Abstract
Approximately 40% of Japanese patients with essential hypertension, including low‐renin hypertension, are inadequately managed. Low‐renin hypertension generally responds poorly to angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers, but may respond more optimally to diuretics, calcium channel blockers, and aldosterone blockers. This multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐ranging study evaluated the efficacy and safety of the selective aldosterone blocker eplerenone in 193 Japanese patients with essential hypertension. Although not a study inclusion criterion, baseline active plasma renin levels were consistently low (5.7–10.1 mU/L); most patients met the criteria for low‐renin hypertension (≤42.5 mU/L; normal range, 7–76 mU/L). Patients received placebo or eplerenone 50, 100, or 200 mg once daily for 8 weeks. Systolic blood pressure decreased significantly (−6.8 to −10.6 mm Hg vs. −2.1 mm Hg; p≤0.0245 vs. placebo) at all eplerenone doses; diastolic pressure decreased significantly at 100 and 200 mg doses (−6.9 to −7.5 mm Hg vs. −3.0 mm Hg; p≤0.0022 vs. placebo). Eplerenone offers significant blood pressure reduction with good tolerability in Japanese patients with hypertension, including those with low‐renin hypertension.
Until recently, the importance of aldosterone in the development of hypertension has been underestimated and the assumption made that aldosterone‐mediated effects could be adequately controlled with angiotensin‐converting enzyme (ACE) inhibitors or angiotensin‐receptor blockers (ARBs). However, long‐term ACE inhibitor or ARB therapy may not adequately protect patients from the effects of aldosterone escape 1 , 2 , 3 —an effect that can be minimized by blocking aldosterone at the mineralocorticoid receptor. 4
A growing body of clinical evidence has linked aldosterone to the development of hypertension, cardiac hypertrophy, cardiac and vascular fibrosis, and ventricular arrhythmias. 3 , 5 , 6 In experimental models of hypertension, mineralocorticoid receptor blockade attenuated proteinuria and renal damage independent of blood pressure (BP) effects and markedly reduced cardiovascular lesions, interstitial cardiac fibrosis, and vascular fibrosis associated with hypertension. 5 , 7 , 8 , 9 , 10 , 11
The nonselective aldosterone blocker spironolactone has been in use for more than 30 years in Japan and in other countries, but its use is often limited by clinical adverse events, such as gynecomastia, menstrual abnormalities, and impotence, attributed to its interactions with progesterone and androgen receptors. The first selective aldosterone blocker, 12 eplerenone, is in development in Japan and in other countries for the treatment of both hypertension and postmyocardial infarction heart failure. Eplerenone is a highly selective, steroid nucleus‐based mineralocorticoid blocker that acts as a competitive and selective inhibitor of aldosterone at mineralocorticoid receptor sites in various tissues throughout the body. The presence of a 9,11 epoxide group in the eplerenone structure confers its high level of specificity for the mineralocorticoid receptor. 13 In placebo‐controlled, dose‐ranging studies, eplerenone monotherapy (50–200 mg/d) 14 , 15 and combination therapy with an ACE inhibitor or ARB (50–100 mg/d) 16 controlled BP in patients with essential hypertension more effectively than placebo. Eplerenone has been demonstrated to have the safety equivalence of placebo in these dose ranges. 14 , 15 , 16 Treatment with this agent also reduced microalbuminuria and left ventricular mass in patients with hypertension and left ventricular hypertrophy. 17 Furthermore, eplerenone has been shown to be safe and effective in patients with low‐renin hypertension and in elderly patients with isolated systolic hypertension, subpopulations that may not optimally respond to ACE inhibitors or ARBs. 18 , 19
Experimental evidence suggests that the combination of excessive salt intake and aldosterone leads to hypertension, 20 vascular inflammation, 21 myocardial and perivascular fibrosis, 20 , 22 myocardial infarction, 23 renal damage, 24 and cerebrovascular lesions. 8 Thus, Japanese hypertensive patients could potentially be at increased risk of these sequelae as a result of their high intake of salt. In addition to the risk posed by high salt intake and aldosterone, Japanese patients also may respond suboptimally to certain antihypertensive drug classes (e.g., ACE inhibitors) and may have a greater incidence of side effects from some antihypertensive drug classes (e.g., ACE inhibitor‐related cough) than whites. 25 This study was designed to confirm that the safety and efficacy of eplerenone 50–200 mg once daily in Japanese patients with hypertension are similar to those observed in previous studies of non‐Japanese populations.
