Abstract
Alteration of autonomic nervous system regulation is known to be present in the persistent vegetative state after traumatic brain injury, termed the dysautonomic syndrome. This study assessed the circadian blood pressure and heart rate pattern and variability in the persistent vegetative state through noninvasive 24‐hour ambulatory blood pressure monitoring. The study was performed in 20 subjects: 10 patients (six men and four women; mean age, 29.5±9.9 years; range, 19–39 years) in a vegetative state (mean, 27.3±5.6 days after trauma) and 10 healthy subjects as controls (six men and four women; mean age, 28±5.7 years; range, 29–37 years). The patients showed a blood pressure nondipper pattern; 24‐hour, daytime, and nighttime values of blood pressure and heart rate were significantly higher in patients than in controls. The day‐night difference in heart rate and blood pressure was also significantly lower in patients. Finally, SD and variation coefficients were significantly lower in patients. The results show changes in the variability and circadian blood pressure and heart rate patterns in persistent vegetative state patients with dysautonomic syndrome, as an expression of the sympathetic‐parasympathetic activity imbalance in the control of vasomotor tone.
In normotensive subjects, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) are characterized by a circadian pattern, with higher values of blood pressure (BP) and HR during the daytime and lower pressure and bradycardia during the nighttime. 1 An important physiologic mechanism behind this circadian BP and HR pattern is the day–night variation in the activity of the autonomic nervous system (ANS), which is under the influence of various intrinsic and extrinsic factors. 2 , 3 BP and HR variability is modulated by both intrinsic cyclic vasomotion and the impact of physical and mental activity 4 and is inversely related to its primary determinant, baroreceptor reflex sensitivity. 5 The role of the ANS in the generation of the BP and HR circadian pattern is also shown when ANS function is affected. In particular, ANS dysfunction is present in patients in a persistent vegetative state after traumatic brain injury. 6 In these patients, autonomic function is worsened by prolonged bed rest and neurosensorial deafferentation, leading to changes including tachycardia, tachypnea, paroxysmal increases in temperature and BP, increased muscle tone, profuse sweating, and decerebrate or decorticate posturing. This syndrome has been termed the autonomic dysfunction syndrome, 7 brainstem attack, 8 or autonomic or sympathetic storming, 9 but the most common term is dysautonomia. 10 Dysautonomia seems to be a distinct syndrome associated with a poorer outcome in patients after traumatic brain injury.
The literature concerning dysautonomic syndrome and traumatic brain injury consists of a small series of case reports. Previous studies have not documented well the natural history, physiopathologic alterations, or outcomes in patients with dysautonomia. A previous report suggests that dysautonomic syndrome can be divided into three phases. 6 In the first phase, dysautonomia is clinically silent in patients with traumatic brain injury; generally it is revealed in the first week after trauma. During the second phase there are the main physiopathologic alterations known as the dysautonomic syndrome, with an average duration of 74 days after injury. In the third phase, dystonia and spasticity of varying severity are seen.
The aim of our study has been to analyze the circadian SBP, DBP, and HR changes and variability in a group of patients in a persistent vegetative state after traumatic brain injury who presented in the second phase of the dysautonomic syndrome.
METHODS
Subjects We evaluated the circadian SBP, DBP, and HR pattern in 20 subjects, 10 patients (six men and four women; mean age, 29.5±9.9 years; range, 19–39 years) who were admitted to our hospital in a persistent vegetative state after traumatic brain injury due to car accidents (mean, 27.3±5.6 days after trauma), compared with 10 healthy normotensive subjects as a control group (six men and four women; mean age, 28±5.7 years; range, 29–37 years). All patients were subjected to a careful clinical history and physical examination. Patients were excluded from the study if they had any major disease and/or clinical manifestations of cardiac or vascular disease.
