Abstract
This prospective, randomized trial evaluated the effect of monotherapy and different combination therapies on cardiovascular target organ damage and metabolic profile in 520 hypertensive patients. Patients were allocated to a single agent: carvedilol 25 mg, amlodipine 10 mg, enalapril 20 mg, or losartan 50 mg (groups C, A, E, and L, respectively). After 2 months (level 2), nonresponders received a low‐dose thiazide diuretic, and after 4 months (level 3), amlodipine (groups E, C, and L) and carvedilol (group A). Twenty‐four‐hour blood pressure was significantly lowered in all treatment groups. Blood pressure control was more pronounced in patients receiving two or three drugs. At the end of the study, the carotid intima‐media thickness decreased in group L (P<.01), left ventricular mass index in groups E and L (P<.05 and P<.001, respectively), with a concomitant reduction in cholesterol in group L (P<.03). Diastolic function improved significantly in group L (P<.05). This study describes the need to control blood pressure with two or more drugs in most hypertensive patients and illustrates good clinical outcomes, independent of blood pressure lowering, using combination therapy with losartan, low‐dose thiazide, and amlodipine.
There is a well‐known relationship between increasing blood pressure (BP) and increasing cardiovascular (CV) morbidity and mortality. 1 , 2 Hypertension trials have emphasized that good BP control is of paramount importance in ensuring optimal prevention of adverse CV events such as strokes and myocardial infarctions. Moreover, it is well documented that only one third of hypertensive patients have BP values <140/90 mm Hg. 3 Part of the reason for this is that a single antihypertensive agent normalizes BP in almost 40% of hypertensive patients. 4 As suggested by treatment guidelines to achieve recommended BP goals, it is often necessary to combine 2 or more antihypertensive agents. 5 Appropriate combination antihypertensive therapy may have a number of advantages, including improved efficacy, tolerability, and sometimes reduced target organ damage. 6 Few studies are available to guide which combination of antihypertensive drugs should be used. In particular, no comparative data suggest when a third drug should be added. The benefit of BP reduction depends not only on the severity of hypertension, but on the global CV risk. 7 “The lower the pressure, the better” is particularly true for patients at high CV risk, in whom the benefit of reducing hypertension complications, such as target organ damage, is largest. 8 Structural and functional changes in the left ventricle and arterial walls are common in hypertension, such as left ventricular hypertrophy (LVH) and high carotid intima‐media thickness (IMT) and plaques. 9 This suggests that in addition to BP control, management of these factors might be necessary for optimal reduction of CV events. 10
The primary objective of this study was to evaluate the efficacy and tolerability of monotherapy and, in the absence of satisfactory BP control, of double and triple antihypertensive combination therapies in a large population of hypertensive patients. We also examined the benefit in reduced CV target organ damage without adverse impact on glycemic and lipid control. 11 , 12
METHODS
Study Population
The study was a prospective, randomized, intervention trial performed on 560 consecutive Caucasian patients (301 men; mean age, 54±11 years; range, 29–90 years) with uncomplicated essential hypertension (systolic BP [SBP] 155–169 mm Hg and diastolic BP 95–109 mm Hg) who were referred to our outpatient clinic. All subjects received a full history and a complete physical examination. Conventional BP readings were used as the basis to screen potential subjects who could enter the study, and 24‐hour ambulatory BP monitoring (ABPM) was used to confirm (as described below) the eligibility of patients for randomization. The clinical diagnosis of hypertension was considered when SBP was ≥140 mm Hg and/or diastolic BP was ≥90 mm Hg on at least 3 visits and when antihypertensive therapy was present. BP was measured with a mercury sphygmomanometer with an appropriate size rubber cuff applied around the nondominant arm. Readings were based on Korotkoff first and fifth phase sounds.
