (95.3%) | 1122 (97.1%) | 1370 (98.6%) | <0.001 |
| Hospitalization for HF | 546 (47.6%) | 526 (47.6%) | 577 (50.0%) | 657 (47.3%) | 0.91 |
| Atrial fibrillation or flutter | 480 (41.9%) | 394 (35.8%) | 346 (30.1%) | 332 (24.0%) | <0.001 |
| Diabetes mellitus | 424 (37.0%) | 461 (41.7%) | 507 (43.9%) | 669 (48.2%) | <0.001 |
| Myocardial infarction | 261 (22.8%) | 241 (21.8%) | 265 (22.9%) | 316 (22.8%) | 0.84 |
| Stroke | 112 (9.8 %) | 116 (10.5%) | 133 (11.5%) | 147 (10.6%) | 0.42 |
| Current smoker | 90 (7.9 %) | 78 (7.1 %) | 87 (7.6 %) | 98 (7.1 %) | 0.55 |
| Medication Use, n (%) | |||||
| ACE-I and/or ARB at screening | 921 (80.4%) | 948 (85.8%) | 1006 (87.1%) | 1264 (91.0%) | <0.001 |
| Beta-blocker | 907 (79.1%) | 886 (80.2%) | 931 (80.6%) | 1096 (78.9%) | 0.89 |
| Calcium channel blocker | 275 (24.0%) | 368 (33.3%) | 414 (35.8%) | 583 (42.0%) | <0.001 |
| Diuretic | 1088 (94.9%) | 1062 (96.1%) | 1114 (96.5%) | 1320 (95.0%) | 0.92 |
| Mineralocorticoid antagonist | 340 (29.7%) | 305 (27.6%) | 304 (26.3%) | 290 (20.9%) | <0.001 |
| Laboratory Testing | |||||
| Estimated glomerular filtration rate (mL/min/1.78 m2) | 60 ± 18 | 62 ± 19 | 64 ± 19 | 64 ± 20 | < 0.001 |
| Hemoglobin (mg/dL) | 13.5 ± 1.6 | 13.5 ± 1.5 | 13.5 ± 1.6 | 13.5 ± 1.6 | 0.97 |
| NT-proBNP (pg/mL)* | 1028 [544, 1680] | 918 [466, 1598] | 852 [432, 1660] | 790 [446, 1556] | <0.001 |
| LV Ejection Fraction | 58 ± 8 | 58 ± 8 | 57 ± 8 | 58 ± 8 | 0.16 |
Presented as median [25th-75th percentile] since values are skewed.
NYHA, New York Heart Association; SBP, systolic blood pressure; DBP, diastolic blood pressure; KCCQ-OSS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; HF, heart failure; LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
In crude analyses of the efficacy outcomes, using quartile 2 (120–129mmHg) as the referent quartile, both the lowest and highest quartiles had a higher risk of the primary outcome (Table 2). However, after multivariable adjustment, only the highest quartile was independently associated with elevated risk for the primary outcome (HR 1.54, 95% CI 1.24, 1.91), which was driven by a greater risk in total HF hospitalizations (HR 1.63, 95% CI 1.28, 2.09). Quartile 2 demonstrated the lowest risk for myocardial infarction or stroke, with greater risk observed in each of the other 3 quartiles after multivariable adjustment. Risk for the renal composite outcome increased in a graded fashion with increasing SBP quartile, while the risk for drug discontinuation was elevated at both the lowest and highest SBP quartiles after adjustment. Thus, quartile 2 demonstrated the lowest risk for all studied outcomes.
TABLE 2.
Event Rates and Crude and Adjusted Hazard Ratios for Efficacy and Safety Outcomes by Baseline Systolic Blood Pressure Quartile
| Efficacy outcomes, n (%) | SBP<120 mmHg N=1146 | 120≤SBP<129 mmHg N=1105 | 130≤SBP<139 mmHg N=1155 | SBP≥140 mmHg N=1389 |
|---|---|---|---|---|
| Composite endpoint | ||||
| • Event rate and 95% CI (per 100 person-years) | 15.2 (13.2, 17.6) | 11.4 (9.7, 13.5) | 12.2 (10.6, 14.1) | 15.6 (13.6, 17.8) |
| • Crude model HR (95% CI) | 1.34 (1.08, 1.67) | Ref | 1.07 (0.86, 1.34) | 1.37 (1.10, 1.69) |
| • Multivariable adjusted model 1 HR (95% CI) | 1.18 (0.94, 1.47) | Ref | 1.17 (0.94, 1.46) | 1.43 (1.16, 1.77) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.11 (0.89, 1.39) | Ref | 1.21 (0.98, 1.51) | 1.54 (1.24, 1.91) |
| Cardiovascular mortality | ||||
| • Event rate and 95% CI (per 100 person-years) | 3.5 (2.9, 4.3) | 2.6 (2.1, 3.3) | 3.0 (2.5, 3.6) | 2.9 (2.4, 3.4) |
| • Crude model HR (95% CI) | 1.35 (1.02, 1.79) | Ref | 1.13 (0.85, 1.51) | 1.08 (0.82, 1.43) |
| • Multivariable adjusted model HR (95% CI) | 1.24 (0.93, 1.65) | Ref | 1.16 (0.87, 1.55) | 1.17 (0.88, 1.55) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.18 (0.88, 1.58) | Ref | 1.19 (0.89, 1.60) | 1.23 (0.92, 1.65) |
