Abstract
The metabolic syndrome, characterized by increases in waist circumference, blood pressure, and triglyceride concentrations combined with reduced high‐density lipoprotein and evidence of glucose intolerance, results from the interaction of visceral or central obesity with insulin resistance. This syndrome presents a clinical situation of systemic inflammation and increased cardiovascular risk. Blood pressure, even if only in the “prehypertensive” range, plays an important role in increasing the risk of cardiovascular disease. Recognition and treatment of each individual component of the metabolic syndrome is critical in reducing cardiovascular risk. Treatment should begin with lifestyle changes, including diet, exercise, and weight reduction. Antihypertensive therapy should be directed toward reduction of blood pressure to levels as close to optimal (<120/80 mm Hg) as feasible, and treatment protocols that do not cause worsening of glucose intolerance should be selected. Therapy for dyslipidemia should be directed at reducing triglycerides and increasing high‐density lipoprotein. Glucose‐lowering agents may be indicated, and drugs such as metformin and thiazolidinediones, which reduce insulin resistance, should form the basis of therapy. Carefully chosen therapy will effectively improve cardiovascular outcomes.
Hypertension may no longer be correctly viewed simply as high blood pressure (BP), but rather must be considered in the context of associated risk factors. More than 80% of individuals with stage 1 or greater hypertension (BP ≥140/90 mm Hg), as defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), 1 have additional comorbidities, increasing the risk of cardiovascular (CV) complications beyond those that result from the increase in BP alone. These comorbidities include obesity, glucose intolerance, hyperinsulinemia, low high‐density lipoprotein (HDL) levels, elevated low‐density lipoprotein (LDL) and triglyceride levels, left ventricular hypertrophy, and tobacco use2; at least 20% of hypertensive individuals have three or more of these comorbidities. The ultimate impact of BP on CV risk is determined by the number of associated risk factors present (Figure I). 3 , 4 , 5
Figure 1.
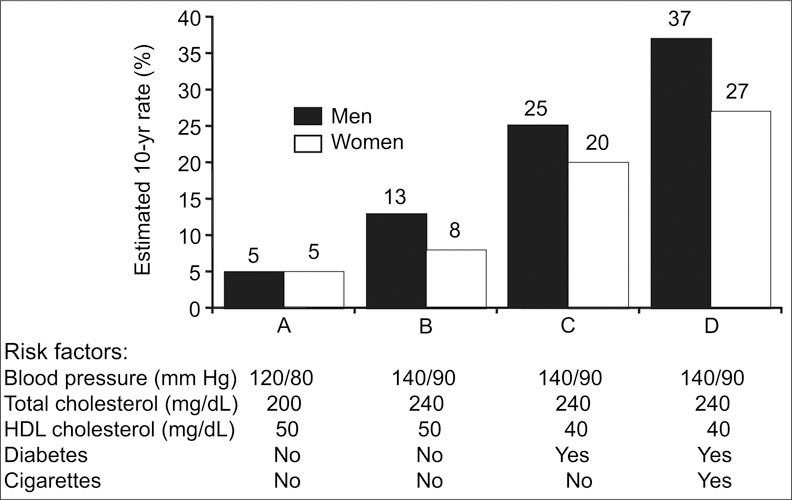
Coronary heart disease risk resulting from interaction of major risk factors. HDL=high‐density lipoprotein. Adapted from Am J Hypertens. 2000;13(suppl 1, pt 2):3S‐10S2 and Circulation. 1998;97:1837–1847. 3
Interestingly, these individual risk factors cluster in what has been defined as the CV metabolic syndrome, a proinflammatory condition associated with an increase in CV risk. The metabolic syndrome may actually be considered a CV disease equivalent. 6 , 7 As an example of this increase in CV risk, participants in the West of Scotland Prevention Study (WOSCOPS) 8 identified as having the metabolic syndrome experienced a 76% increase in the risk of a coronary heart disease (CHD) event and a 3.5‐fold increase in the risk of development of new diabetes over 5 years, compared with individuals without these findings.
CHARACTERISTIC FACTORS
Factors characteristic of the metabolic syndrome are: 1) abdominal visceral obesity; 2) atherogenic dyslipidemia (elevated levels of triglycerides and small LDL particles and low HDL cholesterol levels); 3) elevated BP; 4) insulin resistance (with or without glucose intolerance); and 5) prothrombotic and proinflammatory states. The definition of the metabolic syndrome includes certain key criteria, although some variability remains in the less essential elements as applied by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), 5 the World Health Organization (WHO), 9 and the International Diabetes Foundation 10 (Table I). ATP III criteria focus primarily on environmental causes of the metabolic syndrome, while the WHO emphasizes the importance of insulin resistance. These differences in diagnostic criteria have clinical implications. Investigators found that 28.1% of a group of 2175 subjects free of CV disease at baseline and not taking antihypertensive or lipid‐lowering medications qualified as having the metabolic syndrome by ATP III criteria, but only 21.0% by WHO criteria; the two sets of criteria provided concordant classification for 80.6% of participants. 11 The metabolic syndrome defined with the ATP III criteria, but not with the WHO criteria, was an independent predictor of coronary or cerebrovascular events during a median follow‐up time of 4.1 years and was associated with a 38% increased risk. We will utilize ATP III criteria for this paper because of its apparent greater sensitivity in identifying patients at risk.
