Abstract
Platelet and white blood cell counts are higher among some insulin‐resistant patients and may contribute to atherothromboembolic complications. Metabolic syndrome patients are insulin resistant, often hypertensive, and at high cardiovascular disease risk, yet the relationship of platelets to the metabolic syndrome is unknown. Platelet and white blood cell counts were obtained from 135 volunteers who had measurements of blood pressure, fasting triglycerides, high‐density lipoprotein cholesterol, and glucose. A body mass index >30 kg/m2 served as a surrogate for increased waist circumference. Subjects were subdivided into three groups by the number of metabolic syndrome criteria, i.e., no metabolic syndrome risk factor (MS–0; n=40), one or two metabolic syndrome risk factors (MS1–2; n=61), and three to five metabolic syndrome risk factors (MS3–5; n=34). Platelet counts were increased significantly from 226±8 to 257±8 and 276±10 (×103/mm3) in the MS—0, MS1–2, and MS3–5 groups, respectively (p<0.01), after adjustment for age, gender, ethnicity, total cholesterol, and low‐density lipoprotein cholesterol. White blood cell counts were also increased across the three groups (5.4±0.2, 6.2±0.2, and 6.6±0.3 [×1037/mm3]; p<0.01) after multivariate adjustment. Compared with patients with zero to two metabolic syndrome risk factors, metabolic syndrome patients have higher platelet and white blood counts, which may serve as markers of a prothrombotic and proinflammatory state and contributors to atherothromboembolic risk.
Obesity is associated with a proinflammatory, prothrombotic state that could play a role in accelerating cardiovascular disease (CVD). 1 , 2 , 3 , 4 , 5 Obesity is also associated with insulin resistance, hypertension, and the metabolic syndrome; these are characterized by a clustering of cardiovascular risk factors and elevation of markers indicating a proinflammatory and prothrombotic state.( 6 , 7 , 8 Several inflammatory markers, including C‐reactive protein, 9 fibrinogen, 10 white blood cell (WBC) counts, 11 , 12 , 13 sialic acid, 14 and to a lesser extent factor VIII and von Willebrand factor 10 , 11 , 12 , 13 , 14 , 15 are positively associated with atherosclerosis and an increased incidence of coronary artery disease.
Metabolic syndrome patients have higher leukocyte counts than patients without the metabolic syndrome. 16 Higher platelet and leukocyte counts are associated with atherosclerosis and increased morbidity and mortality from CVD. 17 , 18 WBC counts are also higher in adults who have risk factors such as cigarette smoking and a family history of CVD than in the normal population. 19 , 20 Primary (essential) and secondary thrombocytosis are also linked to thrombotic and/or embolic events in the cerebral, coronary, and peripheral arterial circulation. 21 , 22 Other evidence suggests that platelet counts are higher and contribute to vascular events in patients with other risk factors, e.g., insulin resistance. 23 , 24 , 25 , 26 Thus, platelet counts emerge as another potentially useful marker of CVD risk. Although many reports have focused on platelet function and the benefits of pharmacologic intervention to alter platelet function, the relationship between platelet count and CVD risk has generated less interest.
Given the preceding information, this report examines the relationship of metabolic syndrome risk factors to platelet and leukocyte counts in volunteers with a spectrum of metabolic syndrome risk factors.
METHODS
Subjects
One hundred thirty‐five volunteers were recruited by advertisement to participate in various research protocols. All subjects signed written informed consent documents approved by the Office of Research Protection and Integrity at the Medical University of South Carolina. All volunteers were nonsmokers, 21–9 years old, who underwent screening measurements to determine eligibility for participation in the full protocol. The baseline data in this report were obtained during the screening visit. Patients on medications that could affect platelet counts and/or function were excluded, including 15 women receiving hormonal birth control medications and one patient taking aspirin.
At the screening visit all volunteers had a history, physical examination, electrocardiogram (ECG), and screening laboratory tests in the fasting state. The laboratory tests included complete blood cell count, automated chemistry profile, lipid profile, and urinalysis. Women of child‐bearing potential were required to have a negative urine pregnancy test before participation in this study.