During each visit, 3 consecutive BP readings were obtained with the subject in the sitting position after a rest of at least 10 minutes. The average of the 3 readings was used for the analyses, recorded to the nearest 2 mm on the scale. Measurements were performed early in the morning and carried out by a trained investigator. We also determined lipid profile and fasting glucose in serum with standard laboratory methods. Patients with secondary hypertension, renal failure, diabetes mellitus, congestive heart failure, atrial fibrillation, and severe valvular heart disease were excluded from the study. Patients with severe obesity, defined as a body mass index ≥40 kg/m 2 , and women who were pregnant or lactating were also excluded. None had any evidence or history of myocardial infarction or stroke. Each patient provided informed consent for the study. Previously treated patients (range, 38%–41%) who did not have BP controlled by current medication were asked to suspend therapy under medical supervision for at least 1 week. Moreover, no patient received concomitant medications known to affect BP or interfere with the metabolic parameters.
Patients were randomly allocated in a 1:1 fashion to start with a single agent, and 140 subjects were recruited for each arm. The following classes and drugs were used in the study (level 1): β‐blocker (carvedilol 25 mg once daily, group C), calcium antagonist (amlodipine 10 mg once daily, group A), angiotensin‐converting enzyme inhibitor (enalapril 20 mg once daily, group E), and angiotensin II receptor blocker (losartan 50 mg once daily, group L). The drug doses and daily administration were chosen as those usually employed in clinical practice. Discontinuation depended on the efficacy and tolerability of the first drug. If this was ineffective (fall in SBP <5 mm Hg) and/or not tolerated, the patient was withdrawn from the study. At each scheduled visit throughout the study, seated cuff BP and compliance with study medication were monitored and adverse events were recorded. Response to treatment was defined as mean sitting BP values <140/90 mm Hg or a decrease of 10/10 mm Hg in BP from baseline. When necessary, a second (level 2) or third (level 3) BP‐lowering agent (with low dose, also as fixed dose) by a different mechanism (stepped‐care strategy) was added to lower BP to <140/90 mm Hg. The treatment was adjusted as described below after 2 months (level 2) with an open‐label thiazide diuretic (12.5 mg once daily). After 4 months, nonresponders received the most rational triple‐combination therapy using amlodipine in groups E, C, and L (5 mg once daily) and carvedilol in group A (12.5 mg once daily), according to guideline suggestions. 5 The intermediate visits were planned after 12 and 16 months and the final visit after 24 months.
All patients underwent the following instrumental procedures: 24‐hour ABPM, echocardiography with Doppler tissue imaging (DTI), and carotid ultrasonography at baseline and at the end of the study. We used ABPM because the technique is now recognized as the most effective means of detecting white coat hypertension (average 24‐hour ABPM <130/80 mm Hg) and to confirm the diagnosis of hypertension at baseline (average 24‐hour ABPM >130/80 mm Hg) and the efficacy of antihypertensive medications at the end of the study. 13 We used DTI because the technique is better than conventional Doppler at detecting hypertension‐associated dysfunction. 14 All echocardiographic and ultrasonographic examinations were recorded on videotape and performed by the same experienced physician, who was blinded to the patient's treatment allocation.
Ambulatory BP Monitoring
To perform ABPM, we used a Spacelabs model 90207 monitor (Spacelabs Medical, Inc, Issaquah, WA). This device employs an oscillometric method with a deflation rate of 8 mm Hg/s. The cuff wrapped around at least two thirds of the nondominant upper arm. The device was checked against a mercury sphygmomanometer by a Y‐tube. The patients were asked to undertake their usual activities. BP monitorings were performed over a working day. Recording began between 8:30 AM and 9 AM. The reading frequency was programmed for every 20 minutes from 7 AM to 10 PM (daytime) and every 30 minutes from 10 PM to 7 AM (nighttime). We chose these time intervals because the periods corresponded closely to activity and sleep in most subjects. When interference or error in the reading occurred, the process was automatically repeated after 2 minutes while retaining the preestablished sequence. During the daytime period, an acoustic signal before the measurement was automatically programmed to remind the patients to relax their arms. The ABPMs with 85% or more likely readings were analyzed and recordings with erroneous measurements exceeding 30% were excluded from the analysis.