Table I.
Diagnosis of Metabolic Syndrome
| Source | |||
|---|---|---|---|
| National Cholesterol Education Program (ATP III)5 | World Health Organization 9 | International Diabetes Federation 10 | |
| Criteria* | Three or more of the following: | Glucose intolerance plus two or more of the remaining: | Central obesity plus two or more of the remaining: |
| Waist circumference (in) | BMI >30 kg/m2 and/or: | ||
| Men | >40 | waist/hip ratio >0.90 | ≥43 |
| Women | >35 | waist/hip ratio>0.85 | ≥36 |
| Triglycerides (mg/dL) | ≥150 | >150 | >150 |
| HDL‐C (mg/dL) | |||
| Men | <40 | <35 | <40 |
| Women | <50 | <39 | <50 |
| Blood pressure (mm Hg) | ≥130/85 | >140/90 | ≥130/85 |
| Fasting glucose (mg/dL) | ≥110 | ≥100 <110 | ≥100 |
| Microalbuminuria | NA | albumin/creatinine ratio ≥30 mg/g | NA |
| ATP=Adult Treatment Panel; BMI=body mass index; HDL‐C=high‐density lipoprotein cholesterol; NA=not applicable; *active treatment of any criterion is considered the presence of that criterion, regardless of the value with treatment. | |||
BP is generally elevated in the metabolic syndrome, certainly above “optimal.” Since CV risk increases with systolic Bps >115 mm Hg, even pressures in the “high normal” range of <140/80 mm Hg may contribute to risk. 12 Insulin resistance, observed as hyperinsulinemia with or without glucose intolerance, is a key element, as is a type of atherogenic dyslipidemia commonly seen in diabetes: low HDL and high triglyceride levels. Obesity is abdominal or central, with increased visceral fat, representing the so‐called “apple‐shaped” as differentiated from the “pear‐shaped” configuration; the waist‐to‐hip ratio is specifically addressed in the WHO definition. Waist circumference is a clue to the amount of visceral fat, which while not necessary for the diagnosis, can be quantitated by abdominal CT scanning. Measurement of waist circumference should be made at the level of the umbilicus. A waist circumference of >40 inches for men or >35 inches for women represents an important criterion for the diagnosis of the metabolic syndrome. Additional criteria common to all three definitions include triglyceride concentrations >150 mg/dL and HDL concentrations <40 mg/dL for men and <50 mg/dL for women. There are differences in the requirements for glucose intolerance, with WHO and the International Diabetes Foundation suggesting that glucose concentrations >100 mg/dL indicate potential problems with glucose metabolism. Accurate identification of the extent of glucose intolerance requires measurement of both fasting and 2‐hour postload samples (Table II) 13 ; the 2‐hour sample is perhaps a better indicator of the dynamics of insulin secretion and glucose response.
Table II.
Diagnostic Criteria for Glucose Abnormalities
| Category | Fasting Glucose (mg/dL) | 2 Hour Postload Glucose (mg/dL)* |
|---|---|---|
| Normal | <110 | <140 |
| Impaired fasting glucose (IFG) | 110–125 | <140 |
| Impaired glucose tolerance (IGT) | <110 | 140–199 |
| IGT + IFG | 110–126 | 140–199 |
| Diabetes | ≥126 | >200 |
| *A 75‐g glucose load is used for evaluating 2‐hour postload glucose values. Adapted from Diabetes Care. 2003;26(suppl 1):S5–S20.13 | ||
ATP III and WHO list BP ≥130/85 mm Hg as a criterion for metabolic syndrome; however, there have been suggestions that a more appropriate level would be ≥130/80 mm Hg, or even ≥ 115/75 mm Hg. In diabetic patients, systolic BP is positively associated with the risk for CV death even at systolic pressure levels <120 mm Hg (Figure 2); the risk increases progressively as the level of systolic BP increases. 14
Figure 2.