Measurements. Height was measured with a standard stadiometer to the nearest 0.5 cm or 0.25 inch in subjects without shoes in the upright position. Weight was measured with a calibrated beam balance scale to the nearest 0.2 lb or 0.1 kg. Body mass index (BMI) was calculated in kg/m2. Blood pressure (BP) was measured with a mercury sphygmomanometer after 10 minutes of rest with subjects seated. Three readings were taken in the sitting position with 2 minutes between readings. BP was determined by the average of the second and third values.
Complete Blood Cell Count. Blood was drawn from the antecubital fossa with atraumatic venipuncture into a vacutainer containing ethylenediaminetetraacetic acid. Samples were placed on the CELL‐DYN 4000 System (Abbott Laboratories, Abbott Park, IL), which counts, sizes, and classifies blood cells and platelets by flow cytometry using optical scatter/fluorescence and electrical impedance technologies as a quality check for optical platelet count. On rare occasions when measurements did not match, they were confirmed by manual count of the blood smear, and the number closest to the manual was considered the actual count. Mean platelet volume was measured by electrical impedance.
Glucose and Lipid Profile. Venous blood was drawn after 12 hours' fasting into a serum separator tube and sent for analysis of fasting glucose and lipid profile for every subject simultaneously with the platelet and WBC samples. The quantitative determination of glucose, cholesterol, triglycerides, and high‐density lipoprotein (HDL) in the plasma were done on a SYNCHRON LX System (Beckman Coulter, Inc., Fullerton, CA). Glucose concentration was determined by an oxygen rate method employing the Beckman oxygen electrode. 27 , 28 Specific reagents were used to measure cholesterol and triglyceride concentrations by the timed end point method. 29 , 30 HDL was measured by a direct homogeneous assay in which a unique detergent solubilizes only the HDL particles and releases HDL cholesterol to react with cholesterol esterase and cholesterol oxidase in the presence of chromogens to produce a color product. Low‐density lipoprotein (LDL) cholesterol and very LDL cholesterol were calculated from total cholesterol, HDL, and triglyceride concentrations using the Friedewald equation. 31
Protocol. Patients reported to the General Clinical Research Center after an overnight fast for the initial screening visit. The written informed consent document was reviewed and signed. Bps were measured, and a complete physical examination and ECG were performed. Blood was drawn and sent to the laboratory. All evaluations were performed in the research center clinic in the morning between 8 a.m. and 10 a.m.
Statistical Analyses. Data are presented as mean ± SEM. Statistical analyses were conducted with SAS version 9.1 (SAS Institute Inc., Cary, NC). The chi‐square test was used to analyze the categoric differences between the groups. Multiple linear regression analysis was used to assess the relationships between BMI, platelet counts, and WBC counts. Analysis of variance was used to test for significant difference in means among the three groups. Analysis of covariance was used to test for mean differences between groups, while adjusting for potential confounding variables, and p values <0.05 were considered statistically significant.
RESULTS
As shown in Table I, patients were divided into three groups based on the number of metabolic syndrome criteria they met using the Adult Treatment Panel III (ATP III) criteria: values for BP ≥130/85 mm Hg, fasting glucose ≥110 mg/dL, triglycerides ≥150 mg/dL, and HDL cholesterol <40 mg/dL for men and <50 mg/dL for women. 32 , 33 , 34 , 35 Waist circumference was not available for all subjects. Thus, a BMI >30 kg/m2, which is a component of the World Health Organization definition of the metabolic syndrome, was used. 35 Based on previous research, ATP III waist circumference criteria are likely to be met in men and women when their BMI is ≥28.8 kg/m2. 32 , 33 Patients with a BMI >30 kg/m 2 are likely to meet the ATP III waist circumference criterion for the metabolic syndrome. 35 Forty volunteers did not have any metabolic syndrome risk factors (MS–0), 61 patients had one or two risk factors (MS1–2), and 34 subjects had three or more risk factors (MS3–5) and met the modified metabolic syndrome definition.
Table II.
