Abstract
The authors evaluated, in a community‐based open‐label trial, the effectiveness and safety of perindopril in 13,220 US hypertensive patients and studied how physicians adhere to hypertension treatment guidelines. Patients received perindopril 4 mg q.d. for 6 weeks. Based on physicians' perception of blood pressure response, the patient was either maintained on 4 mg or the dose was increased to 8 mg for an additional 6 weeks. From baseline to week 12, the mean sitting blood pressure significantly declined from 156.9/94.5 mm Hg to 139.2/84.0 mm Hg. Further dose titration resulted in a clinically significant reduction in blood pressure in all patients with inadequate response on 4 mg at week 6. Blood pressure control (<140/<90 mm Hg) was achieved at 12 weeks in 48.8% patients. The subpopulation analyses demonstrated that perindopril monotherapy was effective in both men and women, in patients of all ethnicities, and in patients <65 and ≤65 years of age. Perindopril was safe and well tolerated in all hypertensive subgroups including high‐risk patients. Physicians were more attuned to controlling diastolic than systolic blood pressure, and their adherence to the treatment guidelines was found to be not optimal.
Hypertension is a major risk factor for cardiovascular and cerebrovascular diseases. The Hypertension Optimal Treatment (HOT) trial demonstrated the benefits of lowering diastolic blood pressure (BP) in hypertensive patients. 1 The lowest incidence of major cardiovascular events and mortality occurred at a mean diastolic BP of 82.6 mm Hg and 86.5 mm Hg, respectively. Nevertheless, the majority of diagnosed hypertensive patients in the United States remain undertreated and poorly controlled. 2 Worldwide epidemiologic surveys have found that rates of blood pressure control below 140/90 mm Hg range from as low as 6% to at best 30%. 3
Angiotensin‐converting enzyme (ACE) inhibitors have been shown to be effective in reducing blood pressure and in improving outcomes in a number of cardiovascular disease states. 4 Several controlled clinical trials have shown that perindopril, an ACE inhibitor, administered as monotherapy is effective and well tolerated in patients with mild to moderate essential hypertension. 5 , 6 However, these trials were conducted in selected patients and do not truly reflect the patients seen in a community setting.
Given the large proportion of hypertension in the general population, clinical data in these patients would be invaluable to prescribing clinicians. Previously published community‐based studies in hypertensive patients focused on the efficacy and tolerability of ACE inhibitors in comparison to other classes of antihypertensives. 7 , 8 In this community‐based clinical trial, we evaluated the effectiveness and safety of once‐daily administration of perindopril in patients with essential hypertension seen in general clinical practice and the adherence of physicians to hypertension treatment guidelines in a real‐life setting.
METHODS
Study Design
The clinical trial was a multicenter, practice‐based, open‐label study lasting 12 weeks. The objective of the study was to assess the utility of perindopril monotherapy administered once a day in affecting the sitting systolic blood pressure (SBP) and sitting diastolic blood pressure (DBP). The second objective was to determine whether physicians adhere to hypertension treatment guidelines in real‐life clinical practice.
Patients
Men and women who were at least 18 years of age or older were eligible if they had a sitting SBP of 140–180 mm Hg and/or a sitting DBP of 95–114 mm Hg inclusive and at least one of the following: newly diagnosed hypertension, inability to tolerate other antihypertensive medications, or lack of BP control with any prior antihypertensive monotherapy. Treatment with previous antihypertensive therapy was defined as receiving drugs at the time of enrollment or within 30 days before enrollment. All treated patients, with the exception of those taking drugs that require gradual withdrawal, were directly rolled over to perindopril.
Patients were excluded if they were pregnant or nursing, previously treated with perindopril, known to have allergic reactions to ACE inhibitors, or known to have a history of acute myocardial infarction or a clinically unstable condition in the past 3 months. Before entering the study, all patients signed informed consent forms approved by institutional review boards.
A total of 13,220 patients were enrolled in the study. The subjects in this study represented a cross‐section of patients seen by 2124 primary practice physicians. Patients were not sought by chart search, advertisement, or any other means of enhancing the study recruitment.