The patients included in our study were breathing spontaneously and did not have bulbopontine lesions evaluated through computed tomography or magnetic resonance imaging. Evidence supporting the diagnosis of diffuse axonal injuries included intraventricular hemorrhage, diffuse cerebral edema, and multiple subcortical white matter or brainstem hemorrhages. The hypothalamic and encephalic trunk functions were preserved. The dysautonomic syndrome diagnosis was made by two experienced neurologists, defined as simultaneous, paroxysmal increases in at least five out of the seven reported features of dysautonomia (tachycardia, tachypnea, increases in temperature and BP, posturing, dystonia, and sweating), with episodes persisting for at least 2 weeks after injury. 6 In our patients, no history of hypertension was present and the renal function and plasma electrolytes were normal. The clinical conditions and the levels of activity (vigilance, cognitive ability, functional autonomy) were evaluated through the Disability Rating Scale (DRS) 11 and the severity of head trauma was evaluated by Glasgow Coma Scale (GCS) 12 (Table I). Because hospital protocol was to discontinue medications on admission, patients taking drugs could be included as long as our recordings were made at least 24 hours after the last dose.
Table 1.
Characteristics of Patients and Control Group
| Parameters | Patients (Mean+ SD) | Controls (Mean+ SD) |
|---|---|---|
| Number | 10 | 10 |
| Days after trauma | 27.3+5.6 | NA |
| Age (yr) | 29.5+9.9 | 28+5.7 |
| Male/female ratio | 6/4 | 6/4 |
| Glasgow Coma Scale | 4.4+0.7 | NA |
| Disability Rating Scale | 25.5+0.7 | NA |
| Body mass index (kg/m2) | 23.6+2.1 | 22.6+0.3 |
| NA=not applicable | ||
Ambulatory BP and HR Monitoring All 20 subjects underwent two consecutive ambulatory BP monitoring (ABPM) sessions of at least 24 hours using an automatic auscultatory device, Takeda TM2420 (A&D Medical, Tokyo, Japan), and the average of these measurements was recorded. BP and HR were monitored every 15 minutes between 7 a.m. and 10 p.m. and every 30 minutes during the night. 13 The SBP, DBP, and HR averages were also recorded over 24 hours, with daytime and nighttime values being recorded according to the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. 14 We accepted recordings as valid when the rate of artifacts was <15% and the recording lasted for 20 hours continuously. The circadian rhythm was evaluated by the differences of the mean SBP, DBP, and HR during daytime and nighttime intervals. The SD and the variation coefficient (VC) of the mean values (SD/mean × 100) were taken, respectively, as measures of absolute and normalized short‐term variability of the signals.
Data Analyses Data are expressed as mean ± SD and as the mean of the VC. Statistical comparisons between BP and HR measurements were made with a paired two‐tailed t test, and the mean difference between the measurements compared. A value of p<0.05 was considered to indicate statistical significance. We used the statistical program Microsoft Excel Win Office XP (Microsoft Corporation, Redmond, WA) for the purpose of statistical data analyses.
RESULTS
Clinical characteristics of the patients and controls are given in Table I. As Table II shows, the patients had 24‐hour and nighttime values of SBP significantly higher than the controls, while daytime SBP values was higher in the patients; this difference was not significant. A significant difference in the 24‐hour, daytime, and nighttime values of DBP between control group and patients was observed. The 24‐hour, daytime, and nighttime values of HR were significantly higher in patients than in the controls. Patients did not show a physiologic nocturnal SBP and DBP decrease, which defines these subjects as “nondippers” (Figure 1).
Table 2.