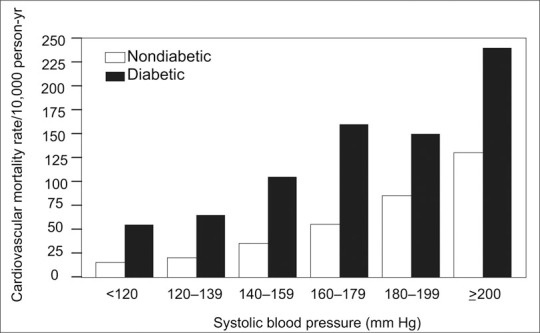
The impact of increasing systolic blood pressure on cardiovascular mortality in type 2 diabetes. Adapted from N Engl J Med. 2001;345:1291–1297. 12
Insulin resistance appears to drive the CV disease process. 15 For example, among individuals with type 2 diabetes, the prevalence of the metabolic syndrome is high, and those with diabetes and metabolic syndrome have the highest prevalence of CHD. Among all individuals with diabetes, the prevalence of CHD is increased compared with those with metabolic syndrome without diabetes. The prevalence of CHD in individuals with diabetes without findings of the metabolic syndrome, however, was no greater than in those with neither diabetes nor the metabolic syndrome. 16 Atherogenic changes in the prediabetic state are mainly seen in insulin‐resistant subjects. 17 Strategies to prevent type 2 diabetes should therefore focus primarily on insulin‐sensitizing interventions rather than those which may increase insulin resistance and may exert a negative impact on CV risk.
It remains unclear whether the metabolic syndrome represents a constellation of individual but etiologically unrelated abnormalities, or an actual “disease” with a central abnormality expressed as the various components. There may well be a genetic component. A mitochondrial transfer RNA mutation has been identified that leads to a syndrome characterized by hypertension, hyper‐cholesterolemia, and hypomagnesemia. 18 Insulin deficiency, central obesity, and insulin resistance are closely related (Figure 3); obesity may be the initiating factor, followed by insulin resistance, and ultimately insulin deficiency that may be either relative or absolute. Alternatively, insulin resistance may represent the initial event, followed by obesity, and subsequently an insulin‐deficient state may result from an inability of pancreatic islet cells to maintain an adequate insulin output.
Figure 3.
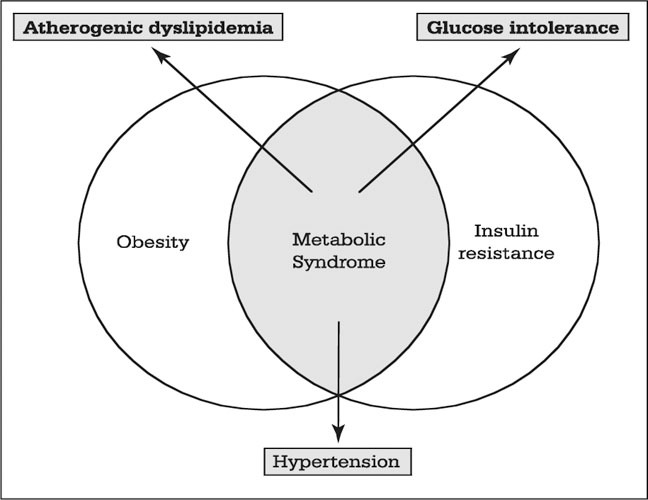
The interrelationship among central obesity, insulin resistance, and the metabolic syndrome
Central obesity is a unique and critical factor in the metabolic syndrome. All fat cells are not created equal; activities in the small adipocytes in the abdominal visceral fat account for many of the metabolic changes noted in the metabolic syndrome. Visceral adipocytes or fat cells are metabolically active. Visceral adipocytes are insulin resistant, with increased expression of adrenergic receptors, increased catecholamine‐mediated lipolysis, and increased insulin‐mediated antilipolysis, all leading to increased release of plasma free fatty acids. Among the other substances produced by these cells are prostaglandins, angiotensinogen, free fatty acids, the prothrombotic plasminogen activator inhibitor‐1, and inflammatory cytokines such as interleukin‐6 and tumor necrosis factor‐α. Other adipocytes (such as those in subcutaneous fat), in contrast, are composed of small insulin‐sensitive cells with a decreased expression of adrenergic receptors.
The pathophysiologic impact of some of these products is illustrated in Figure 4. Increased angiotensinogen, converted to angiotensin I by renin, leads to production of angiotensin II and aldosterone, and ultimately to vascular constriction. Increased free fatty acids lead to decreased hepatic insulin clearance and hyperinsulinemia, in turn increasing renal sodium reabsorption and ultimately elevations in BP. Inflammation and a prothrombotic state are also enhanced.
Figure 4.
