Abstract
To study the effects of device‐guided breathing on office systolic blood pressure (SBP), five centers randomized 149 untrained hypertensives (50% male, age 59±10 years, baseline blood pressure 150±9/86±9 mm Hg, 77% taking drug therapy). One half received a device to guide slow breathing; all received a home blood pressure monitor and only simple, written instructions. The changes in office SBP (adjusted for office‐to‐home difference in baseline SBP and accumulated time spent in slow breathing, guided and measured by the device) were significantly (p<0.001 for trend) correlated with accumulated time spent in slow breathing. Greater decreases in SBP (−15.0±1.8 vs. −7.3±1.9 mm Hg) were observed for those who spent more (vs. less) than 180 minutes over 8 weeks in slow breathing, as well as those who just monitored their blood pressure at home (−9.2±1.6 mm Hg). Thus, even without training, hypertensive patients who receive a device to guide slow breathing significantly lowered their office SBP if the total time spent in slow breathing over 8 weeks exceeded a "threshold" value of 180 minutes.
Elevated blood pressure (BP) is a major risk factor for adverse cardiovascular events, including myocardial infarction, stroke, heart failure, and death. 1 Drug therapy reduces the risk of all these end points, 1 but drugs may cause side effects and are costly. Lifestyle modifications have been recommended by essentially all expert panels on hypertension as either definitive or adjunct methods of lowering BP. 1 , 2 Weight loss and salt restriction are the most effective modalities, 2 but each requires a major sustained change in dietary habits, which is frequently difficult for many patients.
Relaxation and stress‐reduction programs (e.g., yoga, meditation, and biofeedback) can also reduce BP, but the magnitude and duration of the effect and mechanism(s) involved are uncertain. 3 , 4 , 5 , 6 Deep abdominal breathing is common to many of these modalities and has been proposed to elicit "the relaxation response." 3 A reduction in the respiratory rate to <10 beats/min (BPM) from the normal 16.6±2.8 7 with a prolonged expiratory phase may have beneficial effects on the cardiovascular system through multiple mechanisms. 8 , 9 , 10 Acutely, slow breathing is associated with increased baroreflex sensitivity, 11 , 12 heart rate variability, 8 , 12 , 13 and venous return 14 ; some of these effects may be mediated by acutely lowered BP and peripheral resistance. 12
Previous studies using a device to interactively guide the user to slow breathing with prolonged expiration (device‐guided breathing) have shown a significant reduction in clinic, home, and ambulatory BPs 15 , 16 , 17 , 18 in patients with uncontrolled BP, mostly treated with antihypertensive drugs, including resistant hypertensives. 19 Daily use of the device for 10–15 minutes over 2 months produced significantly greater office BP reduction than simply listening to nonrhythmic synthesized music that is promoted commercially for relaxation purposes. 17 , 18
As part of another study in people who had no training or prior expectations about the use of the device to guide slow breathing, we performed analyses to see if there was a minimum duration (or threshhold value) of slow, device‐guided breathing that was associated with significantly lowered office BPs. The existence of such a value and a dose‐response relationship might be expected, as it is generally accepted that clinical benefits associated with physical exercise (e.g., routine walking) are related to the amount of exercise performed. None of the previous studies with the device to guide slow breathing have so far addressed the important question of whether a threshold value exists. It is important to determine the threshold value before firm recommendations can be made for the routine use of the device.