Metabolic Syndrome Risk Factors Present in the Study Groups With Risk Factors
| No. of Risk Factors | ||
|---|---|---|
| Risk Factor | 1 or 2 (n=61) | 3–5 (n= 34) |
| Body mass index ≥30 kg/m2 | 29 (47.5) | 33 (97.1) |
| Systolic BP ≥130 mm Hg | 27 (44.3) | 24 (70.6) |
| Diastolic BP ≥85 mm Hg | 29 (47.5) | 26 (76.5) |
| Triglycerides ≥150 mg/dL | 5 (8.2) | 13 (38.2) |
| High‐density lipoprotein cholesterol | ||
| Men: ≥40 mg/dL | 13 (21.3) | 9 (26.5) |
| Women: ≥50 mg/dL | 13 (21.3) | 20 (58.8) |
| Glucose >110 mg/dL | 0 (0) | 10 (29.4) |
| Data are listed as n (%). BP=blood pressure | ||
Demographic characteristics varied across the three groups (Table I). Mean age and the proportion of African Americans increased significantly across the three groups as shown. Although the proportion of women tended to increase with the number of metabolic syndrome risk factors, the trend was not significant. As expected, the three groups showed significant differences in mean values for the five metabolic syndrome risk factors, including BMI, systolic and diastolic BP, triglycerides, HDL cholesterol, and fasting glucose. Platelet counts showed a significant stepwise increase from the MS–0 to the MS1–2 and MS3–5 groups. WBC counts showed the same significant upward trend across the three study groups. Platelet and leukocyte counts showed a significant positive correlation (r=0.30; p<0.0005); BMI correlated positively with both platelet (r=0.29; p<0.001) and WBC (r=0.26; p=0.002) counts.
Table I.
Clinical Characteristics of the Patients
| No. of Metabolic Syndrome Risk Factors | ||||
|---|---|---|---|---|
| Variable (n or Mean± SEM) | 0 | 1 or 2 | 3–5 | pValue |
| No. of patients | 40 | 61 | 34 | |
| Gender (female/male) | 20/20 | 34/27 | 22/12 | 0.44 |
| Race (black/white) | 14/26 | 30/31 | 23/11 | <0.05 |
| Age (yr) | 34.7±1.3 | 36.2±1.0 | 38.9±1.1 | <0.05 |
| Body mass index (kg/m2) | 23.4±0.4 | 29.6±0.8 | 36.4±1.0 | <0.0001 |
| Systolic BP (mm Hg) | 111.1±1.4 | 127.2±20 | 135.7±2.4 | <0.0001 |
| Diastolic BP (mm Hg) | 72.1±1.2 | 83.1±1.5 | 90.0±1.7 | <0.0001 |
| Total cholesterol (mg/dL) | 182.3±5.6 | 192.1±5.2 | 190.9±7.0 | 0.443 |
| Triglycerides (mg/dL) | 66.3±4.6 | 84.3±5.0 | 130.6±13.9 | <0.0001 |
| High‐density lipoprotein cholesterol (mg/dL) | 56.9±1.9 | 48.5±1.7 | 38.6±1.4 | <0.0001 |
| Low‐density lipoprotein cholesterol (mg/dL) | 112.1±4.8 | 127.0±4.5 | 126.2±5.9 | <0.075 |
| Very low‐density lipoprotein cholesterol (mg/dL) | 13.3±0.9 | 16.9±1 | 26.1±2.8 | <0.0001 |
| Glucose (mg/dL) | 85.0±1.4 | 90.1±1.2 | 104.3±4.8 | <0.0001 |
| White blood cells (×103/mm3) | 5.4±0.2 | 6.2±0.2 | 6.6±0.3 | <0.02 |
| Red blood cells (×106/mm3) | 4.8±0.1 | 4.8±0.1 | 4.8±0.1 | 0.851 |
| Hematocrit (%) | 42.2±0.7 | 41.9±0.6 | 41.7±0.7 | 0.874 |
| Platelets (×103/mm3) | 226±8 | 257±8 | 276±10 | <0.001 |
| Mean platelet volume (fL) | 8.9±0.20 | 8.8±0.1 | 8.5±0.2 | 0.340 |
| BP=blood pressure | ||||
The percentage of metabolic syndrome risk factors present in each of the three groups is shown in Table II. By definition, the MS–0 group had no risk factors. As expected, the MS3–5 group had a higher percentage of every metabolic syndrome risk factor than the MS1–2 group. Five metabolic syndrome patients had uncomplicated type 2 diabetes.
