Abstract
Systolic hypertension is predominant among patients over 50 years of age, is a more important cardiovascular risk factor than diastolic blood pressure, and is more difficult to control than diastolic blood pressure. Consequently, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends combination therapy as first‐line treatment for patients with stage 2 hypertension. In the Systolic Evaluation of Lotrel Efficacy and Comparative Therapies (SELECT) study, 24‐hour ambulatory blood pressure monitoring was used to identify patients with systolic hypertension and to determine the impact of 8 weeks of treatment with either amlodipine besylate/benazepril HCl 5/20 mg combination therapy (n=149), amlodipine besylate 5 mg (n=146), or benazepril HCl 20 mg (n=148). Combination therapy was significantly more effective in reducing systolic blood pressure and pulse pressure than either monotherapy (p<0.0001). Significantly greater percentages of patients in the combination group compared with either monotherapy achieved blood pressure control (p<0.0001). Adverse events were low in all three treatment arms, with less peripheral edema in the combination group than in the amlodipine‐treated group. The combination of amlodipine besylate/benazepril HCl given to patients with stage 2 systolic hypertension resulted in significantly greater reductions in blood pressure and pulse pressure than those seen with monotherapy and was at least as well tolerated as the separate components. This data supports the recommendation of the JNC 7 for the use of combination therapy in patients with stage 2 hypertension.
For many years, diastolic blood pressure (DBP) was regarded as a powerful predictor of cardiovascular (CV) disease, while consideration of systolic blood pressure (SBP) was largely ignored. This is reflected by the first two reports of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure, 1 , 2 which did not include SBP as a criterion for diagnosing hypertension. In recent years, however, epidemiologic studies have clearly demonstrated that SBP is a better predictor of pending CV disease than is DBP, 3 , 4 , 5 especially in patients over 50 years of age. 6 , 7 Small increments in SBP are associated with an increased burden of CV disease, such that an increase in SBP of only 2 mm Hg equates with a 10% increase in fatal stroke and a 7% increase in fatal coronary heart disease. 8 Attention has now shifted to controlling elevated SBP, which is predominant among older persons and is considerably more difficult to control than DBP. 7 Studies suggest that poor control of SBP largely accounts for the low rates of overall blood pressure (BP) control. 9 , 10 In older hypertensives, only approximately 20% achieve BP of <140/90 mm Hg, largely because of poor control of SBP. 11
A large number of outcome studies have been performed in the past decade in patients with systolic hypertension, primarily in older patients. These studies have demonstrated that treating SBP results in significant reductions in CV events. 12 , 13 , 14 , 15 Despite the benefits of treating SBP, however, in several studies most patients continued to have uncontrolled systolic hypertension even when DBP was controlled. 13 , 14 , 15 , 16 , 17 , 18 These studies thus underscore the fact that older patients with systolic hypertension are a difficult‐to‐treat population.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 7 notes that most patients with hypertension will require two or more antihypertensive medications to achieve their BP goals and states that “[w]hen blood pressure is more than 20 mm Hg above systolic goal or 10 mm Hg above diastolic goal, consideration should be given to initiate therapy with two drugs, either as separate prescriptions or in fixed‐dose combinations….” Combination therapy may have several advantages over monotherapy, including improved efficacy, tolerability, and medication adherence. 19 , 20 , 21 , 22 Combination therapy will increase the likelihood of achieving the BP goal in a more timely fashion. 7
Most of our current data on SBP are derived from studies that enrolled patients based on DBP. In such studies, SBP may be lower and may underestimate the impact of antihypertensive agents on SBP. To our knowledge, the Systolic Evaluation of Lotrel Efficacy and Comparative Therapies (SELECT) study is the first trial to enroll patients based on SBP as measured by ambulatory BP monitoring (ABPM).
Amlodipine besylate/benazepril HCl is a fixed‐dose combination antihypertensive containing a dihydropyridine calcium channel blocker and an angiotensin‐converting enzyme (ACE) inhibitor. The SELECT study was conducted to compare the effects of amlodipine besylate/benazepril HCl 5/20 mg combination therapy with those of amlodipine besylate 5 mg monotherapy and benazepril HCl 20 mg monotherapy on mean 24‐hour SBP in patients aged 55 and older during 8 weeks of daily treatment. Additional parameters studied included the effects of these therapies on: 1) mean 24‐hour pulse pressure; 2) mean 24‐hour DBP; 3) BP response and control rates; and 4) the incidence of peripheral edema.