MATERIALS AND METHODS
Study Population
Japanese men and women were eligible for enrollment if they were between the ages of 20 and 80 years, had a history of hypertension that was currently controlled with medication, or were untreated at study entry and had a cuff seated diastolic BP (seDBP) ≥95 mm Hg and <115 mm Hg. Women were required to be either postmenopausal or surgically sterile. Inclusion in the study also required an electrocardiogram (ECG) without evidence of an arrhythmia requiring treatment and a serum potassium level of ≥3.5 to ≤5 mmol/L.
Exclusion criteria included secondary, severe, labile, or malignant hypertension; New York Heart Association class II‐IV heart failure; coronary artery disease; arrhythmia requiring treatment; severe valvular heart disease; cerebrovascular disease; diabetes mellitus; liver disease; or kidney disease. Patients were also excluded if they were taking systemic vasodilators/vasoconstrictors; α or β blockers for treatment of prostatic hypertrophy; other drugs known to affect BP; antiarrhythmics; systemic glucocorticoids; hormonal replacement; immunosuppressive or cytotoxic drugs; nicotine; fluconazole, itraconazole, or erythromycin; or were regularly using nonsteroidal anti‐inflammatory drugs. Patients with a history of alcohol or substance abuse, allergy or sensitivity to the study drug, or who were receiving medications that could alter the gastrointestinal absorption of the study medication also were excluded. Those patients whose blood pressure was being controlled in prestudy with antihypertensive agents were weaned off the agents during the trial run‐in period.
Study Design
This efficacy and safety evaluation study was a randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐ranging study in 193 patients conducted at 22 centers in Japan. The study design was approved by the appropriate institutional review board and conducted in accordance with the Declaration of Helsinki. In addition, this study also complied with Good Clinical Practice and with the Japan Ministry of Health and Welfare Notifications. During a 2‐week pretreatment screening period, patients provided written informed consent; underwent physical examination, including a 12‐lead ECG, heart rate (HR), BP assessment, and medical history; and supplied specimens for routine hematology, clinical chemistry, and urinalysis. The use of antihypertensive medications was allowed during pretreatment screening, but patients were tapered off these drugs during the run‐in period. Following screening, patients entered the single‐blind, placebo run‐in period. Baseline assessments were performed at the end of the run‐in period. At sites where monitoring facilities were available, 24‐hour ambulatory BP monitoring (ABPM) was conducted at the time of the last dose of placebo.
Patients meeting all criteria, including cuff seDBP ≥95 mm Hg and <115 mm Hg and, when available, mean 24‐hour ABPM DBP of ≥85 mm Hg, were randomized to the 8‐week, double‐blind treatment period and received either placebo or eplerenone doses of 50 mg, 100 mg, or 200 mg once daily. In addition to the assessments at baseline (Week 0), assessments were performed at predefined points throughout the duration of treatment. At every visit (Weeks 2, 4, and 8), monitoring included trough cuff BP, HR, fasting clinical laboratory tests, and assessment of adverse events (AEs). During the screening or placebo run‐in phases, if a patient's DBP was ≥115 mm Hg or SBP ≥190 mm Hg on two consecutive occasions 1–3 days apart, the patient was withdrawn from the study. Following randomization, if a patient's DBP was ≥110 mm Hg or SBP was ≥180 mm Hg on two consecutive occasions 1–3 days apart, the patient was withdrawn from the study. If a patient's serum potassium level was >5.5 mEq/L on two consecutive occasions 1–3 days apart, the patient was withdrawn from the study. Total and active plasma renin and serum aldosterone levels were measured at Weeks 4 and 8 to establish the renin‐angiotensin‐aldosterone system (RAAS) hormone profile. At Week 8, a 12‐lead ECG and a physical examination were performed as well as 24‐hour ABPM (at selected sites).
Efficacy Variables
All efficacy variables were measured as change from baseline to the Week 8 end point. The primary efficacy end point was the mean change in trough cuff seDBP. Secondary efficacy variables included mean change in trough cuff seated systolic BP (seSBP), 24‐hour ABPM, and percent change in RAAS hormone profile. An additional, prespecified efficacy end point was change from baseline in pulse pressure (PP), defined as SBP ‐ DBP.
