Abstract
This study evaluated the antihypertensive efficacy and tolerability of a chronotherapeutic formulation of propranolol designed for nighttime dosing (propranolol controlled release [CR]). A total of 434 patients with mild‐to‐moderate hypertension were randomized to placebo or to one of four doses of propranolol CR (80, 120, 160, or 640 mg/d). At baseline, the mean morning blood pressures were similar in each treatment group and averaged 152/101 mm Hg. After 8 weeks of treatment, morning diastolic blood pressure, the primary efficacy measurement, was significantly reduced from baseline in placebo (‐6.98 mm Hg) and all propranolol groups (p<0.001). The decreases ranged from 10.1 mm Hg in the 80‐mg/d group to 11.0 mm Hg in the 120‐mg/d group and were significantly larger than placebo in the 120–, 160‐, and 640‐mg/d groups (p<0.05). Blood pressure measured in the evening (trough) demonstrated similar antihypertensive efficacy. Heart rate and rate‐pressure product were reduced in a dose‐related manner by propranolol CR. The formulation was well tolerated with only fatigue and dizziness being reported more frequently than in the placebo group. Propranolol CR is an effective antihypertensive formulation that May reduce blood pressure during the morning period of maximum cardiovascular risk.
Acute cardiovascular events, such as myocardial infarction, 1 , 2 sudden cardiac death, 2 , 3 or stroke 4 have been reported to occur more commonly in the morning hours than during the remainder of the day. For example, in the Second International Study of Infarct Survival (ISIS‐2), the risk of myocardial infarction (MI) between the hours of 6 a.m. and noon was 1.41 times higher than at other times of day. 1 Others have reported a similar circadian variation in the onset of acute MI. 5 The mechanisms responsible for this increased morning risk are not well understood. Factors that May be implicated in this process include: blood pressure (BP) increases (in association with increased sympathetic nervous system activity), 6 increased platelet aggregability, 7 , 8 and decreases in circulating fibrinolytic factors, 9 all of which are factors known to occur in conjunction with awakening. Of particular interest to the issue of increased morning cardiovascular risk is the increase in sympathetic nervous system activity, 10 , 11 , 12 which increases cardiac contractility and heart rate with a resultant increase in myocardial oxygen demand. Beta blockers are effective antihypertensive agents 13 and have also been shown to reduce morbidity and mortality following acute MI. 14 , 15 , 16 For example, over the 25‐month follow‐up period in the β‐Blocker Heart Attack Trial (BHAT), patients randomized to propranolol following an MI had a 26% reduction in total mortality (p<0.005) 16 and a 14.7% reduction in recurrent MI 14 compared with those receiving placebo. This protective benefit is seen in elderly persons and in patients with diabetes mellitus or congestive heart failure. 17
The observation that the morning peak of MI is less evident in patients treated with β blockers 18 , 19 suggests that these drugs May act to prevent events during the morning hours in those patients, perhaps by reducing the rapid rise in BP or the increase in myocardial oxygen demand that occurs after awakening in hypertensive patients. 6 If true, optimization of the dosing regimen, such that αβ‐blocker formulation has its peak antihypertensive effect and β‐blocking activity in the hours around waking, May increase its ability to reduce major coronary events. However, current formulations and dosing schedules of β blockers are to a degree suboptimal for morning protection because they are typically dosed once daily in the morning. Therefore, the lowest plasma levels and the smallest antihypertensive effect (during a dose interval) occur during the morning hours, when the risk of cardiac events is greatest.
In this article we discuss the efficacy and safety of a new formulation of propranolol intended for the treatment of hypertension and designed for nighttime administration. Following administration of this formulation, propranolol release is delayed for 4–5 hours. After the delay, plasma propranolol concentrations rise steadily, providing maximum β‐adrenergic antagonist activity during the vulnerable morning hours. Drug release and absorption continue for up to 14 hours. 20
MATERIALS AND METHODS
Patients
Patients who were at least 18 years old and had mild‐to‐moderate hypertension (sitting diastolic blood pressure [DBP] between 96 mm Hg and 114 mm Hg and a sitting systolic blood pressure [SBP] ≤200 mm Hg during two consecutive visits during the single‐blind placebo run‐in period) were eligible for randomization. Pregnant or lactating women were excluded, and women of childbearing potential were required to use an effective method of contraception. Other major exclusion criteria included suspected renal artery stenosis; disease of the gastrointestinal tract, kidney, or liver that might unduly influence drug pharmacokinetics; significant cardiac disease (e.g., MI, angina, stroke) within the prior 6 months; congestive heart failure; coronary artery bypass surgery within the previous 6 months; current history of asthma, chronic obstructive pulmonary disease, or nonallergic bronchospasm; insulin‐dependent diabetes mellitus; and concomitant administration of drugs known to affect blood pressure or known to interfere with the activity of propranolol.
Study Design
The trial was a randomized, double‐blind, parallelgroup, placebo‐controlled study conducted at 41 sites within the United States. The study protocol was reviewed and approved by each individual Institutional Review Board and was performed in accordance with the Doctrine of Helsinki. All patients gave written informed consent before participation in the trial.