Values of 24‐Hour, Daytime, and Nighttime Ambulatory SBP, DBP, and HR in Patients and Controls
| Parameters | Patients | Controls | p Value |
|---|---|---|---|
| 24‐hour | |||
| SBP, mean (mm Hg) | 125.8 | 114.3 | <0.01 |
| SBP, SD (mm Hg) | 9.9 | 16.8 | <0.01 |
| DBP, mean (mm Hg) | 81.4 | 69.8 | <0.01 |
| DBP, SD (mm Hg) | 10.4 | 12.8 | NS |
| HR, mean (bpm) | 90.0 | 78.5 | <0.01 |
| HR, SD (bpm) | 8.8 | 14.9 | <0.01 |
| Daytime | |||
| SBP, mean (mm Hg) | 124.3 | 118.4 | NS |
| SBP, SD (mm Hg) | 10.6 | 15.4 | <0.01 |
| DBP, mean (mm Hg) | 82.9 | 72.8 | <0.01 |
| DBP, SD (mm Hg) | 8.5 | 12.3 | <0.03 |
| HR, mean (bpm) | 89.3 | 82.6 | <0.05 |
| HR, SD (bpm) | 7.5 | 13.8 | <0.01 |
| Nighttime | |||
| SBP, mean (mm Hg) | 129.0 | 102.8 | 0.01 |
| SBP, SD (mm Hg) | 9.9 | 11.4 | NS |
| DBP, mean (mm Hg) | 81.5 | 61.0 | <0.01 |
| DBP, SD (mm Hg) | 7.3 | 9.4 | <0.03 |
| HR, mean (bpm) | 87.8 | 66.8 | <0.01 |
| HR, SD (bpm) | 5.0 | 10.7 | <0.01 |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; HR=heart rate; NS=nonsignificant | |||
Figure 1.
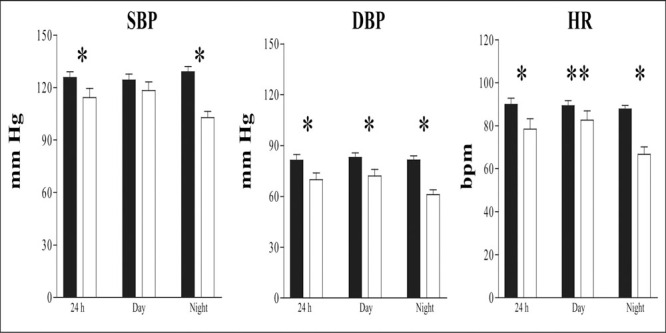
Twenty‐four hour, daytime, and nighttime mean ± SD values for systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) in 20 subjects. Solid bars indicate patients; open bars indicate controls. *p<0.01; **p<0.05
For patients, the SD was significantly lower for SBP 24‐hour and daytime intervals, but was similar for the nighttime period. The SD of 24‐hour DBP values between patients and controls did not differ. The SD for HR was also significantly lower for the patients than for the controls (Figure 2, top). Twenty‐four‐hour, daytime, and nighttime VC were significantly lower in the patients (Figure 2, bottom).
Figure 2.
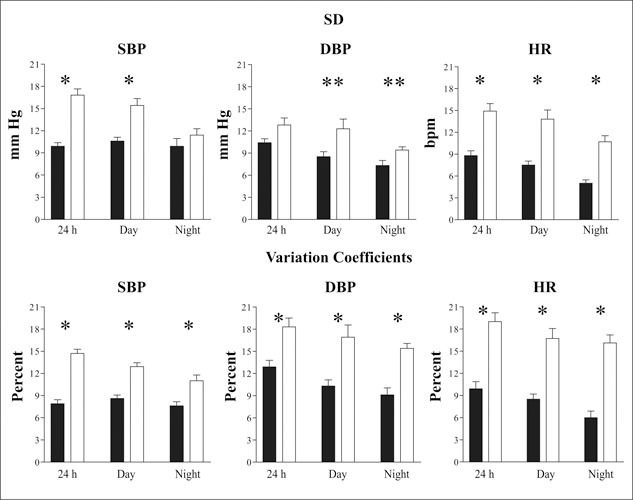
SDs and variation coefficients of 24‐hour, daytime, and nighttime mean ± SD values for systolic blood pressure (SBP), diastolic blood pressure (DBF), and heart rate (HR) in 20 subjects. Solid bars indicate patients; open bars indicate controls. *p<0.01; **p<0.03
Finally,Figure 3 shows circadian rhythm data. Day‐night differences of SBP, DBP, and HR were significantly lower in patients.
Figure 3.