Safety Variables
Safety was assessed by evaluating the incidence of treatment‐emergent AEs based on results of physical examination, vital signs, 12‐lead ECG, and clinical laboratory events. AEs were rated by severity (mild, moderate, or severe); association with study medication (probable, uncertain, or unrelated—designations that were mapped from a four‐level system of attribution used in Japan to facilitate comparison with non‐Japan studies); and seriousness (serious or nonserious). AEs occurring >7 days after the last dose or serious AEs occurring >28 days after the last dose of the study medication were excluded from analyses.
Statistical Analysis
The sample size of 160 patients (40 per group) was selected to provide 80% power to detect a true mean difference of 5.5 mm Hg between the placebo and 200‐mg eplerenone dose group, assuming a standard deviation of 8 mm Hg for the primary efficacy end point. Efficacy analyses other than ABPM were performed on the intent‐to‐treat population, which included all randomized patients with a baseline BP assessment and at least one postbaseline assessment during the double‐blind dosing period. Efficacy analyses of the ABPM variables were based on the intent‐to‐treat population but included only patients randomized at sites that obtained valid baseline and postbaseline ABPM assessments, the latter performed during the period when the study medication was administered. Of the 192 patients evaluated using cuff BP measurements, 58 patients (13–16 patients per treatment group) from sites equipped to perform ABPM measurements during the study met ABPM criteria for randomization. There was a loss of statistical power in the ABPM analysis due to the small sample size. For each efficacy end point analysis, missing values were input using the method of last postbaseline observation carried forward at all time points. Safety analyses included all patients who took ≥1 dose of study medication.
To avoid potential statistical effects resulting from imbalance in the number of patients among centers, data from centers in which the total enrollment was less than one half that of the largest center were pooled into one or more larger centers. Comparability of groups with respect to baseline and demographic factors were examined using one‐way analysis of variance for continuous variables (e.g., age, height) or the Pearson χ 2 tests for categorical variables (e.g., sex, race).
Adjusted mean changes in BP values were compared at each visit by two‐way analysis of covariance, with baseline measurement as the covariate and treatment and center as factors. Based on the assumption that an increase in the eplerenone daily dose would result in a comparable or better antihypertensive effect (i.e., dose‐related response), a sequential step‐down statistical testing method at the 2.5% level (one‐sided) was used. The resulting p values comparing the various doses to placebo were adjusted for monotonicity. As additional information, each dose was compared with placebo at the 5.0% level (two‐sided), with no monotonicity adjustment. Reported AEs were analyzed using the Fisher exact test.
RESULTS
Demographics and Distribution
A total of 193 patients were randomized to treatment (46–50 patients in each group). The groups were similar at baseline (Table I). More than 86% of patients in each group completed the study; one patient in the eplerenone 50‐mg group had no postdose BP measurement due to withdrawal of informed consent and was therefore excluded from the efficacy analysis.
Table I.
Demographics and Baseline Characteristics
| Characteristic | Placebo (n=50) | Eplerenone 50 mg q.d. (n=49) | Eplerenone 100 mg q.d. (n=46) | Eplerenone 200 mg q.d. (n=48) |
|---|---|---|---|---|
| Gender (n [%]) | ||||
| Female | 16 (32.0) | 18 (36.7) | 14 (30.4) | 13 (27.1) |
| Male | 34 (68.0) | 31 (63.3) | 32 (69.6) | 35 (72.9) |
| Age (years) | ||||
| Mean ± SD | 54.3±10.55 | 54.2±11.30 | 52.8±10.02 | 52.6±10.76 |
| Range | 31–70 | 33–76 | 27–68 | 31–75 |
| Race (n [%]) | ||||
| Asian | 50 (100.0) | 49 (100.0) | 46 (100.0) | 48 (100.0) |
| Body mass index (± SD) | ||||
| Female mean | 23.3±3.5 | 24.1±2.6 | 23.0±3.4 | 25.0±3.3 |
| Male mean | 25.8±3.3 | 25.6±2.9 | 25.0±3.3 | 25.1±2.4 |
| BP (mm Hg ± SD) | ||||
| Mean SBP | 150.5±11.67 | 153.5±13.34 | 156.1±14.25 | 152.8±16.10 |
| Mean DBP | 100.6±5.64 | 100.2±4.59 | 101.7±5.72 | 100.9±5.05 |
| 24‐hour ABPM (mm Hg ± SD) | ||||
| Mean SBP | 152.4±15.92* | 150.0±13.76** | 153.1±14.21*** | 155.1±13.49† |
| Mean DBP | 96.1±6.51* | 92.6±5.16** | 96.4±7.77*** | 97.3±6.34† |
| Mean active plasma renin (mU/L) | 10.1†† | 5.7†† | 6.3ɛ | 8.0ɛɛ |
| *n=13; **n=15; ***n=14; †n=16; ††n=48; ɛn=45; ɛɛn=47 | ||||
Of the 189 patients for whom baseline plasma renin levels were available, 188 patients had low active renin values. The normal range for active plasma renin is 7–76 mU/L and the geometric means for the treatment groups ranged from 5.7 mU/L to 10.1 mU/L (see Table I), with no significant differences among the treatment groups.
