Abstract
Background
Advances in diagnostic and therapeutic strategies in oncology have significantly increased the chance of survival of cancer patients, even those with metastatic disease. However, cancer-related cognitive impairment (CRCI) is frequently reported in patients treated for non-central nervous system cancers, particularly during and after chemotherapy.
Design
This review provides an update of the state of the art based on PubMed searches between 2012 and March 2019 on ‘cognition’, ‘cancer’, ‘antineoplastic agents’ or ‘chemotherapy’. It includes the most recent clinical, imaging and pre-clinical data and reports management strategies of CRCI.
Results
Evidence obtained primarily from studies on breast cancer patients highlight memory, processing speed, attention and executive functions as the most cognitive domains impaired post-chemotherapy. Recent investigations established that other cancer treatments, such as hormone therapies and targeted therapies, can also induce cognitive deficits. Knowledge regarding predisposing factors, biological markers or brain functions associated with CRCI has improved. Factors such as age and genetic polymorphisms of apolipoprotein E, catechol-O-methyltransferase and BDNF may predispose individuals to a higher risk of cognitive impairment. Poor performance on neuropsychological tests were associated with volume reduction in grey matter, less connectivity and activation after chemotherapy. In animals, hippocampus-based memory and executive functions, mediated by the frontal lobes, were shown to be particularly susceptible to the effects of chemotherapy. It involves altered neurogenesis, mitochondrial dysfunction or brain cytokine response. An important next step is to identify strategies for managing cognitive difficulties, with primary studies to assess cognitive training and physical exercise regimens.
Conclusions
CRCI is not limited to chemotherapy. A multidisciplinary approach has improved our knowledge of the complex mechanisms involved. Nowadays, studies evaluating cognitive rehabilitation programmes are encouraged to help patients cope with cognitive difficulties and improve quality of life during and after cancer.
Keywords: cancer-related cognitive impairment, cancer treatments, cancer patients, animal model, neuro-imaging, managementof cognitive impairment
Key Message
Recent findings highlight that not only chemotherapy, but also the new generation of medical cancer therapies, may affect cognitive functions in cancer patients. A multidisciplinary approach has to been developed to study pathophysiological mechanisms and potential predisposing factors. Guidelines regarding appropriate strategies for managing cognitive difficulties of cancer patients are needed.
Alt-text: Unlabelled Box
Introduction
Patients with non-central nervous system (CNS) cancers report cognitive symptoms, also called ‘cancer-related cognitive impairment’ (CRCI), mainly studied after chemotherapy, such as impairment of short-term and working memory, attention, executive functions and/or processing speed [1., 2., 3., 4.]. Cognitive complaints are reported by ≥50% of breast cancer patients after chemotherapy; however, only 15%–25% have objective cognitive decline [2, 5]. The relation between objective and subjective troubles is still debated and complaints are often linked with psychological factors [6]. State-of-the-art updates were published in 2011 and 2015 by the International Cancer and Cognition Task Force (ICCTF) [1, 4], primarily focussing on neuropsychological tests and clinical data with chemotherapy. Since then, a growing body of literature has highlighted the potential effects of other cancer treatments and pathophysiological mechanisms.
New generations of hormone therapies, targeted therapies, and immunotherapy have resulted in improved survival rates for some patients with, however, potential impact on cognition [7, 8]. Consequently, the long-term toxic impact of treatment on neurological function is an important issue in terms of quality of life (QoL).
Despite recent large cohort studies utilizing neuropsychological testing and brain imaging in cancer patients treated mainly with chemotherapy, it remains uncertain whether cognitive deficits result from the treatment, the cancer itself, and/or psychological factors. Moreover, studies have suggested that factors such as age, genetic polymorphisms, and psycho-social components may predispose to a higher risk of cognitive impairment.
To better understand the pathophysiology of CRCI and the direct impact of different cancer treatments, animal models have been developed [9]. Animal studies allow for the investigation of selective and combined effects of the disease and treatment on neurocognitive function, the influence of parameters such as stress, mood and aging on cognitive impairment, and for the development of rehabilitation strategies. Brain imaging can also help document mechanisms involved in CRCI, as shown by recent studies [3, 10, 11].
As cognitive difficulties have a negative impact on QoL (autonomy, return to work, social relationships, and self-confidence) in the context of long-term cancer care, there is a growing demand from patients for CRCI management. This has led to studies implementing cognitive rehabilitation in cancer patients.
This review presents an update on CRCI in non-CNS cancers, taking into consideration the increasing use of newer anticancer therapies and the development of multidisciplinary (pre-clinical, imaging) and interventional (management) research strategies.
Search strategy and selection criteria
References are from searches of PubMed between 2012 and March 2019. The terms ‘cognition’ [MeSH Terms] or ‘cognition disorders’ [MeSH Terms], ‘cancer’, ‘antineoplastic agents’ [MeSH Terms], or ‘chemotherapy’ were used. Keywords including ‘cancer’ and ‘brain’ or ‘cerebral’ or ‘central nervous system’ and ‘cognition’ and ‘animal’ or ‘mouse’ or ‘rat’ were used for animal models. We also searched reference lists of identified articles for other relevant reports. Articles related to CNS cancers, childhood cancers, editorials, reviews (except systematic reviews and meta-analyses, and the four recent reviews of the ICCTF), feasibility, and pilot studies or studies with less than 50 patients were excluded. The final reference list was generated based on relevance to the topics covered in this review.
Detecting CRCI in cancer patients
Patient cognitive complaints are clinically important, but neuropsychological testing provides objective assessments of various domains of cognition. The literature indicates that there is considerable variability regarding the severity, duration, or alteration of cognitive domains. The ICCTF [4] recommends using the following criteria for determining cognitive impairment: ≥2 test scores ≤−1.5 standard deviations from the normative mean (or an appropriate control group) or one test score ≤−2.0 standard deviations. Importantly, the more tests administered, the higher the probability of finding cognitive impairment. Ingraham and Aiken [12] provide probability curves and a statistical approach to determine whether the observed frequency of cognitive impairment exceeds the expected rate based upon the number of tests administered.
Cognitive complaints are usually assessed with PROs such as FACT-Cog (time frame: 5 min), especially developed to assess cognitive complaints in cancer patients. To help clinicians and researchers to detect significant complaints, minimal clinically important difference (MCID) can be used [13, 14]. It could be also used as a screening method to assess cognitive difficulties before any further assessments and treatment strategy.
Cognitive complaints and performance on neuropsychological tests often do not correlate very highly [15]; survivors often report cognitive problems but score in the normal range on neuropsychological testing. This pattern is often attributed to psychological factors such as anxiety, depression [15, 16], fatigue, or insomnia [17] that influence perceived cognitive problems to a greater degree than performance on objective testing [18] enhancing the importance of assessing these factors [16]. Imaging studies also suggest that survivors employ compensatory activation of additional brain regions to maintain performance on neuropsychological tests [19, 20]. Therefore, survivors’ perception may be correct: cognitive functioning is affected in day-to-day life, but compensatory mechanisms maintains performance in the structured, distraction-free environment of neuropsychological testing. Finally, this lack of association may be related to concerns about the sensitivity and specificity of traditional neuropsychological tests to detect the relatively subtle cognitive changes experienced by cancer survivors.
The failure to use of a criterion as described above, heterogeneity of study design and lack of consistency of cognitive measures utilized has led to variability in results and interpretations across studies [4, 21, 22]. ICTTF recommend using cognitive tests with adequate sensitivity to assess the cognitive domains most impaired by cancer treatments (Table 1).
Table 1.
Neuropsychological measures recommended by the international cancer and cognition task force (ICCTF) [4]
| Main measures | Administration time (min) | Domains assessed |
|---|---|---|
| Hopkins Verbal Learning Test-Revised (HVLT-R) | 10 | Verbal memory and delayed recall |
| Controlled Oral Word Association Test (COWA) | 5 | Speeded lexical fluency and executive function |
| Trail Making Test (TMT) | 7 | Psychomotor speed and executive function |
| Additional measures | Domains assessed | |
| Auditory Consonant Trigrams | 7 | Working memory,a executive function, complex attention |
| Letter-Number Sequencing (WAIS) | 4 | |
| Paced Auditory Serial Addition Test (PASAT) | 15 | |
| Brief test of attention | 10 |
WAIS, Wechsler Adult Intelligence Scale.
Investigators are encouraged to supplement the core battery with additional tests of working memory capacity, based on their own preferences.
Effect of cancer treatments on cognitive functions
Effect of chemotherapy
Clinical studies
Several studies, mainly in breast cancer but also colorectal, ovarian cancer, and lymphoma, show the impact of chemotherapy on objective cognitive functioning (Table 2) [23, 25, 26]. According to a meta-analysis, CRCI may be related to the duration of treatment with chemotherapy [22]. These cognitive impairments are usually mild to moderate and are often transient. Indeed, a longitudinal study showed a significant cognitive decrease shortly after chemotherapy in breast cancer patients followed by partial recovery 1 year after this treatment in some patients [23].
Table 2.