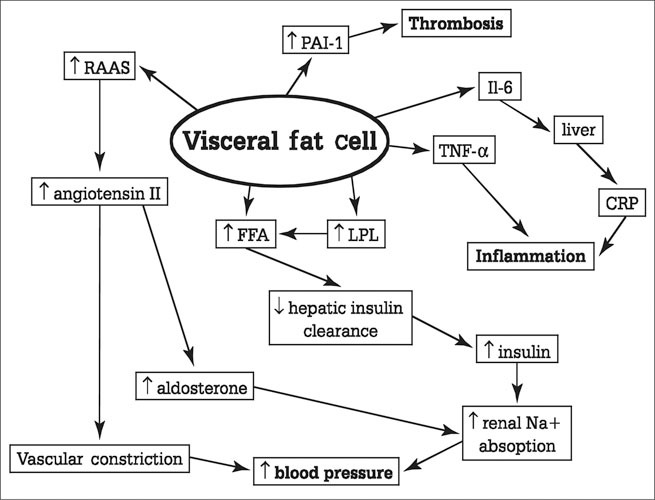
Substances secreted by the visceral fat cell and their relationship to the pathophysiologic features of the metabolic syndrome. ↑=increased; RAAS=renin‐angiotensin‐aldosterone system; PAI‐1=plasminogen activator inhibitor‐1; Il‐6=interleukin‐6; TNF‐α=tumor necrosis factor‐α; CRP=C‐reactive protein; FFA=free fatty acid; LPL=lipoprotein lipase
Inflammation alone contributes significantly to CV risk. C‐reactive protein, produced primarily by the liver when stimulated by interleukin‐6, has a greater predictive value for the development of high BP in middle‐aged normotensive men than does waist girth. 19 This observation is also true for cigarette smoking 20 ; the inhalation of advanced glycosylation end products used in curing tobacco can induce a chronic inflammatory response.
Many individuals with prehypertension, i.e., systolic BP between 120 and 139 mm Hg and diastolic BP between 81 and 89 mm Hg, have increased concentrations of inflammatory markers. 21
MANAGEMENT
The two main goals in the management of patients with the metabolic syndrome are the prevention of progression to overt type 2 diabetes and the prevention of CV disease. ATP III recommendations state that the primary management of the metabolic syndrome should be directed toward reversal of the underlying causes, namely overweight/obesity and physical inactivity. Such therapeutic lifestyle changes, described in more detail in Table III, include caloric restriction, particularly of simple carbohydrates and saturated fats; increase in dietary fiber; increased and regular aerobic exercise; and weight loss. In addition, certain lipid and nonlipid factors (increased BP, the prothrombotic or hypercoagulable state, and dyslipidemia) require specific treatment; such treatment considerations are listed in Table IV and are discussed in detail below.
Table III.
ATP III: Therapeutic Lifestyle Changes
| Dietary regimen |
| • Reduction in saturated fats and cholesterol |
| • Total fat limited to no more than 30% of intake, with specific reduction in trans fatty acids |
| • Add three tablespoons of plant stanols/sterols in margarine form |
| • Add five to nine daily servings of fiber (fruits, vegetables, oat bran, psyllium seed) |
| Regular physical activity—at least 30 minutes daily of such activities as walking, climbing stairs, housework, or yardwork |
| Weight loss |
| Adapted from National Cholesterol Education Program Adult Treatment Panel (ATP) III guidelines. JAMA 2001;285:2486–2497.5 |
Table IV.
Metabolic Syndrome—Pharmacologic Interventions
| Abnormality | Treatment Options |
|---|---|
| Hypertension | Angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker initial therapy, adding calcium channel blocker* or lowdose thiazide as needed |
| Dyslipidemia | Statin or fibrate or combination; fibrate preferred for triglycerides >500 mg/dL |
| Glucose intolerance | Metformin, thiazolidinedione, or combination; avoid insulin if at all possible |
| Prothrombotic state | Aspirin |
| *Nondihydropyridine preferred if microalbuminuria or proteinuria present | |
Lifestyle Modifications
The importance of lifestyle interventions cannot be overestimated. The Diabetes Prevention Program (DPP) 22 demonstrated that in nondiabetic individuals at high risk for development of diabetes, lifestyle modifications reduced the incidence of new diabetes by 58% vs. 31% by metformin. The Finnish Diabetes Prevention Study 23 was able to demonstrate a 58% reduction in the risk of diabetes in a group of overweight subjects with impaired glucose tolerance who underwent extensive lifestyle intervention including individualized counseling aimed at reducing weight and total intake of fat (particularly saturated fat) and increasing intake of fiber, plus increasing physical activity. In a comparative study over a 3‐year period, the Chinese Prevention Trial 24 demonstrated a 43% reduction in the development of diabetes by a diet and exercise program, but there was an 88 % reduction with acarbose and a 77% reduction with metformin. A 5‐lb weight loss in obese subjects has been estimated to produce a 10% reduction in BP. 25
Dyslipidemia