METHODS
Design and Study Population The SELECT study was a multicenter, randomized, double‐blind, parallel‐group study in men and women aged 55 and older with systolic hypertension. Patients who were not currently taking any antihypertensive medication who were newly diagnosed, or who were treated but discontinued their previous antihypertensive medication, were eligible to participate. All patients entered a 2‐ to 4‐week, single‐blind, placebo run‐in period during which they were seen at weekly intervals, followed by 8 weeks of active treatment during which they had clinic visits at biweekly intervals. Written informed consent was obtained from each patient. Women were required to have been postmenopausal for 1 year or to be surgically sterile.
BP Criteria To enter the study, patients were required to have predominantly systolic hypertension by office BP and ABPM criteria. Patients were required to have a mean seated office (average of three seated office BPs) SBP of 160–200 mm Hg and a mean seated DBP of ≤100 mm Hg following 2 weeks of single‐blind placebo run‐in. Patients who did not qualify after 2 weeks of placebo run‐in were allowed a further 2 weeks; after 4 weeks of placebo run‐in, patients who did not qualify by office criteria did not participate in the study. Patients who fulfilled the office BP criteria underwent ABPM.
The second BP criterion was a mean daytime (8 a.m. to 4 p.m.) ambulatory SBP of 150–200 mm Hg and DBP of 60–100 mm Hg. Patients who did not qualify on ABPM after 2 weeks of placebo run‐in were allowed a further 2 weeks of placebo run‐in. If they were requalified based on office readings, the ABPM was repeated. Patients who did not qualify based on ABPM criteria were excluded from the study. Qualifying patients were randomized to receive treatment with either amlodipine besylate/benazepril HCl 5/20 mg, amlodipine besylate 5 mg, or benazepril HCl 20 mg.
Statistical Analyses Continuous variables were summarized by descriptive statistics, and the treatment groups were compared using analysis of covariance. Discrete variables were summarized and compared using the chi‐square test. Adjustments for multiple comparisons were made, and all statistical tests were conducted against a two‐sided alternative hypothesis. Differences were considered significant at p<0.05. All patients randomized to receive at least one dose of assigned medication were included in the safety analysis. All patients who received at least one dose of assigned medication and who had at least one valid postbaseline ABPM assessment were included in the efficacy analysis and comprised the intent‐to‐treat population. The last‐observation‐carried‐forward approach was used to obtain the last postbaseline assessment.
RESULTS
Demographics A total of 813 patients qualified for the study based on office BP criteria. Of this group, 306 patients did not meet the ABPM daytime BP criteria for randomization and two subjects were not randomized for other reasons. Of the 306 patients who did not qualify on ABPM, 208 failed due to a mean daytime SBP of <150 mm Hg and 72 due to a mean daytime DBP of >100 mm Hg. There were 26 patients who had a technical failure on the ABPM, refused to repeat the monitoring, and were excluded from the study. There were 64 patients who qualified by office criteria following 2 weeks of placebo run‐in but who failed to qualify for randomization following ABPM. According to protocol, these patients were allowed to repeat their monitoring 2 weeks later; of this group, 36 patients qualified for randomization (both on office and ABPM criteria).
A total of 505 patients were randomized in the study. At the end of the study, the intent‐to‐treat group included 443 patients (149 in the combination group, 146 in the amlodipine besylate group, and 148 in the benazepril HCl group). A total of 59 patients discontinued the study: 17 (10.2%) in the combination group and 21 (12.4%) in each of the monotherapy groups. The most common reason for study discontinuation was the occurrence of an adverse event: 4.2% for the combination group, 4.1% for the amlodipine besylate group, and 2.4% for the benazepril HCl group. The study population was predominantly female (60.9%) and white (81.7%), with a mean age of approximately 68 years. As shown in the Table, all three treatment groups were comparable with regard to demographic characteristics including sex, age, office BP, and mean daytime ambulatory BP.