The study design and schedule of events are illustrated in Figure 1. Patients were evaluated during a 2‐ to 3‐week single‐blind placebo run‐in period. During this period, patients who were currently treated for hypertension withdrew from their medication according to the manufacturer's recommendation. To qualify for randomization, patients had to meet the BP criteria on two consecutive weekly visits during the placebo run‐in phase (i.e., screening visit 2 [S2] and randomization visit [RO], or screening visit 3 [S3] and RO). If patients qualified for randomization at S2, they returned in 1 week for the RO. Patients failing to qualify at S2 could return in 1 week for evaluation of their blood pressure at S3. Those qualifying at S3 returned in 1 week for RO. At the beginning of week 1 RO, qualified patients were randomized to one of five treatment groups: placebo or one of four doses of propranolol controlled release (CR) (Innopran‐XL; Reliant Pharmaceuticals, Liberty Corner, NJ). The doses of propranolol (80, 120, 160, or 640 mg/d) were selected based on the clinical dose range of sustained release propranolol (Inderal LA; Wyeth‐Ayerst, Philadelphia, PA). 21 Patients were instructed to take their doses once daily between the hours of 9:30 p.m. and 10:30 p.m. Patients in the propranolol CR 80‐, 120‐, or 160‐mg/d groups were started at 80 mg/d and were up‐titrated to the final maintenance dose at the start of week 3. Patients in the propranolol 640‐mg/d group were started at 160 mg/d during Week 1 and titrated to 320 mg/d at the start of Week 2, and to 640 mg/d at the start of Week 3. All patients remained on their final dose for the next 6 weeks. At the end of the dose‐maintenance phase, patients in the propranolol CR 120‐ and 160‐mg/d groups were down‐titrated to 80 mg/d and remained on this dose for the remaining 2 weeks. Prior to discontinuation, patients in the propranolol 640‐mg/d group were down‐titrated to 320 mg/d at the start of Week 8 and to 160 mg/d at the start of Week 9. Patients in the 80‐mg/d group remained on their original dose for the remaining 2 weeks. Placebo substitution was used to blind the identity of the treatment groups.
Figure 1.
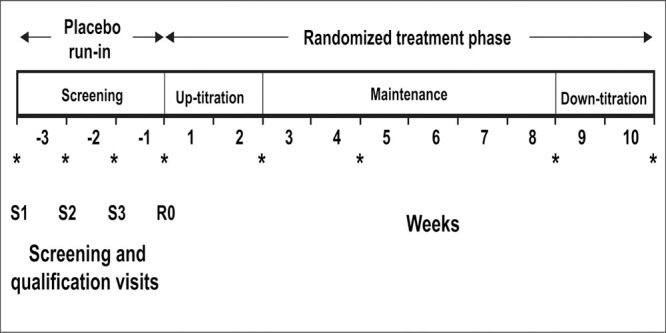
Study design schematic. S1=screening visit 1; S2=screening visit 2; S3=screening visit 3; R0=randomization visit at the start of week 1; *time points at which systolic and diastolic blood pressures were measured
During the randomized treatment phase of the study, seated BP was measured in the morning and evening at the beginning of Week 1 (the R0), and at the end of Weeks 4 and 8. BP was measured in the morning only at the end of Weeks 2 and 10. At each evaluation, seated BP was measured three times with an interval of 1 to 2 minutes between measurements, and the mean of the three SBP and DBP measurements was used as the BP for that time point. During the treatment phase of the study, patients who had DBP >114 mm Hg or SBP >200 mm Hg were evaluated 7 days later and dropped from the study if their BP remained above these proscribed limits. Patients with elevated BP (irrespective of level) and associated symptoms were discontinued immediately from the study. In either case, appropriate antihypertensive therapy was initiated.
Plasma Drug Levels
Trough plasma concentrations of propranolol were measured in 104 patients from eight randomly selected sites. A trough blood sample (within 4 hours of the next dose) was drawn during the evening visit at the end of Weeks 4 and 8. Plasma samples were prepared and analyzed for total propranolol (conjugated and unconjugated) at a central laboratory using a validated high‐performance liquid chromatography method. The lower limit of quantitation was 2.0 ng/mL for this method.
Safety Assessment
Before randomization and at study completion, safety assessments consisted of hematology tests, blood chemistry assays, urinalysis, physical examination, and electrocardiogram (ECG) measurements. Throughout the trial, safety assessments consisted of monitoring and recording all adverse events and the measurement of vital signs. Adverse events were either volunteered by the subjects, discovered during questioning by the investigator, or detected through physical examination or laboratory testing.
STATISTICAL METHODS Sample Size Determination
A sample size of 420 randomized patients (84 per treatment group) was chosen based on an 80% power to detect an expected treatment difference of 4 mm Hg between propranolol CR and the placebo with an expected common standard deviation of 6.8 mm Hg and an expected dropout rate of 20%. In this calculation, the significance level was set to 0.0125 to yield an overall α error of 0.05 for the four planned comparisons and to maintain 80% power.