Study Conduct
At study entry (Visit 1), patients were provided with 4 mg perindopril tablets for 6 weeks from a participating local pharmacy. Patients were not to take any antihypertensive medications other than perindopril and were instructed to take the perindopril tablet at home at the same time every morning. The patients were allowed to take necessary drugs for cardiovascular indications other than hypertension. After 6 weeks on the 4‐mg dose, patients returned to the clinic (Visit 2). After the BP measurement at this visit, the patient's physician—based on clinical judgment—decided whether further up‐titration of perindopril was needed. Patients deemed by the physician as adequately responsive (Group I) were maintained on 4 mg perindopril tablets for an additional 6 weeks (a total of 12 weeks on 4 mg). In patients deemed by the physician as inadequately responsive, the dose of perindopril was increased to 8 mg/d for an additional 6 weeks (Group II). Patients from both groups then returned for the final visit at week 12 (Visit 3).
Study Procedures
At Visit 1, after sitting for 5 minutes, two BP readings, separated by 5 minutes, were taken and averaged for a mean baseline sitting SBP and DBP. Other variables collected at this visit include age, race, gender, duration of hypertension, cardiovascular disease history, previous antihypertensive therapy, and concurrent medications. At Visit 2, in addition to BP measurement, the physician's assessment of responsiveness to perindopril was obtained. At Visit 3, BP measurements were repeated, and patients returned all remaining study drug.
Efficacy Assessments
BP changes from baseline, BP normalization (<130/<85 mm Hg), and BP control (<140/<90 mm Hg) were assessed at week six and week 12. At the end of the study (week 12), the treating physician assessed the effectiveness of perindopril and entered it into the record as either satisfactory or unsatisfactory.
Safety
All patients were questioned by study personnel regarding the occurrence of adverse events (AEs) at week 6 and week 12. AEs were coded using the Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) dictionary (version 3.0, US Food and Drug Administration, Center for Drug Evaluation and Research, Rockville, MD). An AE was counted only once for each body system and preferred term.
Data Analyses
Intent‐To‐Treat (ITT) Patients were all patients who had received at least one dose of perindopril, for whom baseline and at least one postbaseline sitting SBP measurement was recorded, and had a completed safety case report form.
Safety Patients were all patients who had received at least one dose of perindopril and had a completed safety case report form.
Efficacy analysis was done on the ITT population. All statistical tests were two‐tailed, and all analyses were considered statistically significant if p<0.05. Baseline demographic and clinical characteristics, as well as efficacy variables, were compared between Group I and Group II using the Student t test or the χ 2 test for continuous and categorical variables, respectively. A paired t test was used to test the null hypothesis that the mean change from baseline to postbaseline equals zero within the treatment group for each efficacy variable. Subgroup analyses were performed by race, age, gender, presence of diabetes, and patients treated with previous antihypertensives.
RESULTS
Patient Disposition
A total of 13,220 eligible patients were enrolled. Of these, 1430 patients did not have at least one confirmed dose of perindopril and were thus excluded from all analysis. Of the remaining 11,790 patients with confirmed dosing, 10,425 patients met the criteria for the ITT patients and 11,404 patients met the criteria for the safety patients.
Of the 10,425 ITT patients (newly diagnosed hypertensives, n=5144; patients with history of hypertension, n=5224; unknown, n=57) included in the efficacy analysis, 8241 patients completed the study. In the remaining 2184 patients, 2151 did not complete the study due to AE (n=935), withdrawal of consent (n=66), protocol violation (n=145), being lost to follow‐up (n=432), investigator decision (n=141), sponsor discretion (n=6), other reasons (n=201), and unknown reasons (n=225). Completion status was not available for 33 patients.
Baseline Characteristics
ITT Patients (n=10,425). The mean (SD) age was 56.0 (14.3) years. The majority of patients were <65 years (70.9%). Patients were equally distributed by gender. Patient distribution by race in this study was consistent with the race distribution of the US population. Percentages of whites, African Americans, Hispanics, Asians, and others were 74.3%, 13.5%, 8.4%, 2.1%, and 1.0%, respectively.