Table.
Patient Demographics
| Variable | Amlodipine Besylate/ Benazepril HCl | Amlodipine Besylate | Benazepril HCl | All Patients |
|---|---|---|---|---|
| Study population (n) | ||||
| Intent‐to‐treat | 149 | 146 | 148 | 443 |
| Safety | 166 | 169 | 170 | 505 |
| Age (yr)* | 67.4±8.0 | 68.2±7.5 | 67.4±8.1 | 67.7±7.8 |
| Sex (male/female [n/n]) | 61/88 | 55/91 | 57/91 | 173/270 |
| Race (n [%]) | ||||
| White | 124 (83.2) | 119 (81.5) | 119 (80.4) | 362 (81.7) |
| Black | 21 (14.1) | 15 (10.3) | 20 (13.5) | 56 (12.6) |
| Asian | 0 (0.0) | 3 (2.1) | 0 (0.0) | 3 (0.7) |
| Other | 4 (2.7) | 9 (6.2) | 9 (6.1) | 22 (5.0) |
| Office BP (mm Hg)* | ||||
| Systolic | 169.0±8.3 | 168.7±8.8 | 168.8±8.0 | 168.9±8.3 |
| Diastolic | 88.5±7.8 | 87.4±8.2 | 87.8±8.5 | 87.9±8.2 |
| Daytime ambulatory BP (mm Hg)* | ||||
| Systolic | 161.1±9.1 | 159.8±9.4 | 160.0±8.4 | 160.3±9.0 |
| Diastolic | 90.3±8.0 | 89.8±7.8 | 90.0±8.7 | 90.1±8.1 |
| BP=blood pressure; *data are mean ± SD | ||||
Results of ABPM Amlodipine besylate/benazepril HCl combination therapy was significantly more effective than monotherapy with either amlodipine besylate or benazepril HCl in reducing mean 24‐hour SBP, DBP, and pulse pressure. As shown in Figure 1, patients receiving combination therapy had significantly greater mean changes from baseline in mean 24‐hour SBP ± SD (−21.1±9.5 mm Hg) compared with those receiving amlodipine besylate (−12.4±9.8 mm Hg; p<0.0001) or benazepril HCl (−10.8±11.5 mm Hg; p<0.0001). The mean changes from baseline in mean 24‐hour DBP were significantly greater in patients randomized to the combination group compared with those receiving amlodipine besylate or benazepril HCl monotherapy (−10.6±5.7 mm Hg vs. −5.7±5.6 mm Hg and −5.7±6.8 mm Hg, respectively; p<0.0001). Combination therapy was significantly more effective in reducing mean 24‐hour pulse pressure (−10.5±5.9 mm Hg) compared with amlodipine besylate (−6.7±6.1 mm Hg; p<0.0001) or benazepril HCl (−5.0±6.9 mm Hg; p<0.0001).
Figure 1.
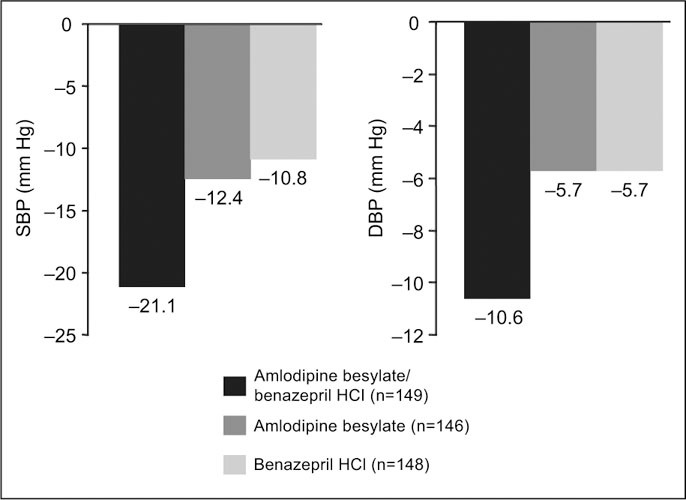
Change from baseline in mean 24‐hour ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the intent‐to‐treat population (p<0.0001 for the combination vs. amlodipine besylate and vs. benazepril HCl)
Office BP and Pulse Pressure Reduction Combination therapy was associated with reductions from baseline in mean seated office SBP and DBP values that were significantly greater than those seen with amlodipine besylate or benazepril HCl (−25.0/8.9 mm Hg vs. −20.2/6.0 mm Hg vs. −12.9/2.9 mm Hg, respectively; p≤0.0016 vs. amlodipine besylate and p<0.0001 vs. benazepril HCl). No significant changes from baseline in mean seated office pulse pressure were seen in any treatment group.