Outcome Measurements
The primary outcome variable was the change in morning mean sitting DBP from baseline (start of Week 1) to end point (end of Week 8). Change in sitting DBP was evaluated on an intent‐to‐treat basis using the last observation carried forward method to impute missing end point values. Change in sitting DBP was analyzed using analysis of covariance with the treatment group and center as factors and the baseline mean sitting DBP as a covariate. The response in each of the four propranolol CR groups was compared with placebo using a modified Dunnett test. Within each group, the hypothesis of no mean change in sitting DBP from baseline to end point was tested using a paired t test.
Secondary efficacy variables were change from baseline to end point in mean sitting SBP, heart rate, and rate‐pressure product (heart rate x mean SBP) measured in the morning and evening, and mean sitting DBP measured in the evening. The secondary efficacy variables were analyzed as described for the primary outcome variable. All statistical analyses were done with SAS (version 6.12, 2000, SAS Institute, Cary, NC).
RESULTS
Patient Disposition
A total of 434 patients were randomized to double‐blind treatment. During the randomized phase of the study, 78 (18%) patients were discontinued, primarily because of adverse events (n=24) or insufficient response (n=12). The reasons for withdrawal were similar in the placebo and propranolol CR groups. In addition, seven randomized patients were removed from the intent‐to‐treat population because of the possibility of unblinding during the study (n=3), or because of data documentation issues (n=4). These seven patients remained part of the safety evaluation.
Demographics and Baseline Characteristics
The demographics and baseline characteristics of the patients in the intent‐to‐treat population are presented in Table I. No statistically significant difference between treatment groups was seen for demographic or baseline characteristics. The average patient age was 53.9 years, and 56% were men. A total of 17.1% were African American, 65.3% were white, 15.9% were Hispanic, and 0.9% were Asian. Baseline morning mean sitting SBP and DBP were approximately 152 mm Hg and 101 mm Hg, respectively, for all treatment groups.
Primary Efficacy Results: Morning DBP
The effect of treatment on mean morning sitting BP is shown in Figure 2. At the end of Week 8, all treatments, including placebo, had significantly reduced morning sitting DBP (p<0.001) compared with baseline. The BP reduction in the placebo therapy group was significant by usual standards (‐6.98 mm Hg [diastolic] and ‐8.19 mm Hg [systolic]) In the intent‐ to‐treat population, the mean change in sitting DBP was significantly greater than placebo in the propranolol 120‐, 180‐, and 640‐mg groups (Table II).
Figure 2.
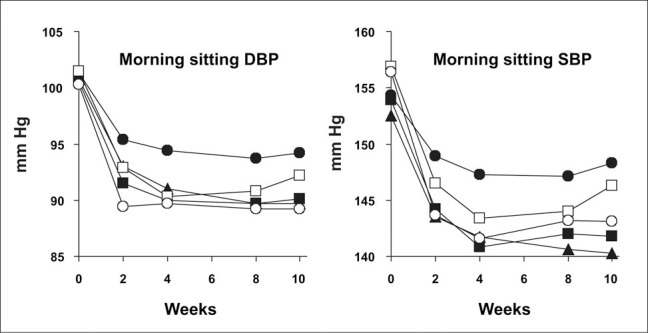
Mean morning sitting diastolic blood pressure (DBP) and systolic blood pressure (SBP). Doses were up‐titrated in the propranolol controlled release (CR) formulation 120‐, 160‐, and 640‐mg groups during Weeks 0©2 and were down‐titrated in the same groups during Weeks 8–10. ???=placebo; ???=propranolol CR 80 mg; ???=propranolol CR 120 mg; ???=propranolol CR 160 mg; ???= propranolol CR 640 mg
Table II.