A multivariable analysis of covariance model was used to adjust for selected variables including age, gender, ethnicity, total cholesterol, LDL cholesterol, and hemoglobin (Table III). The stepwise increase in platelet and WBC counts across the three study groups remained significant in this model, as shown in Table III and Figures 1A and 1B.
Table III.
Multivariable Analysis of Covariance Model to Determine the Effect of the Metabolic State on Platelet and White Blood Cell Counts With Adjustment for the Variables Shown
| Platelets (×103) | White Blood Cells (×103) | |||
|---|---|---|---|---|
| Variable | Coefficient | pValue | Coefficient | pValue |
| No. of risk factors | ||||
| 0 | ||||
| 1 or 2 | 28.6 | 0.01 | 0.82 | 0.02 |
| 3–5 | 42.6 | 0.003 | 1.26 | 0.003 |
| Gender | ||||
| Male | −20.8 | 0.10 | −0.19 | 0.64 |
| Female | ||||
| Race | ||||
| Black | 16.5 | 0.12 | −0.48 | 0.16 |
| White | ||||
| Age | 0.24 | 0.73 | 0.03 | 0.25 |
| Hemoglobin | −3.02 | 0.49 | −0.05 | 0.74 |
| Total cholesterol | 0.60 | 0.08 | 0.02 | 0.08 |
| Low‐density lipoprotein cholesterol | −0.55 | 0.16 | −0.02 | 0.14 |
Figure.
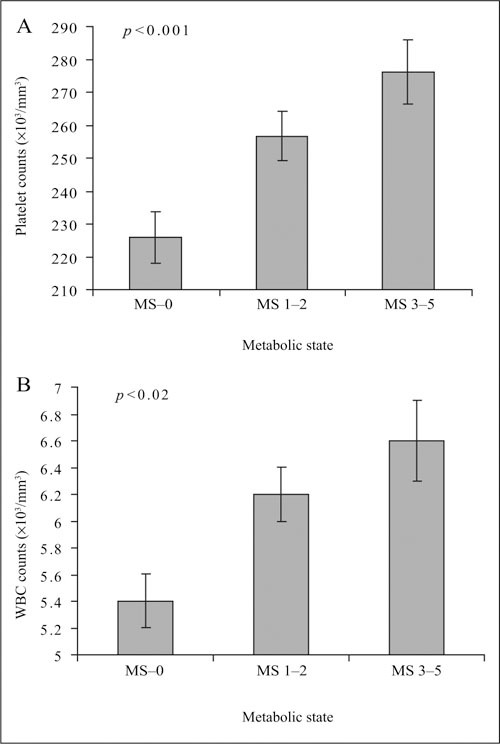
Platelet counts (A) and white blood cell (WBC) counts (B) are depicted (mean ± SEM) for the three groups of patients with no metabolic syndrome risk factors (MS–0), one or two risk factors (MS 1–2), and three to five risk factors (MS 3–5). Platelet and WBC counts increased significantly with the number of metabolic syndrome risk factors.
DISCUSSION
Our study shows that platelet and WBC counts are higher in patients with than without the metabolic syndrome. Both platelet and WBC counts are positively related to the number of metabolic syndrome risk factors (Figure 1A, 1B). The relationships are independent of several non‐metabolic syndrome variables. Moreover, platelet and WBC counts are directly correlated, which suggests that some factor directly or indirectly related to the metabolic syndrome may be driving the elevation of both. BMI, which is strongly related to the metabolic syndrome, 36 is positively correlated with both platelet and WBC counts.
The metabolic syndrome strongly and independently increases risk for heart disease, stroke, and chronic kidney disease. 37 , 38 , 39 The relationship of the metabolic syndrome to CVD risk remains after adjustment for traditional risk factors including age, BP, total cholesterol, and LDL cholesterol. 37 , 38 Moreover, research cited earlier in this paper implicates platelets and leukocytes in the atherothrombotic process and related complications. Thus, elevations of these two markers, which are indicative of a proinflammatory and prothrombotic state, may contribute to the independent risk associated with the metabolic syndrome.
