Abstract
In elderly patients with systolic hypertension resistant to treatment with conventional therapy, increased aortic pulse wave reflection and a high augmentation index are often present. These findings are indicative of endothelial dysfunction and deficient generation of nitric oxide, a potent vasodilator in the arterial tree. In such patients, treatment with the nitric oxide donor extended‐release isosorbide mononitrate characteristically produces prompt and sustained falls in both pulse wave reflection and systolic blood pressure. The adjunct use of this nitrate produces useful additional decreases in systolic blood pressure ranging from 10 to 45 mm Hg, often achieving target blood pressure goals in isolated systolic hypertension. By combining this endothelium‐independent nitric oxide donor with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, the potential exists to address both the nitric oxide deficiency and endothelial dysfunction of the vascular endothelium in these patients. Other possibilities for synergism with this combination include complementary hemodynamic, circadian, and metabolic actions together with prevention of nitrate tolerance. Isosorbide mononitrate may also be used successfully with calcium channel blockers, β blockers, and diuretics.
Systolic hypertension is a risk factor for stroke and heart attack in elderly patients. With the trend for increased longevity among the general population, this form of hypertension has become an important public health issue. The incidence of hypertension in people over the age of 60 years has been estimated as >60%. 1 Isolated systolic hypertension (ISH), which accounts for 65% of the overall incidence of uncontrolled hypertension, 2 is a prevalent form of systolic hypertension in the elderly. Also, systolo‐diastolic hypertension may lead to ISH when the diastolic blood pressure (DBP) responds to therapy but the systolic blood pressure (SBP) does not.
ISH may be resistant to conventional antihypertensive agents. Effects of combination antihypertensive therapy on SBP in older hypertensive patients have been reported in a number of placebo‐controlled, randomized trials. 3 , 4 , 5 , 6 , 7 , 8 These trials have shown significant differences between active treatment and placebo groups for both DBP and SBP values; however, the average SBP reached with long‐term treatment in the active groups was usually above the currently recommended target of 140 mm Hg. In the European Working Party on High Blood Pressure in the Elderly (EWPHE) trial, for example, the average value (±SD) reached at 5 years was 150±20 mm Hg (based on 108 patients). 3 In the Systolic Hypertension in the Elderly Program (SHEP) the corresponding value at 5 years was 155±21 mm Hg (based on 773 patients). 4 In the Swedish Trial in Old Patients with Hypertension (STOP‐Hypertension) the average SBP at 2 years was 166±20 mm Hg (based on 385 patients). 5 In the Systolic Hypertension in Europe (Syst‐Eur) trial at 2 years, SBP was 151 mm Hg. 6 In both SHEP and The Second Australian National Blood Pressure Study (ANBP2) there was a failure to achieve target SBP in approximately 30% of patients. 4 , 7 In Syst‐Eur the corresponding failure rate was 56.5%. 6 It must be kept in mind that these statistics underestimate the true incidence of treatment‐resistant hypertension, because exclusion criteria would have kept some severely hypertensive patients from the trials concerned.
PATHOPHYSIOLOGY OF ISH
Increased Pulse Pressure: Role of Wave Reflection
The high SBP of ISH is characterized by widened arterial pulse pressure, a change that results from the effects of age and age‐associated cardiovascular disease on the arterial tree. 9 With advancing age, there is small artery constriction that boosts the reflected component of the pulse wave. 10 Additionally, there is large artery stiffening that increases the velocity of transmission of the reflected wave so that it moves from diastole to late systole, thereby increasing SBP. 10 The aortic pulse waveform can be quantified by percutaneous applanation tonometry of the radial or carotid artery, and the magnitude of the reflected component may be expressed as the augmentation index (a measure of the increase in pulse pressure caused by wave reflection [Figure 1]). Augmentation by pulse wave reflection is characteristically increased in treatment‐refractory ISH, being responsible for up to 40% of the pulse pressure in this condition. 11
Figure.
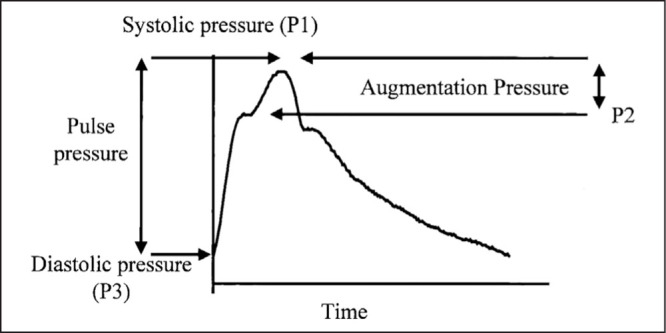
A representative central arterial waveform with pulse pressure and augmentation pressure indicated. In this study, augmentation index (dimensionless) was reported as 100×(P1–P3)/(P2–P3). P2 indicates the first systolic peak and P1 the second (in this case P1 is the systolic pressure).