Efficacy
All doses of eplerenone reduced seSBP and seDBP from baseline after 8 weeks. Compared with placebo, eplerenone 100 mg and 200 mg significantly reduced trough seDBP from baseline (−6.9 and −7.5 mm Hg vs. −3.0 mm Hg; p≥0.0022). Eplerenone 50 mg showed a greater reduction in seDBP compared with placebo, but the difference did not reach statistical significance (−5.1 mm Hg vs. −3.0 mm Hg; p=0.0584) (Figure 1). All doses of eplerenone (50, 100, and 200 mg) significantly reduced trough seSBP from baseline compared with placebo (−6.8 to −10.6 mm Hg vs. −2.1 mm Hg; p≤0.0245) (Figure 1). The reductions in seSBP and seDBP appeared to be dose dependent.
Figure 1.
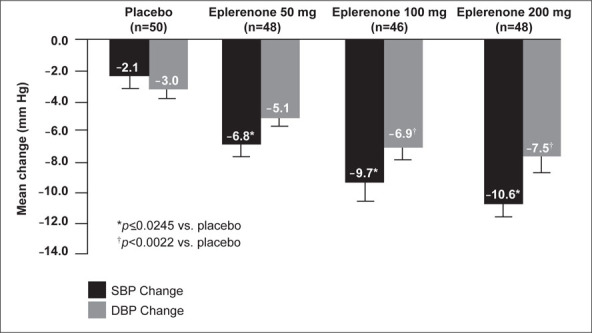
Adjusted mean change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline to Week 8
Of the 192 patients evaluated using cuff BP measurements, 58 patients (13–16 patients in each treatment group) met ABPM criteria for randomization. In this group, mean SBP and DBP increased from baseline in the placebo group. In contrast, eplerenone treatment resulted in a dose‐dependent decrease from baseline (Figure 2). Compared with placebo, eplerenone 200 mg significantly reduced mean 24‐hour DBP and SBP (SBP, −11.9 vs. +2.1 mm Hg; DBP, −5.8 vs. +1.9 mm Hg; p≤0.0009), and eplerenone 100 mg significantly reduced mean 24‐hour DBP (−3.6 vs. +1.9 mm Hg; p≤0.012).
Figure 2.
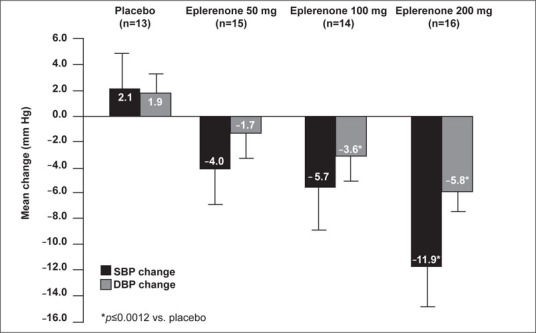
Adjusted mean change in 24‐hour ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline to Week 8
Compared with placebo, the mean change from baseline in PP at Weeks 4 and 8 was significant for the 200‐mg dose (Week 4, −4.4 vs. +0.9 mm Hg; Week 8, −3.2 vs. +0.8 mm Hg; both p≤0.0163) and was significant at Week 4 for the 100‐mg dose (−2.8 vs. 0.9 mm Hg; p=0.0182) (Table II).
Table II.