PATIENTS AND METHODS
Eligible patients included hypertensive men and women, aged 40–75 years, with two measurements of office BP (over 2 weeks): systolic blood pressure (SBP) ranging between 140 and 179 mm Hg and diastolic BP lower than 110 mm Hg. The measurements could not differ by more than 10/5 mm Hg. All patients had stable lifestyles and had taken antihypertensive medications for at least 1 month before enrollment; medications were not changed during the study. Excluded were patients with ischemic heart disease, severe heart failure, chronic atrial fibrillation, chronic renal impairment, previous stroke, respiratory disease (including asthma or chronic obstructive pulmonary disease), pregnancy, obesity (body mass index >35 kg/m2), panic disorder, major psychiatric disorder, or inability to operate a portable music player. All subjects signed written informed consent documents approved by local Institutional Review Boards. These documents did not contain any discussion of the device that guides breathing, its proper use, or its potential effect on BP. Subjects were told only that they would receive, by courier at their homes, one of two systems for assisting in BP control for daily use at home. The system was said to be helpful in measuring, displaying, and recording BP and related parameters, and that the system is simple and convenient, and is not a risk to health. The forms provided the impression that measuring home BP was the major intervention, and no other or additional benefit was expected. Information provided to participants (in advertisements, informed consent documents, and interactions with clinic staff) was carefully worded to minimize "expectation bias" (that use of the device would be expected to lead to lower office BPs).
The protocol was designed to compare two interventions. Treatment patients received a system containing the device to guide slow breathing (RESPeRATE, InterCure, Ltd., Lod, Israel and Fort Lee, NJ) and a BP monitor (Omron 747 IC, Omron Healthcare, Franklin Park, IL). Control patients received only the BP monitor that automatically stores the time and date of use and the oscillometrically‐determined BPs and heart rate. All patients were trained at the clinic to operate the home BP measuring system. Both the home BP measuring system and the device to guide slow breathing came with simple, written instructions about how to use them.
Patients were instructed each day to measure BP three times in the morning and, in the evening, and to either use the device for 15 min/d (treatment) or repeat three BP measurements (control). This was done to create a separation in time between device use and BP measurement, and to maintain blinding (both groups had an equal number of recommended sessions/d). The evening BP measurements from the control group were not used in subsequent data analyses. This study design should bias the results toward the null because the patient, when agreeing to the protocol, would not know that a device to guide slow breathing would or would not be included in the package, and would receive no encouragement or expectation from clinical staff that the BP was likely to be reduced at subsequent visits. To assure the feasibility of self operation of devices at home with only written instructions for use of the device to guide slow breathing, the first 29 enrolled patients all received treatment (both the device to guide slow breathing and a home BP monitor). Subsequently, 123 patients were randomized in a 1:1 ratio between treatment and control groups.
The device to guide slow breathing is about the size and weight of a paperback book and is battery operated. The patient places an elastic belt with a respiration sensor around the torso (typically over clothing), and wears standard headphones. The device monitors and analyzes the respiratory patterns in real time, calculates inspiration and expiration times and, in response, synthesizes a personalized melody comprised of two distinct tones—one for inhalation and one for exhalation. As users listen to the melody and effortlessly synchronize their breathing with the tones, the device gradually prolongs the exhalation tone, guiding the user to a slower respiratory rate (typically <10 BPM) with prolonged exhalation. Performance data, including date, time, breathing pattern characteristics, and exercise quality parameters are stored automatically, allowing for evaluation of patient adherence and performance.
Four visits to the medical office were scheduled for screening, enrollment, and follow‐up at both 4 and 8 weeks after enrollment; the last three visits were scheduled at the same time of day. A mercury sphygmomanometer was used to measure BP during office visits. Three consecutive readings were taken by the same observer (who was unaware of the subject's activities at home) using standard procedures. 20 The overall primary outcome measure was the change in SBP between baseline (average of the first two visits) and at 8 weeks. The outcome measure for the ability to operate the device properly included using the device for at least one session and achieving "slow breathing," defined as <10 BPM at the end of at least one session. The outcome measure for effective exercising was the accumulated time (min) spent in "slow breathing" with the device since enrollment. Home BP monitoring data were downloaded at both the 4‐ and 8‐week visits at the medical office, but were not revealed to clinical personnel. At baseline and at the end of the study period, office‐to‐home BP differences (used as a covariate in the adjusted analyses) were determined from the office BP by subtracting the average of the morning measurements over the prior 2 weeks. Data regarding use of the device that assisted slow breathing were retrieved only after the conclusion of the study, when the devices were returned by the subjects by courier to a central shipping office.