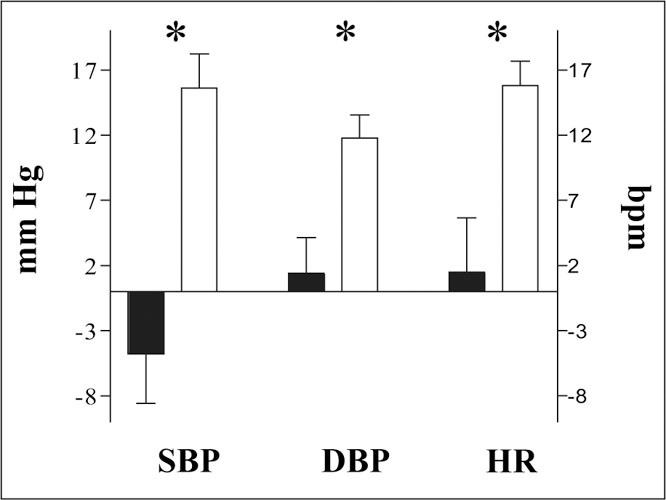
Day‐night differences in systolic blood pressure (SBP), diastolic blood pressure (DBF), and heart rate (HR) in 20 subjects. Solid bars indicate patients; open bars indicate controls. *p<0.01
DISCUSSION
This study shows that the dysautonomic syndrome after traumatic brain injury is associated with an alteration in the circadian BP and HR pattern. The 24‐hour ABPM patients' analysis shows a pattern without a nocturnal BP fall >10%. This nondipper phenomenon is associated with an increased occurrence of cardiovascular events and a higher incidence of end‐organ damage in hypertensive subjects. 15 Although the mechanisms responsible for a nocturnal decrease of BP are not fully understood, withdrawal of sympathetic activity seems to play a pivotal role; the circadian pattern of catecholamines is similar to that of BP. 16 Lack or attenuation of a circadian pattern in BP and HR has been reported in secondary forms of hypertension associated with abnormal sympathetic nervous activities 17 and autonomic nervous failure, 18 further indicating that an autonomic nervous abnormality is associated with the nondipper phenomenon. Previous reports have shown that an advanced impairment of the ANS is responsible for the absence of the nocturnal BP fall. 19 , 20 , 21 , 22 This is also noted in vascular dementia, 23 Alzheimer‐type dementia, 24 cerebral atrophy, 25 cerebrovascular disease, 26 , 27 , 28 , 29 , 30 and ischemic arterial disease after carotid endarterectomy. 31
One might speculate that the balance between sympathetic and parasympathetic activity in the control of vasomotor tone could be shifted toward a vagal decrease with sympathetic predominance during the nighttime hours. This hypothesis is supported by the evidence of a higher HR in patients. This could reflect a decrease in cardiac vagal tone, leading to a predominance of the chronotropic sympathetic tone. An increase in HR has also been observed in the early phase of the autonomic neuropathy of diabetes mellitus. 32 We speculated that in the persistent vegetative state after traumatic brain injury, as in diabetes mellitus, parasympathetic impairment could precede the development of sympathetic dysfunction.
We recorded a very low variability in SBP and DBP (expressed as SD and VC) in our patients. Several studies have shown that end‐organ damage and cardiovascular complications are more closely associated and more severe in subjects with higher 24‐hour BP variability, expressed as mean of the SD and as VC. 33 , 34 , 35 , 36 In a previous report, Kohara and coworkers 37 showed that autonomic dysfunction may be involved in the increased cardiovascular events in nondipper essential hypertensive subjects. In patients, lower BP variability could play a protective role against cardiovascular complications, apparently in contrast with circadian rhythm data. We assumed that the nondipper pattern in patients with persistent vegetative state after traumatic brain injury and dysautonomic syndrome may have a different significance than in hypertensive patients. In our patients, the circadian rhythm was characterized by a lower parasympathetic tone and a predominance of sympathetic tone; this new balance between sympathetic and parasympathetic activity is expressed as a nondipper pattern associated with lower BP and HR variability.
Our study had several limitations. The study involved a small number of patients and did not evaluate the plasma catecholamine concentrations. Future directions for research should be focused on comparing ABPM patterns of severe‐outcome traumatic brain injury patients with ABPM patterns of better‐outcome traumatic brain injury patients to evaluate whether the alteration in circadian BP and HR pattern might represent a prognostic marker.