Effect of Propranolol Controlled Release (CR) Formulation on Morning and Evening Blood Pressure in the Intent‐to‐Treat Population After 8 Weeks of Treatment
| Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Propranolol CR Dose (mg/d) | ||||||
| Parameter | Placebo | 80 | 120 | 160 | 640 | p Value † |
| n | 84 | 88 | 84 | 84 | 87 | |
| Morning DBP†† | –6.98 (–8.89, –5.08) | –10.09 (–11.96, –8.22) | –11.04** (–12.95, –9.13) | –10.43* (–12.36, –8.51) | –10.68* (–12.58, –8.78) | 0.018 |
| Morning SBP†† | ‐8.19 (–11.60, –4.77) | –11.96 (–15.30, –8.63) | –12.43 (–15.85, –9.01) | –12.17 (–15.63, –8.72) | –12.09 (–15.48, –8.70) | 0.345 |
| n‡ | 75 | 79 | 78 | 82 | 80 | |
| Evening DBP†† | –7.76 (–9.70, –5.81) | –11.35* (–13.24, –9.46) | –13.32*** (–15.21, –11.42) | –11.16* (–13.02, –9.30 | –12.23** (–14.13, –10.33) | 0.001 |
| Evening SBP†† | –7.62 (–11.01, –4.22) | –14.00* (–17.30, –10.70) | –13.03 (–16.35, –9.71) | –11.29 (–14.54, –8.03) | –13.47* (–16.78, –10.16) | 0.046 |
| DBP=diastolic blood pressure; SBP=systolic blood pressure † p value for the overall comparison among treatments based on analysis of covariance; ††values are mean change from baseline (mm Hg) with 95% confidence intervals in parentheses; ‡patients who had blood pressure measured in the evening at baseline and at the end of Week 4 or Week 8 or both; * p<0.05, ** p<0.01, and *** p<0.001 for the comparison between the placebo group and that treatment group using the Dunnett test | ||||||
Secondary Efficacy Results
Compared with baseline, all treatments, including placebo, reduced morning sitting SBP and both evening sitting SBP and DBP after 8 weeks of treatment (Table II). Evening sitting DBP was significantly reduced compared with placebo by all doses of propranolol CR, but evening SBP was significantly less than placebo only in the propranolol CR 80‐mg and 640‐mg groups (Table II). Morning SBP was not significantly reduced compared with placebo. At baseline, mean morning heart rates were similar in each group, ranging from 73.3 bpm in the propranolol CR 160‐mg group to 75.4 bpm in the placebo group. Propranolol CR significantly reduced mean morning heart rate in all groups compared with placebo (Figure 3). Similar significant decreases were seen when heart rate was measured in the evening. Propranolol CR significantly reduced the mean morning rate‐pressure product in a dose‐related fashion (Figure 3) and, as was the case with heart rate; similar decreases were observed when rate‐pressure product was determined in the evening.
Figure 3.
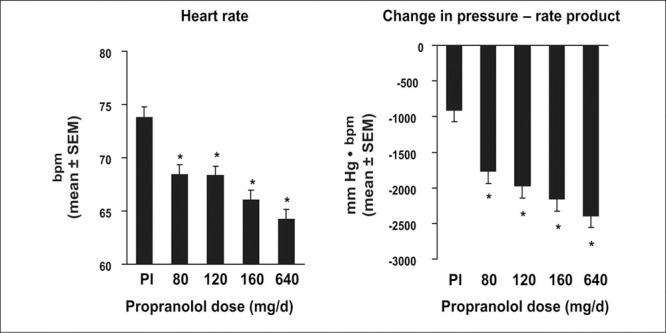
The effect of propranolol controlled‐release formulation on morning heart rate and rate‐pressure product after 8 weeks of treatment. Pl=placebo; *p<0.001 vs. placebo
Plasma Concentration
Trough plasma concentrations of propranolol were measured in 18–23 patients from each treatment group. The demographic composition of this subset was similar to that of the intent‐to‐treat population except that there was a greater percentage of white patients (72%–95% across treatment groups) than in the intent‐to‐treat population (59%–72% across treatment groups). Trough plasma concentrations (Figure 4) were similar at 4 and 8 weeks and increased in a dose‐dependent manner, indicating that the pharmacokinetic pattern of propranolol was consistent and that subject compliance with protocol was maintained. Inter‐ and intrasubject standard deviation estimates were 26.3 ng/mL and 24.3 ng/mL, respectively. These standard deviation estimates are less than what is generally observed with propranolol, which otherwise is a compound characterized by significant first‐pass effect and wide confidence intervals for steady state plasma concentrations. 22 In this subset of patients, linear regression utilizing both absolute and natural log transformed values was used to examine the correlation between trough plasma concentration and the primary and secondary efficacy parameters. Trough plasma concentration was significantly correlated to evening DBP (p=0.026), morning heart rate (p≤0.0001), evening heart rate (p=0.0013), and morning and evening rate‐pressure products (p<;0.0001 and p=0.0039, respectively). No significant correlation was found for morning DBP, morning SBP, or evening SBP (p<;0.0648, p=0.2456, and p=0.0875, respectively). The observed correlations were independent of whether plasma concentrations of propranolol were log‐transformed.
Figure 4.
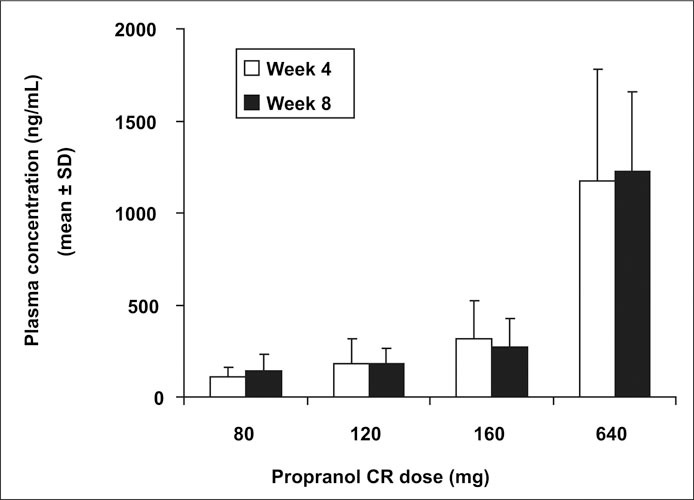
Plasma propranolol levels after 4 and 8 weeks of treatment with propranolol controlled‐release (CR) formulation.