There are a limited number of reports that have focused on platelet number and its possible association with risk factors, inflammatory markers, and atherothrombotic complications. One study linked platelets to insulin resistance. In that study, the homeostasis model assessment index of insulin resistance (HOMA‐IR) was independently predicted by BMI, glycosylated hemoglobin, platelet count, and serum triglycerides. 23 Another investigation reported an increase in total platelet counts in insulin‐dependent diabetics with overt nephropathy compared with those without. 24 One report suggested platelet count and function were prognostic indicators of future vascular complications in type 1 diabetics. 25 Turakhia et al. 26 noted that higher platelet counts may be a marker of coronary or systemic inflammation, which is associated with angiographic outcomes similar to that for C‐reactive protein or WBC counts.
Of potential relevance to the metabolic syndrome, platelet counts can be nutritionally modulated. Rabbits fed a high‐cholesterol diet showed significant increases in platelet production and mean megakaryocyte volume. These changes were associated with the development of fatty plaques in the aorta. 40 In contrast, individuals habitually consuming a diet high in fish have suppressed platelet activity and reduced platelet counts. 41
Platelet counts can also be modified by physical activity. Physical activity is inversely related in a dose‐response fashion to platelet count and leukocyte counts, C‐reactive protein, fibrinogen, blood viscosity, factor VIII and IX, von Willebrand factor, fibrin D‐dimer, and tissue plasminogen activator antigen. 42 These observations suggest that platelets may be one of the transducing factors by which various metabolic and lifestyle factors affect CVD outcomes. The metabolic syndrome reflects an interplay of nutritional, physical activity, behavioral, and genetic factors that may simultaneously impact several metabolic, proinflammatory, and prothrombotic risk factors.
Obesity, insulin resistance, and diabetes are linked to higher levels of several inflammatory markers including interleukin (IL)‐6, which augments hepatic production of C‐reactive protein. 3 , 4 , 5 , 43 , 44 , 45 , 46 Administration of IL‐6 to healthy dogs raises platelet counts in a dose‐dependent fashion, 47 but does not alter the WBC count. Elevated levels of IL‐6 and platelet counts have been reported in diabetic patients with atherosclerosis. These patients also have polyploid megakaryocytes. 48 Collectively, these data raise the possibility that IL‐6 may contribute to the observed relationship between obesity (BMI), insulin resistance, diabetes and elevated platelet counts observed in numerous studies. 23 , 25 , 43 , 48
Previous studies indicate that the WBC count can serve as a surrogate marker for inflammation and increased CVD risk factors and events. 11 , 12 , 13 , 16 , 17 , 18 , 20 , 49 , 50 In patients in this study, leukocyte counts showed a progressive increase from the group with no metabolic syndrome risk factors to those with one or two and three to five metabolic syndrome risk factors. WBC counts correlated positively with metabolic syndrome criteria independent of several other variables (Table III). WBC counts correlated positively with platelet counts, which may suggest that a shared mechanism drives both the elevated platelet and WBC counts in patients with this syndrome. Of note, BMI, which is strongly related to the metabolic syndrome, 37 correlated positively with both platelet and leukocyte counts. The common mechanism underlying the association of obesity, the number of metabolic syndrome factors, and elevations of both platelets and WBCs are not elucidated by our study.
CONCLUSIONS
Platelet and WBC counts are higher in patients with than without the metabolic syndrome and rise in a “dose‐dependent” fashion with the number of metabolic syndrome risk factors. BMI is positively related to the metabolic syndrome and to platelet and WBC counts. Our database does not contain sufficient information to identify the variables affecting the relationship of all three factors, i.e., BMI, platelet count, and WBC count. Our findings and data from the literature, however, raise the possibility that the higher platelet and leukocyte counts in patients with the metabolic syndrome reflect and contribute to the inflammatory and atherothrombotic state in these patients. Elucidating the biologic links between these associations may enhance efforts to ameliorate metabolic syndrome‐related CVD risk. The data suggest that low‐dose aspirin is a reasonable recommendation for most metabolic syndrome patients.