Based on previous studies with this device, 17 , 18 it was expected that 60 subjects per randomized group would afford 80% statistical power to detect a 6.5 mm Hg difference between groups in office SBP after 8 weeks, with a standard deviation of 10 mm Hg. This presumes that 80% of those randomized to device‐guided breathing would use it, but only 50% (overall) would accumulate sufficient slow breathing time to reduce office BP. This is in accord with the hypothesis that achieving a significant BP reduction requires sustained and appropriate use of the device with an unknown threshold. Tabular data are shown as mean±standard deviation. Comparisons for categoric variables were made using Fisher's exact test; those for continuous variables were done using t tests. BPs were compared using analyses of covariance, with clinical site, time, and office‐to‐home SBP difference as covariates. The dependence of individual patients' change in BP on total time spent in slow device‐guided breathing during 8 weeks was evaluated using linear regression modeling, by the significance of the relevant regression coefficient with and without the presence of office‐to‐home difference in BP as a covariate. The dose‐response relationship between BP changes and the total time spent in slow device‐guided breathing was evaluated using the analysis of variance (ANOVA) after grouping according to time spent in slow breathing. The threshold value for change in SBP and time spent in slow breathing was determined by a two‐step method. First, the SBP changes for two groups—high users and low users—defined by differing duration of use of the device, were compared. The resulting p values were then plotted against the putative threshold values and fitted to a nonlinear regression curve using the model p value = A + B (threshold − C), 2 which defined the 95 % confidence intervals of the threshold value that corresponded to the minimum p value(C). For all measurements, the last valid observation during follow‐up was carried forward in time for subjects who did not complete the entire study. Superiority comparisons 21 of the one‐tailed primary outcomes were determined using p values; two‐sided p values were used for all other comparisons. Significance was accepted for p<0.05, unless adjusted for multiple comparisons using Bonferroni's correction.
RESULTS
Patients were recruited by five clinical sites using chart review and staff meetings where the protocol was discussed. Baseline characteristics of the 149 enrolled subjects are shown in Table I. All variables, apart from home SBP, did not show significant differences between the groups, nor were there any significant differences among the first 29 enrolled and the 60 patients actually randomized to treatment. Results from all treatment patients were pooled.
Table I.
Baseline Characteristics of Enrolled Subjects
| Device‐Guided Breathing | |||
|---|---|---|---|
| Characteristic | Plus BP Monitor | BP Monitor Only | p Value |
| Number of subjects | 89 | 60 | — |
| Age (years) | 59.5±9.6 | 58.7±10.5 | 0.61 |
| Male (%) | 55 | 43 | 0.18 |
| BMI (kg/m2) | 29.1±4.3 | 28.3±4.1 | 0.23 |
| Taking antihypertensive drugs (%) | 81 | 70 | 0.17 |
| Office SBP (mm Hg) | 150.4±8.3 | 150.0±9.8 | 0.82 |
| Office DBP (mm Hg) | 85.1±8.9 | 86.7±7.9 | 0.27 |
| Isolated systolic hypertension (%) | 69 | 60 | 0.77 |
| Office heart rate (bpm) | 70.2±8.3 | 72.2±9.2 | 0.16 |
| Initial home SBP (mm Hg)* | 147.8±18.0 | 141.1±15.9 | 0.03 |
| Initial home DBP (mm Hg)* | 86.8±10.7 | 83.5±10.1 | 0.08 |
| Initial home heart rate (bpm)* | 69.4±10.9 | 71.1±10.8 | 0.37 |
| BP=blood pressure; BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure; *home BP measurements were not available from 16 patients from the device‐guided breathing group and four from the BP monitor only group for the following reasons: patients did not take BP readings during the baseline period (n=4 and 0, respectively); did not operate the device at all (n=8 and 3, respectively); did not return the device (n=4 and 0, respectively); or some of the data files could not be downloaded (n=0 and 1, respectively) | |||
In the treatment group, 96% of the patients returned the device and 86% used the device at least once. Although the subjects were not informed at enrollment about the nature of the device and were not trained in its use, 95% of those who operated the device at least once achieved slow breathing using the instructions provided. This was the primary efficacy measurement for the ability to operate the device properly. These data provide strong evidence that the device can be used correctly without guidance from a health care professional. These analyses and conclusions are independent of the effect of the device on office BP.