Adjusted Mean Change in Pulse Pressure* (±SE) From Baseline to Weeks 2, 4, and 8
| Week 2 | Week 4 | Week 8 | |
|---|---|---|---|
| Placebo (n=50) | –2.1±1.23 | 0.9±1.23 | 0.8±1.38 |
| Eplerenone 50 mg q.d. (n=48) | –2.4±1.25 | –2.1±1.24 | –1.4±1.39 |
| Eplerenone 100 mg q.d. (n=46) | –3.2±1.29 | –2.8±1.28† | –2.7±1.44 |
| Eplerenone 200 mg q.d. (n=48) | –4.8±1.24 | –4.4±1.24ɛ | –3.2±1.39ɛ |
| *pulse pressure=systolic blood pressure–diastolic blood pressure; † p=0.0182; ɛ p≤0.0163 | |||
Dose‐dependent increases from baseline in active plasma renin levels were observed across all eplerenone treatment groups (34.9%–80.7%) compared with placebo (14.5%) at the end of the study (Week 8). The difference was statistically significant for the 200‐mg dose (p=0.0068) (Figure 3). Significant increases from baseline in serum aldosterone also were observed across all eplerenone treatment groups (54.6%–78.2%) compared with placebo (10.8%) at the Week 8 end point (p<0.0005).
Figure 3.
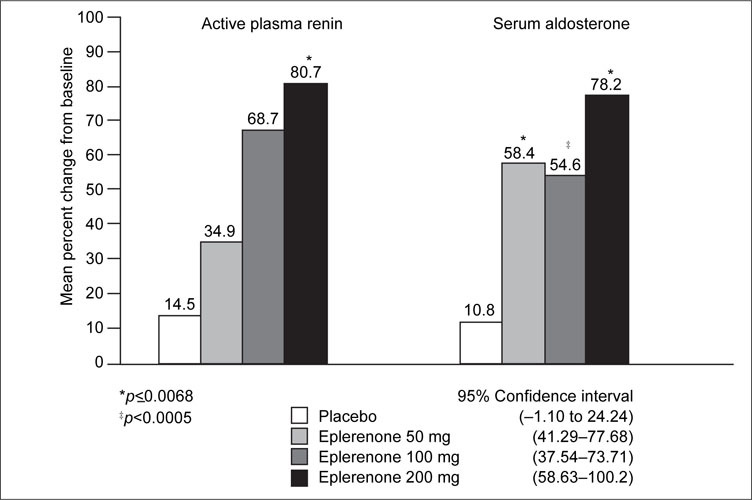
Adjusted mean percent change in renin‐angiotensin‐aldosterone system hormone profile from baseline to Week 8
Safety and Tolerability
Overall treatment‐emergent AEs in all active treatment arms were reported at rates equal to or less than placebo. AEs that were reported by >5% of patients in any group are summarized in Table III. There were no clinically relevant differences between the placebo group and any eplerenone group in the incidence of AEs. However, in the eplerenone groups, 6.1%–6.5% of patients experienced AEs of probable relationship to study medication, compared with 2.0% in the placebo group. No patient experienced a serious AE. Four patients withdrew from the study due to an AE (eplerenone 50 mg, aggravated hypertension; eplerenone 200 mg, increased aspartate transaminase [baseline 30 IU/L, last visit 78 IU/L] and alanine transaminase [baseline 77 IU/L, last visit 124 IU/L]; increased γ‐glutamyl transferase [baseline 30, last visit 129]; and cholelithiasis).
Table III.
Treatment‐Emergent Adverse Events Reported in >5% of Patients in Any Group
| Placebo (n=50) | Eplerenone 50 mg (n=49) | Eplerenone 100 mg (n=46) | Eplerenone 200 mg (n=48) | |||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event | n | % | n | % | n | % | n | % |
| Influenza‐like symptoms | 3 | 6.0 | 4 | 8.2 | 3 | 6.5 | 6 | 12.5 |
| AST increased | 1 | 2.0 | 2 | 4.1 | 0 | 0.0 | 4 | 8.3 |
| ALT increased | 0 | 0.0 | 3 | 6.1 | 0 | 0.0 | 4 | 8.3 |
| Eosinophilia | 0 | 0.0 | 1 | 2.0 | 1 | 2.2 | 4 | 8.3 |
| GGT increased | 0 | 0.0 | 1 | 2.0 | 2 | 4.3 | 3 | 6.3 |
| Back pain | 1 | 2.0 | 1 | 2.0 | 0 | 0.0 | 3 | 6.3 |
| Headache | 6 | 12.0 | 8 | 16.3 | 1 | 2.2 | 2 | 4.2 |
| Hypertriglyceridemia | 3 | 6.0 | 2 | 4.1 | 2 | 4.3 | 2 | 4.2 |
| Hyperuricemia | 1 | 2.0 | 3 | 6.1 | 3 | 6.5 | 2 | 4.2 |
| Pharyngitis | 1 | 2.0 | 2 | 4.1 | 3 | 6.5 | 2 | 4.2 |
| Malaise | 3 | 6.0 | 0 | 0.0 | 1 | 2.2 | 1 | 2.1 |
| Upper RTI | 3 | 6.0 | 2 | 4.1 | 1 | 2.2 | 1 | 2.1 |
| Lymphocytosis | 3 | 6.0 | 1 | 2.0 | 1 | 2.2 | 0 | 0.0 |
| Specific ECG abnormality | 3 | 6.0 | 0 | 0.0 | 1 | 2.2 | 0 | 0.0 |
| Hypertension aggravated | 3 | 6.0 | 2 | 4.1 | 0 | 0.0 | 0 | 0.0 |
| Peripheral pain | 3 | 6.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| AST=aspartate transaminase; ALT=alanine transaminase; GGT=γ‐glutamyl transferase; RTI=respiratory tract infection; ECG=electrocardiogram | ||||||||