Safety and Tolerability
Overall, propranolol CR was safe and well tolerated. During the double‐blind treatment phase of the study, about half of the patients in any treatment group experienced an adverse event with most classified as mild or moderate in severity. The adverse events reported by 5% or more of the patients in any treatment group are presented in Table III. There did not appear to be any clinically relevant differences in adverse events recorded for women and men. Fatigue and dizziness were somewhat more common in the patients treated with propranolol CR, but did not appear to be dose related. Although the numbers of patients with fatigue and insomnia were small there appeared to be a relationship between these side effects and the high‐end dose of 640 mg/d. Two patients treated with propranolol CR (one in the 120‐mg group and one in the 160‐mg group) experienced postural hypotension, as did one patient in the placebo group.
A total of 23 patients discontinued the study because of an adverse event. Of these, five were in the placebo group (5.7%), six were in the 80‐mg group (6.7%), and 10 were in the 640‐mg group (11.5%). The 120‐mg and 160‐mg groups had one discontinuation each (1.2%). The most common reasons for discontinuation from the 640‐mg group were insomnia (n=3, 3.4%) and fatigue (n=2, 2.3%). Serious adverse events resulting in discontinuation occurred in four patients during the double‐blind treatment phase of the study. One male patient in the 80‐mg group was hospitalized for moderate musculoskeletal chest discomfort. In the 120‐mg group, one male patient suffered a severe gastrointestinal disorder (diverticulitis, ruptured sigmoid) for which he was hospitalized and treated surgically, and another male patient was hospitalized for chest pressure and difficulty breathing. In the placebo group, a male patient suffered a stroke. In none of these four cases was the serious adverse event deemed to be related to the study treatment by the investigator.
No clinically significant changes from screening to the termination of the study were seen in any mean clinical laboratory values. Mean plasma glucose increased slightly in all treatment groups from screening to study termination, with the largest increase being seen in the 160‐mg/d group (7.3+5.1 mg/dL). However none of the increases was statistically significant. The number of patients who had a change from a normal ECG at screening to an abnormal ECG at study termination was comparable across treatment groups and ranged from 12% to 17%. ECG changes were not associated with definable clinical sequelae.
DISCUSSION
This study demonstrated the efficacy and safety of a novel delivery system of propranolol specifically designed for nighttime dosing. Administered around bedtime, propranolol CR at doses of 120, 160, and 640 mg/d significantly reduced morning DBP, the primary outcome variable, compared with placebo in patients with mild‐to‐moderate hypertension (Table II). The reduction in BP with propranolol CR was comparable in the dose range from 120 to 640 mg. This is not an unexpected observation in that β blockade typically has a flat dose‐response curve once an effect dose has been established 23
In addition, all doses of propranolol CR provided effective 24‐hour antihypertensive control, as shown by the evening measurements of DBP (which occurred at the trough of the dosing cycle). As shown in Table II, the decreases in DBP measured in the evening were approximately the same as those measured in the morning and these changes were generally more prominent in women than men (data not shown). The reduction in BP with propranolol CR occurred to a similar degree in all ethnic groups. This was particularly so in the African‐American cohort, where mean DBP reductions of 11.33 mm Hg and 11.67 mm Hg were noted in the 120‐mg and 160‐mg groups, respectively. These are interesting findings suggesting a time‐of‐delivery component to the BP response to propranolol in African Americans; however, the number of African Americans in this study was small (Table II) and this observation requires additional confirming studies.
The changes in morning SBP in the active treatment groups were not significantly different than those observed in the placebo group (Table II). The failure to reduce SBP in the morning hours likely related to the large placebo effect (regression to the mean) masking the anticipated SBP reduction and/or study specific conditions that May have complicated interpretation of the morning SBP change. The latter BP readings were obtained in the morning hours in a office‐based setting and May have missed an SBP/ DBP effect in the early morning hours before arrival at the study facility. Such changes would have been more likely to have been identified with ambulatory BP monitoring, which unfortunately was not part of the design for this study.
This persistence of DBP effect at trough is relevant in that it establishes propranolol in this delivery system as a true once‐a‐day compound, whereas several other β blockers provide less than complete coverage at the end of their dose interval. 24 It is unclear whether propranolol in this delivery system provides >24 hours coverage because subjects were not studied with ambulatory BP monitoring. Despite the absence of such data the pharmacokinetic profile of this compound as well as the residual blood levels at trough (Figure 4) would suggest a meaningful BP reduction beyond 24 hours.