BP Response and Control Rates BP response was defined as a reduction from baseline in mean daytime (8 a.m. to 4 p.m.) ambulatory SBP of ≥10% of the baseline value, and BP control was defined as mean daytime ambulatory SBP of ≤140 mm Hg. As shown in Figure 2, combination therapy resulted in a significantly greater proportion of patients who responded to treatment compared with patients who received monotherapy (73.2% of patients in the combination group vs. 38.4% in the amlodipine besylate group and 37.2% in the benazepril HCl group; p<0.0001). The percentage of patients achieving goal SBP of ≤140 mm Hg was significantly greater in the combination group (65.1%) compared with the amlodipine besylate group (28.1%; p<0.0001) and the benazepril HCl group (33.8%; p<0.0001).
Figure 2.
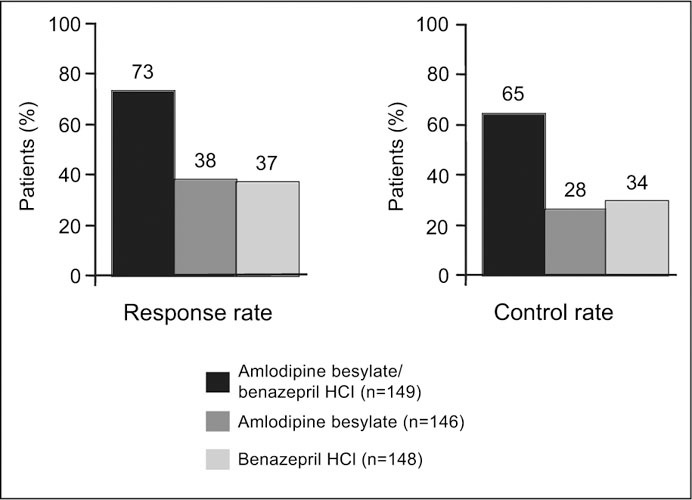
Blood pressure response and control rates in the intent‐to‐treat population (p<0.0001 for the combination vs. amlodipine besylate and vs. benazepril HCl)
Adverse Events During the 8 weeks of the active treatment period, 48.2% of the patients randomized to the combination group, 52.1% of patients randomized to the amlodipine besylate group, and 55.9% of patients randomized to the benazepril HCl group experienced adverse events. These events were generally of mild‐to‐moderate intensity. The most frequently reported adverse events were peripheral edema, headache, and cough. Cough was more commonly reported with combination therapy (9.0%) than with either amlodipine besylate or benazepril HCl (1.2% and 6.5%, respectively). The incidence of new‐onset edema (edema that developed during the study) was lowest in the combination group (3.8%) compared with either monotherapy group (9.4% and 5.6%, respectively). The incidences of dizziness, fatigue, and nausea were low and similar in the three groups (<5% in any group). No clinically significant changes from baseline were noted in any laboratory parameter in any treatment group, and no deaths occurred during the study.
DISCUSSION
It is well known that SBP is more difficult to treat than DBP. Consequently, surveys such as the Third National Health and Nutrition Examination Survey (NHANES III) have shown that the vast majority of inadequately controlled hypertension is explained by poor SBP control. In the SELECT study we have studied a group of elderly hypertensive patients with stage 2 systolic hypertension diagnosed by ABPM. Treating these patients with a fixed‐dose combination of amlodipine besylate 5 mg and benazepril HCl 20 mg resulted in an additive reduction in SBP compared with each of the components. Using combination therapy as initial treatment in this group of patients resulted in a control rate (BP <140/90 mm Hg) of 65.1% as compared with 28% with amlodipine besylate 5 mg or 33% with benazepril HCl 20 mg. This control rate is substantially better than those reported in elderly patients in national surveys.