Cognitive impairment induced by chemotherapy in patients
| Study | Study design Country | Patients, control group Age | Cognitive assessment | Main CT type (% of patients) | Cognitive measures | Other measures | Statistical analysis | Main outcome |
|---|---|---|---|---|---|---|---|---|
| Objective cognition (neuropsychological tests) | ||||||||
| Collins et al. [23] |
|
Breast cancer (n=56, 52 years old ±7.8) versus HC (n=56) | Before, shortly and 1 year post-CT | FEC-T: 70% | Digit-symbol coding, symbol search, TMT, digit span, letter-number sequencing, PASAT, Auditory consonant trigrams, COWA, BVMT, HVLT and CNS-Vital signs tests | BDI, POMS | Multilevel growth modelling (controlled for age, education, baseline BDI score) and SRB scores |
|
| Vardy et al. [24] |
|
Colorectal cancer with CT (n=173, 57 years old [23–75]), without (n=116), or metastatic (n=73) versus HC (n=72) | Before and 6, 12 and 24 months after CT |
|
Standard cognitive tests, CANTAB, six elements test | FACT-Cog, FACT-G, FACT-F, GHQ | Linear mixed models |
|
| Wouters et al. [25] |
|
Lymphoma (n=106, 47 years old ±12.6) versus HC (n=53) | After CT (median months since completion: 54.5) | CHOP/MOPP-ABV : 76% | Stroop, verbal fluency, digit symbol substitution, TMT, WMS visual memory, verbal learning and memory test, finger tapping, Eriksen task | HSCL, EORTC QLQ-C30 | Regressions analysis and Ingraham and Aiken probability curves | Patients: cognitive impairment in 16% (with lower education and pre-morbid IQ) |
| Hess et al. [26] | LongitudinalUSA | Ovarian cancer (n=231, 40–79 years old) | Before CT, before cycle 4, after cycle 6, and 6 months after completion of primary CT | NK | HeadMinder Clinical Research Tool (processing speed, motor reaction time, attention) | PAF, FACT-O, FACT-Ntx, HADS | Cognitive impairment: ≥2 cognitive domain impaired at each time based on reliable cognitive index | At cycle 4: 25% of patients had cognitive impairment in at least one domain, 21% and 18% at cycle 6 and 6 months post-CT, respectively |
| Cognitive complaints (self-report questionnaire) | ||||||||
| Ganz et al. [27] |
|
Breast cancer (n=189, 52 years old) | Before HT and after CT±RT | Anthracycline: 25% | PAOFI (cognitive complaints), CVLT, WMS, BVMT, ROCF, digit symbol, TMT, Stroop, letter-number sequencing, grooved pegboard |
|
Multivariable regression models (age and IQ controlled) | 1/5 patients had higher memory and/or executive complaints related to CT+RT |
| Amidi et al. [28] |
|
Breast cancer after systemic treatment (n=1503, 63 years old ±8.2) versus without systemic treatment (n=386) | 7–9 year follow-up after primary surgery | Mainly CEF and CMF | CFQ | None | ANOVAs and ANCOVA (menopausal status, sociodemographic and clinical covariates) | No significant difference between the two groups |
| Janelsins et al. [29] |
|
Breast cancer with CT (n=581, 53 years old ± 0.4) versus HC (n=364) | Before and after CT and at 6 months | Anthracycline: 48% | FACT-Cog | WRAT, STAI | Linear mixed model (age, education, race, menopausal status, reading ability, anxiety and depression controlled) | Patients: significant increase of cognitive complaints after CT (45.2 versus 10.4%) and 6 months after CT (36.5% versus 13.6%) |
| Ng et al. [17] |
|
Breast cancer (n=166, 51 years old ± 9.2) | Before CT, 6, 12 and 15 months post-CT initiation | Anthracycline: 65% | FACT-Cog, Headminder | BAI, BFI, EORTC QLQ-C30 | Linear mixed model (age, anxiety, fatigue, CT regimen, insomnia, menopausal status, years of education) | 5 distinct cognitive trajectories were established |
| Impact of cognitive dysfunctions | ||||||||
| Galica et al. [30] |
|
Colorectal cancer (n=74, age not known) | Pre- or post-CT or post-surgery | 5-FU and oxaliplatin : 52% | CANTAB | Psychosocial Adjustment to Illness Scale–Self-Report (PAIS-SR) | Regression model | Cognitive changes do not influence patients’ relationships and functional roles |
| Pereira et al. [31] |
|
Breast cancer (n=506, 55 years old ±11.2) | Before any treatment (including surgery) and 1 year | 5-FU, epirubicin, cyclophosphamide, docetaxel: 59% | MoCA | Neurological complications | Cumulative incidence estimates with corresponding 95% confidence intervals | Cognitive decline: frequent side-effect Cognitive decline during the first year after diagnosis: 8.1% |
5-FU, 5-fluorouracil; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BVMT, Brief Visuospatial Memory Test; CANTAB, Cambridge Neuropsychological Test Automated Battery; CEF, cyclophosphamide, epirubicin, and fluorouracil; CFQ, Cognitive Failures Questionnaire; CHOP, doxorubicin, cyclophosphamide, vincristine, prednisone; CMF, cyclophosphamide, methotrexate, and fluorouracil; COWA, Controlled Oral Word Association Test; CT, chemotherapy; CVLT, California Verbal Learning Test; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FACT- (O; Ntx; G; F; Cog), Functional Assessment of Cancer Therapy (Ovarian cancer; Neurotoxicity; General; Fatigue; Cognitive Function); FEC-T, FEC plus taxotere; FU, fluorouracil or capecitabine; GHQ, General Health Questionnaire; HADS, Hospital Anxiety and Depression Scale; HC, healthy controls; HSCL, Hopkins Symptoms Checklist; HT, hormone therapy; HVLT, Hopkins Verbal Learning Test; MFSI, Multidimensional Fatigue Symptom Inventory; MoCA, Montreal Cognitive Assessment; MOPP-ABV, mechlorethamine, oncovin, procarbazine, prednisone-doxorubicin, bleomycin, vinblastine; PAF, patients' perceptions of their own cognitive function; PAOFI, Patient’s Assessment of Own Functioning Inventory; PASAT, Paced Auditory Serial Addition Task; POMS, Profile of Mood States; ROCF, Rey Osterreith Complex Figure; RT, radiotherapy; SRB, Standardized Regression-Based; STAI, Spielberger State-Trait Anxiety Inventory; TMT, Trail Making Test; WMS, Wechsler Memory Scale; WRAT, Wide Range Achievement Test; WTAR, Wechsler Test of Adult Reading.
Memory, processing speed, attention, and executive functions are the domains most impaired post-chemotherapy [1, 4, 21., 22., 23., 24.]. In addition to objective cognitive impairment, subjective cognitive complaints are one of the major side-effects reported by patients [31] (especially in breast cancer patients) and suggest a temporary negative effect of chemotherapy on cognition. Breast cancer patients treated with chemotherapy had more cognitive complaints, particularly memory and executive functions (1/5 of patients) [6], and reported more cognitive difficulties than before treatment (45.2% versus 10.4%) [29]. However, heterogeneous trajectories of cognitive complaints exist in breast cancer survivors [17]. Furthermore, according to a long-term follow-up study (7–9 years post-surgery), breast cancer survivors treated with systemic treatment did not have more cognitive complaints than those who did not receive treatment [28].
Colorectal patients treated with chemotherapy had also more cognitive complaints particularly at 6 months than patients without chemotherapy (32% versus 16%) [24]. In a small sample of colorectal cancer patients, CRCI had an impact on patient’s QoL, but cognitive impairment did not seem to influence patients’ relationships and their functional roles [30].
Animal models
Animal model research has been instrumental in validating CRCI as a legitimate medical condition in cancer survivors who receive chemotherapy. In line with the clinical literature, numerous studies involving rodents, tested on a wide range of behavioural tasks, have confirmed that commonly used anticancer drugs produce moderately severe, and often long-lasting, cognitive deficits [32., 33., 34., 35., 36., 37.]. Memory loss induced by chemotherapy is related to hippocampal and frontal lobes dysfunction and is often reported with attention, working memory, and strategic learning deficits [9, 38].
Animal research has helped to identify critical biological mechanisms that account for CRCI. Indeed, cyclophosphamide, doxorubicin, and 5-fluorouracil prevent the production of new cells in the hippocampus, and suppression of neurogenesis is directly related to accompanying loss of hippocampus-dependent cognitive functions [32, 39]. Furthermore, mitochondrial dysfunction [40] and dysregulation of cytokine activity contribute to at least some of the cognitive deficits seen after chemotherapy. Particular attention has focussed on increased levels of chemotherapy-induced pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF-α, IL-10) which, in cancer patients, have been related to impairment, especially on tests of frontal lobe function [41, 42].
Impact of other oncological therapies and new treatment strategies in clinical studies and animal models
Beyond the impact of chemotherapy, hormone therapies and targeted therapies, such as antiangiogenics, can induce cognitive deficits (Tables 3 and 4).
Table 3.