The mean (SD) duration of hypertension was 3.3 (6.4) years. A total of 1600 (15.3%) patients were reported to have a significant cardiovascular disease history. These include angina (3.2%), arrhythmia (2.7%), stroke or transient ischemic attack (2.7%), myocardial infarction (2.1%), congestive heart failure (1.2%), and other (9.2%). In addition, 876 patients (8.4%) entered the study on oral hypoglycemic agents suggesting the concomitant presence of diabetes. A total of 3159 (30.3%) patients were not responsive to previous antihypertensive therapy. Of these, 970 patients were on previous ACE inhibitors. The baseline mean (SD) sitting SBP and DBP of ITT patients was 156.9 (14.4) mm Hg and 94.5 (9.5) mm Hg, respectively.
Group I and Group II Patients
The percentage of men (51.1% vs. 46.8%) and African Americans (16.1% vs. 11.9%) was significantly higher in Group II than in Group I (p<0.001). Similarly, the mean duration of hypertension (3.8 years vs. 3.0 years) and baseline mean BP (159.4/95.8 mm Hg vs. 155.2/93.6 mm Hg) was significantly higher in Group II than in Group I (p<0.001). The mean age, percentage of elderly, and diabetes and cardiovascular history were similar in both groups.
Effect on Blood Pressure
ITT patients (Group I and Group II). From baseline to week 12, the mean sitting BP declined from 156.9/94.5 mm Hg to 139.2/84.0 mm Hg (p<0.001). The mean reduction in BP at weeks 6 and 12 was 14.6/8.7 mm Hg and 17.7/10.5 mm Hg, respectively. The mean sitting BP from baseline to week 12 in newly diagnosed hypertensives declined from 156.3/95.6 mm Hg to 136.8/83.9 mm Hg (p<0.001), and in previous hypertensives, the mean BP declined from 157.4/93.4 mm Hg to 141.5/84.2 mm Hg (p<0.001).
The mean changes from baseline in SBP and DBP at week 6 and 12 for Group I and Group II are illustrated in Figure 1. In Group I, the mean BP from baseline to week 6 and to week 12 declined from 155.2/93.6 mm Hg to 135.9/82.3 mm Hg and 134.9/81.8 mm Hg, respectively. In Group II, the mean BP from baseline to week 6 and to week 12 declined from 159.4/95.8 mm Hg to 151.8/91.1 mm Hg and 144.6/86.9 mm Hg, respectively. In this group the reduction in SBP and DBP on 4‐mg dose at week 6 was markedly less than in Group I. Increasing the dose to 8 mg resulted in a clinically significant total reduction in both SBP and DBP at week 12. The BP reduction from baseline at week 6 and week 12 was statistically significant (p<0.001) in both groups.
Figure 1.
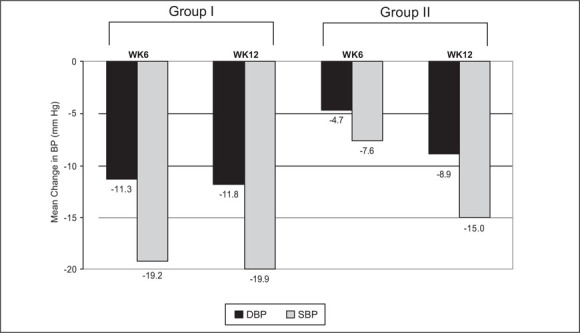
Mean change in blood pressure (BP) from baseline for systolic blood pressure (SBP) and diastolic blood pressure (DBP); WK=week
Blood Pressure Normalization (<130/85 mm Hg)
The percent of patients with BP normalized during the study increased over time during the 12‐week study period. At baseline, week 6, and week 12, the percent of patients with BP normalization were 0.6%, 15.7%, and 21.1%, respectively.