Specific pharmacologic therapy includes statins and fibrates. A recently published meta‐analysis of data from 25 studies evaluating the effect of statin therapy in CHD patients demonstrated a 16% reduction in all‐cause mortality, a 23% reduction in CHD mortality, and a 25% reduction in CHD mortality or nonfatal myocardial infarction. 26 The Heart Protection Study (HPS) 27 demonstrated that major acute coronary events are reduced as much in diabetic subjects as in nondiabetics. These studies, however, focused primarily on reducing LDL cholesterol levels. The particular dyslipidemia of the metabolic syndrome is not, however, generally characterized by high LDL levels, but rather by average‐to‐low LDL levels with low levels of HDL and high levels of triglycerides and atherogenic small dense lipoprotein particles, including very low‐density lipoprotein remnants rich in apolipoprotein C‐III content. The fibrates, as peroxisome proliferator‐activated receptor (PPAR)‐α activators, may be particularly suited pharmacologically to treat this profile of dyslipidemia. Gemfibrozil was tested in the Veterans Affairs Cooperative Studies Program High‐Density Lipoprotein Cholesterol Intervention Trial (VA‐HIT) 28 in patients with coronary events but relatively low LDL levels. At 1 year, the mean HDL level was 6% higher, the mean triglyceride level was 31% lower, and the mean total cholesterol level was 4% lower in the gemfibrozil group than in the placebo group; LDL levels did not differ significantly between the groups. After a median of 5.1 years, the investigators observed a 24% reduction in the combined outcome of death from CHD, nonfatal myocardial infarction, and stroke. Fenofibrate has been demonstrated to be more effective than atorvastatin in increasing HDL levels in nondiabetic subjects with low HDL levels (13.3% vs. 5.3%, respectively). 29
The Diabetes Atherosclerosis Intervention Study (DAIS) evaluated the ability of micronized fenofibrate to reduce the angiographic progression of coronary artery disease in type 2 diabetic subjects with dyslipidemia but with reasonably good glycemic control (mean glycosylated hemoglobin level, 7.5%). 30 The fenofibrate group showed a significantly smaller increase in diameter stenosis, a smaller decrease in minimal luminal diameter, and a nonsignificant, smaller decrease in mean segment diameter. Although the trial was not powered to detect clinical end points, there were fewer in the fenofibrate group than in the placebo group. Fenofibrate significantly reduced levels of total cholesterol, LDL, and triglycerides, and increased HDL levels, relative to placebo. Combination therapy with a statin and a fibrate may yield even greater benefits. When patients with mixed hyperlipidemias were randomized to receive either atorvastatin, fenofibrate, or the combination, lipoproteins were changed to a greater extent with combined therapy than with either drug alone.
Flow‐mediated dilator responses to hyperemia and plasma high‐sensitivity C‐reactive protein and fibrinogen levels were changed to a greater extent with combined therapy when compared with atorvastatin or fenofibrate alone. The effects of combined therapy or fenofibrate alone on plasma adiponectin levels and insulin sensitivity were greater than those of atorvastatin alone. 31
Hypertension
Effective reduction of BP is perhaps the most important element in reducing CV risk. Results from the United Kingdom Diabetes Prevention Study (UKDPS) 32 indicated that each 10 mm Hg decrease in mean systolic BP reduced all CV event rates, both microvascular and macrovascular, by 12%; there was also a 12% reduction for any complication related to diabetes, 15% for deaths related to diabetes, 11% for myocardial infarction, and 13% for microvascular complications. As further demonstrated in UKPDS data, “tight” BP reduction (an achieved BP of 144/82 mm Hg compared with 154/87 mm Hg, a difference of 10/5 mm Hg) is more effective than “less tight” glucose reduction in achieving event reduction. 33 Results of treatment of the diabetic cohort in the Systolic Hypertension in the Elderly Program (SHEP) 34 demonstrate the significant benefit of BP reduction, despite the fact that the target pressures were considerably above what we now consider appropriate. The 5‐year major CV disease event rate was lower by 34% for active treatment compared with placebo, both for diabetic patients and nondiabetic patients; the absolute risk reduction, however, with active treatment compared with placebo, was twice as great for diabetic compared with nondiabetic patients (101/1000 vs. 51/1000 randomized participants at the 5‐year follow‐up), reflecting the higher risk present with diabetes. The Systolic Hypertension in Europe (SYS‐Eur) trial, 35 utilizing a treatment regimen based on the calcium channel blocker (CCB) nitrendipine, demonstrated a 76% reduction in CV mortality in diabetic patients as compared with a 13% reduction in nondiabetics.