Echocardiography
The M‐mode echocardiogram was performed using 3.5‐MHz phased array placed on the III–IV left intercostal space along the parasternal line, with patients supine, in left lateral decubitus position, with the head of the bed kept at a 30° angle. The end‐diastolic measurements of left ventricular (LV) internal dimension, left interventricular septum, and posterior wall thickness at the QRS peak using the Penn convention were taken. The LV mass was calculated according to the Devereux formula. Patients with an LV mass index (LVMI) >130 g/m2 in men and >110 g/m2 in women, based on the upper 90th percentile from 150 apparently healthy normotensive adults, were classified as having LVH.
Doppler Mitral Inflow Velocities
The pulsed Doppler sample volume was placed at the mitral valve tips and 5–10 cardiac cycles were recorded from the apical window on super VHS videotape at a velocity of 100 mm/s. The following measurements of LV diastolic function were determined: E and A peak velocities (m/s) and their ratio and E‐wave deceleration time (milliseconds) by placing a continuous‐wave Doppler sample volume between LV outflow tract and the mitral valve.
Doppler Tissue Imaging
The DTI program was set to the pulsed‐wave Doppler mode. Filters were set to exclude high‐frequency signals, and the Nyquist limit was adjusted to a velocity range of 15–20 cm/s; gains were minimized to allow for a clear tissue signal with minimal background noise. All DTI recordings were obtained during normal respiration. A 5‐mm sample volume was placed at the apical 4‐chamber view on the lateral corner of the mitral annulus. The resulting velocities were recorded for 5–10 cardiac cycles at a sweep speed of 100 mm/s and stored on a 0.5‐inch VHS videotape for later playback and analysis. The following measurements were determined as indexes of myocardial function: peak myocardial systolic velocities (cm/s), myocardial early and late diastolic peak velocities and their ratio (cm/s), and E‐wave deceleration time.
Carotid Ultrasonography
Ultrasound examination of the carotid was performed with a transducer frequency of 7.5 MHz. Measurements involved a primary transverse and longitudinal scanning of the common carotid artery, bifurcation, and internal carotid. The end‐diastolic IMT of the far wall of the middle segment of both common carotid arteries, defined by a simultaneous electrocardiographic recording, was measured 1 cm caudal to the bulb, as the distance between the lumen‐intima interface and the media‐adventitia interface. 15 , 16 Each measurement was calculated by taking the average of 3 readings. Intima‐media thickening of the common carotid arteries was defined as an average IMT ≥0.9 mm. All measurements were made at a site without plaque. The near and far walls of the carotid were scanned longitudinally and transversely to assess the presence of plaques. The presence of plaques was defined as localized echo structures encroaching into the vessel lumen for which the distance between the media‐adventitia interface and internal side of the lesion was ≥1.3 mm or as the presence of calcification.
Statistical Analyses
Statistical analyses were carried out with GB‐STAT version 6.50 (Dynamic Microsystems, Inc, Silver Spring, MD). Comparison among groups was performed using analysis of variance plus the Bonferroni t test for unpaired data. Comparisons of categoric data were made using the Fisher exact test. Significance was defined as P<.05. The results are expressed as mean ± SD. Kappa statistic was used to assess inter‐reader and intrareader variability for echocardiographic and ultrasonographic parameters.