Although in Group I the increase in BP normalization from baseline was from 24.8% at week 6 to 28.1% at week 12, in Group II the increase with up‐titration was from 0.7% at week 6 to 10.7% at week 12.
Blood Pressure Control
The percent of patients with overall BP control (<140/90 mm Hg), SBP control (<140 mm Hg), and DBP control (<90 mm Hg) is shown in Table I.
Table I.
Blood Pressure Control
| Percent Achieving Blood Pressure Control | |||
|---|---|---|---|
| Patients | SBP <140 mm Hg, DBP <90 mm Hg | SBP <140 mm Hg | DBP <90 mm Hg |
| Total | |||
| Week 6 (n=10,360) | 37.8% | 45.8% | 64.2% |
| Week 12 (n=8788) | 48.8% | 55.7% | 73.3% |
| Group I | |||
| Week 6 (n=6206) | 58.3% | 64.3% | 81.6% |
| Week 12 (n=4933) | 60.4% | 66.6% | 81.9% |
| Group II | |||
| Week 6 (n=4154) | 7.1% | 18.2% | 38.2% |
| Week 12 (n=3855) | 34.0% | 41.8% | 62.3% |
| SBP=systolic blood pressure; DBP=diastolic blood pressure | |||
BP Control (<140/90 mm Hg). Perindopril treatment improved systolic and diastolic BP control from 1.7% at baseline to 37.8% at week 6 and 48.8% at week 12 in total ITT patients. Whereas in Group I the increase in BP control from week 6 to week 12 was minimal (from 58.3% to 60.4%), in Group II with up‐titration from 4 mg to 8 mg, the increase from week 6 to week 12 was substantial (from 7.1% to 34.0%).
SBP Control (<140 mm Hg). Perindopril treatment improved SBP control from 7.6% at baseline to 45.8% at week 6 and to 55.7% at week 12 in total ITT patients. In Group I the increase in SBP control from week 6 to week 12 was minimal (from 64.3% to 66.6%), but in Group II with up‐titration from 4 mg to 8 mg the increase was more than two‐fold (from 18.2% to 41.8%).
DBP Control (<90 mm Hg). Perindopril treatment improved DBP control from 22.9% at baseline to 64.2% at week 6 and to 73.3% at week 12 in total ITT patients. Whereas in Group I the DBP control was similar at week 6 (81.6%) and week 12 (81.9%), in Group II with up‐titration from 4 mg to 8 mg this was increased from 38.2% at week 6 to 62.3% at week 12.
Subpopulation Analyses. All of the subpopulations (age, gender, race) analyzed had statistically significant reductions from baseline in mean sitting BP at week 12 (p<0.001) consistent with ITT population as shown in Table II. In addition, the percent of patients with BP control (<140/90 mm Hg) was comparable in all groups except African Americans and elderly.
Table II.
Effect of Perindopril on Blood Pressure (BP) in Subpopulations
| BP (mm Hg) | ||||
|---|---|---|---|---|
| Demographic Subgroups | n | Baseline | Week 12 | BP Control* |
| Age | ||||
| <65 | 7332 | 154.7±13.7/96.8±8.0 | 137.2±15.3/85.5±9.2 | 51.9% |
| ≥65 | 3010 | 162.2±14.6/88.9±10.4 | 143.8±17.9/80.4±9.7 | 41.4% |
| Race | ||||
| White | 7745 | 157.0±14.4/94.1±9.6 | 138.8±16.3/83.5±9.4 | 50.2% |
| African American | 1412 | 156.3±14.0/96.5±8.8 | 142.0±17.3/87.5±10.5 | 38.9% |
| Hispanic | 877 | 157.0±15.0/95.0±9.4 | 138.3±15.8/83.6±9.2 | 50.6% |
| Asian | 214 | 156.5±13.4/95.3±9.1 | 137.5±16.1/84.2±9.7 | 54.1% |
| Gender | ||||
| Male | 5056 | 154.8±13.9/95.7±9.0 | 138.3±15.7/85.1±9.6 | 49.5% |
| Female | 5209 | 158.8±14.6/93.3±9.8 | 139.9±16.9/83.0±9.6 | 48.2% |
| *<140/90 mm Hg at week 12, BP reduction from baseline to week 12 was significant between <65 vs. ≥65 (p<0.05) and African American vs. white (p<0.001) | ||||
Patients on Previous Antihypertensives
The effect of perindopril was evaluated in the subgroup of patients who were nonresponsive to previous antihypertensives (as indicated by treating physicians). These include diuretics (n=929), β blockers (n=663), calcium‐channel blockers (n=972), α blockers (n=200), ACE inhibitors (n=970), and angiotensin‐receptor blockers (n=376).