Cognitive impairment induced by medical oncology treatments other than chemotherapy in patients
| Study | Study design Country | Patients, control group Age | Cognitive assessment | Cognitive measures | Other measures | Statistical analysis | Main outcomes |
|---|---|---|---|---|---|---|---|
| Hormone therapy—breast cancer | |||||||
| Schilder et al. [43] |
|
Breast cancer, tamoxifen users (n=80, 69 years old ±7.6), exemestane users (n=99, 68 years old ±6.8) versus HC (n=120) | Before and after 1 year of HT (CT naïve) | CFQ and interview questions about cognitive complaints | Cognitive tests (only to assess the relation with cognitive complaints), HSCL, fatigue subscale (EORTC-QLQ-C30), FACT-B-ES | ANOVA, ANCOVA and logistic regressions (anxiety, depression covariates) | In tamoxifen users: increased attention/concentration complaints (not in exemestane users) |
| Danhauer et al. [44] |
|
Breast cancer (n=1479, 67 years old ±4.3), tamoxifen and raloxifen groups (randomized) | Baseline, 2 and 3 years follow-up | 3MSE, PMA vocabulary, verbal fluencies, BVRT, CVLT, digit span, card rotations, finger tapping | PANAS, GDS | Linear mixed models for repeated measures | No significant interactions between treatment and any of the cognitive test results |
| Ganz et al. [27] |
|
Breast cancer with HT (n=122, 52 years old ±7.9, tamoxifen or aromatase inhibitor) versus without (n=51) | Before HT and 6 months later | WTAR, CVLT, WMS, BVMT, ROCF, block design, digit symbol, TMT, Stroop, letter-number sequencing, grooved pegboard | PAOFI, BDI, SF-36 | Bivariate analyses and multivariable linear regression models | Cognitive complaints (language and communication) increase in HT patients but no significant changes in cognitive scores |
| Bender et al. [45] |
|
Breast cancer with CT and anastrozole (n=114, 59 years old ± 5.5) versus anastrozole alone (n=173) versus HC (n=110) | Before, 6, 12, and 18 months after the start of HT | CANTAB, digit vigilance, rivermead story, ROCF, RAVLT, verbal fluency, Stroop, grooved pegboard, digit symbol substitution, NART | BDI, POMS | Linear mixed effects modelling (age and estimated verbal intelligence controlled) |
|
| Le Rhun et al. [46] |
|
Breast cancer (n=74, 61 years old [49–69]), with tamoxifen (n=37) versus AI (n=37) | Before, 6 and 12 months after the start of HT | MMSE, RAVLT, BVRT, digit and spatial span, TMT, Stroop, verbal fluency, Wisconsin card sorting test | CDS, IADL, EORTC QLQ-C30, HADS | Mixed design analysis models of variance (adjusted for baseline performance) | No significant difference between groups on cognitive scores for all the follow-up |
| Phillips et al. [47] |
|
Breast cancer with ovarian function suppression + tamoxifen or exemestane (n=54, 44 years old) versus tamoxifen alone (n=20, 46 years old) | Before HT and 1 year later | CogState (seven tasks) | CFQ, GHQ, Brief fatigue inventory | Wilcoxon rank-sum tests to compare the change between the t groups (CT treatment and baseline characteristics controlled) | No significant difference in the changes of objective cognitive scores between all groups |
| Van Dyk et al. [48] |
|
Breast cancer with HT (n=126, 52 years old ±7.9) versus without (n=63, 52 years old ± 9.2) | Before, 6 and 12 months and 3–6 years after the start of HT | CVLT, BVMT, Digit span, coding, letter-number sequencing, PASAT, ROCF, block design, TMT, Verbal fluency, Grooved pegboard, Stroop | BDI, State Anxiety Inventory, WTAR | Linear mixed effect models for repeated measures, analysis of covariance and binary logistic regression | No detrimental effect of HT on cognition |
| Androgen deprivation therapy—prostate cancer | |||||||
| Wiechno et al. [49] |
|
Prostate cancer with ADT (n=88, 67 years old [50–80]) and without (n=61) | After RT and LH-RH analogue | MMSE | HADS, questions on QoL |
|
No significant difference between groups for MMSE |
| Gonzalez et al. [50] | LongitudinalUSA | Prostate cancer with ADT (n=58, 67 years old ±8.9 versus without (n=84) and HC (n=88) | Before, 6 and 12 months after the start of ADT | HVLT, WMS logical memory, digit and spatial spans, BVMT, colour trails, COWA, NART | TIADL | Mixed models and logistic regressions |
|
| Morote et al. [51] |
|
Prostate cancer (n=308, 71 years old ±8.1) | Before and 6 months after the start of LHRH analogues | Digit span, visual memory, judgment of line orientation and mental rotation tests, matrix reasoning test | None | Changes outside the baseline 95% confidence intervals were considered significant | 20% of patients had significant decline on ≥1 test |
| Thiery-Vuillemin [52] |
|
Prostate cancer with AAP (n=46, 73 years old [53–90]) versus ENZ (n=59, 76 years old [60–92]) | 1, 2, and 3 months | FACT-Cog | EORTC QLQ-C30, BFI-SF, BPI-SF | Multivariate repeated measures logistic models and clinically meaningful change | More cognitive complaints in patients with ENZ than AAP and higher risk to develop depression |
| Antiangiogenic | |||||||
| Joly et al. [7] |
|
Renal cancer (n=75, 65 years old [28–81]) | Before, 3 and 6 months after the start of AATs | Grober–Buschke test, ROCF, arithmetic, digit-span, letter-number sequencing, TMT, verbal fluencies | MFI, FACT-G and FKSI, BDI, STAI | Fisher’s exact test, Wilcoxon test, and Spearman correlation coefficient | Cognitive decline in one-third of patients post-AATs independently of fatigue |
AAP, Abiraterone Acetate plus Prednisone; AATs, antiangiogenic therapies; AI, Aromatase inhibitors; ADT, Androgen Deprivation Therapy; BDI, Beck Depression Inventory; BFI-SF, Brief Fatigue Inventory-Short Form; BPI-SF, Brief Pain Inventory-Short Form; BVMT, Brief Visuospatial Memory Test; BVRT, Benton Visual Retention Test; CANTAB, Cambridge Neuropsychological Test Automated Battery; CDS, cognitive difficulties scale; CFQ, Cognitive Failures Questionnaire; CT, chemotherapy; CVLT, California Verbal Learning Test; ENZ, Enzalutamide; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FACT- (B-ES; G; FKSI), Functional Assessment of Cancer Therapy (Breast-Endocrine Subscale; General; Kidney Symptom Index); GDS, Geriatric Depression Scale; GHQ, General Health Questionnaire; HADS, Hospital Anxiety and Depression Scale; HC, healthy controls; HSCL, Hopkins Symptoms Checklist; HT, hormone therapy; QoL, quality of life; LHRH, luteinizing hormone-releasing hormone; MFI, Multidimensional Fatigue Inventory; MMSE, Mini Mental State Examination; NART, National Adult Reading; PANAS, positive and negative affect schedule; PASAT, Paced Auditory Serial Addition Task; PMA, primary mental abilities vocabulary test; PAOFI, Patient’s Assessment of Own Functioning Inventory; POMS, Profile of Mood States; ROCF, Rey Osterreith Complex Figure; RT, radiotherapy; STAI, Spielberger State-Trait Anxiety Inventory; TIADL, Instrumental Activities of Daily Living Test; TMT, Trail Making Test; WMS, Wechsler Memory Scale; WTAR, Wechsler Test of Adult Reading.
Table 4.
Suspected mechanisms involved in cognitive impairment induced by cancer treatments
| Cancer treatments Main studied drugs | Brain functions (excluding complaints) | Suspected mechanisms |
|---|---|---|
|
|
|
|
Clinical studies: executive functions, working memory, concentration (Anastrozole), visuomotor functions |
|
|
Clinical studies: fatigue, one main domain of cognition in a subpopulation of patients, working memory |
|
|
|
|
VEGF, vascular endothelial growth factor; BBB, blood–brain barrier.
Although the results of clinical studies generally are inconclusive, CRCI has been shown with aromatase inhibitors [1]. Breast cancer patients treated with anastrozole had lower executive function scores than healthy controls up to 18 months after the start of treatment [45]. In the same study, women receiving anastrozole alone exhibited deterioration in working memory and concentration 12–18 months after initiation of therapy. Nevertheless, another study did not show a significant difference on cognitive scores post-hormone therapy initiation (tamoxifen or aromatase inhibitors) compared with patients without hormone therapy [27]. Nevertheless, cognitive complaints increased in patients with hormone therapy. More recently, in breast cancer patients treated with hormone therapy, no detrimental effect was described at 6-years follow-up [48]. Two studies [44, 46] compared the effects of antiestrogens and aromatase inhibitors on cognition. Both found no significant difference on objective cognitive scores. After randomization for hormone therapy, there was no difference between breast cancer patients treated with aromatase inhibitors or tamoxifen at 6 months and 1-year follow-up [48]. Conversely, cognitive complaints (attention/concentration) significantly increased in tamoxifen users but not in women on exemestane [43].