RESULTS
Of the 560 patients examined at the beginning of the study, the diagnosis of hypertension was not confirmed after the baseline 24‐hour ABPM in 40 subjects because they were considered white coat hypertensives. Of the remaining 520 patients who were included at the baseline treatment period, 54 (10.4%) did not complete the study. Of these, 40 (7.7%) patients had adverse events and no BP control. The reasons for the withdrawals are shown in Table I. Clinical and laboratory characteristics of the study population are reported in Table II. Age and sex distribution, body mass index, family history of hypertension, 24‐hour ABPM, and glucose and serum lipids did not differ significantly among groups. There were no significant differences in the 4 arms regarding systolic and diastolic function indexes, the average of IMT of the common carotid artery, and the percentage of plaques. LVMI alone was significantly higher in groups L and A (P<.05) (Table III). The myocardial early and late diastolic peak velocity ratios were lower in the 4 groups compared with standard Doppler E/A ratios (Table III). Twenty‐four‐hour SBP and diastolic BP were significantly reduced in all treatment groups, and two thirds of patients were on combination therapy (Table IV). The BP normalization (24‐hour BP <130/80 mm Hg) rate was more pronounced in patients receiving 2 or 3 drugs than in those randomized to the monotherapy group, and at the end of the study, it was similar in all groups (Table IV). Echocardiographic and carotid ultrasonographic findings after 24 months are shown in Figure 1 and Figure 2. The greatest and most significant decrease in LVMI was found in the 3 levels of groups E and L (P<.05 and P<.001, respectively). At the end of the study, the carotid IMT decreased only in group L (P<.01). Group L also had a significant reduction in cholesterol (‐10 mg/dL; P<.03). No significant changes in total cholesterol were observed after treatment in the other 3 groups (group C, +1 mg/dL; group A, −3 mg/dL; group E, +1 mg/dL; P=not significant). Similarly, no significant changes in fasting glucose were noted (group C, +3 mg/dL; group A, 0 mg/dL; group E, +3 mg/dL; group L, 0 mg/dL; P=not significant). There were no significant differences in overall diastolic parameters, assessed by Doppler mitral inflow velocities, after 24 months in the four treatment strategies. On the contrary, diastolic function, evaluated by TDI, was improved in all groups, with a small significant improvement in group L (P<.05).
Table I.
Discontinuation During the Study in 4 Groups at All Levels, n (%)
| Group C (n=131) | Group A (n=130) | Group E (n=130) | Group L (n=129) | |
|---|---|---|---|---|
| Patients who completed the trial | 113 (86) | 114 (88) | 118 (91) | 121 (94) |
| Reason for patient withdrawal | ||||
| Adverse events | 9 (6.9) | 9 (7.0) | 5 (3.7) | 2 (1.6) |
| No blood pressure control | 5 (3.8) | 2 (1.6) | 4 (3.1) | 4 (3.2) |
| Lost to follow‐up | 4 (3.1) | 5 (3.8) | 3 (2.3) | 2 (1.6) |
Table II.
Clinical Characteristics of Patients Randomly Allocated to Groups at Baseline
| Group C (n=131) | Group A (n=114) | Group E (n=118) | Group L (n=121) | |
|---|---|---|---|---|
| Age, y | 54±11 | 55±10 | 54±10 | 53±11 |
| Men, % | 59 | 60 | 59 | 62 |
| Body mass index, kg/m2 | 29±3 | 29±4 | 28±4 | 29±3 |
| Family history of hypertension, % | 48 | 50 | 51 | 50 |
| 24‐hour systolic blood pressure, mm Hg | 155±17 | 156±18 | 154±17 | 155±18 |
| 24‐hour diastolic blood pressure, mm Hg | 97±7 | 98±8 | 98±6 | 98±8 |
| Heart rate, bpm | 74±5 | 73±5 | 75±6 | 74±5 |
| Total cholesterol, mg/dL | 206±34 | 207±35 | 205±33 | 202±37 |
| Fasting glucose, mg/dL | 93±13 | 95±11 | 93±11 | 94±11 |
| Previous treatment, % | 38 | 40 | 38 | 41 |
| All data are mean ± SD unless otherwise indicated. No significant differences were found among groups. | ||||
Table III.