Use of the device was hypothesized to be effective in lowering office SBP only for those patients who actually achieved slow breathing during the exercise (Table II). In simple linear regression analyses including all patients who received the device and had BPs measured (Figure 1), a significant direct relationship was found. A greater duration of slow breathing was associated with a lower office SBP (slope=−0.015±0.005 mm Hg/min, r=0.34, p<0.01), even after adjustment using the office‐to‐home BP difference (slope=−0.012±0.004 mm Hg/min, r=0.54, p<0.01). After stratifying the patients into three distinct subgroups (Figure 2) according to the total time spent in slow breathing over 8 weeks, a significant dose‐response relationship (p=0.01 for trend) was found. The best‐fit threshold separating high users from low users was determined to be 180 minutes, with a 95% confidence interval of 135–275 minutes over 8 weeks (Figure 1). This threshold had the lowest value of the nonlinear regression curve and was 50% of the 45 min/wk for 8 weeks that was originally recommended in the user's manual.
Table II.
Blood Pressures (BPs) (Office vs. Home, Average ± Standard Deviation) for Subjects With Outcomes Before and After 8 Weeks of Study According to Randomized Group and Duration of Use of the Device to Guide Slow Breathing
| Office BPs (mm Hg) | Home BPs (mm Hg) | |||||
|---|---|---|---|---|---|---|
| Group | n | Baseline | After 8 Weeks | n | Baseline | After 8 Weeks |
| Device provided | 79 | 150.3±8.3/ | 139.7±13.7/ | 57 | 145.8±17.0/ | 145.3±15.8/ |
| 84.7±9.0 | 81.5±9.6 | 85.9±10.7 | 85.3±9.8 | |||
| BP monitoring only | 57 | 149.8±9.4/ | 140.6±15.0/ | 47 | 141.3±15.8/ | 141.9±17.4/ |
| 86.8±8.1 | 83.6±9.0 | 83.7±9.7 | 83.5±10.7 | |||
| High users | 33 | 150.3±6.5/ | 135.3±12.0/ | 29 | 145.9±15.9/ | 147.6±16.9/ |
| 83.4±9.0 | 78.9±9.2 | 85.0±9.4 | 85.4±8.9 | |||
| Low users | 43 | 150.7±9.6/ | 143.3±14.4*/ | 28 | 145.7±18.2/ | 143.1±14.6/ |
| 86.0±9.1 | 83.3±9.0** | 86.9±12.0 | 85.2±10.9 | |||
| *p=0.005, high users vs. low users; **p=0.025, high users vs. low users | ||||||
Figure 1.
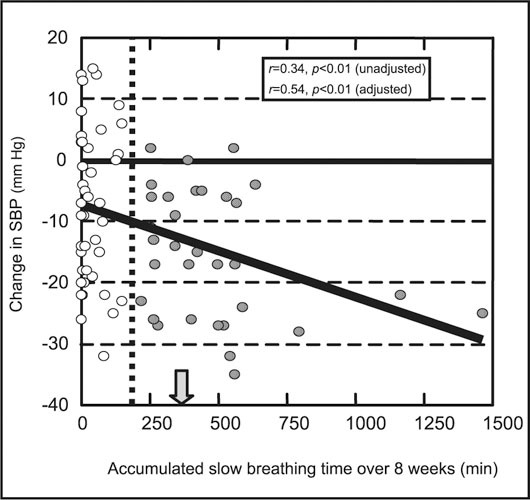
Correlation of individual patient changes in office systolic blood pressure (SBP) (y‐axis) on total time spent in slow device‐guided breathing (x‐axis) in 76 patients. The vertical dotted line marks the 180‐minute threshold value that best separates low users (empty circles) from high users (filled circles), and the filled arrow marks the minimum slow breathing time recommended by the user's manual. The adjusted analysis refers to a regression analysis that incorporated the office‐to‐home blood pressure (BP) differences for each participant.