In patients with diabetes, antihypertensive therapy should include agents that inhibit the renin‐angiotensin system 1 , 36 ; this recommendation is based on the established activation of the renin‐angiotensin system in diabetes and supported by data from numerous clinical trials. Such agents include the angiotensin‐converting enzyme (ACE) inhibitors and the angiotensin receptor antagonists (ARBs). The Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) 37 demonstrated that, although an amlodipine‐based regimen was more effective than a fosinopril‐based regimen in reducing systolic BP (−19 mm Hg vs. −13 mm Hg, respectively), the fosinopril group showed a significant reduction in the combined end point of myocardial infarction, stroke, and hospitalization for angina. The Appropriate Blood Pressure Control in Diabetes Trial (ABCD), 38 comparing treatments with nisoldipine‐ and enalapril‐based regimens, demonstrated similar findings. ACE inhibitors and ARBs appear to be equivalent in reduction of CV and renal risk, as demonstrated in a comparison of the ACE enalapril with the ABR telmisartan in a 5‐year trial in diabetic patients. 39 In another study, both an ACE inhibitor, an ARB, or their combination significantly reduced C‐reactive protein and oxidized LDL cholesterol serum levels in type 2 diabetes patients who were free of coronary artery disease, demonstrating a positive effect on inflammation and lipid peroxidation. 40
A number of trials have demonstrated that treatment with an ACE inhibitor‐ or ARB‐based regimen reduces the incidence of new diabetes relative to treatment with thiazides and β blockers, particularly when these latter drugs are combined. The differing effects of these drugs on progression to diabetes are revealed by analysis of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 41 data. Chlorthalidone, although more effective than lisinopril in reducing BP, was associated with a higher incidence of new diabetes after 4 years. CV outcome, was not, however, affected by this occurrence. A review of data from 58,010 patients treated in hypertension trials demonstrated that ACE inhibitors and ARBs reduced new diabetes by 20%, and CCBs by 16%, relative to thiazides and β blockers. 42 The absolute risk increase is 1%–3.5% between an ACE inhibitor and diuretic and about 1%–1.5% between a CCB and diuretic plus β blocker treatment. 43 It has been demonstrated in one study that diabetes occurring during treatment has the same adverse prognostic implications as does new‐onset diabetes in the absence of antihypertensive treatment, especially in higher‐risk patients, 44 although a follow‐up of the SHEP study did not confirm this. 45 The converse of this observation might be that treatment with thiazides is associated with an increased rate of new diabetes, beginning in individuals with a fasting glucose as low as 90–95 mg/dL; in other words, in the normal range. Placebo‐controlled diuretic studies demonstrated a <10% increase in hyperglycemia or new‐onset diabetes. 46 Data from the International Verapamil‐Trandolapril Study (INVEST) 47 suggested that hydrochlorothiazide doses as small as 12.5 mg/d could offset the protective effect of ACE inhibition against the onset of new diabetes. It may be prudent therefore, despite the fact that the occurrence of new‐onset diabetes has not affected outcome in the clinical trials, to utilize thiazides and especially β blockers with caution in patients with the metabolic syndrome, and then only when necessary to achieve optimal BP reduction and only in combination with ACE inhibitors or ARBs to offset the hyperglycemic effect.
Avoidance of Progression to Diabetes
Studies discussed earlier that compared lifestyle modifications with treatment with active hypoglycemic agents such as metformin and acarbose demonstrated benefit for these pharmacologic agents. Another class of agents, the thiazolidinediones (pioglitazone and rosiglitazone), may deserve special consideration. These agents are PPAR‐γ agonists that exert not only hypoglycemic effects via insulin‐sensitizing effects, but exert significant effects on lipid metabolism and inflammation. 48 The PPARs are a family of nuclear receptors that exert profound effects on vascular function and lipid metabolism. The fibrates, as PPAR‐α agonists, increase apolipoprotein A‐I, apolipoprotein A‐II, lipoprotein lipase, and the activity of the scavenger receptor class B type 1 receptors and adenosine triphosphate‐binding cassette Al receptors; their efficacy has been considered earlier in this paper. The DPP was a randomized clinical trial to determine prevention of type 2 diabetes in high‐risk individuals; treatment arms included troglitazone, a PPAR‐γ agonist and insulin‐sensitizing agent no longer available for clinical use; metformin; lifestyle modifications; and placebo. 49 Troglitazone was withdrawn after a mean treatment period of 0.9 year. At that interval, however, the diabetes incidence rate with troglitazone was 3.0 cases/100 person‐years, compared with 12.0, G.I, and 5.1 cases/100 person‐years in the placebo, metformin, and intensive lifestyle‐moderation groups, respectively. This effect of troglitazone was in part due to improved insulin sensitivity with maintenance of insulin secretion. During the 3 years after troglitazone withdrawal, the diabetes incidence rate was almost identical to that of the placebo group. Due to the essential contribution of insulin resistance to the development of the metabolic syndrome, treatment with insulin should be avoided.