Echocardiographic and Carotid Ultrasonographic Parameters of Patients Randomly Allocated to Groups at Baseline
| Group C (n=113) | Group A (n=114) | Group E (n=118) | Group L (n=121) | |
|---|---|---|---|---|
| Left ventricular mass index, g/m2 | 106±25* | 113±23 | 106±21* | 114±25 |
| Left ventricular hypertrophy, % | 30 | 35 | 30 | 33 |
| Peak velocity E wave, cm/s | 69±18 | 66±19 | 69±18 | 65±17 |
| Peak velocity A wave, cm/s | 74±17 | 73±18 | 72±19 | 70±18 |
| E/A ratio | 0.99±0.38 | 0.97±0.41 | 1.02±0.40 | 0.99±0.36 |
| E‐wave deceleration time, ms | 230±37 | 229±35 | 223±33 | 225±38 |
| Em, cm/s | 19±5 | 19±9 | 19±5 | 19±6 |
| Am, cm/s | 24±8 | 27±15 | 23±7 | 25±15 |
| Em/Am ratio | 0.90±0.53 | 0.86±0.47 | 0.92±0.46 | 0.86±0.40 |
| Carotid intima‐media thickness, mm | 0.73±0.18 | 0.74±0.18 | 0.73±0.17 | 0.74±0.16 |
| Plaques, % | 15 | 17 | 15 | 17 |
| All data are mean ± SD unless otherwise indicated. Em indicates early diastolic peak velocity; Am, late diastolic peak velocity. *P<.05 vs group L and group A. | ||||
Table IV.
Changes in Systolic and Diastolic Blood Pressure (BP) After 24 Months
| Group C (n=113) | Group A (n=114) | Group E (n=118) | Group L (n=121) | |
|---|---|---|---|---|
| 24‐hour systolic BP, mm Hg | 133±8 | 132±9 | 132±8 | 132±8 |
| 24‐hour diastolic BP, mm Hag | 85±5 | 86±6 | 85±5 | 85±5 |
| Responders with one drug, % | 25 | 30 | 25 | 23 |
| Responders with two drugs, % | 40 | 50 | 49 | 50 |
| Responders with three drugs, % | 35 | 20 | 26 | 27 |
| A in BP at 24 months, systolic BP/diastolic BP, mm Hag | −22/−12 | −24/−12 | −22/−13 | −23/−13 |
| 24‐hour BP <130/80 mm Hag, % | 71 | 73 | 71 | 72 |
| All data are mean ± SD unless otherwise indicated. No significant differences were found among groups. A indicates change. | ||||
Figure 1.
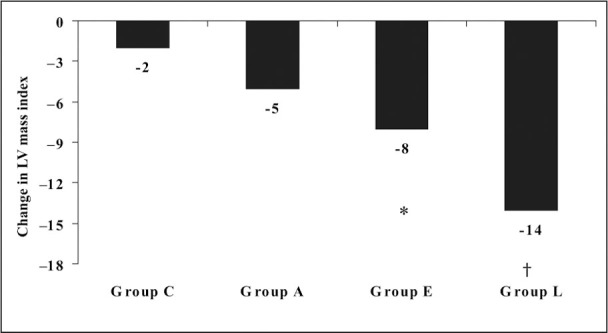
Treatment effect on left venticular (LV) mass index at the end of study. *P<01;†P<001.
Figure 2.
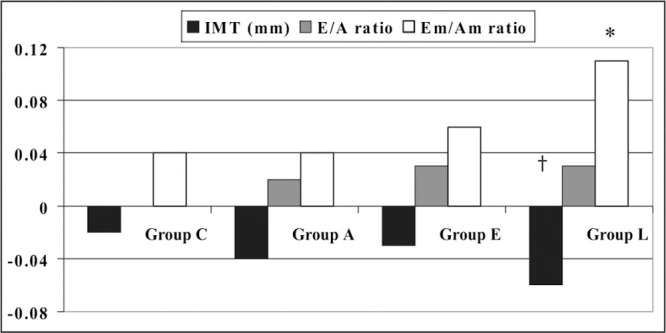
Treatment effect on diastolic function and carotid intima‐media thickness (IMT) at the end of study. Em/Am indicates ratio of early to late diastolic velocities. *P<.05;†P<.01.