There were no significant ECG or HR changes associated with the study medication, nor were there any clinically significant changes in laboratory tests other than noted above or in physical examinations. The safety assessment also included events of special interest related to agents that act on the RAAS. Importantly, no patients met the predefined criteria for hyperkalemia (>5.5 mmol/L on two consecutive measures, 1–3 days apart). There were no reports of gynecomastia or erectile dysfunction, or of female breast pain. There was one report of menstrual abnormality unrelated to study medication in the eplerenone 50‐mg group. Nine patients (one placebo, three eplerenone 50 mg, three eplerenone 100 mg, and two eplerenone 200 mg) had asymptomatic hyperuricemia; however, no patients had uric acid levels that met the laboratory criteria for an event of special interest (baseline less than or equal to upper limit of normal and >594.8 μmol/L on any occasion or baseline greater than upper limit of normal and >1.5 times baseline on any occasion).
DISCUSSION
This study demonstrates that the safety and efficacy of the selective aldosterone blocker eplerenone in Japanese patients with hypertension are similar to those observed in non‐Japanese patients. Notably, although the populations had different baseline characteristics, particularly with regard to renin status, salt intake, and race, the results of this study are remarkably comparable to that of the previous study of similar design. 15 The safety and efficacy of eplerenone for the treatment of essential hypertension has been demonstrated in clinical trials that included more than 3000 patients worldwide in a variety of patient populations. 14 , 15 , 16 , 17 , 18 , 19 , 26 , 27 , 28 Similar dose‐dependent efficacy and good safety profiles were observed in this study. The finding that all but one of the patients who completed the study had low‐renin hypertension was unexpected. However, the results of this study further support the use of eplerenone as one option in these patients who frequently do not respond well to ACE inhibitors and ARBs.
Dose‐dependent reductions in the primary end point of mean change from baseline of trough cuff DBP were observed at Week 8. The differences were significant at the 100‐mg and 200‐mg doses; at the 50‐mg dose, DBP decreased from baseline, although the change was not statistically significant. The significant reductions in seSBP, the secondary end point, achieved at all three once‐daily doses of eplerenone (50, 100, and 200 mg) are consistent with a previous dose‐ranging study demonstrating that eplerenone is effective in isolated systolic hypertension. 19 Consistent with earlier findings, this report demonstrated that eplerenone 200 mg was more effective than placebo in lowering PP at the Week 8 end point. The earlier efficacy trial of eplerenone conducted in 269 older patients with essential hypertension found that eplerenone was as effective as amlodipine in reducing PP. 19
Japanese patients with hypertension appear to have circadian changes in blood pressure similar to those seen in other groups worldwide. Therefore, loss of efficacy at the end of the dosing interval is a potential concern among this population as well. In the Ohasama Study, 29 more than 25% of patients treated for hypertension were reported to have high morning blood pressure that was attributed to insufficient duration of antihypertensive medications. Although the number of individuals assessed for 24‐hour blood pressure was small (15–16 per treatment group) in our study, eplerenone treatment achieved significant reductions in mean 24‐hour DBP using once‐daily doses within the typical dosing range; 100 mg and 200 mg once daily significantly decreased the mean 24‐hour DBP. At the once‐daily 200‐mg dose, reduction in mean 24‐hour SBP was incrementally greater than that observed at 50‐mg or 100‐mg doses, and reductions were significantly greater compared with placebo. Furthermore, trends for daytime and nighttime ABPMs were identical (data not shown). Similar results were attained in two non‐Japan eplerenone dose‐ranging studies that used ABPM. 15 , 28
In the studied dose range, treatment with eplerenone significantly increased serum aldosterone levels above baseline, indicating blockade by eplerenone at the mineralocorticoid receptor. This increase in aldosterone levels supports the mechanism of action of the drug.