Ovarian function suppression may also increase cognitive complaints in breast cancer patients but without significant impact on objective cognitive functions [47].
In prostate cancer, the impact of androgen deprivation therapy (ADT) has also been assessed. Overall, little effect on cognition was found (effect size, g = −0.67) and, according to a meta-analysis, visuomotor functions are likely the most impaired domain [53]. On the Mini Mental State Examination (MMSE), prostate cancer patients treated with luteinizing hormone-releasing hormone (LH-RH) analogues did not score statistically differently than patients without LH-RH [49]. Otherwise, according to cognitive tests, 20% of patients had cognitive decline 6 months after the start of LH-RH analogues, a majority deteriorating only on one test, without alteration in one domain in particular [51]. Also, patients with ADT had more CRCI, 6 and 12 months after the start of treatment, compared with patients without ADT and healthy controls, but there was no difference between groups in changes of mean-level cognitive performance [50]. Results at 3 months indicate that more prostate cancer patients receiving enzalutamide experienced cognitive complaints than patients taking acetate abiraterone and prednisone [52].
Initial results with targeted therapies suggested an impact on cognition in a subgroup of patients [7]. Cognitive decline was observed after antiangiogenic therapies (AAT), independently of fatigue, in 31% of metastatic renal cell carcinoma patients, the majority of whom did not have cognitive impairment before treatment [7]. In agreement, one study tested the effects of the mTOR inhibitor everolimus in mouse brain functions [54]. No alteration was found in cognitive performance, associated with the absence of modification in hippocampal neural cell proliferation, but weight loss and modification of neural activity in brain areas involved in the sleep/wake cycle suggest AAT-evoked fatigue [7].
Although there are no clinical data on the impact of immunotherapy on cognition, some data are starting to emerge from animal model research. Radiotherapy combined with immunotherapy (checkpoint inhibitor and anti-CTL4 antibody) in an animal model showed behavioural and cognitive altered performances associated with proinflammatory cytokines [55].
Associated psychological, sociodemographic and genetic factors involved in CRCI
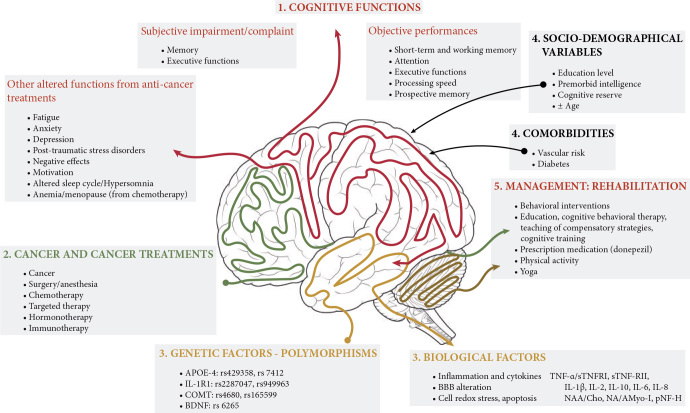
Many factors increase risk for CRCI (Figure 1), including psychological and sociodemographic variables and genetic predisposition.
Figure 1.
Schema outlining the complexity of cancer-related cognitive impairment. In cancer patients and survivors, the effect of chemotherapy on cognitive functions has been shown to impact different brain areas involved in attention, processing speed, memory, and executive functions. Recently, newly developed therapies involving targeted therapy, hormone therapy, and immunotherapy also appear to affect cognitive functions. The cancer treatments were associated with changes in brain volume, metabolic, or network modifications potentially related to direct neuronal toxicity and inflammation and genetic polymorphism combined with the aging process, patients’ emotional status, co-morbidities, or lifestyle. Cancer patients can be affected in multiple aspects, highlighting the urgency of initiating specific onco-neuro-psychological patient care. APOE, Apolipoprotein E; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; Cho, choline; COMT, catechol-O-methyltransferase; IL1-R1, interleukin-1-receptor1; Myo-I, Myo-inositol; NAA, N-acetylaspartate; pNF-H, phosphorylated neurofilament subunit H; TNF-α, tumour necrosis factor-alpha; sTNF-RII, tumour necrosis factor-receptor type II.
Fatigue, psychological and socio-demographic factors
Anxiety, depression, and fatigue are frequent in cancer patients and should be taken into account for cognitive assessment. Several studies showed an association between cognitive complaints and anxiety [15, 16, 24, 29, 43, 56., 57., 58., 59.], depression [6, 15, 16, 24, 29, 43, 44, 57, 58, 60], post-traumatic stress disorder symptoms [58], negative affect (e.g. distress and negative mood) [44] and motivation [61].
Fatigue was also frequently associated with CRCI [24, 43, 57., 58., 59.] and insomnia [17]. Risk factors for these difficulties included education level [25, 58], premorbid intelligence [25, 62], or cognitive reserve [29, 63]. Patient information about cognitive side-effects associated with cancer treatments could induce cognitive complaints [64, 65].
Aging
Although aging is a risk factor for cancer and cognitive impairment, and despite the potential impact of these impairments on patient’s autonomy, few studies have focussed on CRCI in cancer patients over 60–65 years old [61, 66., 67., 68., 69., 70., 71., 72., 73.]. Although several studies showed an impact of age on CRCI, others did not support age as a risk factor. Until now, it is not proved that cancer and/or its treatments (particularly hormone-therapy) induce Alzheimer disease [74]. However, ADT may increase the risk of dementia [75]. Among elderly cancer patients, the difficulty of isolated early signs and symptoms of dementia in relation with the age and cognitive decline induced by cancer treatments is an issue.
Using a cognitive screening tool, a prospective cohort in older patients with breast or colorectal cancer did not show cognitive decline to be associated with chemotherapy [76]. Nevertheless, an interaction was found between age and chemotherapy treatment on memory functioning [66]. Furthermore, in breast cancer patients ≥65 years old, 49% had objective cognitive decline after adjuvant treatment [72] a higher proportion than that reported in younger patients. Among patients with decline, 12% of patients had a non-pathological decline, 31% without initial cognitive impairment developed impairment, and 6% experienced accelerated cognitive decline [71]. Furthermore, the oldest patients were more likely to have cognitive decline with chemotherapy, particularly with docetaxel [72]. Chemotherapy in cancer patients 60–64 years old seemed to be associated with faster memory decline compared with older patients treated with chemotherapy, patients without chemotherapy and healthy controls [73]. In addition, results suggest a significant association between measures of biological aging and cognition in breast cancer survivors [77].
Markers, genetic predisposition/polymorphism
Biomarkers of cognitive decline post-cancer treatment, and/or risk factors such as inflammatory status or predisposing genetic factors have been investigated recently [78]. Results on correlations between levels of cytokines or inflammation [79, 80], neurobiological status and genetic polymorphisms are conflicting. However, the most reliable biomarkers associated with CRCI were cytokines (IL-6, TNF-α, etc.), cytokine receptors (sTNRFII, sTNFRI, etc.) and inflammation components [41, 42, 57, 81., 82., 83.], while mitochondrial DNA content in peripheral blood can be specifically associated with fatigue during chemotherapy [84].
Neurological markers or factors also detected during cancer treatments included decreased N-acetylaspartate (NAA)/choline (Cho) and NAA/myo-inositol (myo-I) ratios [85]. The breast cancer group with the lowest ratios reported reduced cognition, highlighting defects in the neurobiological status post-chemotherapy [85]. The serum axonal phosphorylated neurofilament subunit H pNF-H level in patients undergoing chemotherapy for breast cancer increased in a cumulative dose-dependent manner, suggesting its potential application as a biomarker of neural damage post-chemotherapy [86].
The potential role of genetic single nucleotide polymorphisms (SNPs) has been explored in other recent studies. Based on the inflammation-associated cognitive dysfunctions, a protective relationship between the SNP IL1R1 rs2287047 and cognitive complaints was established in breast cancer survivors. However, the SNP IL1R1 rs949963 was shown to be a significant genotypic predictor with breast cancer patients carrying the rare ‘A’ allele (e.g. GA + AA) having lower perceived attentional function [87], highlighting the complexity of cytokine SNPs. One of the first candidates suspected was the gene encoding apolipoprotein E (APOE). The allelic variants APOE-4 is a well-known risk factor for Alzheimer’s disease. APOE-4 may also contribute to poorer cognitive performance following chemotherapy and/or hormonal therapy in breast cancer patients [88]. Furthermore, an association between APOE status, breast cancer treatment, and cognition were found and moderated by smoking history [78]. Prefrontal volume reductions specific to patients treated with chemotherapy were associated with poorer cognitive performance related to an increase in TNF-α and in APOE-4 carriers, providing a strong relationship between inflammation, brain functions and cognitive impairment post-chemotherapy [10].