Acknowledgments and disclosure: The authors are grateful to the General Clinical Research Center administrative, nursing, and laboratory staff for their expert assistance with the clinical research activities described in this report. This research was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute HL58794, which included a minority training supplement (ECO), HL04290, P60‐MD00267 (EXPORT), and General Clinical Research Center RR‐01070 from the National Center for Research Resources.
References
- 1. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999; 340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003; 91:3A–6A. [DOI] [PubMed] [Google Scholar]
- 3. Hukshorn CJ, Lindeman JH, Toet KH, et al. Leptin and the proinflammatory state associated with human obesity. J Clin Endocrinol Metab. 2004; 89:1773–1778. [DOI] [PubMed] [Google Scholar]
- 4. You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004; 89:5517–5522. [DOI] [PubMed] [Google Scholar]
- 5. Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003; 289:1799–1804. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism. 1996; 45:699–706. [DOI] [PubMed] [Google Scholar]
- 7. Egan BM, Papademetriou V, Wofford M, et al. Metabolic syndrome and insulin resistance: contrasting views in patients with high normal blood pressure. Am J Hypertens. 2005; 18:3–12. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults. (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353:1649–1652. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men [published correction appears in N Engl J Med. 1997;337:356]. N Engl J Med. 1997;336:973—979. [DOI] [PubMed] [Google Scholar]
- 10. Folsom AR, Wu KK, Rosamond WD, et al. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997; 96:1102–1108. [DOI] [PubMed] [Google Scholar]
- 11. Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974; 290:1275–1278. [DOI] [PubMed] [Google Scholar]
- 12. Yarnell JW, Baker IA, Sweetnam PM. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation. 1991; 83:836–844. [DOI] [PubMed] [Google Scholar]
- 13. Kostis JB, Turkevich D, Sharp J. Association between leukocyte count and the presence and extent of coronary atherosclerosis as determined by coronary angiography. Am J Cardiol. 1984; 53:997–999. [DOI] [PubMed] [Google Scholar]
- 14. Lindberg G, Rastam L, Gullberg B, et al. Serum concentrations of total sialic acid and sialoglycoproteins in relation to coronary heart disease risk markers. Atherosclerosis. 1993; 103:123–129. [DOI] [PubMed] [Google Scholar]
- 15. Meade TW, Cooper JA, Stirling Y, et al. Factor VIII, ABO blood group and the incidence of ischaemic heart disease. Br J Haematol. 1994; 88:601–607. [DOI] [PubMed] [Google Scholar]
- 16. Nagasawa N, Tamakoshi K, Yatsuya H, et al. Association of white blood cell count and clustered components of metabolic syndrome in Japanese men. Circ J. 2004; 68:892–897. [DOI] [PubMed] [Google Scholar]
- 17. Brown DW, Giles WH, Croft JB. White blood cell count: an independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol. 2001; 54:316–322. [DOI] [PubMed] [Google Scholar]
- 18. Lee CD, Folsom AR, Nieto FJ, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African‐American and white men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001; 154:758–764. [DOI] [PubMed] [Google Scholar]
- 19. Pannacciulli N, Giorgino F, Martina RA, et al. Effect of family history of type 2 diabetes on white blood cell count in adult women. Obesity Research. 2003; 11:1232–1237. [DOI] [PubMed] [Google Scholar]
- 20. Petitti DB, Kipp H. The leukocyte count: association with intensity of smoking and persistence of effect after quitting. Am J Epidemiol. 1986; 123:89–95. [DOI] [PubMed] [Google Scholar]
- 21. Bennett CL, Weinberg PO, Colub RM. Cost‐effectiveness model of a phase II clinical trial of a new pharmaceutical for essential thrombocythemia: is it helpful to policy makers? Semin Hematol. 1999;36(1 suppl 2):26–29. [PubMed] [Google Scholar]
- 22. Christenson JT. Preoperative lipid control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg. 1999; 16:399–400. [DOI] [PubMed] [Google Scholar]
- 23. Taniguchi A, Fukushima M, Seino Y, et al. Platelet count is independently associated with insulin resistance in non‐obese Japanese type 2 diabetic patients. Metabolism. 2003; 52:1246–1249. [DOI] [PubMed] [Google Scholar]