Adapted with permission from Dart AM, Reid CM, McGrath B, et al. Effects of ACE inhibitor therapy on derived central arterial waveforms in hypertension. Am J Hypertens. 2001;14(8 pt 1):804–810.
Augmentation Index Is Related to Endothelial Dysfunction
There is considerable evidence that increase in the augmentation index denotes deficient vasodilative endogenous nitric oxide (NO) generation associated with endothelial dysfunction. 12 , 13 Endothelial dysfunction is found with aging, 14 chronic hypertension, 15 atherosclerosis, 16 diabetes, 17 long‐term cigarette smoking, 18 and obstructive sleep apnea. 19 Likewise, decreased vascular NO production has been reported in chronic hypertension, diabetes, and obstructive sleep apnea. 19 , 20 , 21 In normal arteries, fluid shear stress and pulsatile stretch are responsible for the release of NO and other autacoids, 22 which maintain the constrictor and dilator functions of the vessel wall. 23 , 24 We have suggested that the increase in pulse wave reflection in ISH results from impairment of these functions and particularly from a loss of NO‐dependent arterial vasodilatation. 25 , 26
Implications for Choice of Therapy
Not all patients with ISH have a high augmentation index. White coat hypertension, characterized by a discrepancy between office blood pressure (BP) readings and automated ambulatory or home BP recordings, is more prevalent in the elderly. 27 Tachycardia, cutaneous flush, and other features of a high output state are sometimes present. In such patients, the primary or ejection peak of the arterial pulse waveform is increased rather than the wave reflection, and the augmentation index may be normal. The prognosis and the role of pharmacotherapy in this group are controversial. Conversely, finding a raised augmentation index in patients with ISH has important implications for their prognosis and for selection of an appropriate drug regimen. Such patients frequently have other risk factors for cardiovascular disease, such as hyperlipidemia or diabetes mellitus, and a history of vasculopathy. Augmentation index is strongly correlated with cardiovascular risk. 28 Indeed, ISH with a high augmentation index may be considered a final common pathway to which vascular disease due to age, hypertension, atherosclerosis, or diabetes all lead. Whatever the primary etiology, the presence of endothelial dysfunction tends to increase pulse wave reflection, and thereby pulse pressure and SBP.
THERAPEUTIC MANAGEMENT OF ISH
General Approach
This review will not attempt to provide a comprehensive account of the clinical management of ISH, which has been dealt with recently in subsections of the seventh report of the Joint National Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the 2003 European Society of Hypertension‐European Society of Cardiology guidelines. 29 , 30 Current management is based on evidence from large‐scale clinical trials, which showed that reduction of SBP to <140 mm Hg (and reduction of DBP to <90 mm Hg) decreases the incidence of cardiovascular morbid events in elderly hypertensive subjects up to the 9th decade of life. 31 Thus, 140 mm Hg should be the general therapeutic goal for SBP in ISH, with a lower goal of 130 mm Hg being set for patients with diabetes mellitus or renal disease. In the elderly, the SBP goal is more difficult to reach than the DBP goal and commonly requires combination therapy employing two or more drug classes in addition to lifestyle measures.
The following drug classes, given singly or in combinations, have been shown to be effective for long‐term BP reduction: diuretics (thiazide or thiazide‐like), β blockers, angiotensin II inhibitors (angiotensin‐converting enzyme [ACE] inhibitors or angiotensin II receptor blockers), and calcium channel blockers. For many patient groups (possibly excluding some with diabetes, renal disease, or heart failure) there is no proven rationale for preferring one drug class over another, and the most important criterion is the ability of the regimen chosen to achieve goal BP with minimal side effects. However, some findings suggest that diuretics and calcium channel blockers may have a greater effect in the elderly. Patients who do not reach goal BP based on office readings may require automated or home BP recording to exclude the possibility of a white coat effect. The preferred arbiter of effective BP control (although not yet recommended for general use) is 24‐hour ambulatory monitoring showing acceptable daytime and nighttime average BP values with an absence of sustained peaks. The path forward for managing patients who fail to reach goal despite trials of various combinations of conventional antihypertensive drugs is the subject of the remainder of this review.