Figure 2.
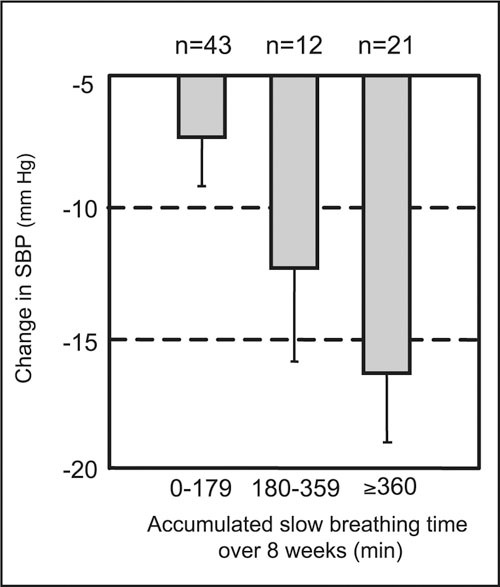
Dose‐response relationship between mean office systolic blood pressure (SBP) and the total time (minutes) spent in slow breathing after grouping the data of Figure 1 (p<0.02 for trend). Error bars denote one standard error of the mean.
Individuals who used the device to guide slow breathing for at least 180 min/8 wk (high users, n=33) experienced a significant lowering of office SBP of −15.0±1.8 mm Hg (mean±standard error [SE], compared with baseline, p<0.001). This change in office SBP was significantly greater than the −7.3 ±1.9 mm Hg lowering seen in low users (<180 min/8 wk, n=43, p=0.005). The change in office SBP among high users was also significantly greater than those who received only home BP monitoring (−9.2±1.6 mm Hg, p=0.012). In all three comparisons, significant differences persisted after adjusting for office‐to‐home SBP difference by ANOVA. The change in office SBP among low users was numerically, but not significantly, less than that seen in non users, and is consistent with previous work demonstrating that more frequent home monitoring results in lower office BP readings: non users were told to take home BPs twice daily, as opposed to once‐daily measurements in the low users.
Further analysis of the data comparing high users and low users revealed an interesting difference regarding the time course of use of the device. The high users achieved slow breathing (<10 BPM) approximately 61±4 min/wk (mean±SE) during the first week of use, and were consistent across the 8 weeks of treatment, with approximately 57±4 min/wk during the last week of use (p=0.98, ANOVA). The low users achieved slow breathing approximately 22±3 min/wk during the first week of use, but gradually declined in minutes of use during the remainder of the 8‐week treatment period (p=0.02, ANOVA), such that, during the last week, 78% did not use the device at all. These results are consistent with a lack of persistent motivation among the low users, which might be an artifact of the study design, since the subjects were not told about the expected results and received no coaching by study personnel during the treatment period.
Because of the public health importance of isolated systolic hypertension (SBP ≥140 mm Hg, but diastolic BP <90 mm Hg), 22 , 23 , 24 the responses of patients fitting this diagnosis were analyzed separately in a prespecified subgroup analysis. Sixty‐one subjects received the device and a home BP monitor and 36 patients received only the home BP monitor. After adjusting for the difference in office‐to‐home BP readings, there was a significant difference in office SBPs between the groups of −7.4±3.4 mm Hg (mean±SE, p=0.02). The mean reduction in SBP for the 25 high users was −14.7±2.3 mm Hg (p<0.001 compared with the low users). These data suggest that isolated systolic hypertension, which may be the most difficult form of hypertension to control, 24 can be significantly improved by device‐guided slow breathing when performed as recommended.