In addition to the antihypertensive effects, propranolol in this new formulation resulted in dose‐related decreases in morning and evening heart rate and rate‐pressure product, an indicator of myocardial oxygen consumption. The dose dependency in the reduction in rate‐pressure product related to a drop in pulse rate more so than an incremental reduction in BP with propranolol CR dose escalation. The fact that propranolol incrementally reduced pulse rate without significant additional falls in BP suggest that these study subjects were not pulse rate dependent in their BP. Pulse‐rate dependent hypertensives typically experience a more brisk fall in BP with β blockade; thus, the magnitude BP drop in these subjects can be viewed as being broadly representative of a population‐based response to β blockade.
Chronotherapeutics refers to the concept of matching the pharmacologic delivery of drugs to the natural circadian rhythms of disease/body functions and is a relatively new concept in cardiovascular therapy. One potential target for chronotherapy is hypertension. Both systolic and diastolic BP exhibit substantial and regular changes during a 24‐hour period in both normotensive and hypertensive subjects. In most subjects, BP reaches a nadir during the hours of sleep, shows a substantial increase (20–30 mm Hg) in the hours immediately before and just after waking, and remains relatively constant until late afternoon and evening when it trends downward. 6 , 25 , 26 This circadian pattern is modified by a range of environmental factors and is oftentimes difficult to identify with so many extraneous factors at play. In this regard, the delivery characteristics of most currently available antihypertensive drugs are mismatched with the circadian pattern of BP. Most antihypertensive medications are dosed once daily in the morning with the end result being that the lowest plasma concentration of drug is present when morning BP values begin to rise. The failure to adequately control hypertension during this time period May be of critical importance, as indicated by the temporal association of this morning surge in BP with the increased morning incidence of MI, sudden cardiac death, and stroke. 1 , 2 , 3 , 4
The increased morning incidence of acute MI is well established and is clearly illustrated by the time of onset of symptoms for patients in the ISIS‐2 trial. 1 In this study, the time of MI could be estimated in 71% of the study population (12,163 of 17,187 patients), and the analysis showed that between the hours of 6 a.m. and noon, the relative risk of MI was 1.41 (95% confidence interval [CI], 1.36, 1.46) times higher than it was at any other time during the day. The linkage of time to the occurrence of MI showed the sharpest increase between 6 a.m. and 8 a.m. Other studies have confirmed these observations. 5 , 18 , 19 Of particular interest to our study was the observation that patients treated with β blockers do not exhibit this circadian distribution of time of onset of MI. 5 , 18 , 19 In a study reported by Muller and colleagues, 5 the risk for the onset of MI in patients who were not taking β blockers was 34% higher (p<0.01) between the 6‐hour time period between 6 a.m. and noon than during the other three 6‐hour periods. However, in patients who were treated with β blockers, the increased morning risk was only 3% higher (p=not significant). Because of the retrospective design of these studies of the circadian distribution of the incidence of MI, it is not possible to determine if prior use of β blockers actually decreased the absolute incidence of morning MI or if it only shifted the distribution of events to other time periods. However, the extensive clinical evidence that β blockers are effective in the primary and secondary reduction of morbidity and mortality from coronary heart disease 14 , 16 , 17 , 27 , 28 , 29 strongly suggests that attenuation of the circadian distribution of the incidence of MI occurred because of an overall reduction in morning events.
The mechanisms by which propranolol could reduce the peak in morning cardiovascular events is not fully understood, but likely involves a combination of its antihypertensive action, as well as its direct antagonism of sympathetic nervous system activity. Reduction in BP in at‐risk patients with hypertension has long been recognized as a means to reduce cardiovascular morbidity and mortality, 30 , 31 , 32 , 33 , 34 but by itself May be insufficient to account for the reduction in morning cardiovascular events in patients treated with β blockers.