MANAGEMENT OF PATIENTS WITH REFRACTORY ISH
Diagnostic Considerations
In patients with ISH who fail to reach goal BP after a thorough trial of appropriate therapy, a second look at the diagnosis is advisable even if routine assessment for underlying causes was undertaken earlier. Tests for renal or renovascular disease, primary hyperaldosteronism, and obstructive sleep apnea may be required in selected cases. Additionally, noninvasive evaluation of arterial stiffness may have a role in therapeutic design for elderly individuals with difficult‐to‐manage hypertension. This approach, advocated by an expert committee of the European Society of Hypertension, 32 but not by some other national guidelines committees, incurs expense and may not be justified for routine clinical assessment. The evaluation, which includes determination of central augmentation index, can be performed by one of several commercially available tonometric devices that record pulse wave contour. Based on a comparison of pulse wave reflectance between normotensive, normocholesterolemic elderly volunteers and vasculopathic hypertensive patients, 11 it is suggested that values for aortic augmentation index >120% should be regarded provisionally as abnormal.
Systolic hypertension associated with a raised augmentation index may prove refractory to antihypertensive agents. Some antihypertensive agents (such as diuretics and negative inotropes) reduce the ejection component of the pulse pressure by decreasing cardiac stroke volume without reducing pulse wave reflection. Others have the capacity to decrease wave reflection by restoring impaired vascular NO generation but may take a long time to produce this effect fully. For example, it has been shown that ACE inhibitors may take up to 3 years to reverse impaired acetylcholine‐responsive vasorelaxation in essential hypertensives. 33 , 34 , 35 With such agents some continued elevation of augmentation index and SBP may be expected unless adjunctive treatment is given with a direct, endothelium‐independent vasodilator. A nitrate (or an NO donor) is a logical choice. 36
Role of Extended‐Release Nitrate
Patients with ISH found to have an inadequate response to conventional combination antihypertensive treatment and a raised augmentation index have responded well to adjunctive therapy with extended‐release nitrates. 26 , 37 , 38 Characteristically, within 1–3 hours of administration of a single 60 mg dose of isosorbide mononitrate (ISMN), SBP fell by 10–45 mm Hg and augmentation index decreased by 50% of baseline value. The change in augmentation index exceeded corresponding values found for captopril and eprosartan in placebo‐controlled comparative studies against the nitrate and was greater than values reported in the literature for various ACE inhibitors, angiotensin II receptor blockers, diuretics, and β blockers. 26 In a placebo‐controlled study in which ambulatory BP was measured during short‐term withdrawal of chronic once‐daily administration of ISMN, we showed that the antihypertensive effect of a regular morning dose lasted 10–12 hours. 37 BP remained controlled without cumulative or rebound effects during continued once‐daily dosing. 37 , 39
Role of Angiotensin II Inhibitors: Separate and in Combination With ISMN
Short‐term treatment with moderate‐to‐high doses of ACE inhibitors or angiotensin II receptor blockers has been associated with relatively minor decreases in augmentation index 26 ; however, these drugs are known to have beneficial effects on NO bioavailability. 40 Also, they can improve endothelium‐dependent vasodilatation with long‐term use 33 , 41 and can repair resistance artery structure. 42 , 43 , 44 Moreover, the vasodepressor actions of ISMN and angiotensin II inhibitors in systolic hypertension of the elderly appear to be additive. In acute studies, we have shown that effects of ISMN on SBP and augmentation index were undiminished by therapy at baseline with angiotensin II inhibitors. 26 , 38 In an acute study, the effect of ISMN on SBP was shown to be augmented by coadministration of L‐arginine (a substrate for endogenous NO formation) if angiotensin II inhibitors were not also used. 38 However, if angiotensin II inhibitors were used, L‐arginine had no effect on the BP response to ISMN in the presence of either acute 38 or chronic 45 ISMN therapy. This could indicate that angiotensin II inhibition augmented the vasodilator effect of ISMN through a mechanism similar to that exerted by L‐arginine, perhaps involving limitation of superoxide production. 46
It is not known whether tolerance to nitrates, long recognized as a problem in the treatment of coronary vascular disease with nitrates, 46 can complicate nitrate use for systolic hypertension. Follow‐up studies of 14 patients with ISH who were treated for up to 9 years with an extended‐release formulation of ISMN as an adjunct to conventional antihypertensive therapy suggested that there was no major loss of effect with the elapse of time (G.S.S., unpublished data, 2004). The finding may have resulted from once‐daily dosing. When used for the relief of angina pectoris, the extended‐release formulation has been reported to cause tolerance if given twice daily, but not if given once daily. 47 Another factor that could have prevented tolerance development was the coadministration of ACE inhibitors, known to counter nitrate tolerance in cardiac patients. 48 More data from prospective, long‐term studies of nitrates in ISH are needed to determine whether nitrate tolerance develops and if so, whether it can be modified by appropriate dosing schedules or cotherapies.