Many patients with low‐renin hypertension have evidence of significant mineralocorticoid excess. 30 , 31 , 32 Unexpectedly, patients in this study had relatively low active plasma renin levels at baseline (geometric mean ranged from 5.7 mU/L to 10.1 mU/L in the different treatment groups). ACE inhibitors and ARBs are not particularly effective in patients with low‐renin hypertension. 18 , 33 In a previous study, eplerenone monotherapy was significantly more effective than losartan monotherapy in reducing mean DBP and SBP in patients with confirmed low‐renin hypertension (active plasma renin ≤42.4 mU/L). 18 Therefore, one might have expected the consistent efficacy profile of eplerenone in the population of this study as well.
Eplerenone was generally safe and well tolerated; no serious or severe AEs were attributable to the study drug. No patients met the predefined criteria for hyperkalemia or reported events of special interest related to neurohormonal activity or progestational or androgenic antagonism leading to sexual side effects such as those that often occur with spironolactone therapy. There were no disturbances of cardiac rate and rhythm, ECG abnormalities, adverse laboratory results other than changes in enzymes in four patients on the highest dose, or changes in vital signs or physical examination. This suggests that monitoring for liver enzyme changes should be part of follow‐up examinations.
CONCLUSIONS
Eplerenone is the first antihypertensive agent to become available that selectively blocks aldosterone at the mineralocorticoid receptor. The findings reported here contribute to the expanding evidence that eplerenone treatment reduces DBP, SBP, and PP, even in patients with low baseline active renin levels. ABPM results also support the use of once‐daily dosing with eplerenone. Eplerenone has been shown to be an efficacious and well tolerated agent and should be considered as one of the options for the management of patients with essential hypertension.
Disclosure:
This research was supported by a grant from Pfizer Inc.
References
- 1. MacFadyen RJ, Lee AF, Morton JJ, et al. How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart. 1999;82:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKelvie R, Yusuf S, Pericak D, et al. Comparison of candesartan, enalApril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation. 1999;100:1056–1064. [DOI] [PubMed] [Google Scholar]
- 3. Swedberg K, Eneroth P, Kjekshus J, et al., for the CONSENSUS Trial Study Group. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation. 1990;82:1730–1736. [DOI] [PubMed] [Google Scholar]
- 4. Stier CT Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev. 2002;10:97–107. [DOI] [PubMed] [Google Scholar]
- 5. Frierdich G, Schuh J, Brown M, et al. Effects of the selective mineralocorticoid receptor antagonist, eplerenone, in a model of aldosterone‐induced hypertension and cardiac fibrosis [abstract B004]. Am J Hypertens. 1998;11:94A. [Google Scholar]
- 6. Muller J. Spironolactone in the management of congestive heart failure: a review. Clin Ther. 1986;9:63–76. [PubMed] [Google Scholar]
- 7. Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res. 1992;26:671–677. [DOI] [PubMed] [Google Scholar]
- 8. Rocha R, Chander PN, Khanna K, et al. Mineralocorticoid blockade reduces vascular injury in stroke‐prone hypertensive rats. Hypertension. 1998;31(1 pt 2):451–458. [DOI] [PubMed] [Google Scholar]
- 9. Rocha R, Chandler PN, Zuckerman A, et al. Mineralocorticoid antagonism reduces Ang II‐induced renal injury in stroke‐prone hypertensive rats [abstract B001]. Am J Hypertens. 1998;11:94A. [DOI] [PubMed] [Google Scholar]
- 10. Rocha R, Stier CT Jr, Kifor I, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. [DOI] [PubMed] [Google Scholar]
- 11. Young M, Head G, Funder J. Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol. 1995;269(4 pt 1):E657–E662. [DOI] [PubMed] [Google Scholar]
- 12. Funder JW. Eplerenone, a new mineralocorticoid antagonist: in vitro and in vivo studies. Curr Opin Endocrinol Diabet. 2000;7:138–142. [Google Scholar]