The role of neurotransmitter metabolism as a potential genetic risk was reported with the catechol-O-methyltransferase (COMT) which catalyses the metabolic breakdown of catecholamine. Rs165599 in the COMT gene was correlated with impaired retrospective memory in patients receiving chemotherapy, suggesting that the COMT metabolic pathway is a determinant in CRCI [89]. Furthermore, the BDNF polymorphism (rs6265) [Val66Met] was implicated in the decreased susceptibility of cognitive complaints in breast cancer patients receiving some chemotherapy regimens [90].
Imaging of brain changes after cancer treatments
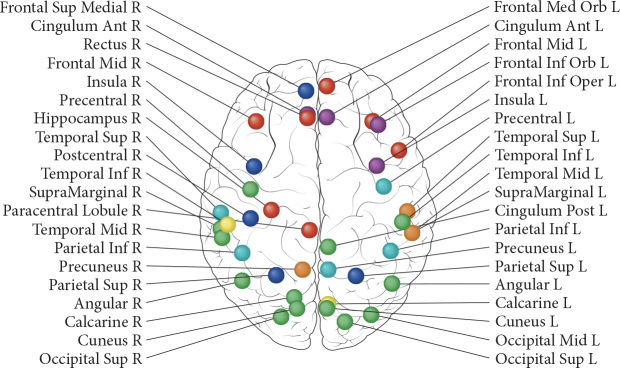
Many studies have reported reductions in grey matter volume or density, reductions in white matter microstructure, changes in brain activation and connectivity post-chemotherapy [3] (Figure 2) [11]. Findings of functional hyperactivation and hyperconnectivity of brain regions that support cognition have been interpreted as compensatory processes for treatment-induced brain injury [19, 20].
Figure 2.
Brain regions that show changes in brain morphology, perfusion and/or activation after chemotherapy as reported by longitudinal studies issued from Li & Caeyenberghs, 2018 [11]. Changes in morphology [voxel-based morphometry (VBM) of grey matter or diffusion tensor imaging of white matter] are indicated in red. Changes in perfusion [arterial spin labelling (ASL)] are indicated in green. Changes in brain activation (fMRI) are indicated in blue. Several brain regions reveal overlap between modalities: magenta for overlap between brain morphology and activation, cyan for overlap between activation and perfusion, yellow for overlap between morphology and perfusion, orange for overlap between the three modalities. White matter regions are not included in the figure, nor was the cerebellum. However, two fMRI studies have revealed changes in the right cerebellum (lobule 4–5 and Crus- 2). The longitudinal studies are including cancer types, sample sizes, and chemotherapeutic agents: morphological changes—VBM: [10] 22 TC+, 43 TC−, 25 HC; bleomycin, etoposide, and cisplatin; [91] 8 BC+, 6 BC−; doxorubicin and cyclophosphamide/fluorouracil, epirubicin, cyclophosphamide, with or without docetaxel. In two patients, trastuzumab was also administered; [92] 14 GC+, 11 HC; capecitabine, oxaliplatin; [93] 19 BC+, 19 HC; fluorouracil, epirubicin, cyclophosphamide, docetaxel/cyclophosphamide, docetaxel/cyclophosphamide, doxorubicin; [94] 16 BC+, 12 BC−, 15 HC; doxorubicin/cyclophosphamide/paclitaxel, docetaxel/cyclophosphamide, docetaxel/carboplatin, docetaxel/doxorubicin/cyclophosphamide, docetaxel/cisplatin, paclitaxel. DTI: [95] 22 TC+, 43 TC−, 25 HC; bleomycin, etoposide, and cisplatin; [96] 34 BC+, 16 BC−, 19 HC; fluorouracil, epirubicin, and cyclophosphamide with or without paclitaxel; [97] 26 BC+, 23 BC−, 30 NC; doxorubicin, cyclophosphamide with or without docetaxel or paclitaxel, fluorouracil, epirubicin, cyclophophamide. Changes in perfusion: [98] 31 BC+, 34 HC; doxorubicin, cyclophosphamide, docetaxel; [99] 27 BC+, 26 BC−, 26 HC; doxorubicin, cyclophosphamide, paclitaxel/docetaxel, cyclophosphamide//docetaxel, doxorubicin, cyclophosphamide/docetaxel, cisplatin/doxorubicin, cyclophosphamide/paclitaxel. Changes in activation: [100] 18 BC+, 12 HC; doxorubicin, cyclophosphamide with or without docetaxel. One patient also received trastuzumab; [101] 18 BC+, 16 BC−, 18 HC; fluorouracil, epirubicin, cyclophophamide, with or without paclitaxel; [92] 14 GC+, 11 HC; capecitabine, oxaliplatin; [102] 21 BC+, 21 HC, fluororacil, epirubicin, cyclophosphamide, docetaxel/cyclophosphamide, docetaxel/cyclophosphamide, doxorubicin; [103] 16 BC+, 12 BC−, 15 HC; doxorubicin/cyclophosphamide/paclitaxel/docetaxel/doxorubicin, cyclophosphamide/doxorubicin/cyclophosphamide [20] 28 BC+, 24 BC−, 31 HC; doxorubicin, cyclophosphamide with or without docetaxel or paclitaxel/fluorouracil, epirubicin, cyclophophamide. BC, breast cancer; TC, testicular cancer; GC, gastric cancer; HC, healthy controls. Reprinted from [11], Copyright (2019), with permission from Elsevier.
A decrease in white matter microstructure specific for chemotherapy-exposed patients 3–4 months post-treatment were observed in breast cancer patients, associated with performance decline in attention and verbal memory [96]. Three to four years of follow-up suggested cognitive and brain recovery [104].
A study evaluating breast cancer survivors 20 years post-chemotherapy showed worse performance on neuropsychological tests and global reductions in grey matter volume relative to a control group, comparable to 4 years of normal brain aging [105].
A prospective study evaluating early effects of BEP chemotherapy (bleomycin, etoposide, cisplatin) exposure on brain structure assessed testicular cancer patients after surgery but before further treatment and again 6 months later [10]. Widespread reductions in grey matter density occurred, with prefrontal reductions specific for the chemotherapy-treated patients (3 months post-completion) associated with cognitive decline. Network analyses carried out by DTI revealed altered global and local brain networks that were also associated with cognitive decline [106]. Ten years post-BEP chemotherapy, testicular cancer survivors had changes in white matter structure and cognitive decline compared with non-exposed survivors [107].
Some evidence indicates that the type and extent of neurotoxicity depend on the type of chemotherapy. In breast cancer survivors 2 years post-treatment, resting-state functional magnetic resonance imaging showed anthracycline-based chemotherapy was associated with lower connectivity of the default mode network in addition to showing more cognitive impairments than patients treated with other cytotoxic regimens [108]. In breast cancer survivors 10 years post-treatment, multimodality MRI revealed treatment dose-dependent effects on grey matter volume, white matter microstructure and task activation [109, 110].
In addition to providing an assay for neurotoxicity, neuroimaging measures might provide a means to identify patients at high risk for treatment-induced cognitive decline, such as those with suboptimal brain network characteristics [63, 111].
Management strategies
A survey conducted in ∼1600 survivors (>85% breast cancer survivors), at a median of ∼3 years after cancer treatment, found 75% of participants self-reported cognitive symptoms related to cancer treatments [5]. Three quarters of respondents reported cognitive symptoms impacted their ability to return to work. Most participants (75%) wished to receive support, particularly cognitive training (72%). This highlights the importance of monitoring for CRCI. Strategies of management of CRCI have been studied [112., 113., 114., 115., 116., 117., 118., 119., 120., 121., 122., 123., 124., 125., 126., 127.].
Physical activity
A few studies have shown exercise programmes can improve cognitive complaints [118, 126] but most have not assessed objective cognitive function [116]. However, a recent study evaluates cognition in sedentary breast cancer survivors randomized to a 12-week exercise programme compared with a wait-list control group. The exercise group had improvement in processing speed in those diagnosed within the previous 2 years, and reduction in cognitive symptoms [126]. Another analysis of survivors randomized to eight sessions of yoga versus controls found improvement in cognitive symptoms in the yoga group [118]. In rat models, physical exercise has been shown to reduce cognitive deficits induced by chemotherapy by preventing diminished hippocampal neurogenesis [119].
Behavioural
Behavioural interventions generally focus on education, cognitive behaviour therapy and/or teaching of compensatory strategies, or cognitive training. Cognitive behaviour therapy and cognitive rehabilitation studies in cancer survivors consistently report improvement in cognitive complaints but show variable results for objective cognitive tests [117, 120].
The largest cognitive training study randomized cancer survivors (n = 242) with cognitive symptoms 6–60 months after adjuvant chemotherapy, to a web-based rehabilitation programme done at home, or a control group [112]. There was significant improvement in self-reported cognitive problems, anxiety/depression, and fatigue, but no significant differences among the groups on neuropsychological testing. Two smaller cognitive training studies in breast cancer survivors also found improvement in cognitive complaints but reported improvement in some aspects of objective cognitive function [121, 122].
Many of the studies included few participants and did not have a therapeutic control group making it difficult to determine whether any improvement seen was due to an expectancy effect.