The Possibility of Utilizing Additive/Synergistic Actions Between ISMN and Angiotensin II Inhibitors in Therapy
Potential additive/synergistic antihypertensive effects exist with respect to combined therapy with ISMN and angiotensin II inhibitors in an appropriately selected elderly patient population. The principal basis of the combined action is improvement of endothelium‐dependent NO production by angiotensin II inhibitors together with endothelium‐independent NO donation by ISMN. Another consideration is that ISMN has selective hemodynamic effects different from angiotensin II inhibitors. ISMN produces a greater fall in SBP than in DBP and a greater decrease in the reflection component of the pulse wave than in the ejection component. 26 Also, ISMN produces its effect principally on vascular tone, whereas angiotensin II inhibitors have cardiac, renal, and metabolic activity independent of antihypertensive activity. Finally, angiotensin II inhibitors are known to have an antihypertensive effect overnight 49 and thus should cover the period 12–24 hours after dosing with ISMN (given once daily to avoid tolerance).
Further research is necessary to determine whether the long‐term process of restoring vascular NO generation by treatment with angiotensin II inhibitors might gradually reduce the capacity for NO donors to lower the augmentation index in these patients. If this were so, the resulting diminution in nitrate effectiveness over time would need to be distinguished from true nitrate tolerance.
Combination Therapy With ISMN and Other Antihypertensive Agents
ISMN has been shown to produce an additive antihypertensive effect when combined with calcium channel blockers, β blockers, or diuretics. 11 , 26 , 37 , 38 Some grounds exist also for expecting synergistic activity between these agents and nitrates. Thus, calcium channel blockade has been shown to restore endothelium‐dependent vasodilation in essential hypertension 50 and to enhance the effects of an ACE inhibitor on endogenous NO production. 51 Beta blockade may prevent any tendency for ISMN‐induced cardioacceleration. Diuretics may oppose nitrate‐induced plasma volume expansion, thought at one stage to be a counter‐regulatory mechanism in nitrate tolerance. 52
Introduction of Adjunct Therapy With ISMN
A starting dose of 30–60 mg (as an isosorbide mononitrate extended‐release formulation) is recommended to be given once daily in the morning. This may be increased rapidly to 120 mg once daily if required to reach target SBP. If the patient has a history of headaches, the 30‐mg starting dose should be used in conjunction with oral paracetamol 1 g. If headache occurs, it often abates after the first few doses. The dosage can usually be increased to 120 mg daily without reappearance of headache. Apart from headache, which is reported in about 10% of patients, side effects are rare. Although orthostatic hypotension from nitrates may be encountered in patients with low cardiac output states, it has not occurred in our ambulant study population.
An important contraindication to the introduction of nitrates in this, as in other clinical settings, is the concurrent use of sildenafil for erectile dysfunction.
In summary, there is evidence to indicate that the use of once‐daily, long‐acting nitrate preparations have a role as adjunct therapy in achieving target SBP for ISH patients. It is suggested that such nitrate therapy should be undertaken in ISH patients who have failed to achieve target SBP after an adequate trial of combined therapy with conventional antihypertensive agents.