| Total HF hospitalization | ||||
| • Event rate and 95% CI (per 100 person-years) | 11.7 (9.9, 13.8) | 8.8 (7.2, 10.6) | 9.2 (7.8, 10.9) | 12.7 (10.9, 14.8) |
| • Crude model HR (95% CI) | 1.34 (1.04, 1.72) | Ref | 1.06 (0.82, 1.36) | 1.45 (1.14, 1.86) |
| • Multivariable adjusted model HR (95% CI) | 1.17 (0.91, 1.51) | Ref | 1.18 (0.91, 1.51) | 1.51 (1.18, 1.92) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.10 (0.85, 1.42) | Ref | 1.22 (0.95, 1.57) | 1.63 (1.28, 2.09) |
| Myocardial infarction or stroke | ||||
| • Event rate and 95% CI (per 100 person-years) | 3.3 (2.7, 4.0) | 1.9 (1.5, 2.5) | 3.0 (2.5, 3.7) | 3.7 (3.1, 4.3) |
| • Crude model HR (95% CI) | 1.70 (1.24, 2.33) | Ref | 1.56 (1.31, 2.15) | 1.88 (1.39, 2.54) |
| • Multivariable adjusted model HR (95% CI) | 1.53 (1.10, 2.12) | Ref | 1.66 (1.20, 2.30) | 1.98 (1.45, 2.70) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.54 (1.10, 2.15) | Ref | 1.66 (1.20, 2.30) | 1.97 (1.43, 2.70) |
| All-cause mortality | ||||
| • Event rate and 95% CI (per 100 person-years) | 5.8 (5.1, 6.7) | 5.0 (4.3, 5.8) | 4.5 (3.9, 5.3) | 4.7 (4.1, 5.4) |
| • Crude model HR (95% CI) | 1.19 (0.96, 1.46) | Ref | 0.91 (0.73, 1.14) | 0.93 (0.76, 1.15) |
| • Multivariable adjusted model HR (95% CI) | 1.12 (0.90, 1.39) | Ref | 0.95 (0.76, 1.19) | 1.00 (0.80, 1.23) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.07 (0.86, 1.33) | Ref | 0.97 (0.78, 1.22) | 1.05 (0.84, 1.31) |
| Renal composite outcome | ||||
| • Event rate and 95% CI (per 100 person-years) | 0.3 (0.2, 0.6) | 0.5 (0.3, 0.8) | 0.8 (0.5, 1.1) | 1.1 (0.9, 1.5) |
| • Crude model HR (95% CI) | 0.67 (0.30, 1.49) | Ref | 1.67 (0.88, 3.15) | 2.44 (1.37, 4.38) |
| • Multivariable adjusted model HR (95% CI) | 0.63 (0.28, 1.41) | Ref | 1.74 (0.92, 3.29) | 2.47 (1.37, 4.43) |
| • Multivariable adjusted model 2 HR (95% CI) | 0.60 (0.27, 1.37) | Ref | 1.77 (0.93, 3.36) | 2.58 (1.42, 4.71) |
| Drug discontinuation | ||||
| • Event rate and 95% CI (per 100 person-years) | 11.8 (10.6, 13.1) | 8.8 (7.8, 10.0) | 8.7 (7.8, 9.9) | 10.7 (9.7, 11.8) |
| • Crude model HR (95% CI) | 1.34 (1.14, 1.57) | Ref | 0.99 (0.83, 1.18) | 1.21 (1.03, 1.42) |
| • Multivariable adjusted model HR (95% CI) | 1.27 (1.08, 1.50) | Ref | 1.04 (0.87, 1.23) | 1.23 (1.04, 1.45) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.28 (1.09, 1.52) | Ref | 1.03 (0.87, 1.23) | 1.22 (1.03, 1.44) |
HR, hazard ratio; CI, confidence interval; HF, heart failure.
Model 1 covariates include region, atrial fibrillation, creatinine, diabetes mellitus, New York Heart Association class, heart rate, sex, age, race, current smoking, number of anti-hypertensive medications, and treatment group.
Model 2 includes model 1 covariates and additionally adjusts for diastolic blood pressure.
In a complementary analysis modeling SBP as a continuous variable, a J-shaped relationship was observed between SBP and the primary outcome (as well as the other cardiovascular outcomes) (Figure 1); p<0.05 for overall relationship and for non-linearity). Baseline SBP did not modify the relationship between sacubitril/valsartan and the primary outcome (interaction p=0.50) or any other outcome (p>0.20 for all interaction terms).
Figure 1: Relationship between Baseline Continuous Systolic Blood Pressure and Efficacy and Safety Outcomes.
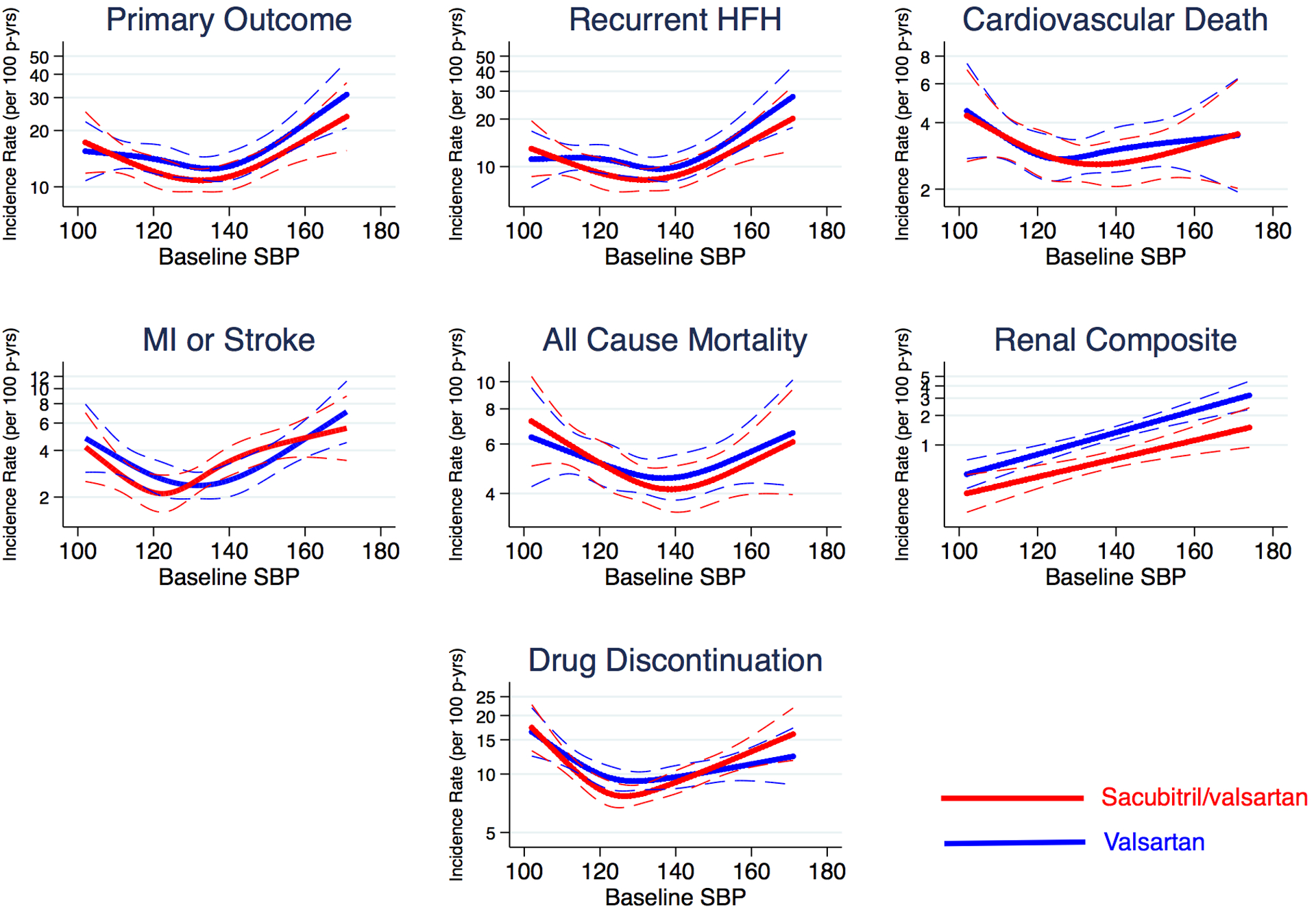
Incidence rates for the primary endpoint, cardiovascular death, total heart failure hospitalizations, all-cause death, myocardial infarction or stroke, a renal composite outcome, and study drug dose reduction or discontinuation among all patients according to systolic blood pressure at baseline. Sacubitril/valsartan shown in red, and valsartan depicted in blue. The interrupted lines are 95% confidence limits. MI, myocardial infarction; p-yrs, person-years.