CONCLUSIONS
Our study shows an altered circadian BP and HR pattern in patients in a persistent vegetative state after traumatic brain injury, with higher SBP, DBP, and HR values and lower variability when compared with healthy subjects. In persistent vegetative patients after traumatic brain injury, ABPM can study the changes in the circadian BP and HR pattern and could represent a useful adjunct to the commonly used tests for the quantification, pharmacotherapy, and rehabilitation management of patients in a persistent vegetative state with dysautonomic syndrome.
Acknowledgment: This study was possible thanks to the cooperation of the Centro di Riabilitazione per Traumatizzati Cranici Cardinal Ferrari, Fontanellato PR, Italy.
References
- 1. Richardson DW, Honour AJ, Fenton GW, et al. Variation in arterial pressure throughout the day and night. Clin Sci. 1964;26:445–460. [PubMed] [Google Scholar]
- 2. Tuck ML, Stern N, Sowers JR. Enhanced 24‐hour norepinephrine and renin secretion in young patients with essential hypertension: relation with the circadian pattern of arterial blood pressure. Am J Cardiol. 1985;55:112–115. [DOI] [PubMed] [Google Scholar]
- 3. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha‐sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. [DOI] [PubMed] [Google Scholar]
- 4. Pickering TG. Variability of blood pressure. Blood Press Monit. 1998;3:141–145. [PubMed] [Google Scholar]
- 5. Floras JS, Hassan MO, Jones JV, et al. Factors influencing blood pressure and heart rate variability in hypertensive humans. Hypertension. 1988;11:273–281. [DOI] [PubMed] [Google Scholar]
- 6. Baguley IJ, Nicholls JL, Felmingham KL, et al. Dysautonomia after traumatic brain injury: a forgotten syndrome? J Neurol Neurosurg Psychiatry. 1999;67:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossitch E, Bullard DE. The autonomic dysfunction syndrome: aetiology and treatment. Br J Neurosurg. 1988;2:471–478. [DOI] [PubMed] [Google Scholar]
- 8. Scott JS, Ockey RR, Holmes GE, et al. Autonomic dysfunction associated with locked‐in syndrome in a child. Am J Phys Med Rehabil. 1997;76:200–203. [DOI] [PubMed] [Google Scholar]
- 9. Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boeve BF, Wijdicks EF, Benarroch EE, et al. Paroxysmal sympathetic storms (diencephalic seizures) after severe diffuse axonal head injury. Mayo Clin Proc. 1998;73:148–152. [DOI] [PubMed] [Google Scholar]
- 11. Rappaport M, Hall KM, Hopkins K, et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 12. Teasdale G, Jennet B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. [DOI] [PubMed] [Google Scholar]
- 13. Di Rienzo M, Grassi G, Pedotti A, et al. Continuous vs intermittent blood pressure measurements in estimating 24‐hour average blood pressure. Hypertension. 1983;5:264–269. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens. 1995;13:1377–1390. [PubMed] [Google Scholar]
- 15. Mancia G, Parati G. The role of blood pressure variability in end‐organ damage. J Hypertens Suppl. 2003;21:S17–S23. [DOI] [PubMed] [Google Scholar]
- 16. Imai Y, Abe K, Miura Y, et al. Hypertensive episodes and circadian fluctuations of blood pressure in patients with phaeochromocytoma: studies by long‐term blood pressure monitoring based on a volume‐oscillometric method. J Hypertens. 1988;6:9–15. [PubMed] [Google Scholar]
- 17. Padfield PL, Stewart MJ. Ambulatory blood pressure monitoring in secondary hypertension. J Hypertens Suppl. 1991;9:S69–S71. [PubMed] [Google Scholar]
- 18. Mann S, Altman DG, Raftery EB, et al. Circadian variation of blood pressure in autonomic failure. Circulation. 1983;68:477–483. [DOI] [PubMed] [Google Scholar]