References
- 1. Weber MA. Introduction: hypertension in the elderly. Am J Geriatr Cardiol. 2000;9:11. [Google Scholar]
- 2. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives. Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 3. Amery A, Birkenhager W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet. 1985;1:1349–1354. [DOI] [PubMed] [Google Scholar]
- 4. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 5. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP‐Hypertension). Lancet. 1991;338:1281–1285. [DOI] [PubMed] [Google Scholar]
- 6. Staessen JA, Fagard R, Thijs L, et al. Randomised, double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 7. Reid CM, Ryan P, Wing LMH. The 2nd Australian National Blood Pressure Study (ANBP2). In: Black HR, ed. Clinical Trials in Hypertension. New York , NY : Marcel Dekker. 2001;587–604. [Google Scholar]
- 8. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouyrie P, Benetos A. Pathophysiology of hypertension in the elderly. Am J Geriatr Cardiol. 2002;11:34–39. [DOI] [PubMed] [Google Scholar]
- 10. Nichols WW, O'Rourke MF, Avolio AP, et al. Effects of age on ventricular‐vascular coupling. Am J Cardiol. 1985;55:1179–1184. [DOI] [PubMed] [Google Scholar]
- 11. Stokes GS, Ryan M. Can extended‐release isosorbide mononitrate be used as adjunctive therapy for systolic hypertension? An open study employing pulse‐wave analysis to determine effects of antihypertensive therapy. Am J Geriatr Cardiol. 1997;6:11–19. [PubMed] [Google Scholar]
- 12. Wilkinson IB, Hall IR, MacCallum H, et al. Pulse‐wave analysis. Clinical evaluation of a non‐invasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–152. [DOI] [PubMed] [Google Scholar]
- 13. Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens. 1999;17(suppl 5):S41–S44. [PubMed] [Google Scholar]
- 14. Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on changing arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities. Circulation. 1985;71:202–210. [DOI] [PubMed] [Google Scholar]
- 15. Panza JA, Quyyumi AA, Brush JE, et al. Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 16. Heistad DD, Armstrong ML, Baumbach GL, et al. Sick vessel syndrome. Recovery of atherosclerotic and hypertensive vessels. Hypertension. 1995;26:509–513. [DOI] [PubMed] [Google Scholar]
- 17. Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium‐dependent vasodilation in patients with insulin‐dependent diabetes mellitus. Circulation. 1993;88:2510–2516. [DOI] [PubMed] [Google Scholar]
- 18. McVeigh GE, Morgan DJ, Finkelstein SM, et al. Vascular abnormalities associated with long‐term cigarette smoking identified by arterial waveform analysis. Am J Med. 1997;102:227–231. [DOI] [PubMed] [Google Scholar]
- 19. Ip MSM, Lam B, Chan L‐Y, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. [DOI] [PubMed] [Google Scholar]
- 20. Forte P, Copland M, Smith LM, et al. Basal nitric oxide synthesis in essential hypertension. Lancet. 1997;349:837–842. [DOI] [PubMed] [Google Scholar]
- 21. Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. [DOI] [PubMed] [Google Scholar]
- 22. Archer SL, Gragasin FS, Wu X, et al. Endothelium‐derived hyperpolarizing factor in human internal mammary artery is 11,12‐epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK (Ca) channels. Circulation. 2003;107:769–776. [DOI] [PubMed] [Google Scholar]
- 23. Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium‐derived relaxing factors. J Vasc Res. 1998;35:73–84. [DOI] [PubMed] [Google Scholar]
- 24. Macha M, Yamasaki K, Gordon LM, et al. The vasoregulatory role of endothelium derived nitric oxide during pulsatile cardiopulmonary bypass. ASAIO J. 1996;42: M800–M804. [DOI] [PubMed] [Google Scholar]
- 25. Stokes GS. A dynamic role of NO in the small artery conduits of the systemic circulation. Physiol Res. 2002;51:57P. [Google Scholar]
- 26. Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41:297–301. [DOI] [PubMed] [Google Scholar]
- 27. Staessen JA, O'Brien ET, Atkins N, et al. Short report: ambulatory blood pressure in normotensive compared with hypertensive subjects. J Hypertens. 1993;11:1289–1297. [PubMed] [Google Scholar]
- 28. Nurnberger J, Keflioglu‐Scheiber A, Saez AMO, et al. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. [DOI] [PubMed] [Google Scholar]