As shown in Figure 2, perindopril treatment for 12 weeks achieved significant reduction from baseline in SBP and DBP in patients who were nonresponsive to different classes of previous antihypertensives (p<0.001). At week 12, the reduction from baseline in SBP and DBP ranged from 9.8 to 16.5 mm Hg and from 5.3 mm Hg to 9.7 mm Hg, respectively.
Figure 2.
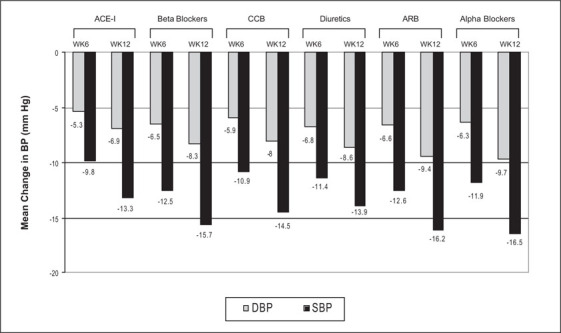
Mean change in blood pressure (BP) with perindopril in patients nonresponsive to previous antihypertensives ACE‐I=angiotensin‐converting enzyme inhibitor; CCB=calcium channel blocker; ARB=angiotensin receptor blocker; WK=week; DBP=diastolic blood pressure; SBP=systolic blood pressure
Patients With Diabetes
For this subgroup analysis, patients maintained on oral hypoglycemic agents were considered diabetics. A total of 876 patients were included in this subgroup analysis. The BP values of diabetic patients and the rest of patients (others) included in the study were assessed by treating physicians as satisfactory or unsatisfactory at week 12 and are shown in Table III. The mean BP in diabetics and others assessed by treating physicians as satisfactory was 137.6/80.7 mm Hg and 135.3/82.4 mm Hg, respectively. From these data, it appears that physicians did not set lower BP goals for diabetics than for the rest of the patients.
Table III.
Physician Assessment of Perindopril Effect on Blood Pressure (BP)
| Baseline | Week 12 | |||||
|---|---|---|---|---|---|---|
| BP/P Assessment | Diabetics* | Others† | p Value‡ | Diabetics* | Others† | p Value‡ |
| Systolic BP (mm Hg) | ||||||
| Satisfactory | 156.8±14.8 | 156.0±14.0 | 0.175 | 137.6±14.7 | 135.3±13.4 | <0.001 |
| Unsatisfactory | 161.5±14.1 | 159.8±15.3 | 0.167 | 159.5±17.5 | 156.6±17.4 | 0.082 |
| Diastolic BP (mm Hg) | ||||||
| Satisfactory | 91.0±10.7 | 94.6±9.1 | 0.002 | 80.7±9.4 | 82.4±8.3 | <0.001 |
| Unsatisfactory | 93.0±10.6 | 95.6±10.2 | 0.002 | 90.4±12.9 | 93.0±10.3 | 0.033 |
| *Diabetics (based on patients maintained on oral hypoglycemic agents): satisfactory (n=672), unsatisfactory (n=172); †others: satisfactory (n=7158), unsatisfactory (n=1866); ‡p value using t test comparing diabetics vs. others | ||||||
Safety Assessment
The overall percent of patients with adverse events was 22.9%. Cough, the most frequent adverse event, was experienced by 8.1% of patients, and total edema had the lowest incidence (1.1%). The percentage of patients who discontinued due to adverse events was 10.4%. The most frequent adverse events (>1%) that led to discontinuation included cough (4.1%), headache (1.2%), and dizziness (1.2%).