DISCUSSION
This controlled, double‐blind study was designed to relate office BP response to device‐guided slow breathing while avoiding subjects' prior knowledge or expectations about the modality used to assist in BP reduction. The results confirm and extend previous work with this device as a safe and effective adjunctive treatment for BP reduction. Nearly all hypertensive subjects were able to use the device to assist in slow breathing without prior advice or training. Patients who received the device (and a BP monitor) and performed slow breathing routinely had a significant (p<0.02) lowering in office SBP, compared with subjects who received only a home BP monitor, even when taking antihypertensive drug therapy. There was a significant correlation between SBP reduction and the accumulated time of slow breathing during 8 weeks in the individual patients (Figure 1). A dose‐response relationship (Figure 2) was noted after grouping that defined a threshold of approximately 180 minutes of slow breathing exercise over 8 weeks to achieve a significant reduction in office SBP.
This study verifies several previous assumptions and recommendations for the use of the device to assist with slow breathing. Although the subjects were not given any information about the device, its mechanism, or its use when volunteering for the study, 95% were able to achieve slow breathing by following the simple included instructions. On average, subjects who used the device spent 45% of each session taking <10 BPM, which was similar to the manufacturer's recommended use of 43%, or slow breathing 45 min/wk. Most subjects were able to properly mount the belt‐type sensor on the torso, as there was a detectable respiration signal during 87% of the total session time. Most subjects who used the device effectively and according to the included instructions persisted in use over 8 weeks. Lastly, the average duration for each breathing session in this study was 14.5±1.9 minutes, which is very close to the recommended duration of 15 min/d.
There are a number of potential limitations to our conclusions. The data are necessarily limited by the design of the study. Ten subjects who received the device to guide slow breathing did not complete the protocol or return their device and home BP monitor, as was the case for three control patients. These individuals did not have their office BP response to the protocol assessed and could not be included in the final analyses. In telephone follow‐up, four of these patients admitted that they had not used the device as directed, if at all; this would likely be less of a problem if the device and the results expected from its proper use were explained to the subjects before receiving it. Similarly, 14% of the patients who received the device never used it; this is probably an artifact of the study design and is unlikely to occur in clinical practice, as only motivated patients are likely to purchase and then use a device. It is conceivable that a motivated individual could perform breathing exercises and lower office BPs without using a device to guide slow breathing, 3 , 4 , 5 , 6 but the current study design does not allow us to address this possibility.
In summary, these data indicate that device‐guided slow breathing, when performed as recommended, reduces office SBP significantly better than home BP monitoring alone. This effect can be discerned in subjects who receive no specific information or training about the device from a health care provider. The threshold for achieving a significantly lower office SBP was approximately 180 minutes of achieved slow breathing over 8 weeks or, on average, approximately 23 min/wk. As with treatment of any chronic condition, better results were seen when patients complied with the recommended use of the device. Larger and longer studies will be needed to demonstrate the utility of device‐guided slow breathing in the long‐term control of hypertension.
Acknowledgment:
This research was supported by grants and contracts from Intercure, Ltd., Lod, Israel and Fort Lee, NJ.