Pharmacological
There is no evidence to support the use of pharmacological agents, such as erythropoietin or methylphenidate, in randomized controlled trials for the treatment of CRCI [1], as reported in a recent review [128]. At this time, clinical trials testing diverse neurostimulating, neuroprotectants or antineuroinflammatory therapeutic agents are currently in test phase, with the objective to prevent or treat CRCI. Efficacy of neurostimulants such as methylphenidate or modafinil is diverse and clinical experience concerning antidementia drugs (e.g. donepezil, memantine) is limited [128].
Work in animals suggests that several drugs, including fluoxetine [115], donepezil [38], and cotinine, the main derivative of nicotine [113], can improve cognitive performance as well as emotional state following chemotherapy, but further research is required.
Discussion
Conclusions and future directions
Cognitive impairments are reported during and after treatment in cancer patients receiving chemotherapy. Even if other treatments, such as hormone therapies in breast and prostate cancer patients, seem to have lower impact on cognition, the real current difficulty is to assign selective cognitive disorders to specific treatments because surgery, anaesthesia, and radiotherapy are often part of treatment. Further studies are needed to take into account the care trajectory and investigate the impact of newer therapies such as the new generation of hormone therapies in prostate cancer and immunotherapy, with preliminary data supporting cognitive alterations.
While there has been progress in identifying factors involved in CRCI (psychological and sociodemographic variables or biomarkers and genetic predisposition), complementary studies, including work with animal models and neuroimaging, are needed to define precisely which factors predispose to a higher risk of cognitive impairment.
Translational research, including clinical, imaging, and animal models has improved knowledge about CRCI. Studies on animal models have helped identify neurobiological mechanisms, highlighting a strong translational role for animal models in this field. Neuroimaging studies have provided valuable insights into functional and structural brain regions and networks affected by cancer treatment. As imaging methods continue to develop, we expect these will aid in uncovering the biological mechanisms involved and identifying patients at high risk for cognitive decline.
Significant progress in the field has been made utilizing traditional neuropsychological tests and validated self-report measures of cognition. However, concerns have been raised about the sensitivity and specificity of traditional neuropsychological tests to detect the relatively subtle cognitive changes often experienced by cancer survivors. Some investigators are advocating the addition of tests based on cognitive neuroscience, which may be more sensitive and assess specific subcomponents of cognitive processes [129] with the use of more ecological tests.
There is limited high-quality evidence guiding how best to help cancer survivors with cognitive complaints. No pharmacological agents have been approved to reduce CRCI and, despite encouraging results with animals, none of the drugs examined in animal models have been subjected to clinical trials. For those with sustained impairment, or for whom impairment impacts daily function, referral to a neuropsychologist is recommended. Due to the association of cognitive symptoms with anxiety/depression, fatigue, and sleep disorders, it is essential to assess patients with CRCI for common symptom clusters and to treat these symptoms if present. The most promising strategy is likely cognitive rehabilitation but its impact on improvement in daily function remains unclear. Although only preliminary data are available, physical activity programmes could also be considered. More studies and especially robust clinical trials are needed to find adequate strategies of management of CRCI for routine oncology supportive care, to respond to demands and improve QoL of patients.
Multidisciplinary cooperation between oncologists, neurologists, imaging researchers, and neuroscientists is encouraged to define mechanisms of CRCI and to optimise medical care and patients’ rehabilitation. Early detection of cognitive impairment is needed, especially in elderly patients who could be referred to an onco-geriatrician and/or neurologist to screen for cognitive impairment before and during treatment. Management of CRCI should be incorporated into clinical practice as for patients with neurodegenerative disease.
Acknowledgements
The Cancer and Cognition Platform is supported by the French Ligue contre le Cancer.
Funding
None declared.
The Cancer and Cognition Platform
French Ligue contre le Cancer
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Joly F., Giffard B., Rigal O. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Ahles T.A., Root J.C., Ryan E.L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deprez S., Kesler S.R., Saykin A.J. International cognition and cancer task force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. J Natl Cancer Inst. 2018;110(3):223–231. doi: 10.1093/jnci/djx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wefel J.S., Vardy J., Ahles T., Schagen S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 5.Lange M., Licaj I., Clarisse B. Cognitive complaints in cancer survivors and expectations for support: results from a web-based survey. Cancer Med. 2019;8(5):2654–2663. doi: 10.1002/cam4.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz P.A., Kwan L., Castellon S.A. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joly F., Heutte N., Duclos B. Prospective evaluation of the impact of antiangiogenic treatment on cognitive functions in metastatic renal cancer. Eur Urol Focus. 2016;15:642–649. doi: 10.1016/j.euf.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Joly F., Castel H., Tron L. Potential impact of immunotherapy agents on cognitive function in cancer patients. JNCI. 2019 doi: 10.1093/jnci/djz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winocur G., Johnston I., Castel H. Chemotherapy and cognition: international cognition and cancer task force recommendations for harmonising preclinical research. Cancer Treat Rev. 2018;69:72–83. doi: 10.1016/j.ctrv.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Amidi A., Agerbæk M., Wu L.M. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11(3):769–783. doi: 10.1007/s11682-016-9552-3. [DOI] [PubMed] [Google Scholar]
- 11.Li M., Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: a systematic review. Neurosci Biobehav Rev. 2018;92:304–317. doi: 10.1016/j.neubiorev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Ingraham L.G., Aiken C.B. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10(1):120–124. [Google Scholar]
- 13.Cheung Y.T., Foo Y.L., Shwe M. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67(7):811–820. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Bell M.L., Dhillon H.M., Bray V.J., Vardy J.L. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACTCog) J Patient Rep Outcomes. 2018;2(1):1–11. [Google Scholar]
- 15.Dhillon H.M., Tannock I.F., Pond G.R. Perceived cognitive impairment in people with colorectal cancer who do and do not receive chemotherapy. J Cancer Surviv. 2018;12(2):178–185. doi: 10.1007/s11764-017-0656-6. [DOI] [PubMed] [Google Scholar]
- 16.Pullens M.J., De V.J., Van Warmerdam L.J. Chemotherapy and cognitive complaints in women with breast cancer. Psychooncology. 2013;22(8):1783–1789. doi: 10.1002/pon.3214. [DOI] [PubMed] [Google Scholar]
- 17.Ng T., Dorajoo S.R., Cheung Y.T. Distinct and heterogeneous trajectories of self-perceived cognitive impairment among Asian Breast Cancer Survivors. Psychooncology. 2018;27(4):1185–1192. doi: 10.1002/pon.4635. [DOI] [PubMed] [Google Scholar]
- 18.Bray V.J., Dhillon H.M., Vardy J.L. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J Cancer Surviv. 2018;12(4):537–559. doi: 10.1007/s11764-018-0692-x. [DOI] [PubMed] [Google Scholar]
- 19.Apple A.C., Schroeder M.P., Ryals A.J. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin. 2018;20:110–118. doi: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menning S., de Ruiter M.B., Veltman D.J. Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS One. 2017;12(3):e0171724. doi: 10.1371/journal.pone.0171724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner O.C., Phillips B., McCabe M.G. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014;28(5):726–740. doi: 10.1037/neu0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson K.D., Hutchinson A.D., Wilson C.J., Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39(3):297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Collins B., Mackenzie J., Tasca G.A. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. J Int Neuropsychol Soc. 2014;20(4):370–379. doi: 10.1017/S1355617713001215. [DOI] [PubMed] [Google Scholar]
- 24.Vardy J.L., Dhillon H.M., Pond G.R. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. doi: 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wouters H., Baars J.W., Schagen S.B. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 2016;55(9-10):1121–1125. doi: 10.1080/0284186X.2016.1189092. [DOI] [PubMed] [Google Scholar]
- 26.Hess L.M., Huang H.Q., Hanlon A.L. Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2015;139(3):541–545. doi: 10.1016/j.ygyno.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz P.A., Petersen L., Castellon S.A. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol. 2014;32(31):3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amidi A., Christensen S., Mehlsen M. Long-term subjective cognitive functioning following adjuvant systemic treatment: 7-9 years follow-up of a nationwide cohort of women treated for primary breast cancer. Br J Cancer. 2015;113(5):794–801. doi: 10.1038/bjc.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janelsins M.C., Heckler C.E., Peppone L.J. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017;35(5):506–514. doi: 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galica J., Rajacich D., Kane D., Pond G.R. The impact of chemotherapy-induced cognitive impairment on the psychosocial adjustment of patients with nonmetastatic colorectal cancer. Clin J Oncol Nurs. 2012;16(2):163–169. doi: 10.1188/12.CJON.163-169. [DOI] [PubMed] [Google Scholar]