Despite the significantly greater reductions in SBP in the patients treated with combination therapy, there were no significant differences in the side effect profile when compared with that of each of the components. The rate of new‐onset peripheral edema was lower in the patients on combination therapy than in those treated with amlodipine besylate given as monotherapy (3.8% vs. 9.4%), confirming what has been reported in previous studies. This highlights one of the important advantages of combination therapy. When two complimentary drugs are given in combination they tend to reduce BP in a more physiologic manner. One of the problems with unopposed dihydropyridine calcium channel blockers is that they only vasodilate the arterial side of the vascular tree. This results in adverse consequences such as peripheral edema 23 and may increase proteinuria in patients with diabetic nephropathy. 24 The addition of an ACE inhibitor or an angiotensin receptor blocker opens up the venous side of the vascular tree, resulting in a more physiologic reduction in BP and a reduction or disappearance of the edema. 22 , 25 , 26 In the case of the kidney, the ACE inhibitor or angiotensin receptor blocker results in a reduction in glomerular pressure and a decrease in proteinuria. In addition, the greater reduction in systemic BP in patients treated with combination therapy vs., for example, ACE inhibitors alone results in a greater reduction in proteinuria.
One unexpected finding in this study was the higher incidence of cough in patients treated with combination therapy than those treated with ACE inhibitor monotherapy. This is contrary to what has been reported in previous studies with amlodipine besylate/benazepril HCT or in ACE inhibitor‐diuretic combinations in which cough (which is not dose dependent) was no different in the combination‐treated patients than in the monotherapy‐treated patients. There were differences in symptoms of hypotension in the treatment groups (dizziness or fatigue), underscoring the safety of combination therapy in elderly stage 2 hypertensive patients.
A very important differentiating feature of the SELECT study was the use of a mean SBP as measured by ABPM as an inclusion criterion. SBP measured in the office environment is extremely variable and, as a result, the incidence of white coat systolic hypertension is common. This group of hypertensive patients constitutes a significant percentage of patients enrolled in studies using office criteria. Since it is well known that the magnitude of SBP reduction is highly dependent on the baseline BP (the higher the BP, the greater the reduction), the inclusion of white coat hypertensives will dilute the effect of the study drug on SBP. Another problem is that many of the studies reporting changes in SBP were enrolled based on DBP inclusion criteria, which are frequently associated with lower baseline SBPs. In the SELECT study, mean daytime BP was used as an inclusion criterion, providing true baseline pressures and enabling us to exclude white coat hypertensives. Mean office SBP in the SELECT study was 169 mm Hg, and mean daytime SBP was 160 mm Hg (Table).
It is well known that SBP is an extremely variable measurement. The SELECT study has clearly demonstrated that systolic hypertension is difficult to diagnose in the clinical setting and that it may frequently be overdiagnosed. We have shown that approximately one third of patients with a mean office SBP of ≥160 mm Hg had a mean daytime SBP of <150 mm Hg. This would suggest that additional caution should be exercised in the diagnosis of systolic hypertension and, where possible, ABPM should be performed.
Another important advantage of combination therapy is the ability to achieve more rapid BP control. The active treatment phase of the SELECT study was 8 weeks. Most of the BP reductions seen in patients in the combination group were achieved in 4 weeks. This may have an important impact on CV disease. Recent data from the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) 27 and from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 28 have shown greater reductions in CV disease in patients who achieved goal BP after the first month of treatment compared with those who achieved goal at a later date.
The SELECT study design incorporated the JNC 7 recommendations that combination therapy should be considered as initial treatment in patients with stage 2 hypertension. Using this approach, 65.1% of elderly hypertensive patients with stage 2 hypertension achieved a BP goal of <140/90 mm Hg when treated with a combination of amlodipine besylate and benazepril HCl, which was significantly greater than that which was achieved with each of the components given as monotherapy. Furthermore, despite having received initial combination therapy, patients had a side‐effect profile that was at least as good as that seen with monotherapy. These results support an important change to the recommendations made by the Joint National Committee for patients with stage 2 hypertension and represents a change to our traditional approach to the management of hypertension.