In the Intravenous Streptokinase in Acute Myocardial Infarction (ISAM) study, patients reporting the use of β blockers had a reduced frequency of morning events, whereas those citing the use of calcium channel blockers did not. 19 It seems likely that the ability of β blockers to directly interfere with the morning increase in sympathetic nervous system activity through the blockade of P‐adrenergic receptors contributes substantially to the reduction in morning events. β blockade could attenuate the increase in heart rate and myocardial contractility seen upon waking and thus reduce myocardial oxygen demand and limit the hemodynamic changes that May be responsible for triggering plaque rupture. Although the ability of platelets to aggregate has been reported to increase in the hours after waking, 7 , 8 the role of β blockers to influence platelet aggregation is not well established. β blockers have little effect on in vitro aggregation of platelets taken from normal subjects 35 but May reduce aggregation in vivo in patients with ischemic heart disease. 36 , 37 , 38 , 39 For example, in a small subset of patients in the β‐Blocker Heart Attack study, 16 fewer circulating aggregates of platelets were found in patients treated with propranolol than in those treated with placebo. 39
It is well established that reduction of BP in populations of hypertensive patients reduces the incidence of cardiovascular morbidity and mortality, 40 " 41 but it has not yet been proven that using a chronotherapeutic formulation that provides maximum antihypertensive efficacy in the morning hours will improve these benefits. One study of antihypertensive chronotherapy was recently completed. The Controlled Onset Verapamil Investigation of Cardiovascular End points (CONVINCE) trial 42 was scheduled to be a 5‐year randomized, double‐blind study comparing a delayed‐onset, controlled‐release formulation of the calcium channel blocker verapamil to standard morning‐dosed antihypertensive therapy (diuretic or β blocker) in 16,602 hypertensive patients. At the early termination of the study (after about 3 years of follow‐up), the number of major events that occurred in each treatment group was nearly identical (364 in the verapamil group, and 365 in the standard care group). 42 There was no difference in the occurrence of morning events. Because the number of events was smaller than anticipated, the investigators were unable to conclude that chronotherapeutically delivered verapamil was equivalent to the standard of care in reducing major cardiovascular events in hypertensive patients. Whether a chronotherapeutic formulation of propranolol like that tested in our study has an enhanced ability to reduce cardiovascular morbidity and mortality will require further evaluation. In this study, no morning events were reported that might allow end point discrimination between placebo and propranolol. Finally, chronotherapeutics remains a poorly studied area in relationship to the pattern of drug delivery, possibly influencing side‐effect profiles to medications. For example, prior studies with nocturnally delivered forms of verapamil 43 and diltiazem 44 have been associated with fewer symptoms (e.g., less peripheral edema) than might be the case with daytime administration of these compounds. This seems to also be the case with nocturnally administered propranolol, which seemed to be somewhat better tolerated in women than is routinely the case for this compound when it is administered in the daytime.
CONCLUSIONS
In patients with mild‐to‐moderate hypertension, propranolol CR taken once nightly around bedtime provides effective and safe 24‐hour control of BR It is the first delayed‐release β blocker specifically formulated for nighttime administration to provide maximum drug concentration during the early morning rise in BR This morning antihypertensive activity combined with effective morning β blockade limits several of the well established triggers for morning events; however, whether propranolol in this formulation specifically confers morning cardiovascular protection above and beyond that of conventional β‐blocker formulations that could be administered on a b.i.d. basis remains to be determined.
Disclosure:
This study was funded by Reliant Pharmaceuticals, Liberty Corner, NJ.
References
- 1. Morning peak in the incidence of myocardial infarction: experience in the ISIS‐2 trial. Eur Heart J. 1992;13:594–598. [PubMed] [Google Scholar]
- 2. Cohen MC, Rohtla KM, Lavery CE, et al. Meta‐analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. [DOI] [PubMed] [Google Scholar]
- 3. Willich SN, Levy D, Rocco MB, et al. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. [DOI] [PubMed] [Google Scholar]
- 4. Elliott WJ. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke. 1998;29:992–996. [DOI] [PubMed] [Google Scholar]
- 5. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 6. Baumgart P. Circadian rhythm of blood pressure: internal and external time triggers. Chronobiol Int. 1991;8:444–450. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Manson JE, Buring JE, et al. Circadian variation of acute myocardial infarction and the effect of low‐dose aspirin in a randomized trial of physicians. Circulation. 1990;82:897–902. [DOI] [PubMed] [Google Scholar]
- 8. Muller JE. Morning increase of onset of myocardial infarction. Implications concerning triggering events. Cardiology. 1989;76:96–104. [DOI] [PubMed] [Google Scholar]
- 9. Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62:635–637. [DOI] [PubMed] [Google Scholar]
- 10. Feng DL, Tofler GH. Diurnal physiologic processes and circadian variation of acute myocardial infarction. J Cardiovasc Risk. 1995;2:494–498. [DOI] [PubMed] [Google Scholar]