The inter‐reader and intrareader variability was good (κ>0.75). Medication compliance was adequate, with more than 90% of pills having been taken in each treatment group.
Safety Evaluation
During the study, approximately 25% of the patients in each treatment group had at least 1 adverse event that was generally of mild‐to‐moderate intensity and did not require the cessation of therapy. The most frequently reported adverse events were dizziness, headache, asthenia, cough, peripheral edema, and hot flushes. However, 25 (4.8%) patients withdrew from the study because of adverse events. There were more patients in groups C (6.9%) and A (7.0%) who withdrew from the study than in groups E (3.7%) and L (1.6%), but differences were not significant.
Reasons for discontinuing therapy in group C were asthenia/fatigue (3 patients), bradycardia (3 patients), and dizziness and cold extremities (3 patients); in group A they were peripheral edema (5 patients), headache (3 patients), and hot flushes (1 patient); in group E they were cough (4 patients) and dizziness (1 patient); and in group L it was dizziness (2 patients).
DISCUSSION
The main findings of this study confirm that a combined, well‐tolerated antihypertensive drug regimen is required to reach the recommended BP goal in most hypertensive patients. The hypertension was controlled by monotherapy in only 30% of patients. Moreover, our results have important clinical implications since the complementary use of agents from different classes, such as in the losartan group on monotherapy and in combination with low‐dose thiazide and amlodipine, can provide positive CV effects by reducing LVMI and IMT, and improving LV diastolic function. The inclusion only of patients with confirmed hypertension in our trial has been shown to minimize the diluting effect of the white coat phenomenon in patients receiving antihypertensive therapy.
The choice of antihypertensive classes in our study follows the general trends in antihypertensive prescriptions in Italy. Although diuretics are recommended in all hypertension treatment guidelines, in our study they were not given on their own but in combination with other drugs to improve the BP‐lowering effect and reduce the risk of an unfavorable metabolic impact. Recent landmark trials showed that CV events, mostly strokes, are prevented more often in patients who take more than 1 antihypertensive agent, in particular, when a diuretic was the added medication at low dose. 17 , 18 Although compliance decreases with the number of tablets taken daily, if more than 1 antihypertensive is used, there may be a place for fixed‐dose combinations. This could improve compliance through simplicity of use. Here, compliance was good and no significant differences were found in adverse events among the treatment groups. Similarly, the number of discontinuations due to adverse events is not significantly different, but the overall rate of discontinuation was lower in the losartan group.