To understand the relationship between change in BP and subsequent risk, we analyzed the relationship between time-updated, mean achieved SBP for all study outcomes, using quartile 2 (120–129mmHg) as the referent arm (Table 3) (17). Similar to the baseline SBP analysis, quartile 4 was associated with higher risks of the primary outcome and total HF hospitalizations after multivariable adjustment. In contrast, compared to quartile 2, quartile 1 was associated with a higher risk of mortality (HR 1.29, 95% CI 1.04, 1.62), and both quartiles 3 and 4 were associated with a higher risk of myocardial infarction or stroke as well as the renal composite outcome. Therefore quartile 2 was again associated with the lowest risk for all outcomes.
TABLE 3.
Event Rates and Crude and Adjusted Hazard Ratios for Efficacy and Safety Outcomes by Time-Updated Mean Achieved Systolic Blood Pressure Quartile
| Efficacy outcomes, n (%) | SBP<120 mmHg | 120≤SBP<129 mmHg | 130≤SBP<139 mmHg | SBP≥140 mmHg |
|---|---|---|---|---|
| Composite endpoint | ||||
| • Events/person-years | 397/2564 | 474/3776 | 499/4013 | 532/3494 |
| • Event rate and 95% CI (per 100 person-years) | 15.5 (14.0, 17.1) | 12.6 (11.5, 13.7) | 12.4 (11.4, 13.6) | 15.2 (14.0, 16.6) |
| • Crude model HR (95% CI) | 1.25 (1.04, 1.42) | Ref | 0.99 (0.87, 1.12) | 1.22 (1.07, 1.38) |
| • Multivariable adjusted model 1 HR (95% CI) | 1.10 (0.96, 1.27) | Ref | 1.05 (0.92, 1.19) | 1.24 (1.10, 1.41) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.07 (0.93, 1.23) | Ref | 1.07 (0.94, 1.22) | 1.30 (1.14, 1.47) |
| Cardiovascular mortality | ||||
| • Events/person-years | 89/2559 | 112/3780 | 117/4015 | 98/3505 |
| • Event rate and 95% CI (per 100 person-years) | 3.5 (2.8, 4.3) | 3.0 (2.5, 3.6) | 2.9 (2.4, 3.5) | 2.8 (2.3, 3.4) |
| • Crude model HR (95% CI) | 1.20 (0.91, 1.58) | Ref | 0.98 (0.76, 1.27) | 0.95 (0.72, 1.24) |
| • Multivariable adjusted model HR (95% CI) | 1.15 (0.86, 1.53) | Ref | 1.01 (0.78, 1.31) | 1.01 (0.77, 1.34) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.12 (0.84, 1.50) | Ref | 1.03 (0.79, 1.34) | 1.05 (0.79, 1.39) |
| Total HF hospitalizations | ||||
| • Events/person-years | 312/2564 | 356/3776 | 385/4013 | 433/3494 |
| • Event rate and 95% CI (per 100 person-years) | 12.2 (10.9, 13.6) | 9.4 (8.5, 10.5) | 9.6 (8.7, 10.6) | 12.4 (11.3, 13.6) |
| • Crude model HR (95% CI) | 1.30 (1.12, 1.51) | Ref | 1.02 (0.88, 1.18) | 1.32 (1.14, 1.51) |
| • Multivariable adjusted model HR (95% CI) | 1.13 (0.97, 1.32) | Ref | 1.09 (0.94, 1.26) | 1.33 (1.15, 1.54) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.09 (0.94, 1.28) | Ref | 1.11 (0.96, 1.29) | 1.39 (1.20, 1.60) |
| Myocardial infarction or stroke | ||||
| • Events/person-years | 78/2467 | 84/3647 | 116/3857 | 125/3318 |
| • Event rate and 95% CI (per 100 person-years) | 3.2 (2.5, 3.9) | 2.3 (1.9, 2.9) | 3.0 (2.5, 3.6) | 3.8 (3.2, 4.5) |
| • Crude model HR (95% CI) | 1.33 (0.98, 1.82) | Ref | 1.31 (0.98, 1.73) | 1.62 (1.23, 2.14) |
| • Multivariable adjusted model HR (95% CI) | 1.21 (0.87, 1.66) | Ref | 1.39 (1.05, 1.85) | 1.70 (1.28, 2.25) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.21 (0.88, 1.69) | Ref | 1.39 (1.04, 1.84) | 1.68 (1.26, 2.24) |
| All-cause mortality | ||||
| • Events/person-years | 161/2559 | 179/3780 | 180/4015 | 170/3505 |
| • Event rate and 95% CI (per 100 person-years) | 6.3 (5.4, 7.3) | 4.7 (4.1, 5.5) | 4.5 (3.9, 5.2) | 4.9 (4.2, 5.6) |
| • Crude model HR (95% CI) | 1.38 (1.11, 1.71) | Ref | 0.94 (0.77, 1.16) | 1.03 (0.84, 1.27) |
| • Multivariable adjusted model HR (95% CI) | 1.33 (1.07, 1.66) | Ref | 0.95 (0.77, 1.17) | 1.07 (0.87, 1.33) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.29 (1.04, 1.62) | Ref | 0.97 (0.79, 1.20) | 1.11 (0.89, 1.38) |
| Renal composite outcome | ||||
| • Events/person-years | 13/2543 | 17/3762 | 34/3979 | 33/3467 |
| • Event rate and 95% CI (per 100 person-years) | 0.5 (0.3 0.9) | 0.5 (0.3, 0.7) | 0.9 (0.6, 1.2) | 1.0 (0.7, 1.3) |
| • Crude model HR (95% CI) | 1.19 (0.58, 2.46) | Ref | 1.89 (1.06, 3.38) | 2.15 (1.20, 3.38) |
| • Multivariable adjusted model HR (95% CI) | 1.26 (0.60, 2.61) | Ref | 1.91 (1.06, 3.43) | 1.95 (1.08, 3.54) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.30 (0.62, 2.71) | Ref | 1.87 (1.04, 3.38) | 1.87 (1.02, 3.43) |
| Drug discontinuation | ||||
| • Events/person-years | 274/2261 | 318/3412 | 315/3584 | 327/3046 |
| • Event rate and 95% CI (per 100 person-years) | 12.1 (10.8, 13.6) | 9.3 (8.3, 10.4) | 8.8 (7.9, 9.8) | 10.7 (9.6, 12.0) |
| • Crude model HR (95% CI) | 1.29 (1.10, 1.52) | Ref | 0.94 (0.81, 1.10) | 1.14 (0.98, 1.33) |
| • Multivariable adjusted model HR (95% CI) | 1.20 (1.02, 1.41) | Ref | 0.96 (0.82, 1.13) | 1.09 (0.93, 1.28) |
| • Multivariable adjusted model 2 HR (95% CI) | 1.21 (1.02, 1.43) | Ref | 0.96 (0.82, 1.12) | 1.08 (0.92, 1.27) |
HR, hazard ratio; CI, confidence interval; HF, heart failure.