- 19. Reeves RA, Shapiro AP, Thompson ME, et al. Loss of nocturnal decline in blood pressure after cardiac transplantation. Circulation. 1986;73:401–408. [DOI] [PubMed] [Google Scholar]
- 20. Schalekamp MA. Man in't Veld AJ, Wenting GJ. The second Sir George Pickering memorial lecture. What regulates whole body autoregulation? Clinical observations. J Hypertens. 1985;3:97–108. [DOI] [PubMed] [Google Scholar]
- 21. Franklin SS, Sowers JR, Batzdorf U. Relationship between arterial blood pressure and plasma norepinephrine levels in a patient with neurogenic hypertension. Am J Med. 1986;81:1105–1107. [DOI] [PubMed] [Google Scholar]
- 22. Carvalho MJ, van Den Meiracker AH, Boomsma F, et al. Diurnal blood pressure variation in progressive autonomic failure. Hypertension. 2000;35:892–897. [DOI] [PubMed] [Google Scholar]
- 23. Tohgi H, Chiba K, Kimura M. Twenty‐four‐hour variation of blood pressure in vascular dementia of the Binswanger type. Stroke. 1991;22:603–608. [DOI] [PubMed] [Google Scholar]
- 24. Otsuka A, Mikami H, Katahira K, et al. Absence of nocturnal fall in blood pressure in elderly persons with Alzheimer‐type dementia. J Am Geriatr Soc. 1990;38:973–978. [DOI] [PubMed] [Google Scholar]
- 25. Tominaga M, Tsuchihashi T, Kinoshita H, et al. Disparate circadian variations of blood pressure and body temperature in bedridden elderly patients with cerebral atrophy. Am J Hypertens. 1995;8:773–781. [DOI] [PubMed] [Google Scholar]
- 26. Stoica E, Enulescu O. Inability to deactivate sympathetic nervous system in brainstem infarct patients. J Neurol Sci. 1983;58:223–234. [DOI] [PubMed] [Google Scholar]
- 27. Shimada K, Kawamoto A, Matsubayashi K, et al. Silent cerebrovascular disease in the elderly. Correlation with ambulatory blood pressure. Hypertension. 1990;16:692–699. [DOI] [PubMed] [Google Scholar]
- 28. Shimada K, Kawamoto A, Matsubayashi K, et al. Diurnal blood pressure variation and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 29. Matsumura K, Abe I, Fukuhara M, et al. Attenuation of nocturnal BP fall in essential hypertensives with cerebral infarction. J Hum Hypertens. 1993;7:309–310. [PubMed] [Google Scholar]
- 30. Sander D, Klingelhofer J. Changes of circadian blood pressure patterns after hemodynamic and thromboembolic brain infarction. Stroke. 1994;25:1730–1737. [DOI] [PubMed] [Google Scholar]
- 31. Asmar RG, Julia PL, Mascarel VL, et al. Ambulatory blood pressure profile after carotid endarterectomy in patients with ischaemic arterial disease. J Hypertens. 1994;12:697–702. [PubMed] [Google Scholar]
- 32. Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years' experience in diabetes. Diabetes Care. 1985;8:491–498. [DOI] [PubMed] [Google Scholar]
- 33. Parati G, Pomidossi G, Albini F, et al. Relationship of 24‐hour blood pressure mean and variability to severity of target‐organ damage in hypertension. J Hypertens. 1987;5:93–98. [DOI] [PubMed] [Google Scholar]
- 34. Frattola A, Parati G, Cuspidi C, et al. Prognostic value of 24‐hour blood pressure variability. J Hypertens. 1993;11:1133–1137. [DOI] [PubMed] [Google Scholar]
- 35. Palatini P, Penzo M, Racioppa A, et al. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med. 1992;152:1855–1860. [PubMed] [Google Scholar]
- 36. Dawson SL, Manktelow BN, Robinson TG, et al. Which parameters of beat‐to‐beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke. 2000;31:463–468. [DOI] [PubMed] [Google Scholar]
- 37. Kohara K, Nishida W, Maguchi M, et al. Autonomic nervous function in non‐dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26:808–814. [DOI] [PubMed] [Google Scholar]