Given the relationship between raised BP and CV events, improvement of hypertension control in the population is expected to have a large impact on CV morbidity and mortality. Why then is BP so poorly controlled? The reasons include disease severity, drug‐related factors, and the fact that hypertension is usually asymptomatic and complications appear only after time. Treatment failure may also be due to suboptimal drug therapy, inappropriate or wrong dosing schedules, or poor use of therapeutic options. All currently available antihypertensive drugs lower BP, but the response varies from patient to patient, in particular when combining drugs on an empirical basis. A rational combination uses drugs with different and complementary modes of action. It is generally believed that some drugs protect beyond BP reduction. LVH is a common manifestation of elevated BP, an initially useful compensatory process to abnormal loading conditions, but it is also the first step toward the development of overt CV disease. 19 Moreover, as recently demonstrated in the Assessment of Prognostic Risk Observational Survey (APROS), 20 there is a positive relationship between LVMI and carotid IMT. This is in line with the evidence that the alterations in cardiac structure induced by hypertension tend to proceed with analogous alterations in the arterial wall structure. 21 , 22 Hemodynamic and nonhemodynamic mechanisms, such as angiotensin II via angiotensin II type 1 receptors, can explain the increase of IMT and cardiac hypertrophy. Recent studies revealed that antihypertensive treatment could reverse LVH and reduce the risk for subsequent CV disease. 23 , 24 However, it is well known that even the full normalization of BP may be unable to entirely regress LVH. 25
Recent studies evaluating the reversal of LVH with the use of different antihypertensive drugs have indicated that angiotensin II receptor blockers, angiotensin‐converting enzyme inhibitors, and calcium antagonists may be more effective than β‐blockers and diuretics in reducing LV mass, and their use is associated with fewer cases of diabetes mellitus. 26 , 27 , 28 , 29 Our study confirmed these findings. The similar reduction of the BP in the 4 groups was not linked to the same decrease in LVMI and IMT in all groups, but particularly in the losartan group in monotherapy and in combination with low‐dose thiazide and amlodipine. The coadministration of losartan and amlodipine exerted antihypertensive and CV protective effects with a good impact on metabolic parameters, producing a low percentage of side effects. A slight reduction in cholesterol and fasting glucose, as well as an improvement in diastolic function detected by DTI, was also observed in this group. It is important to emphasize this aspect because it is well known that impaired LV relaxation has been associated with increased CV mortality in patients with essential hypertension. 30 Moreover, the early diastolic mitral annulus velocity measured by TDI that is relatively preload and heart rate insensitive provides prognostic information in hypertensive patients with echocardiographic evidence of LVH. 31
There are some limitations to the study, particularly related to the impact that the choice of “per protocol” analysis could have on our findings. The view that only patients who complied with the trial's protocol should be considered in the analysis leads to over‐optimistic treatment effects. Moreover, the absence of blinding in our protocol is, in part, compensated by the investigator directly associated with the instrumental procedures, who was blinded to treatment. This guaranteed that the main outcomes were unbiased. In addition, the efficacy of different treatment groups was evaluated by ABPM. Finally, our follow‐up was too short to know what would happen after a lapse of time.
CONCLUSIONS
Recent guidelines have strongly supported the use of combination treatment in stage 2 hypertension as a means to best achieve BP control. 5 , 32 In accordance with guidelines, this study informs physicians on the need to control BP with 2 or more drugs in most hypertensive patients and on the positive effect on clinical outcomes, which is independent of BP lowering, using combination therapy involving losartan with low‐dose thiazide and amlodipine. These latter observations stress the importance of different mechanisms of the various antihypertensive drugs and the opportunity of a well‐considered choice before starting any therapy. Long‐term follow‐up would be needed to demonstrate the predominant role of certain combination therapies.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–19. [DOI] [PubMed] [Google Scholar]
- 2. Sytkowski PA, D'Agostino RB, Belanger AJ, et al. Secular trends in long‐term sustained hypertension, long‐term treatment, and cardiovascular mortality. The Framingham Heart Study 1950 to 1990 . Circulation. 1996;93:697–703. [DOI] [PubMed] [Google Scholar]