Disclosure: This study was sponsored by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
References
- 1. Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. A cooperative study. JAMA. 1977;237:255–261. [PubMed] [Google Scholar]
- 2. The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140:1280–1285. [PubMed] [Google Scholar]
- 3. Flack JM, Neaton J, Grimm R Jr, et al., for the Multiple Risk Factor Intervention Trial Research Group . Blood pressure and mortality among men with prior myocardial infarction. Circulation. 1995;92:2437–2445. [DOI] [PubMed] [Google Scholar]
- 4. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 5. Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. [DOI] [PubMed] [Google Scholar]
- 6. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 7. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 8. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 9. Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–486. [DOI] [PubMed] [Google Scholar]
- 10. Lloyd‐Jones DM, Evans JC, Larson MG, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. [DOI] [PubMed] [Google Scholar]
- 11. Burt VL, Cutler JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–69. [DOI] [PubMed] [Google Scholar]
- 12. Sutton‐Tyrrell K, Wildman R, Newman A, et al. Extent of cardiovascular risk reduction associated with treatment of isolated systolic hypertension. Arch Intern Med. 2003;163:2728–2731. [DOI] [PubMed] [Google Scholar]
- 13. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 14. Kostis JB, Davis BR, Cutler J, et al., for the SHEP Cooperative Research Group . Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 15. Staessen JA, Fagard R, Thijs L, et al., for the Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators . Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 16. Hansson L, Zanchetti A, Carruthers SG, et al., for the HOT Study Group . Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 17. Hansson L, Lindholm LH, Ekbom T, et al., for the STOP‐Hypertension‐2 Study Group . Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular morbidity and mortality: the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet 1999;354:1751–1756. [DOI] [PubMed] [Google Scholar]
- 18. Wing LM, Reid CM, Ryan P, et al., for the Second Australian National Blood Pressure Study Group . A comparison of outcomes with angiotensin‐converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 19. Skolnik NS, Beck JD, Clark M. Combination antihypertensive drugs: recommendations for use. Am Fam Physician. 2000;61:3049–3056. [PubMed] [Google Scholar]
- 20. Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- 21. Weir MR, Rosenberger C, Fink JC. Pilot study to evaluate a water displacement technique to compare effects of diuretics and ACE inhibitors to alleviate lower extremity edema due to dihydropyridine calcium antagonists. Am J Hypertens. 2001;14:963–968. [DOI] [PubMed] [Google Scholar]
- 22. Messerli FH, Weir MR, Neutel JM. Combination therapy of amlodipine/benazepril versus monotherapy of amlodipine in a practice‐based setting. Am J Hypertens. 2002;15:550–556. [DOI] [PubMed] [Google Scholar]
- 23. Kuschnir E, Acuna E, Sevilla D, et al. Treatment of patients with essential hypertension: amlodipine 5 mg/benazepril 20 mg compared with amlodipine 5 mg, benazepril 20 mg, and placebo. Clin Ther. 1996;18:1213–1224. [DOI] [PubMed] [Google Scholar]
- 24. Fogari R, Zoppi A, Mugellini A, et al. Effect of benazepril plus amlodipine vs. benazepril alone on urinary albumin excretion in hypertensive patients with type II diabetes and microalbuminuria. Clin Drug Invest. 1997;13(suppl 1):50–55. [Google Scholar]
- 25. Valentino VA, Wilson MD, Weart W, et al. A perspective on converting enzyme inhibitors and calcium channel antagonists in diabetic renal disease. Arch Intern Med. 1991;151:2367–2372. [PubMed] [Google Scholar]
- 26. Vivian EM, Goebig ML. Slowing the progression of renal disease in diabetic patients. Ann Pharmacother. 2001;35:452–463. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Kjeldsen SE, Weber M, et al., for the VALUE trial Group . Outcomes in hypertensive patients at high cardiovascular risk treated with a regimen based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 28. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published correction in JAMA. 2003;289:178. JAMA. 2004;291:2196]. JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]