Time-updated, mean achieved systolic blood pressure uses average SBP as a time-updated covariate, which is updated at each BP ascertainment to represent the average observed blood pressure up to that time point.
Model 1 covariates include region, atrial fibrillation, creatinine, diabetes mellitus, New York Heart Association class, heart rate, sex, age, race, current smoking, number of anti-hypertensive medications, and treatment group.
Model 2 includes model 1 covariates and additionally adjusts for diastolic blood pressure.
During the run-in period, SBP decreased 5.5 (95% CI 5.1, 6.0) mmHg. After randomization, sacubitril/valsartan further reduced SBP by a maximum of 5.2 (95% CI 4.4, 6.0) mmHg, when compared with valsartan, by the 4-week visit (Table 4). Sacubitril/valsartan had a similar BP lowering effect across the 4 SBP baseline quartiles at the 4-week visit (interaction p=0.61) (Table 4 and Central Illustration). However, during follow-up, the difference in SBP among the treatment arms in the highest quartile diminished over time. In addition, the distribution of time-updated, mean achieved SBP quartile for the entire cohort is shown at randomization, 16 weeks, and 48 weeks by treatment arm in Figure 3, demonstrating a greater distribution of participants in lower SBP categories in the sacubitril/valsartan arm compared to the valsartan arm; this distribution is also shown in Supplementary Table 1 among those with baseline SBP ≥ 130 mmHg.
TABLE 4.
Valsartan-adjusted Change in Systolic Blood Pressure at the 4 Week Visit by Treatment Arm
| Baseline Systolic Blood Pressure Group | Sacubitril/Valsartan Arm (N=2379) | Valsartan Arm (N=2366) | Treatment Effect of Sacubitril/valsartan vs. Valsartan | |
|---|---|---|---|---|
| 4-week Change in SBP (95% CI) | 4-week Change in SBP (95% CI) | 4-week Change in SBP (95% CI) | P-value | |
| All Patients | −1.8 (−2.4, −1.2) | +3.4 (+2.8, +4.1) | −5.2 (−6.0, −4.4) | <0.001 |
| SBP < 120 mmHg | +5.5 (+4.4, +6.6) | +10.7 (+9.5, +11.9) | −5.3 (−6.9, −3.6) | <0.001 |
| 120 mmHg ≤ SBP <129 mmHg | +1.4 (+0.3, +2.5) | +6.3 (+5.2, +7.4) | −4.9 (−6.5, −3.3) | <0.001 |
| 130 mmHg ≤ SBP < 139 mmHg | −3.8 (−4.9, −2.7) | +1.3 (+0.1, +2.4) | −5.1 (−6.7, −3.5) | <0.001 |
| SBP ≥ 140 mmHg | −8.5 (−9.6, −7.4) | −3.1 (−4.3, −2.0) | −5.4 (−7.0, −3.9) | <0.001 |
SBP, systolic blood pressure.
Analyses adjust for baseline blood pressure.
Figure 2 (Central Illustration): Treatment effects on systolic blood pressure over time by baseline systolic blood pressure quartile.
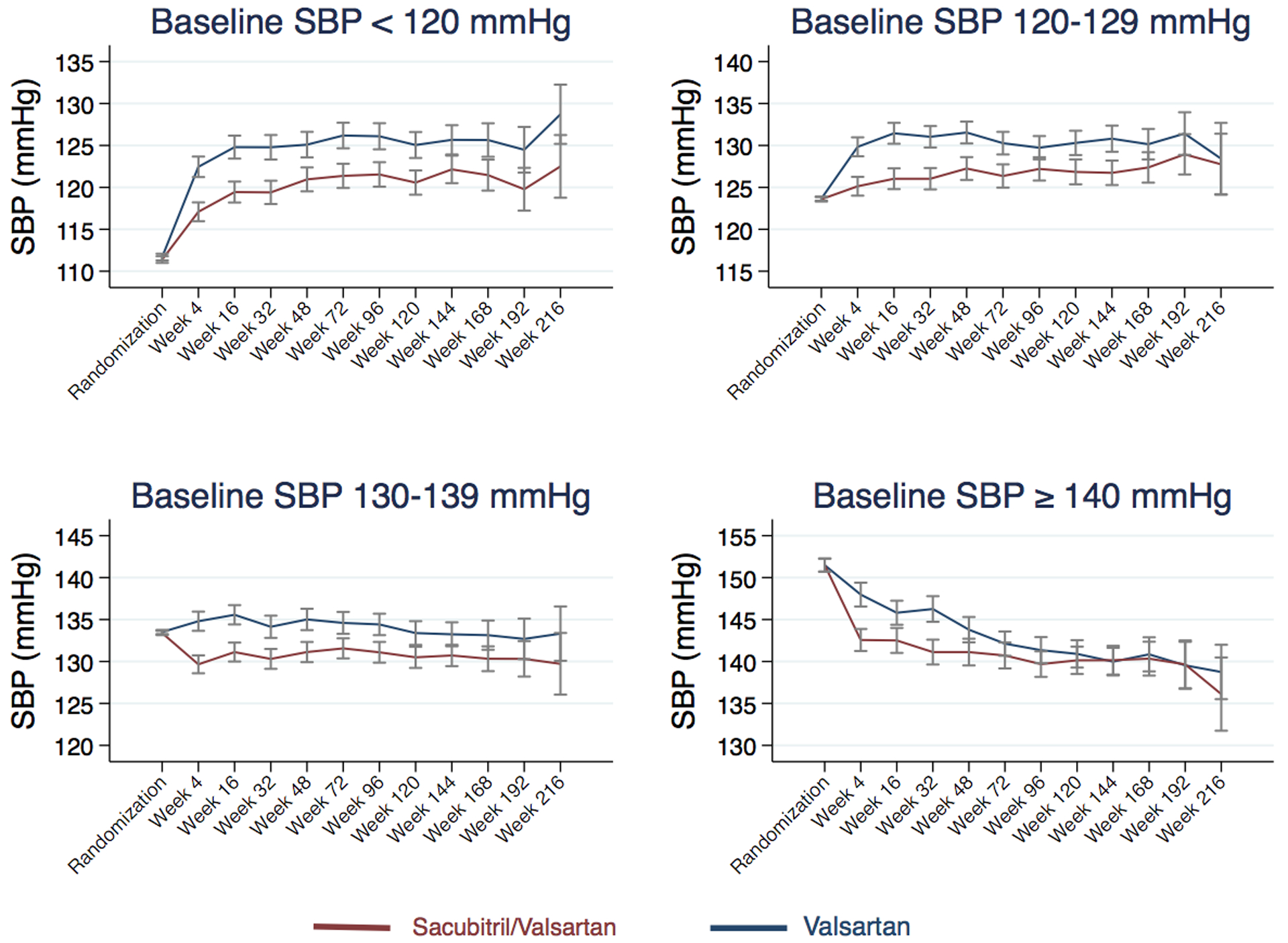
Systolic blood pressure during follow-up for valsartan (blue) and sacubitril/valsartan (red) treated patients shown separately for each systolic blood pressure quartile at baseline. Visits are truncated after week 216. Bars represent 95% confidence interval. SBP, systolic blood pressure.