- 3. Marques‐Vidal P, Tuomilehto J. Hypertension awareness, treatment and control in the community: is the “rule of halves” still valid? J Hum Hypertens. 1997;11:213–220. [DOI] [PubMed] [Google Scholar]
- 4. Dickerson JE, Hingorani AD, Ashby MJ, et al. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet. 1999;353:2008–2013. [DOI] [PubMed] [Google Scholar]
- 5. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 6. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group . Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 7. Zanchetti A, Hansson L, Dahlof B, et al. Effects of individual risk factors on the incidence of cardiovascular events in treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group . J Hypertens. 2001;19:1149–1159. [DOI] [PubMed] [Google Scholar]
- 8. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 9. Roman MJ, Saba PS, Pini R, et al. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. [DOI] [PubMed] [Google Scholar]
- 10. Cuspidi C, Lonati L, Macca G, et al. Cardiovascular risk stratification in hypertensive patients: impact of echocardiography and carotid ultrasonography. J Hypertens. 2001;19:375–380. [DOI] [PubMed] [Google Scholar]
- 11. Verdecchia P, Reboldi G, Angeli F, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43:963–969. [DOI] [PubMed] [Google Scholar]
- 12. Thomas F, Bean K, Guize L, et al. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002;23:528–535. [DOI] [PubMed] [Google Scholar]
- 13. Dolan E, Stanton A, Atkins N, et al. Determinants of whitecoat hypertension. Blood Press Monit. 2004;9:307–309. [DOI] [PubMed] [Google Scholar]
- 14. Pela G, Bruschi G, Cavatorta A, et al. Doppler tissue echocardiography: myocardial wall motion velocities in essential hypertension. Eur J Echocardiogr. 2001;2:108–117. [DOI] [PubMed] [Google Scholar]
- 15. Kanters SD, Elgersma OE, Banga JD, et al. Reproducibility of measurements of intima‐media thickness and distensibility in the common carotid artery. Eur J Vase Endovasc Surg. 1998;16:28–35. [DOI] [PubMed] [Google Scholar]
- 16. Bots ML, Evans GW, Riley WA, et al. Carotid intima‐media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985–2994. [DOI] [PubMed] [Google Scholar]
- 17. Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. [DOI] [PubMed] [Google Scholar]
- 18. PROGRESS collaborative group . Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. [DOI] [PubMed] [Google Scholar]
- 19. Schillaci G, Verdecchia P, Porcellati C, et al. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–586. [DOI] [PubMed] [Google Scholar]
- 20. Cuspidi C, Ambrosioni E, Mancia G, et al. Role of echocardiography and carotid ultrasonography in stratifying risk in patients with essential hypertension: the Assessment of Prognostic Risk Observational Survey. J Hypertens. 2002;20:1307–1314. [DOI] [PubMed] [Google Scholar]
- 21. Cuspidi C, Mancia G, Ambrosioni E, et al. Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: results from the assessment prognostic risk observational survey (APROS). J Hum Hypertens. 2004;18:891–896. [DOI] [PubMed] [Google Scholar]
- 22. Vaudo G, Schillaci G, Evangelista F, et al. Arterial wall thickening at different sites and its association with left ventricular hypertrophy in newly diagnosed essential hypertension. Am J Hypertens. 2000;13(4 pt 1):324–331. [DOI] [PubMed] [Google Scholar]
- 23. Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens. 2003;16(11 pts 1):895–899. [DOI] [PubMed] [Google Scholar]
- 24. Koren MJ, Ulin RJ, Koren AT, et al. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. 2002;15:1021–1028. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G, Carugo S, Grassi G, et al. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control. Data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni . Hypertension. 2002;39:744–749. [DOI] [PubMed] [Google Scholar]
- 26. Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients. A meta‐analysis of 109 treatment studies. Am J Hypertens. 1992;5:95–110. [DOI] [PubMed] [Google Scholar]
- 27. Tedesco MA, Ratti G, Aquino D, et al. Effects of losartan on hypertension and left ventricular mass: a long‐term study. J Hum Hypertens. 1998;12:505–510. [DOI] [PubMed] [Google Scholar]
- 28. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 29. Weir MR. Providing end‐organ protection with reninangiotensin system inhibition: the evidence so far. J Clin Hypertens (Greenwich). 2006;8:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schillaci G, Pasqualini L, Verdecchia P, et al. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol. 2002;39:2005–2011. [DOI] [PubMed] [Google Scholar]
- 31. Wang M, Yip GW, Wang AY, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens. 2005;23:183–191. [DOI] [PubMed] [Google Scholar]
- 32. Bakris GL. Who should be treated with combination therapy as initial treatment for hypertension? J Clin Hypertens (Greenwich). 2003;5(4 suppl 3):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