References
- 1. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560 – 2572. [DOI] [PubMed] [Google Scholar]
- 2. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882 – 1888. [DOI] [PubMed] [Google Scholar]
- 3. Benson H, Rosner BA, Marzetta BR, et al. Decreased blood pressure in pharmacologically treated hypertensive patients who regularly elicited the relaxation response. Lancet. 1974;1:289 – 291. [DOI] [PubMed] [Google Scholar]
- 4. Patel C. 12‐month follow‐up of yoga and bio‐feedback in the management of hypertension. Lancet. 1975;1:62 – 64. [DOI] [PubMed] [Google Scholar]
- 5. Irvine J, Johnson DW, Jenner D, et al. Relaxation and stress management in the treatment of essential hypertension. J Psychosom Res. 1986;30:437 – 450. [DOI] [PubMed] [Google Scholar]
- 6. Bernardi L, Sleight P, Bandinelli G, et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ. 2001;323:1446 – 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tobin MJ, Chadha TS, Jenouri G, et al. Breathing patterns. 1. Normal subjects. Chest. 1983;84:202 – 205. [DOI] [PubMed] [Google Scholar]
- 8. Novak V, Novak P, De Champlain J, et al. Altered cardiorespiratory transfer in hypertension. Hypertension. 1994;23:104 – 113. [DOI] [PubMed] [Google Scholar]
- 9. Daly MB. Interactions between respiration and circulation. In: Cheniack NS, Widdicombe JG, eds. Handbook of Physiology. Bethesda, MD: American Physiological Society; 1986:529 – 594. [Google Scholar]
- 10. Bernardi L, Gabutti A, Porta C, et al. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens. 2001;19:2221 – 2229. [DOI] [PubMed] [Google Scholar]
- 11. Parati G, Izzo JL Jr, Gavish B. Respiration and blood pressure. In: Izzo JL Jr, Black HR, eds. Hypertension Primer. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins;2003:117 – 120. [Google Scholar]
- 12. Pitzalis MV, Mastropasqua F, Massari F, et al. Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: a frequency‐dependent phenomenon. Cardiovasc Res. 1998;38:332 – 339. [DOI] [PubMed] [Google Scholar]
- 13. Cooke WH, Cox JF, Diedrich AM, et al. Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol. 1998;274:H709 – H718. [DOI] [PubMed] [Google Scholar]
- 14. Amoore JN, Santamore WP. Venous collapse and the respiratory variability in systemic venous return. Cardiovasc Res. 1994;28:472 – 479. [DOI] [PubMed] [Google Scholar]
- 15. Rosenthal T, Alter A, Peleg E, et al. Device‐guided breathing exercises reduce blood pressure ambulatory and home measurement. Am J Hypertens. 2001;14:74 – 76. [DOI] [PubMed] [Google Scholar]
- 16. Giannattasio C, Failla M, Meles E, et al. The response of self‐monitored home blood pressure of hypertensives to self‐treatment with device‐guided breathing exercises: interim results [abstract]. J Hypertens. 2001;19(suppl 2):S190 – S191. [Google Scholar]
- 17. Grossman E, Grossman A, Schein MH, et al. Breathing‐control lowers blood pressure. J Hum Hypertens. 2001;15:263 – 269. [DOI] [PubMed] [Google Scholar]
- 18. Schein M, Gavish B, Herz M, et al. Treating hypertension with a device that slows and regularizes breathing: a randomized double‐blind controlled study. J Hum Hypertens. 2001;15:271 – 278. [DOI] [PubMed] [Google Scholar]
- 19. Viskoper R, Shapira I, Priluck R, et al. Non‐pharmacological treatment of resistant hypertensives by device‐guided slow breathing exercises. Am J Hypertens. 2003;16:484 – 487. [DOI] [PubMed] [Google Scholar]
- 20. Perloff D, Grim C, Flack J, et al. Special report: human blood pressure determination by sphygmomanometry [AHA Medical/Scientific Statement]. Circulation. 1993;88:2460 – 2470. [DOI] [PubMed] [Google Scholar]
- 21. Blackwelder WC. Similarity/equivalence trials for combination vaccines. Ann N Y Acad Sci. 1995;754:321 – 328. [DOI] [PubMed] [Google Scholar]
- 22. Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479 – 486. [DOI] [PubMed] [Google Scholar]
- 23. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: metaanalysis of outcome trials. Lancet. 2000;355:865 – 872. [DOI] [PubMed] [Google Scholar]
- 24. Izzo JL Jr, Levy D, Black HR. Clinical advisory statement: importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021 – 1024. [DOI] [PubMed] [Google Scholar]