SUMMARY
Although the metabolic syndrome may be defined in several ways, certain key features include central obesity and insulin resistance leading to glucose intolerance and atherogenic dyslipidemia. The presence of metabolic syndrome predicts the development of both overt type 2 diabetes and CV disease with such accuracy that it should be considered a CV disease equivalent. Treatment should be directed at all aspects of the syndrome, beginning with lifestyle modifications, particularly for adolescents and young adults, and continuing with pharmacologic interventions as indicated to reduce BP, dyslipidemia, and glucose abnormalities. Although not specifically addressed in this paper, aspirin should be included as an antithrombotic agent; doses between 75 mg/d and 325 mg/d appear equally efficacious. 50 A clinical trial has now clearly demonstrated that aggressive treatment of individual abnormalities markedly improves clinical outcomes. Steno‐2, 51 a trial in type 2 diabetic subjects, randomly assigned 80 patients to receive conventional treatment in accordance with national guidelines and 80 to receive intensive treatment, with a stepwise implementation of behavior modification and pharmacologic therapy that targeted hyperglycemia, hypertension, dyslipidemia, and microalbuminuria, along with secondary prevention of CV disease with aspirin. Treatment goals included glycosylated hemoglobin concentrations <6.5%, BP <130/80 mm Hg, total cholesterol levels <175 mg/dL, and triglyceride levels <150 mg/dL. Patients receiving intensive therapy had a significantly lower risk of CV disease (hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.24–0.73), nephropathy (HR, 0.39; 95% CI, 0.17–0.87), retinopathy (HR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (HR, 0.37; 95% CI, 0.18–0.79).
The critical importance of recognizing and treating the metabolic syndrome is now clear. Guidelines are available to appropriately characterize individuals with this syndrome, as well as to suggest appropriate treatment protocols. BP control should probably begin with, or certainly include, antihypertensive agents such as ACE inhibitors and ARBs that block the renin‐angiotensin‐aldosterone system. Dyslipidemias are addressed primarily with statins and fibrates, with fibrates perhaps preferred in the presence of very high triglyceride levels (>500 mg/dL) and low HDL levels; combinations of these drugs may have additional benefits over monotherapy. Early treatment for glucose intolerance might well include the insulin‐sensitizing agent metformin and, in some instances, thiazolidinediones; metformin presents an initial advantage of associated weight loss. Insulin should be avoided if at all possible. The message is to treat aggressively and to treat to targets; this will result in maximal patient benefit.
References
- 1. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13(suppl 1, pt 2):3S–10S. [DOI] [PubMed] [Google Scholar]
- 3. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 4. American Heart Association. Heart Disease and Stroke Statistic–2005 Update. Available at: http://www.americanheart.orgpresenter.jhtml?identifier=3018163. Accessed June 15, 2005.
- 5. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 6. Wilson PW, Grundy SM. The metabolic syndrome: practical guide to origins and treatment, I. Circulation. 2003; 108:1422–1424. [DOI] [PubMed] [Google Scholar]
- 7. Wilson PW, Grundy SM. The metabolic syndrome: a practical guide to origins and treatment, II. Circulation. 2003; 108:1537–1540. [DOI] [PubMed] [Google Scholar]
- 8. Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003; 108:414–419. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva , Switzerland : World Health Organization; 1999. Available at: http:whqlibdoc.who.inthq1999WHO_NCD_NCS_99.2.pdf. Accessed June 15, 2005. [Google Scholar]
- 10. International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels , Belgium : International Diabetes Federation; 2004. Available at: http:www.idf.orgwebdatadocsMetabolic_syndrome_ definition.pdf. Accessed June 15, 2005. [Google Scholar]
- 11. Scuteri A, Najjar SS, Morrell CH, et al. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005; 28:882–887. [DOI] [PubMed] [Google Scholar]
- 12. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001; 345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 14. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16:434–444. [DOI] [PubMed] [Google Scholar]
- 15. Hanley AJ, Williams K, Stern MP, et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002; 25:1177–1184. [DOI] [PubMed] [Google Scholar]
- 16. Third National Health and Nutrition Examination Survey (NHANES III). National Cholesterol Education Program (NCEP). NCEP‐defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003; 52:1210–1214. [DOI] [PubMed] [Google Scholar]
- 17. Haffner SM, Mykkanen L, Festa A, et al. Insulin‐resistant prediabetic subjects have more atherogenic risk factors than insulin‐sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000; 101:975–980. [DOI] [PubMed] [Google Scholar]
- 18. Wilson FH, Hariri A, Farhi A, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004; 306:1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niskanen L, Laaksonen DE, Nyyssönen K, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004; 44:859–865. [DOI] [PubMed] [Google Scholar]