Figure 3: Stacked bar graphs of time-updated, mean achieved systolic blood pressure quartile over time by treatment group.
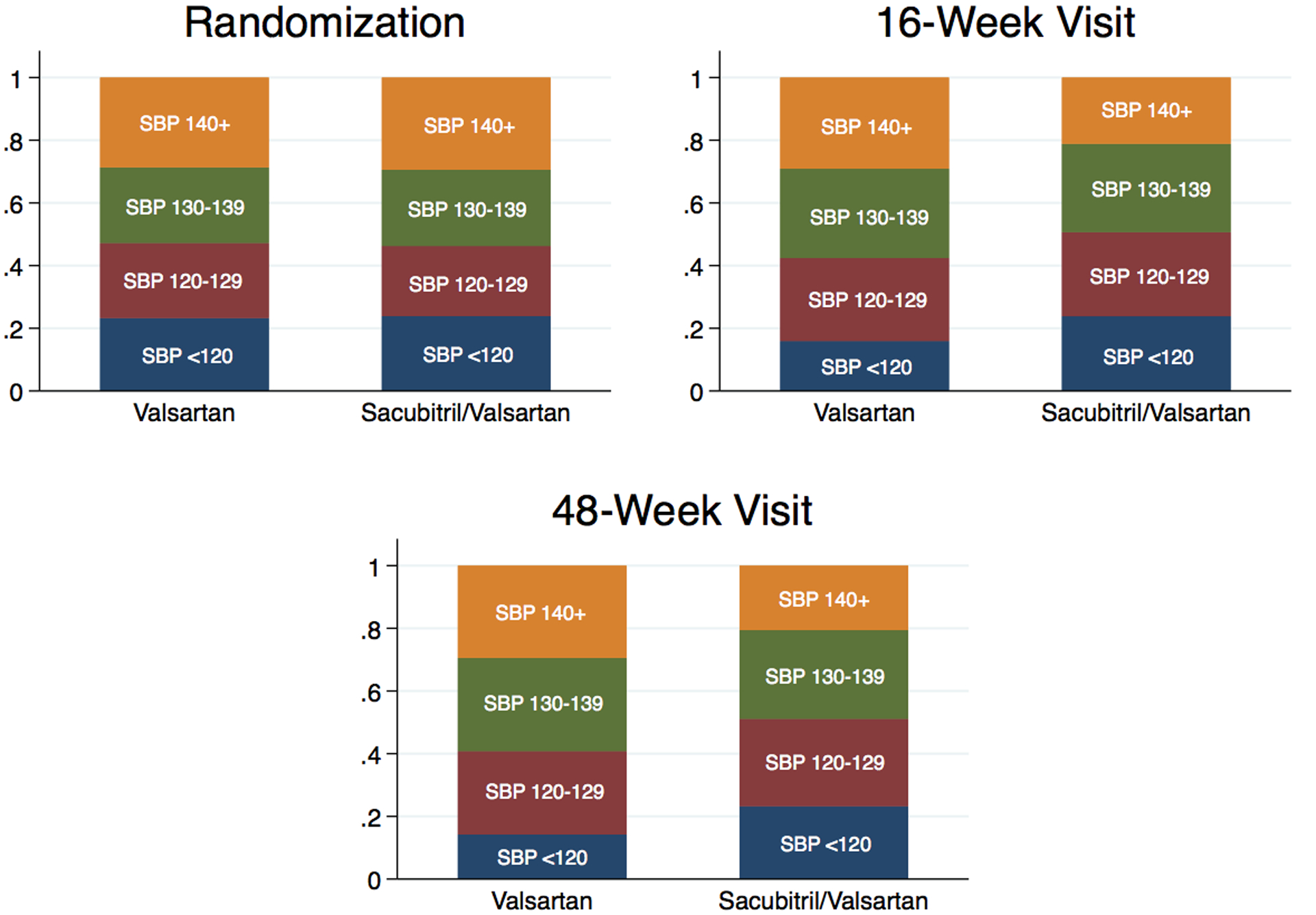
Percent of participants in each time-updated, mean achieved systolic blood pressure quartile at randomization, 16-week, and 48-week visits by treatment randomization to valsartan or sacubitril/valsartan. Systolic blood pressure expressed in mmHg.
We noted significant effect modification by gender for the SBP lowering effect of sacubitril/valsartan, such that sacubitril/valsartan reduced SBP at the 4-week visit more in women (6.3 mmHg, 95% CI 5.2, 7.4 mmHg) than in men (4.0 mmHg, 95% CI 2.9, 5.1 mmHg) (interaction p=0.005), which was driven by higher SBP among women in the valsartan arm (Figure 4). A marginal treatment effect by EF was observed (interaction p=0.08), such that sacubitril/valsartan reduced SBP more in with EF>57% (5.8 mmHg, 95% CI 4.6, 7.0 mmHg) than in those with EF≤57% (4.6 mmHg, 95% CI 3.6, 5.7 mmHg).
Figure 4: Systolic blood pressure over time by treatment arm and gender.