- 31.Pereira S., Fontes F., Sonin T. Neurological complications of breast cancer: a prospective cohort study. Breast. 2015;24(5):582–587. doi: 10.1016/j.breast.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Dubois M., Lapinte N., Villier V. Chemotherapy-induced long-term alteration of executive functions and hippocampal cell proliferation: role of glucose as adjuvant. Neuropharmacology. 2014;79:234–248. doi: 10.1016/j.neuropharm.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Fardell J.E., Vardy J., Johnston I.N. The short and long term effects of docetaxel chemotherapy on rodent object recognition and spatial reference memory. Life Sci. 2013;93(17):596–604. doi: 10.1016/j.lfs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Winocur G., Henkelman M., Wojtowicz J.M. The effects of chemotherapy on cognitive function in a mouse model: a prospective study. Clin Cancer Res. 2012;18(11):3112–3121. doi: 10.1158/1078-0432.CCR-12-0060. [DOI] [PubMed] [Google Scholar]
- 35.Fardell J.E., Vardy J., Monds L.A., Johnston I.N. The long-term impact of oxaliplatin chemotherapy on rodent cognition and peripheral neuropathy. Behav Brain Res. 2015;291:80–88. doi: 10.1016/j.bbr.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Fardell J.E., Zhang J., De S.R. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology (Berl) 2014;231(5):841–852. doi: 10.1007/s00213-013-3301-8. [DOI] [PubMed] [Google Scholar]
- 37.Seigers R., Loos M., Van T.O. Neurobiological changes by cytotoxic agents in mice. Behav Brain Res. 2016;299:19–26. doi: 10.1016/j.bbr.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 38.Lim I., Joung H.Y., Yu A.R. PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. Biomed Res Int. 2016;2016:1. doi: 10.1155/2016/6945415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christie L.A., Acharya M.M., Parihar V.K. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 40.Lomeli N., Di K., Czerniawski J. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic Biol Med. 2017;102:274–286. doi: 10.1016/j.freeradbiomed.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pomykala K.L., Ganz P.A., Bower J.E. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyon D.E., Cohen R., Chen H. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schilder C.M., Seynaeve C., Linn S.C. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21(5):479–487. doi: 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- 44.Danhauer S.C., Legault C., Bandos H. Positive and negative affect, depression, and cognitive processes in the Cognition in the Study of Tamoxifen and Raloxifene (Co-STAR) Trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(5):532–552. doi: 10.1080/13825585.2012.747671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender C.M., Merriman J.D., Gentry A.L. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121(15):2627–2636. doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Rhun E., Delbeuck X., Lefeuvre-Plesse C. A phase III randomized multicenter trial evaluating cognition in post-menopausal breast cancer patients receiving adjuvant hormonotherapy. Breast Cancer Res Treat. 2015;152(3):569–580. doi: 10.1007/s10549-015-3493-1. [DOI] [PubMed] [Google Scholar]
- 47.Phillips K.A., Regan M.M., Ribi K. Adjuvant ovarian function suppression and cognitive function in women with breast cancer. Br J Cancer. 2016;114(9):956–964. doi: 10.1038/bjc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van D.y., Crespi C.M., Bower J.E. The cognitive effects of endocrine therapy in survivors of breast cancer: a prospective longitudinal study up to 6 years after treatment. Cancer. 2019;125:681–689. doi: 10.1002/cncr.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiechno P.J., Sadowska M., Kalinowski T. Does pharmacological castration as adjuvant therapy for prostate cancer after radiotherapy affect anxiety and depression levels, cognitive functions and quality of life? Psychooncology. 2013;22(2):346–351. doi: 10.1002/pon.2095. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez B.D., Jim H.S., Booth-Jones M. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33(18):2021–2027. doi: 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morote J., Tabernero A.J., Alvarez Ossorio J.L. Cognitive function in patients with prostate cancer receiving luteinizing hormone-releasing hormone analogues: a prospective, observational, multicenter study. Int J Radiat Oncol Biol Phys. 2017;98(3):590–594. doi: 10.1016/j.ijrobp.2017.02.219. [DOI] [PubMed] [Google Scholar]
- 52.Thiery-Vuillemin A., Poulsen M.H., Lagneau E. Impact of abiraterone acetate plus prednisone or enzalutamide on fatigue and cognition in patients with metastatic castration-resistant prostate cancer: initial results from the observational AQUARiUS study. ESMO Open. 2018;3(5):e000397. doi: 10.1136/esmoopen-2018-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGinty H.L., Phillips K.M., Jim H.S. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22(8):2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubois M., Le J.V., Tonon M.C. Evaluation of the impact of the cancer therapy everolimus on the central nervous system in mice. PLoS One. 2014;9(12):e113533. doi: 10.1371/journal.pone.0113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGinnis G.J., Friedman D., Young K.H. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017;8(6):9155–9173. doi: 10.18632/oncotarget.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramalho M., Fontes F., Ruano L. Cognitive impairment in the first year after breast cancer diagnosis: a prospective cohort study. Breast. 2017;32:173–178. doi: 10.1016/j.breast.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Vardy J.L., Stouten-Kemperman M.M., Pond G. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2019;13(1):15–26. doi: 10.1007/s11682-017-9728-5. [DOI] [PubMed] [Google Scholar]
- 58.Li J., Yu L., Long Z. Perceived cognitive impairment in Chinese patients with breast cancer and its relationship with post-traumatic stress disorder symptoms and fatigue. Psychooncology. 2015;24(6):676–682. doi: 10.1002/pon.3710. [DOI] [PubMed] [Google Scholar]
- 59.Cheung Y.T., Shwe M., Chui W.K. Effects of chemotherapy and psychosocial distress on perceived cognitive disturbances in Asian breast cancer patients. Ann Pharmacother. 2012;46(12):1645–1655. doi: 10.1345/aph.1R408. [DOI] [PubMed] [Google Scholar]
- 60.Seliktar N., Polek C., Brooks A., Hardie T. Cognition in breast cancer survivors: hormones versus depression. Psychooncology. 2015;24(4):402–407. doi: 10.1002/pon.3602. [DOI] [PubMed] [Google Scholar]
- 61.Libert Y., Borghgraef C., Beguin Y. Cognitive compensatory processes of older, clinically fit patients with hematologic malignancies undergoing chemotherapy: a longitudinal cohort study. Psychooncology. 2017;26(12):2086–2093. doi: 10.1002/pon.4424. [DOI] [PubMed] [Google Scholar]
- 62.Menning S., de Ruiter M.B., Kieffer J.M. Cognitive impairment in a subset of breast cancer patients after systemic therapy-results from a longitudinal study. J Pain Symptom Manage. 2016;52(4):560–569. doi: 10.1016/j.jpainsymman.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Kesler S.R., Rao V., Ray W.J., Rao A. Probability of Alzheimer's disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement (Amst) 2017;9:67–75. doi: 10.1016/j.dadm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schagen S.B., Das E., Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psychooncology. 2012;21(10):1132–1135. doi: 10.1002/pon.2011. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs W., Das E., Schagen S.B. Increased cognitive problem reporting after information about chemotherapy-induced cognitive decline: the moderating role of stigma consciousness. Psychol Health. 2017;32(1):78–93. doi: 10.1080/08870446.2016.1244535. [DOI] [PubMed] [Google Scholar]
- 66.Morin R.T., Midlarsky E. Treatment with chemotherapy and cognitive functioning in older adult cancer survivors. Arch Phys Med Rehabil. 2018;99(2):257–263. doi: 10.1016/j.apmr.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Chen B.T., Jin T., Patel S.K. Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat. 2018;172(2):363–370. doi: 10.1007/s10549-018-4911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rambeau A., Beauplet B., Laviec H. Prospective comparison of the Montreal Cognitive Assessment (MoCA) and the Mini Mental State Examination (MMSE) in geriatric oncology. J Geriatr Oncol. 2019;10(2):235–240. doi: 10.1016/j.jgo.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Mandelblatt J.S., Small B.J., Luta G. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol. 2018 doi: 10.1200/JCO.18.00140. doi:JCO1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandelblatt J.S., Clapp J.D., Luta G. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance) Cancer. 2016;122(22):3555–3563. doi: 10.1002/cncr.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange M., Heutte N., Noal S. Cognitive changes after adjuvant treatment in older adults with early-stage breast cancer. Oncologist. 2019;24(1):62–68. doi: 10.1634/theoncologist.2017-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lange M., Heutte N., Rigal O. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist. 2016;21(11):1337–1348. doi: 10.1634/theoncologist.2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anstey K.J., Sargent-Cox K., Cherbuin N., Sachdev P.S. Self-reported history of chemotherapy and cognitive decline in adults aged 60 and older: the PATH through life project. Gerona. 2015;70(6):729–735. doi: 10.1093/gerona/glt195. [DOI] [PubMed] [Google Scholar]
- 74.Okereke O.I., Meadows M.E. More evidence of an inverse association between cancer and alzheimer disease. JAMA Netw Open. 2019;2(6):e196167.. doi: 10.1001/jamanetworkopen.2019.6167. [DOI] [PubMed] [Google Scholar]