There were 39 cases (0.3%) of angioedema reported in these patients; 25 of these were in women, 13 in African Americans, 24 in whites, and two in Hispanics; mean age was 59.3±16.0 years. Three of these patients (all white women) required hospitalization and recovered completely.
During the course of the study, 11 patients died. The cause of death was myocardial infarction, two patients; hypertensive and atherosclerotic heart disease, two patients; asystole, one patient; subarachnoid hemorrhage, one patient; pulmonary embolism, two patients; noncardiovascular cause, three patients (two died as the result of motor vehicle accidents, one died of multidrug toxicity). According to the treating physicians, the causal relationship to perindopril was either unlikely or unrelated in all patients.
DISCUSSION
This community‐based trial was undertaken to determine the effectiveness and safety of perindopril in real‐life practice settings with hypertensive patients from various demographic subpopulations. This study also evaluated how physicians adhered to treatment guidelines when given to average patients, seen in regular clinics, in the course of regular clinical practice. We purpusely did not define a specific BP goal that must be reached during the trial or the conditions under which the dose of perindopril ought to be increased.
Whereas usefulness of perindopril was established in previous controlled trials, 9 , 10 , 11 this large‐scale clinical experience trial was successful in confirming the antihypertensive effectiveness and safety of once‐daily administration of perindopril in hypertensive patients treated in uncontrolled conditions. The subpopulation analyses demonstrated that perindopril monotherapy was effective in both men and women, in patients of all ethnicities, and in patients younger and older than age 65 years. In addition, this community trial established that perindopril was effective and safe in high‐risk hypertensive subgroups such as African Americans and diabetics.
The titration approach adopted in this study achieved clinically significant reduction in BP in additional numbers of patients. Support for the titration approach from 4 to 8 mg is corroborated by the following evidence seen in Group II patients: increase in the reduction of SBP/DBP from 7.6/4.7 mm Hg at week 6 to 15.0/8.9 mm Hg at week 12, increase in the percent of patients achieving BP normalization (<130/<85 mm Hg) from 0.7% at week 6 to 10.7% at week 12, and increase in the percent of patients achieving blood pressure control (<140/<90 mm Hg) from 7.1% at week 6 to 34.0% at week 12. Study patients who needed up‐titration (Group II) tended to be more often male, African American, to have longer duration of hypertension, and to have had higher blood pressure at entry. Overall, our results indicate that uptitration of perindopril monotherapy resulted in a further decrease of BP. Thus, titration to higher doses of perindopril before addition of other antihypertensive drugs is a viable clinical option.
In the recently published Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), 12 it was shown that a large proportion of patients required more than one drug to adequately control their BP; that is, multidrug therapy resulted in achieving BP control (<140/<90 mm Hg) in more than 60% of patients. In this community trial consisting of a broad range of hypertensive patients including African Americans and diabetics (similar to ALLHAT), perindopril monotherapy achieved BP control in 49% of patients. This could be attributed to the up‐titration of perindopril to the maximum dose.
In our study, up‐titration of the perindopril dose was left to the physicians' decision, and no specific target goals were preset. This permitted us to gain some insight into physicians' adherence to hypertension treatment guidelines. Generally, the physicians seemed to follow the guidelines better than expected based on the National Health and Nutrition Examination Survey (NHANES) report that 70% of patients in the United States have inadequate BP control. From Table I it could be inferred that physicians accepted inadequate BP control (<140/90 mm Hg) without further upward drug titration at week 6 in 41.7% of patients (Group I). Table I also suggests that physicians were more attuned to controlling DBP than SBP. In Group I the physicians decided not to increase the dose of perindopril at week 6 in 35.7% of patients who had inadequate SBP values compared with 18.4% of patients whose DBP was not at the goal defined by the sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI). 13
Our data also suggest that practicing physicians' adherence to JNC VI guidelines for intensive lowering of BP in high‐risk groups like diabetics was not optimal. As shown in Table III, the physicians assessed BP response to be satisfactory at similar BP levels in diabetics and other patients.