- 29. The seventh report of the Joint National Committee on Prevention , Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 30. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hyperten. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 31. Gueyffier F, Bulpitt C, Boissel JP, et al. Antihypertensive drugs in very old people: a subgroup analysis of randomised clinical trials. INDANA Group. Lancet. 1999;353:793–796. [DOI] [PubMed] [Google Scholar]
- 32. Safar ME, London GM. Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. J Hypertens. 2000;18:1527–1535. [DOI] [PubMed] [Google Scholar]
- 33. Taddei S, Virdis A, Ghiadoni L, et al. Effects of angiotensin‐converting enzyme inhibition on endothelial‐dependent vasodilatation in essential hypertensive patients. J Hypertens. 1998;16:447–456. [DOI] [PubMed] [Google Scholar]
- 34. Schiffrin EL, Deng Li‐Y. Comparison of effects of angiotensin I‐converting enzyme inhibition and beta‐blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703. [DOI] [PubMed] [Google Scholar]
- 35. Rizzoni D, Muiesan ML, Porteri E, et al. Effects of long‐term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ventricular hypertrophy. J Hypertens. 1997;15:197–204. [DOI] [PubMed] [Google Scholar]
- 36. Safar ME. Antihypertensive effects of nitrates in chronic human hypertension. J Appl Cardiol. 1990;5:69–81. [Google Scholar]
- 37. Stokes GS, Ryan M, Brnabic A, et al. A controlled study of the effects of isosorbide mononitrate on arterial blood pressure and pulse wave form in systolic hypertension. J Hypertens. 1999;17:1767–1773. [DOI] [PubMed] [Google Scholar]
- 38. Stokes GS, Barin ES, Gilfillan KL, et al. Interactions of L‐arginine, isosorbide mononitrate and angiotensin II inhibition on arterial pulse wave. Am J Hypertens. 2003;16:719–724. [DOI] [PubMed] [Google Scholar]
- 39. Stokes GS, Barin E, Gilfillan K. Superiority of hypotensive effect of nitrate over that of angiotensin inhibition in patients with refractory systolic hypertension. Am J Hypertens. 2001;14:119A. [Google Scholar]
- 40. Brosnan MJ, Hamilton CA, Graham D, et al. Irbesartan lowers superoxide levels and increases nitric oxide bioavailability in blood vessels from spontaneously hypertensive stroke‐prone rats. J Hypertens. 2002;20:281–286. [DOI] [PubMed] [Google Scholar]
- 41. Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery function. Hypertension. 2003;41:1281–1286. [DOI] [PubMed] [Google Scholar]
- 42. Chen R, Iwai M, Wu L, et al. Important role of nitric oxide in the effect of angiotensin‐converting enzyme inhibitor imidApril on vascular injury. Hypertension. 2003;42:542–547. [DOI] [PubMed] [Google Scholar]
- 43. Kiowski W, Linder L, Nuesch R, et al. Effect of cilazopril on vascular structure and function in essential hypertension. Hypertension. 1996;27:371–376. [DOI] [PubMed] [Google Scholar]
- 44. Schiffrin EL, Park JB, Pu Q. Effect of crossing over hypertensive patients from a beta‐blocker to an angiotensin receptor antagonist on resistance artery structure and on endothelial function. J Hypertens. 2002;20:71–78. [DOI] [PubMed] [Google Scholar]
- 45. Stokes GS, Kaesemeyer WH, Smith G. Can L‐arginine act as an adjunct to nitrate in therapy for systolic hypertension? Am J Hypertens. 2003;16:198A. [Google Scholar]
- 46. Parker JO, Parker JD, Caldwell RW, et al. The effect of supplemental L‐arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39:1199–1203. [DOI] [PubMed] [Google Scholar]
- 47. Nordlander R, Walter M, for the Swedish Multicentre Group . Once‐ versus twice‐daily administration of controlled‐release isosorbide‐5‐mononitrate 60 mg in the treatment of stable angina pectoris. A randomized, double‐blind, crossover study. Eur Heart J. 1994;15:108–113. [DOI] [PubMed] [Google Scholar]
- 48. Stork T, Eichstadt H, Mockel M, et al. Hemodynamic action of captopril in coronary patients with heart failure tolerant to nitroglycerin. Clin Cardiol. 1997;20:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan TO, Anderson A. Different drug classes have variable effects on blood pressure depending on the time of day. Am J Hypertens. 2003;16:46–50. [DOI] [PubMed] [Google Scholar]
- 50. Taddei S, Virdis A, Gwhiadoni L, et al. Lacidipine restores endothelium‐dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30:1606–1612. [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Xu X, Nasjletti A, et al. Amlodipine enhances NO production induced by an ACE inhibitor through a kinin‐mediated mechanism in canine coronary microvessels. J Cardiovasc Pharmacol. 2000;35:195–202. [DOI] [PubMed] [Google Scholar]
- 52. Gori T, Parker JD. Nitrate tolerance. A unifying hypothesis. Circulation. 2002;106:2510–2513. [DOI] [PubMed] [Google Scholar]