- 75.Cherrier M.M., Higano C.S. Impact of androgen deprivation therapy on mood, cognition, and risk for AD. Urol Oncol. 2019 doi: 10.1016/j.urolonc.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 76.Shaffer V.A., Merkle E.C., Fagerlin A. Chemotherapy was not associated with cognitive decline in older adults with breast and colorectal cancer: findings from a prospective cohort study. Med Care. 2012;50(10):849–855. doi: 10.1097/MLR.0b013e31825a8bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carroll J.E., Van D.K., Bower J.E. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer. 2019;125(2):298–306. doi: 10.1002/cncr.31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahles T.A., Li Y., McDonald B.C. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology. 2014;23(12):1382–1390. doi: 10.1002/pon.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Willik K.D., Koppelmans V., Hauptmann M. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20(1):135. doi: 10.1186/s13058-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheung Y.T., Ng T., Shwe M. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams A.M., Shah R., Shayne M. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chae J.W., Ng T., Yeo H.L. Impact of TNF-alpha (rs1800629) and IL-6 (rs1800795) polymorphisms on cognitive impairment in Asian breast cancer patients. PLoS One. 2016;11(10):e0164204. doi: 10.1371/journal.pone.0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andreano J.M., Waisman J., Donley L., Cahill L. Effects of breast cancer treatment on the hormonal and cognitive consequences of acute stress. Psychooncology. 2012;21(10):1091–1098. doi: 10.1002/pon.2006. [DOI] [PubMed] [Google Scholar]
- 84.Chae J.W., Chua P.S., Ng T. Association of mitochondrial DNA content in peripheral blood with cancer-related fatigue and chemotherapy-related cognitive impairment in early-stage breast cancer patients: a prospective cohort study. Breast Cancer Res Treat. 2018;168(3):713–721. doi: 10.1007/s10549-017-4640-7. [DOI] [PubMed] [Google Scholar]
- 85.Kesler S.R., Watson C., Koovakkattu D. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2013;7(4):501–510. doi: 10.1007/s11682-013-9228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Natori A., Ogata T., Sumitani M. Potential role of pNF-H, a biomarker of axonal damage in the central nervous system, as a predictive marker of chemotherapy-induced cognitive impairment. Clin Cancer Res. 2015;21(6):1348–1352. doi: 10.1158/1078-0432.CCR-14-2775. [DOI] [PubMed] [Google Scholar]
- 87.Merriman J.D., Aouizerat B.E., Cataldo J.K. Association between an interleukin 1 receptor, type I promoter polymorphism and self-reported attentional function in women with breast cancer. Cytokine. 2014;65(2):192–201. doi: 10.1016/j.cyto.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koleck T.A., Bender C.M., Sereika S.M. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum. 2014;41(6):E313–E325. doi: 10.1188/14.ONF.E313-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng H., Li W., Gan C. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am J Transl Res. 2016;8(11):5087–5097. [PMC free article] [PubMed] [Google Scholar]
- 90.Ng T., Teo S.M., Yeo H.L. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro Oncol. 2016;18(2):244–251. doi: 10.1093/neuonc/nov162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenkins V., Thwaites R., Cercignani M. A feasibility study exploring the role of pre-operative assessment when examining the mechanism of ‘chemo-brain’ in breast cancer patients. Springerplus. 2016;5(1):390.. doi: 10.1186/s40064-016-2030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim H.G., Shin N.-Y., Bak Y. Altered intrinsic brain activity after chemotherapy in patients with gastric cancer: a preliminary study. Eur Radiol. 2017;27(7):2679–2688. doi: 10.1007/s00330-016-4578-x. [DOI] [PubMed] [Google Scholar]
- 93.Lepage C., Smith A.M., Moreau J. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3(1):444. doi: 10.1186/2193-1801-3-444. doi:101186/2193-1801-3-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McDonald B.C., Conroy S.K., Smith D.J. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amidi A., Hosseini S.M.H., Leemans A. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109(12):9041–9044. doi: 10.1093/jnci/djx085. [DOI] [PubMed] [Google Scholar]
- 96.Deprez S., Amant F., Smeets A. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 97.Menning S., de Ruiter M.B., Veltman D.J. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav. 2018;12(2):324–334. doi: 10.1007/s11682-017-9695-x. [DOI] [PubMed] [Google Scholar]
- 98.Chen X., He X., Tao L. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: evidence from an arterial spin labeling perfusion study. Sci Rep. 2017;7:1–9. doi: 10.1038/srep42684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nudelman K.N.H., Wang Y., McDonald B.C. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLoS One. 2014;9(5):e96713.. doi: 10.1371/journal.pone.0096713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conroy S.K., McDonald B.C., Ahles T.A. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav. 2013;7(4):491–500. doi: 10.1007/s11682-013-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deprez S., Vandenbulcke M., Peeters R. Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol. 2014;32(19):2031–2038. doi: 10.1200/JCO.2013.53.6219. [DOI] [PubMed] [Google Scholar]
- 102.López Zunini R.A., Scherling C., Wallis N. Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: a prospective fMRI study. Brain Imaging Behav. 2013;7(4):460–477. doi: 10.1007/s11682-012-9213-0. [DOI] [PubMed] [Google Scholar]
- 103.McDonald B.C., Conroy S.K., Ahles T.A. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Billiet T., Emsell L., Vandenbulcke M. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav. 2018;12(1):64–77. doi: 10.1007/s11682-016-9665-8. [DOI] [PubMed] [Google Scholar]
- 105.Koppelmans V., de Ruiter M.B., van der Lijn F. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 106.Amidi A., Hosseini S.M.H., Leemans A. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109(12) doi: 10.1093/jnci/djx085. [DOI] [PubMed] [Google Scholar]
- 107.Stouten-Kemperman M.M., de Ruiter M.B., Caan M.W. Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp. 2015;36(11):4638–4647. doi: 10.1002/hbm.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kesler S.R., Blayney D.W. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016;2(2):185–192. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stouten-Kemperman M.M., de Ruiter M.B., Koppelmans V. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9(2):275–284. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
- 110.Stouten-Kemperman M.M., de Ruiter M.B., Boogerd W. Very late treatment-related alterations in brain function of breast cancer survivors. J Int Neuropsychol Soc. 2015;21(1):50–61. doi: 10.1017/S1355617714001015. [DOI] [PubMed] [Google Scholar]
- 111.Jung M.S., Zhang M., Askren M.K. Cognitive dysfunction and symptom burden in women treated for breast cancer: a prospective behavioral and fMRI analysis. Brain Imaging Behav. 2017;11(1):86–97. doi: 10.1007/s11682-016-9507-8. [DOI] [PubMed] [Google Scholar]
- 112.Bray V.J., Dhillon H.M., Bell M.L. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol. 2017;35(2):217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 113.Iarkov A., Appunn D., Echeverria V. Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemother Pharmacol. 2016;78(5):1033–1039. doi: 10.1007/s00280-016-3161-0. [DOI] [PubMed] [Google Scholar]
- 114.Lawrence J.A., Griffin L., Balcueva E.P. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10(1):176–184. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lyons L., ElBeltagy M., Bennett G., Wigmore P. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS One. 2012;7(1):e30010.. doi: 10.1371/journal.pone.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bedillion M.F., Ansell E.B., Thomas G.A. Cancer treatment effects on cognition and depression: the moderating role of physical activity. Breast. 2019;44:73–80. doi: 10.1016/j.breast.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 117.King S., Green H.J. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol. 2015;5:72. doi: 10.3389/fonc.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Janelsins M.C., Peppone L.J., Heckler C.E. YOCAS(c)(R) yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther. 2016;15(3):263–271. doi: 10.1177/1534735415617021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winocur G., Wojtowicz J.M., Huang J., Tannock I.F. Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology (Berl) 2014;231(11):2311–2320. doi: 10.1007/s00213-013-3394-0. [DOI] [PubMed] [Google Scholar]
- 120.Ercoli L.M., Petersen L., Hunter A.M. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015;24(11):1360–1367. doi: 10.1002/pon.3769. [DOI] [PubMed] [Google Scholar]
- 121.Von A.D., Carpenter J.S., Saykin A. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kesler S., Hadi Hosseini S.M., Heckler C. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knobf M.T., Thompson A.S., Fennie K., Erdos D. The effect of a community-based exercise intervention on symptoms and quality of life. Cancer Nurs. 2014;37(2):E43–E50. doi: 10.1097/NCC.0b013e318288d40e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmidt M.E., Wiskemann J., Armbrust P. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137(2):471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 125.Galiano-Castillo N., Arroyo-Morales M., Lozano-Lozano M. Effect of an Internet-based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Support Care Cancer. 2017;25(11):3551–3559. doi: 10.1007/s00520-017-3782-9. [DOI] [PubMed] [Google Scholar]
- 126.Hartman S.J., Nelson S.H., Myers E. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124:192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Damholdt M.F., Mehlsen M., O'Toole M.S. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology. 2016;25(11):1293–1300. doi: 10.1002/pon.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karschnia P., Parsons M.W., Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 2019;20(2):e92–e102. doi: 10.1016/S1470-2045(18)30938-0. [DOI] [PubMed] [Google Scholar]
- 129.Ahles T.A., Root J.C. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14(1):425–451. doi: 10.1146/annurev-clinpsy-050817-084903. [DOI] [PMC free article] [PubMed] [Google Scholar]