This large, clinical‐experience trial established that overall, patients tolerated perindopril well in the dose range of 4–8 mg with a low withdrawal rate due to AE (10.4%) and a very low incidence of hypotension (<1%) and angioedema (0.3%). Cough was reported in 8.1%, an incidence similar to others in its class.
The strength of this study is the large sample size and prospective design. However, this study has limitations due to the lack of control group and its non‐randomized design, although comparison of age, race, and gender breakdown of the sample suggests that the sample was representative. A limitation of a study of this size is management (monitoring and data collection) of the study, which corresponded to some unavoidable loss of data from patients. In addition, the study design did not include a wash‐out period, which could have compromised baseline BP due to influence of previous antihypertensives.
Success of a study of this type is not only gauged by the results of the study occurring within the study period, but also by the reaction of the investigators to the study drug after the study is completed. Overall, the investigators assessed the effectiveness and safety of perindopril as satisfactory in 79% of the patients based on their experience.
Our findings in this study confirm that perindopril monotherapy in the dose range of 4–8 mg is effective and safe in a large, diverse, US hypertensive patient population.
Acknowledgment: The authors thank Sherrill Martin for secretarial help in preparing the manuscript.
References
- 1. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. HOT Study Group. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 2. Oliveria SA, Lapuerta P, McCarthy BD, et al. Physician‐related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–420. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Sega R, Milesi C, et al. Blood‐pressure control in the hypertensive population. Lancet. 1997;349:454–457. [DOI] [PubMed] [Google Scholar]
- 4. Halkin A, Keren G. Potential indications for angiotensin‐converting enzyme inhibitors in atherosclerotic vascular disease. Am J Med. 2002;112:126–134. [DOI] [PubMed] [Google Scholar]
- 5. Hurst M, Jarvis B. Perindopril: an updated review of its use in hypertension. Drugs. 2001;61:867–896. [DOI] [PubMed] [Google Scholar]
- 6. Chrysant SG, McDonald RH, Wright JT, et al. Perindopril as monotherapy in hypertension: a multicenter comparison of two dosing regimens. The Perindopril Study Group. Clin Pharmacol Ther. 1993;53:479–484. [DOI] [PubMed] [Google Scholar]
- 7. Gregoire JP, Moisan J, Guibert R, et al. Tolerability of antihypertensive drugs in a community‐based setting. Clin Ther. 2001;23:715–726. [DOI] [PubMed] [Google Scholar]
- 8. Messerli F, Weir MR, Neutel JM. Combination therapy of amlodipine/benazepril versus monotherapy of amlodipine in a practice‐based setting. Am J Hypertens. 2002;15:550–556. [DOI] [PubMed] [Google Scholar]
- 9. Zannad F, Bernaud CM, Fay R. Double‐blind, randomized, multicentre comparison of the effects of amlodipine and perindopril on 24 h therapeutic coverage and beyond in patients with mild to moderate hypertension. General Physicians Investigators' Group. J Hypertens. 1999;17:137–146. [DOI] [PubMed] [Google Scholar]
- 10. Overlack A, Adamczac M, Bachmann W, et al. ACE‐inhibition with perindopril in essential hypertensive patients with concomitant diseases. Am J Med. 1994;97:126–134. [DOI] [PubMed] [Google Scholar]
- 11. Morgan TO, Louis WT, MacDonald GJ, et al. Antihypertensive efficacy and safety of perindopril in mild‐to‐moderate essential hypertension: results of a double‐blind multicenter study versus atenolol. Am J Med. 1992;92(suppl 4B):73S–78S. [DOI] [PubMed] [Google Scholar]
- 12. The ALLHAT officers and coordinators for the ALLHAT collaborative research group . Major cardiovascular events in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic. The antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 13. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]