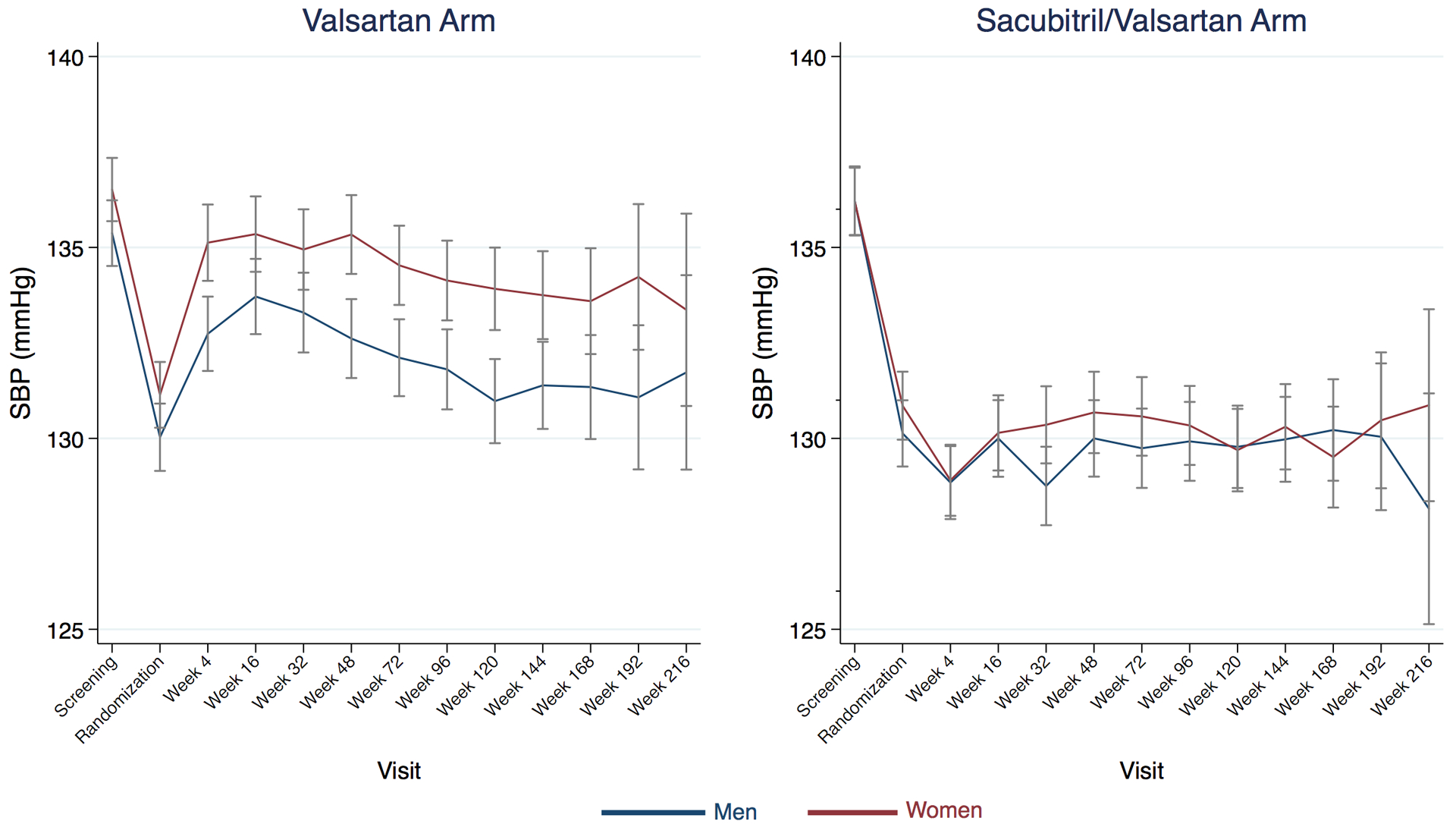
Women (maroon) had higher blood pressure than men (navy) on valsartan treatment, but there was no gender difference in patients randomized to sacubitril/valsartan. Visits are truncated after week 216. Bars represent 95% confidence interval. SBP, systolic blood pressure.
In a mechanistic analysis, we assessed the relationship between change in SBP between baseline to the 16 and 48-week visits with quality of life (KCCQ-OSS) and biomarkers (NT-proBNP and high-sensitivity troponin T) (Table 5). Change in SBP was not associated with change in KCCQ-OSS or high-sensitivity troponin T after multivariable adjusted analysis. Change in SBP was associated with a modest change in log transformed NT-proBNP after multivariable (−3.8% per 10 mmHg lowering in SBP, p<0.001) analysis at 16 weeks, which was similar at 48 weeks (−2.1% per 10 mmHg lowering in SBP, p=0.027).
TABLE 5.
Relationship between Change in Systolic Blood Pressure and Changes in Quality of Life and Biomarkers from Baseline to the 16 and 48-Week Visit.
| Total N | Change in Parameter per 10 mmHg Reduction in Systolic Blood Pressure from Baseline to the 16-Week Visit Fully-adjusted Model Beta-coefficient (95% CI)*^ | P for treatment interaction | Change in Parameter per 10 mmHg Reduction in Systolic Blood Pressure from Baseline to the 48-Week Visit Fully-adjusted Model Beta-coefficient (95% CI)*^ | P for treatment interaction | |
|---|---|---|---|---|---|
| Quality of Life | |||||
| • KCCQ-OSS (change in score) | 4507 | +0.1 (−0.2, +0.4), p=0.40 | 0.10 | +0.1 (−0.3, +0.3), p=0.92 | 0.30 |
| Biomarkers | |||||
| • NT-proBNP (% change) | 3222 | −3.8% (−5.4%, −2.2%), p<0.001 | 0.75 | −2.1% (−3.8%, −0.2%), p=0.027 | 0.72 |
| • High-sensitivity troponin T (% change) | 1205 | +0.9% (−0.3%, +2.0%), p=0.13 | 0.98 | +1.0% (−0.4%, +2.4%), p=0.16 | 0.98 |
CI, confidence interval; KCCQ-OSS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score.
Expressed as change in the parameter (KCCQ-OSS, NT-proBNP, or high-sensitivity troponin T) per 10 mmHg decrease in systolic blood pressure from baseline to the specified visit (16 or 48 week visit). All analyses controlled for and baseline blood pressure.
Additionally adjusted for baseline covariates including region, atrial fibrillation, creatinine, diabetes mellitus, New York Heart Association class, heart rate, sex, age, race, current smoking, number of anti-hypertensive medications, and treatment group.
To determine whether the treatment effects of sacubitril/valsartan were mediated by BP reduction, we performed Cox regression adjusting for baseline SBP and time-updated SBP for the primary outcome, total HF hospitalizations, and the renal composite outcome. As shown in Table 6, adjustment had little effect on the hazard ratios for any endpoint. Further, analyses were consistent regardless of sex (interaction p=0.49) or EF (continuous interaction p=0.80).
Table 6.
Effect of Change in Systolic Blood Pressure on Treatment Effect of Sacubitril/valsartan
| Efficacy outcomes | Unadjusted Hazard Ratio Sacubitril/Valsartan vs. Valsartan (95% CI) | P-value | Multivariable-Adjusted Hazard Sacubitril/Valsartan vs. Valsartan (95% CI)* | P-value |
|---|---|---|---|---|
| Composite endpoint | 0.87 (0.75, 1.01) | 0.058 | 0.87 (0.75, 1.00) | 0.056 |
| Total HF hospitalizations | 0.85 (0.72, 1.00) | 0.051 | 0.85 (0.72, 1.01) | 0.059 |
| Renal composite outcome | 0.50 (0.33, 0.77) | 0.001 | 0.52 (0.34, 0.79) | 0.002 |
CI, confidence interval; HF, heart failure.
Adjusted for baseline systolic blood pressure and time-updated systolic blood pressure.