- 13. De Gasparo M, Joss U, Ramjoue HP, et al. Three new epoxy‐spironolactone derivatives: characterization in vivo and vitro. J Pharmacol Exp Ther. 1987;240:650–656. [PubMed] [Google Scholar]
- 14. Flack JM, Oparil S, Pratt JH, et al. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J Am Coll Cardiol. 2003;41:1148–1155. [DOI] [PubMed] [Google Scholar]
- 15. Weinberger MH, Roniker B, Krause SL, et al. Eplerenone, a selective aldosterone blocker, in mild‐to‐moderate hypertension. Am J Hypertens. 2002;15:709–716. [DOI] [PubMed] [Google Scholar]
- 16. Krum H, Nolly H, Workman D, et al. Efficacy of eplerenone added to renin‐angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–123. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalApril, and eplerenone/enalApril in patients with essential hypertension and left ventricular hypertrophy. The 4E‐Left Ventricular Hypertrophy Study. Circulation. 2003;108(15)1831–1838. [DOI] [PubMed] [Google Scholar]
- 18. Weinberger M, MacDonald TM, Conlin PR, et al. Comparison of eplerenone and losartan in patients with low‐renin hypertension [abstract]. Am J Hypertens. 2002;15:24A. 11824855 [Google Scholar]
- 19. White WB, Duprez D, St Hillaire R, et al. Effects of the selective aldosterone blocker eplerenone versus the calcium channel antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021–1026. [DOI] [PubMed] [Google Scholar]
- 20. Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. [DOI] [PubMed] [Google Scholar]
- 21. Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. [DOI] [PubMed] [Google Scholar]
- 22. Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 23. Schmidt BM, Schmieder RE. Aldosterone‐induced cardiac damage: focus on blood pressure independent effects. Am J Hypertens. 2003;16:80–86. [DOI] [PubMed] [Google Scholar]
- 24. Rocha R, Chander PN, Zuckerman A, et al. Role of aldosterone in renal vascular injury in stroke‐prone hypertensive rats. Hypertension. 1999;33(1 pt 2):232–237. [DOI] [PubMed] [Google Scholar]
- 25. Chan JCN, Leung WYS, Critchley JAJH. Drug responsiveness and side effects: lessons from Chinese populations. In: Epstein M, ed. Calcium Antagonists in Clinical Medicine. 3rd ed. Philadelphia , PA : Hamley and Belfus; 2002;447–468. [Google Scholar]
- 26. Inspra [prescribing information]. Chicago , IL : GD Searle, LLC; 2002. [Google Scholar]
- 27. Epstein M, Buckalew V, Martinez F, et al. Antiproteinuric efficacy of eplerenone, enalApril, and eplerenone/enalApril combination in diabetic hypertensives with microalbuminuria[abstract]. Am J Hypertens. 2002;15(4 suppl 1):24A. 11824855 [Google Scholar]
- 28. White WB, Carr AA, Krause S, et al. Assessment of the novel selective aldosterone blocker eplerenone using ambulatory and clinical blood pressure in patients with systemic hypertension. Am J Cardiol. 2003;92:38–42. [DOI] [PubMed] [Google Scholar]
- 29. Chonan K, Hashimoto J, Ohkubo T, et al. Insufficient duration of action of antihypertensive drugs mediates high blood pressure in the morning in hypertensive population: the Ohasama study. Clin Exp Hypertens. 2002;24:261–275. [DOI] [PubMed] [Google Scholar]
- 30. Komiya I, Yamada T, Aizawa T, et al. Inappropriate elevation of the aldosterone/plasma renin activity ratio in hypertensive patients with increases of 11‐deoxycorticosterone and 18‐hydroxy‐11‐deoxycortisterone: a subtype of essential hypertension? Cardiology. 1991;78:99–110. [DOI] [PubMed] [Google Scholar]
- 31. Griffing GT, Dale SL, Holbrook MM, et al. The regulation of urinary free 19‐nor‐deoxycorticosterone and its relation to systemic arterial blood pressure in normotensive and hypertensive subjects. J Clin Endocrinol Metab. 1983;56:99–103. [DOI] [PubMed] [Google Scholar]
- 32. Laragh JH, Sealey J, Brunner HR. The control of aldosterone secretion in normal and hypertensive man: abnormal renin‐aldosterone patterns in low renin hypertension. Am J Med. 1972;53:649–663. [DOI] [PubMed] [Google Scholar]
- 33. Preston RA, Materson BJ, Reda DJ, et al. Age‐race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. JAMA. 1998;280:1168–1172. [DOI] [PubMed] [Google Scholar]