- 24. Sterner G, Carlson J, Ekberg G. Raised platelet levels in diabetes mellitus complicated with nephropathy. J Intern Med. 1998; 244:437–441. [PubMed] [Google Scholar]
- 25. Cho NH, Becker DJ, Ellis D, et al. Spontaneous whole blood platelet aggregation, hematological variables and complications in insulin‐dependent diabetes mellitus: the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications. 1992; 6:12–18. [DOI] [PubMed] [Google Scholar]
- 26. Turakhia MP, Murphy SA, Pinto TL, et al; Thrombolysis in Myocardial Infarction Study Group. Association of platelet count with residual thrombus in the myocardial infarctrelated coronary artery among patients treated with fibrinolytic therapy for ST‐segment elevation acute myocardial infarction. Am J Cardiol. 2004;94:1406–410. [DOI] [PubMed] [Google Scholar]
- 27. Kadish AH, Hall DA. A new method for the continuous monitoring of blood glucose by measurement of dissolved oxygen. Clin Chem. 1965; 11:869–875. [PubMed] [Google Scholar]
- 28. Kadish AH, Little RL, Sternberg JC. A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem. 1968; 144:116–131. [Google Scholar]
- 29. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973; 19:476–482. [PubMed] [Google Scholar]
- 30. Pinter JK, Hayashi JA, Watson JA. Enzymic assay glycerol dihydroxyacetone and glyceraldehyde. Arch Biochem Biophys. 1965; 121:404–414. [DOI] [PubMed] [Google Scholar]
- 31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. [PubMed] [Google Scholar]
- 32. Scuteri A, Najjar SS, Morrell CH, et al. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005; 28:882–887. [DOI] [PubMed] [Google Scholar]
- 33. Goodman E, Dolan LM, Morrison JA, et al. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005; 111:1970–1977. [DOI] [PubMed] [Google Scholar]
- 34. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 35. The European Group for the Study of Insulin Resistance. (EGIR) . Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002; 28:364–376. [PubMed] [Google Scholar]
- 36. Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003; 163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–689. [DOI] [PubMed] [Google Scholar]
- 38. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002; 288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 39. Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004; 140:167–174. [DOI] [PubMed] [Google Scholar]
- 40. Martin JF, Slater DN, Kishk YT, et al. Platelet and megakaryocyte changes in cholesterol‐induced experimental atherosclerosis. Arteriosclerosis. 1985; 5:604–612. [DOI] [PubMed] [Google Scholar]
- 41. Imano H, Kudo M, Ohira T, et al. The effects of fish supplementation on platelet function, count and metabolism in healthy Japanese [in Japanese]. Nippon Eiseigaku Zasshi. 1999; 53:601–610. [DOI] [PubMed] [Google Scholar]
- 42. Wannamethee SG, Lowe GD, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002; 105:1785–1790. [DOI] [PubMed] [Google Scholar]
- 43. Brown AS, Hong Y, De Belder A, et al. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol. 1997; 17:802–807. [DOI] [PubMed] [Google Scholar]
- 44. Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin‐6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. [DOI] [PubMed] [Google Scholar]
- 45. Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003; 108:414–419. [DOI] [PubMed] [Google Scholar]
- 46. Ridker PM, Buring JE, Cook NR, et al. C‐reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8‐year follow‐up of 14,719 initially healthy American women. Circulation. 2003; 107:391–397. [DOI] [PubMed] [Google Scholar]
- 47. Burstein SA, Downs T, Friese P, et al. Thrombocytopoiesis in normal and sublethally irradiated dogs: response to human interleukin‐6. Blood. 1992; 80:420–428. [PubMed] [Google Scholar]
- 48. Klover PJ, Zimmers TA, Koniaris LG, et al. Chronic exposure to interleukin‐6 causes hepatic insulin resistance in mice. Diabetes. 2003; 52:2784–2789. [DOI] [PubMed] [Google Scholar]
- 49. Ford ES. The metabolic syndrome and C‐reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003; 168:351–358. [DOI] [PubMed] [Google Scholar]
- 50. Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004; 44:1945–1956. [DOI] [PubMed] [Google Scholar]