- 20. Ikonomidis I, Lekakis J, Vamvakou G, et al. Cigarette smoking is associated with increased circulating proinflammatory and procoagulant markers in patients with chronic coronary artery disease. Am Heart J. 2005; 149:832–839. [DOI] [PubMed] [Google Scholar]
- 21. Chae CU, Lee RT, Rifai N, et al. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001; 38:399–403. [DOI] [PubMed] [Google Scholar]
- 22. Diabetes Prevention Trial‐Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002; 346:1685–1691. [DOI] [PubMed] [Google Scholar]
- 23. Tuomilehto J, Lindstrom J, Eriksson JG, et al, for the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 24. Yang W, Lin L, Qi J, et al. The preventive effect of acarbose and metformin on the progression to diabetes mellitus in the impaired glucose tolerance population: a 3‐year multicenter prospective study. Chin J Endocrinol Metab. 2001; 17:131–136. [Google Scholar]
- 25. Reisin E, Frohlich ED, Messerli FH, et al. Cardiovascular changes after weight reduction in obesity hypertension. Ann Intern Med. 1983; 98:315–319. [DOI] [PubMed] [Google Scholar]
- 26. Wilt TJ, Bloomfield HE, MacDonald R, et al. Effectiveness of statin therapy in adults with coronary heart disease. Arch Intern Med. 2004; 164:1427–1436. [DOI] [PubMed] [Google Scholar]
- 27. Collins R, Armitage J, Parish S, et al, for the Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003; 361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 28. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high‐density lipoprotein cholesterol. Veterans Affairs High‐Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999; 341:410–418. [DOI] [PubMed] [Google Scholar]
- 29. Despres JP, Lemieux I, Salomon H, et al. Effects of micronized fenofibrate versus atorvastatin in the treatment of dyslipidemic patients with low plasma HDL‐cholesterol levels: a 12‐week randomized trial. J Intern Med. 2002; 251:490–499. [DOI] [PubMed] [Google Scholar]
- 30. Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary‐artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001; 357:905–909. [PubMed] [Google Scholar]
- 31. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005; 45:1649–1653. [DOI] [PubMed] [Google Scholar]
- 32. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000; 321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998; 317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 34. Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996; 276:1886–1892. [PubMed] [Google Scholar]
- 35. Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effects of calcium‐channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999; 340:677–684. [DOI] [PubMed] [Google Scholar]
- 36. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000; 36:646–661. [DOI] [PubMed] [Google Scholar]
- 37. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998; 21:597–603. [DOI] [PubMed] [Google Scholar]
- 38. Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. N Engl J Med. 1998; 338:645–652. [DOI] [PubMed] [Google Scholar]
- 39. Barnett A, Bain SC, Bouter P, et al, for the Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004; 351:1952–1961. [DOI] [PubMed] [Google Scholar]
- 40. Koulouris S, Symeonides P, Triantafyllou K, et al. Comparison of the effects of ramipril versus telmisartan in reducing serum levels of high‐sensitivity C‐reactive protein and oxidized low‐density lipoprotein cholesterol in patients with type 2 diabetes mellitus. Am J Cardiol. 2005; 95:1386–1388. [DOI] [PubMed] [Google Scholar]
- 41. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in highrisk hypertensive patients randomized to angiotensinconverting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 42. Opie LH, Schall R. Old antihypertensives and new diabetes. J Hypertens. 2004; 22:1453–1458. [DOI] [PubMed] [Google Scholar]
- 43. Moser M. New‐onset diabetes in the hypertension treatment trials: a point of view. J Clin Hypertens (Greenwich). 2004; 6:610–613. [DOI] [PubMed] [Google Scholar]
- 44. Verdecchia P, Reboldi G, Angeli F, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004; 43:963–969. [DOI] [PubMed] [Google Scholar]
- 45. Kostis JB, Wilson AC, Freudenberger RS, et al. Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005; 95:29–35. [DOI] [PubMed] [Google Scholar]
- 46. Moser M. Suppositions and speculations—their possible effects on treatment decisions in the management of hypertension. Am Heart J. 1989; 118:1362–1369. [DOI] [PubMed] [Google Scholar]
- 47. Pepine CJ, Handberg EM, Cooper‐DeHoff RM, et al, for the INVEST Investigators. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003; 290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 48. Nagashima K, Lopez C, Donovan D, et al. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005; 115:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knowler WC, Hamman RF, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the diabetes prevention program. Diabetes. 2005; 54:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Antiplatelet Trialist Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994; 308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 51. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003; 348:383–393. [DOI] [PubMed] [Google Scholar]