DISCUSSION
In patients with HFpEF, both baseline and time-updated, mean achieved SBP 120–129 mmHg independently identified patients at the lowest risk for cardiovascular and renal outcomes prespecified in PARAGON-HF. Change in SBP related to change in NT-proBNP, though not with quality of life. Sacubitril/valsartan reduced SBP by a maximum of 5.2 mmHg, compared with valsartan, by the 4-week visit; this effect was consistently observed across baseline SBP quartiles, though the BP lowering effect was greater in women than men. However, the treatment effect of sacubitril/valsartan was not modified by baseline SBP and was independent of change in SBP, irrespective of sex. Our analyses provide new insight into the relationship of baseline, and mean achieved, SBP and outcomes in HFpEF, and further suggest that SBP reduction with sacubitril/valsartan is not responsible for its treatment benefits in HFpEF in both women and men.
A lack of a relationship between SBP quartiles and outcomes in HFpEF was shown in a TOPCAT analysis restricted to the Americas, though when SBP was analyzed continuously, a J-shaped relationship was observed (13). This difference in SBP quartile findings relative to the current study may simply relate to the significantly larger sample size in PARAGON. Additionally, in a post-hoc analysis of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, a randomized trial of sacubitril/valsartan in HF and reduced EF, the relationship between SBP and several outcomes was also noted to be J-shaped (18). Low SBP in HFpEF could be causally related to worse outcomes, but might also signify a sicker patient population with confounding conditions, supported by attenuation of the association between lower SBP and worse outcomes after the multivariable adjustment which included comorbidities and other variables predictive of higher risk. For example, there was a higher percentage of patients with atrial fibrillation or flutter in the lowest SBP quartile, as demonstrated in another HFpEF analysis (13), which could reflect greater use of therapies for rate control that additionally lower SBP or loss of atrial contraction that could also reduce BP.
The anti-hypertensive properties of sacubitril/valsartan in HFpEF in PARAGON-HF are similar to findings observed in HF with reduced EF. Vasodilators including sacubitril/valsartan in PARADIGM-HF (though not hydralazine and nitrates), reduce SBP across the baseline SBP range (18–21). Similar to these former studies in HFrEF, the beneficial effects of the therapies tested were independent of baseline SBP or change in SBP in PARAGON-HF. Among HFpEF patients, the BP lowering of sacubitril-valsartan in PARAGON-HF is similar in magnitude to spironolactone in TOPCAT (13). Compared specifically to studies of sacubitril-valsartan in other settings, the magnitude of BP reduction observed in PARAGON-HF was greater than that seen in mild to moderate hypertension (22) and acutely decompensated HFrEF (23), but similar to chronic kidney disease (24), HFrEF (18), and the phase II trial of sacubitril-valsartan in HFpEF (25). Interestingly, women achieved greater SBP reduction than men, which was driven by worse BP control with valsartan and equivalent BP control with sacubitril/valsartan compared to men. This differential gender effect does not appear to be observed in other cardiovascular conditions for which valsartan has been studied (26). However, the BP lowering effect still failed to explain the beneficial treatment effect of sacubitril/valsartan observed in women (14). In addition, a marginal treatment interaction by EF was observed, such that those with lower baseline EF had less BP reduction. It is possible the physiology in these participants behaves more similarly to HFrEF, whereby some vasodilators may increase cardiac output, attenuating the BP lowering effects of therapy (19,21).
In a mechanistic analysis, we assessed the change in SBP with change in biomarkers and quality of life. Change in SBP was associated with change in NT-proBNP, but not high-sensitivity troponin T or quality of life. Interestingly, the phase II trial of sacubitril/valsartan in HFpEF did not identify a significant relationship between change in BP and change in NT-proBNP (25). Our findings of a modest relationship may relate to larger sample or larger range of SBP allowed for study entry in PARAGON-HF. The lack of change with high sensitivity troponin T and quality of life could reflect a longer time course needed to achieve these findings.
As a result of the SPRINT Trial, professional society guidelines have recommended an SBP goal <130 mmHg (8). Notably, SPRINT excluded patients with prevalent HF. Our analysis shows that a mean achieved SBP of 120–129 mmHg was associated with the lowest risk for all study outcomes, supporting the current recommendation for blood pressure management. Given the observational nature of this analysis, a randomized trial of achieved SBP targets in HFpEF would be useful to confirm these findings.
Interestingly, the SBP reduction of sacubitril/valsartan in PARAGON-HF did not account for the treatment effect of sacubitril/valsartan (irrespective of sex), similar to findings of spironolactone in HFpEF (13). It is possible that the time course needed for BP reduction to reflect in the treatment effect could be much longer, since a longer duration of BP control may improve arterial stiffness, diastolic function, or cardiac remodeling. In addition, greater SBP control in the valsartan arm over time, particularly evident in the highest quartile, may have diminished the relevance of SBP reduction to the treatment effect. The Prospective comparison of ARNI with ARB on Management of HFpEF (PARAMOUNT) trial also demonstrated the independence of sacubitril/valsartan BP lowering with left atrial size and NYHA class (25). In addition, in HFrEF, sacubitril/valsartan did not affect central aortic stiffening compared to enalapril (27). These data suggest that the potential benefits of sacubitril/valsartan in HFpEF are likely multifactorial and go beyond its hemodynamic effects on blood pressure. Sacubitril/valsartan, for instance, increases availability of natriuretic peptides and a number of other vasoactive peptides which induce diuresis, natriuresis, and improve myocardial relaxation (5). Sacubitril/valsartan also specifically improves left atrial remodeling, a strong predictor of adverse events in HFpEF (28).
There are some limitations of the study. Ambulatory BP monitoring, rather than office BPs, may provide a more accurate assessment of BP as well as the treatment effect of sacubitril/valsartan. A previous analysis of sacubitril/valsartan in hypertension showed a significant reduction in ambulatory BP (29), but its relationship to outcomes was not studied. In addition, the specific inclusion/exclusion criteria for blood pressure in PARAGON-HF may limit generalizability to a broad HFpEF population. Finally, although PARAGON-HF is the largest trial in HFpEF to date, we may have been underpowered to detect more subtle relationships of SBP quartiles with some outcomes.
In summary, baseline and mean achieved SBP of 120–129 mmHg was associated with the lowest risk of adverse outcomes after multivariable adjustment. Lowering SBP was associated with a modest reduction in NT-proBNP, however without relationship to quality of life. Sacubitril/valsartan consistently reduced SBP across baseline SBP quartiles by 5.2 mmHg, an effect greater in women than men at the 4-week visit compared to valsartan. though BP reduction failed to account for its treatment effects on outcomes irrespective of sex. The potential benefits of sacubitril/valsartan in HFpEF may thus be mediated through other mechanisms.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In patients with HFpEF, SBP 120 to 129 mm Hg is associated with the lowest risk. Sacubitril/valsartan lowers blood pressure by ~5 mm Hg more than valsartan, and more in women than men.