- 11. Furlan R, Guzzetti S, Crivellaro W, et al. Continuous 24‐hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. [DOI] [PubMed] [Google Scholar]
- 12. van de Borne P, Nguyen H, Biston P, et al. Effects of wake and sleep stages on the 24‐h autonomic control of blood pressure and heart rate in recumbent men. Am J Physiol. 1994;266:H548–H554. [DOI] [PubMed] [Google Scholar]
- 13. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. [DOI] [PubMed] [Google Scholar]
- 14. A randomized trial of propranolol in patients with acute myocardial infarction. II. Morbidity results. JAMA. 1983;250:2814–2819. [DOI] [PubMed] [Google Scholar]
- 15. Timolol‐induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. [DOI] [PubMed] [Google Scholar]
- 16. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714. [DOI] [PubMed] [Google Scholar]
- 17. Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta‐blockade on mortality among high‐risk and low‐risk patients after myocardial infarction. N Engl J Med. 1998;339:489–497. [DOI] [PubMed] [Google Scholar]
- 18. Sayer JW, Wilkinson P, Ranjadayalan K, et al. Attenuation or absence of circadian and seasonal rhythms of acute myocardial infarction. Heart. 1997;77:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willich SN, Linderer T, Wegscheider K, et al. Increased morning incidence of myocardial infarction in the ISAM Study: absence with prior beta‐adrenergic blockade. Circulation. 1989;80:853–858. [DOI] [PubMed] [Google Scholar]
- 20. Sica DA, Frishman WH, Manowitz N. Pharmacokinetics of propranolol following single and multiple dosing with sustained release or propranolol CR, a new chronotherapeutic formulation. Heart Dis. 2003;5:176–181. [DOI] [PubMed] [Google Scholar]
- 21. Inderal [package insert]. Philadelphia , PA : Wyeth‐Ayerst Co; 2000. [Google Scholar]
- 22. Frishman WH, Alwarshetty M. Beta‐adrenergic blockers in systemic hypertension: pharmacokinetic considerations related to the current guidelines. Clin Pharmacokinet. 2002;41:505–516. [DOI] [PubMed] [Google Scholar]
- 23. Serlin MJ, Orme ML, Baber NS, et al. Propranolol in the control of blood pressure: a dose‐response study. Clin Pharmacol Ther. 1980;27:586–592. [DOI] [PubMed] [Google Scholar]
- 24. Neutel JM, Schnaper H, Cheung DG, et al. Antihypertensive effects of β‐blockers administered once‐daily: 24‐hour measurements. Am Heart J. 1990;120:166–171. [DOI] [PubMed] [Google Scholar]
- 25. Lemmer B. The clinical relevance of chronopharmacology in therapeutics. Pharmacol Res. 1996;33:107–115. [DOI] [PubMed] [Google Scholar]
- 26. Anwar YA, White WB. Chronotherapeutics for cardiovascular disease. Drugs. 1998;55:631–643. [DOI] [PubMed] [Google Scholar]
- 27. Rabkin R, Stables DP, Levin NW, et al. The prophylactic value of propranolol in angina pectoris. Am J Cardiol. 1966;18:370–383. [DOI] [PubMed] [Google Scholar]
- 28. The Beta‐Blocker Pooling Project (BBPP): subgroup findings from randomized trials in post infarction patients . The Beta‐Blocker Pooling Project Research Group. Eur Heart J. 1988;9:8–16. [PubMed] [Google Scholar]
- 29. Wikstrand J, Berglund G, Tuomilehto J. Beta blockade in the primary prevention of coronary heart disease in hypertensive patients. Review of present evidence. Circulation. 1991;84(6 suppl):VI93–VI100. [PubMed] [Google Scholar]
- 30. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 31. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. [DOI] [PubMed] [Google Scholar]
- 32. Dahlöf B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP‐Hypertension). Lancet. 1991;338:1281–1285. [DOI] [PubMed] [Google Scholar]
- 33. Hansson L, Hedner T, Lund‐Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365. [DOI] [PubMed] [Google Scholar]
- 34. Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension‐2 study. Lancet. 1999;354:1751–1756. [DOI] [PubMed] [Google Scholar]
- 35. Hjemdahl P, Larsson PT, Wallen NH. Effects of stress and beta‐blockade on platelet function. Circulation. 1991;84: VI44–VI61. [PubMed] [Google Scholar]
- 36. Mehta J, Mehta P, Pepine CJ. Differences in platelet aggregation in coronary sinus and aortic blood in patients with coronary artery disease: effect of propranolol. Clin Cardiol. 1978;1:96–100. [DOI] [PubMed] [Google Scholar]
- 37. Mikhailidis DP, Barradas MA, Mier A, et al. Platelet function in patients admitted with a diagnosis of myocardial infarction. Angiology. 1987;38:36–45. [DOI] [PubMed] [Google Scholar]
- 38. Frishman WH, Weksler B, Christodoulou JP, et al. Reversal of abnormal platelet aggregability and change in exercise tolerance in patients with angina pectoris following oral propranolol. Circulation. 1974;50:887–896. [DOI] [PubMed] [Google Scholar]
- 39. Green D, Rossi EC, Haring O. The Beta‐Blocker Heart Attack Trial: studies of platelets and factor VIII. Thromb Res. 1982;28:261–267. [DOI] [PubMed] [Google Scholar]
- 40. Effects of ACE inhibitors, calcium antagonists, and other blood‐pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;355:1955–1964. [DOI] [PubMed] [Google Scholar]
- 41. Staessen JA, Wang J‐G, Thijs L. Cardiovascular protection and blood pressure reduction: a meta‐analysis. Lancet. 2001;358:1305–1315. [DOI] [PubMed] [Google Scholar]
- 42. Black HR, Elliott WJ, Grandits G, et al. CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. [DOI] [PubMed] [Google Scholar]
- 43. White WB, Anders RJ, MacIntyre JM, et al. Nocturnal dosing of a novel delivery system of verapamil for systemic hypertension. Verapamil Study Group. Am J Cardiol. 1995;76:375–380. [DOI] [PubMed] [Google Scholar]
- 44. Glasser SP, Neutel JM, Gana TJ, et al. Efficacy and safety of a once daily graded‐release diltiazem formulation in essential hypertension. Am J Hypertens. 2003;16:51–58. [DOI] [PubMed] [Google Scholar]