TRANSLATIONAL OUTLOOK:
Because SBP lowering does not explain the treatment effects of sacubitril/valsartan, more research is needed to expose the mechanisms responsible.
FUNDING SOURCE
PARAGON-HF was funded by Novartis.
DISCLOSURES
Dr. Selvaraj is supported by the National Institutes of Health (Training Grant 5-T32HL007843-23). Dr. Claggett has received consultancy fees from Boehringer Ingelheim, Gilead, AOBiome, and Corvia. Dr. Böhm is supported by the Deutsche Forschungsgemeinschaft (DFG, TTR 219) and reports support from Abbott, Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Medtronic, Novartis, ReCor, Servier and Vifor. Dr. Anker reports grants from Vifor International and Abbott Vascular. He also personal fees from Bayer, Boehringer Ingelheim, Brahms GmbH, Impulse Dynamics, Novartis, Servier, St. Jude Medical, and Vifor International. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541) and serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, and Relypsa. Dr. Zannad reports receiving fees for serving on a steering committee from Janssen, Bayer, Boston Scientific, CVRx, and Boehringer Ingelheim, consulting fees from Amgen, Vifor Pharma-Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, and Merck, and consulting fees and fees for serving on a steering committee from AstraZeneca and serving as founder of cardiorenal and CVCT. Dr. Pieske reports receiving fees for serving on a steering committee, fees for serving on an advisory board, and lecture fees from Bayer HealthCare Pharmaceuticals and MSD, lecture fees from AstraZeneca, fees for serving on an advisory board and lecture fees from Bristol-Myers Squibb, fees for serving on an advisory board from Daiichi Sankyo, and lecture fees and honoraria from Medscape. Dr. Lam reports receiving grant support and fees for serving on an advisory board from Boston Scientific and Roche Diagnostics, grant support, fees for serving on an advisory board, and fees for serving on steering committees from Bayer, grant support from Medtronics, grant support and fees for serving on a steering committee from Vifor Pharma, fees for serving on an advisory board and fees for serving on steering committees from AstraZeneca and Novartis, fees for serving on an advisory board from Amgen, Boehringer Ingelheim, and Abbott Diagnostics, consulting fees from Merck and Stealth BioTherapeutics, fees for serving on a steering committee from Janssen Research and Development, lecture fees and consulting fees from Menarini, and fees for serving on a scientific committee from Corvia Medical and holding a pending patent (PCT/SG2016/050217) on a method regarding diagnosis and prognosis of chronic heart failure. Dr. Anand reports receiving fees for serving on a steering committee from AstraZeneca, ARCA Biopharma, Amgen, and LivaNova, fees for serving as chair of a data and safety monitoring board from Boston Scientific, fees for serving on an end-point committee from Boehringer Ingelheim, and fees for serving on an advisory board from Zensun. Drs. Lefkowitz and Shi are salaried employees of Novartis and ARR owns Novartis stock. Dr. McMurray has served as an executive committee member and coprincipal investigator of ATMOSPHERE and coprincipal investigator of the PARADIGM-HF and PARAGON-HF trials; and his employer, Glasgow University, has been paid by Novartis for his time spent in these roles. Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardior, Corvia, Cytokinetics, Gilead, GSK, Ironwood, Merck, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions.
ACRONYMS AND ABBREVIATIONS
- eGFR
estimated glomerular filtration rate
- HFpEF
heart failure with preserved ejection fraction
- KCCQ-OSS
Kansas City Cardiomyopathy Questionnaire overall summary score
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- PARAGON-HF
Prospective Comparison of Angiotensin receptor–neprilysin inhibitor with Angiotensin-receptor blockers Global Outcomes in HF with Preserved Ejection Fraction
- PARAMOUNT
Prospective comparison of ARNi with ARB on Management Of heart failUre with preserved ejectioN fracTion
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
Footnotes
The other authors report no conflicts.
ClinicalTrials.gov Identifier: NCT01920711
REFERENCES
- 1.Yusuf S, Pfeffer MA, Swedberg K et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777–81. [DOI] [PubMed] [Google Scholar]
- 2.Massie BM, Carson PE, McMurray JJ et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Pfeffer MA, Assmann SF et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 4.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA 2008;300:431–3. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Rizkala AR, Gong J et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC Heart Fail 2017;5:471–482. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Verma A, Desai A et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010;55:241–8. [DOI] [PubMed] [Google Scholar]
- 7.Lam CS, Shah AM, Borlaug BA et al. Effect of antihypertensive therapy on ventricular-arterial mechanics, coupling, and efficiency. Eur Heart J 2013;34:676–83. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017. [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 10.Group SR, Wright JT Jr., Williamson JD et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleland JG, Tendera M, Adamus J et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338–45. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, McMurray JJV, Anand IS et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraj S, Claggett B, Shah SJ et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail 2018;20:483–490. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV, Jackson AM, Lam CSP et al. Effects of Sacubitril-Valsartan, versus Valsartan, in Women Compared to Men with Heart Failure and Preserved Ejection Fraction: Insights from PARAGON-HF. Circulation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon SD, Vaduganathan M, Claggett BL et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2019. [Google Scholar]
- 16.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 17.Bohm M, Schumacher H, Teo KK et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet 2017;389:2226–2237. [DOI] [PubMed] [Google Scholar]
- 18.Bohm M, Young R, Jhund PS et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J 2017;38:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand IS, Rector TS, Kuskowski M, Thomas S, Holwerda NJ, Cohn JN. Effect of baseline and changes in systolic blood pressure over time on the effectiveness of valsartan in the Valsartan Heart Failure Trial. Circ Heart Fail 2008;1:34–42. [DOI] [PubMed] [Google Scholar]
- 20.Meredith PA, Ostergren J, Anand I et al. Clinical outcomes according to baseline blood pressure in patients with a low ejection fraction in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) Program. J Am Coll Cardiol 2008;52:2000–7. [DOI] [PubMed] [Google Scholar]
- 21.Anand IS, Tam SW, Rector TS et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007;49:32–9. [DOI] [PubMed] [Google Scholar]
- 22.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010;375:1255–66. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 24.Haynes R, Judge PK, Staplin N et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation 2018;138:1505–1514. [DOI] [PubMed] [Google Scholar]
- 25.Jhund PS, Claggett B, Packer M et al. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: an analysis of the PARAMOUNT trial. Eur J Heart Fail 2014;16:671–7. [DOI] [PubMed] [Google Scholar]
- 26.Tamargo J, Rosano G, Walther T et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017;3:163–182. [DOI] [PubMed] [Google Scholar]
- 27.Desai AS, Solomon SD, Shah AM et al. Effect of Sacubitril-Valsartan vs Enalapril on Aortic Stiffness in Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon SD, Zile M, Pieske B et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. [DOI] [PubMed] [Google Scholar]
- 29.Kario K, Sun N, Chiang FT et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension 2014;63:698–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


