Abstract
Elevated plasma homocysteine (Hcy) concentration is considered a risk factor for cardiovascular disease and may also be associated with hypertension. Although links have been established between hyperhomocysteinemia and elevated risk for cardiovascular events, the precise role of plasma Hcy in cardiovascular disease is unclear. Plasma Hcy increases with aging and is associated with other health‐related behaviors, including smoking and diet patterns. Both epidemiologic and longitudinal clinical investigations have investigated the possible contribution of plasma Hcy to cardiovascular disease, and most report an association of plasma Hcy with the risk for cardiovascular and cerebral events. Some reports describe a significant relationship between Hcy and blood pressure levels, as well as higher Hcy in hypertensives compared to normotensives. Other studies find that the effect of Hcy disappears following adjustment for other risk factors. Because Hcy cosegregates with other risk factors, it has been difficult to identify an independent effect of Hcy on cardiovascular disease or hypertension. Hcy can be modified to some extent by vitamin supplementation. Hcy reduction may have some benefit in reducing cardiovascular risk in some patients, particularly the elderly. Because the question of an independent role of Hcy on risk for cardiovascular disease has not been determined, the assessment and treatment of Hcy should be approached in the context of other modifiable risk factors.
Elevated plasma homocysteine concentration ([Hcy]) is considered a risk factor for cardiovascular disease (CVD) and may also be associated with hypertension. Although links have been established between hyperhomocysteinemia and elevated risk for CVD, the precise role of plasma homocysteine (Hcy) in cardiovascular events is unclear. Plasma homocysteine (Hcy) is affected by health‐related behaviors, including diet, smoking, and sedentary lifestyle. Genetic factors also contribute to plasma [Hcy]. This review will discuss the clinical data and reports that have examined the contribution of Hcy to hypertension and CVD.
HOMOCYSTEINE METABOLISM
Hcy is a sulfhydryl amino acid compound that is generated from protein breakdown and the essential amino acid methionine as it is metabolized to cysteine. 1 Hcy can be metabolized by two major pathways. When methionine is in excess, Hcy is directed to the transulphuration pathway, where it is irreversibly sulfoconjugated to cysteine by cystathionine B‐synthase with vitamin B6 as a cofactor. Hcy is also remethylated in a methionine‐conserving pathway. This process requires methionine synthase, vitamin B12 as a cofactor, and methyltetrahydrofolate as a cosubstrate. The methionine‐conserving pathway requires folic acid and methyltetrahydrofolate reductase (MTHFR). 2 There is a strong inverse correlation of plasma Hcy with plasma folate concentration. In contrast to folate, serum vitamin B12 or vitamin B6 levels show only a weak correlation with plasma Hcy. 3 Deficiencies in any of these above enzymes, folic acid, or the cofactors may lead to some degree of hyperhomocysteinemia. 1 Metabolic pathways are summarized in the Figure 1.
Figure 1.
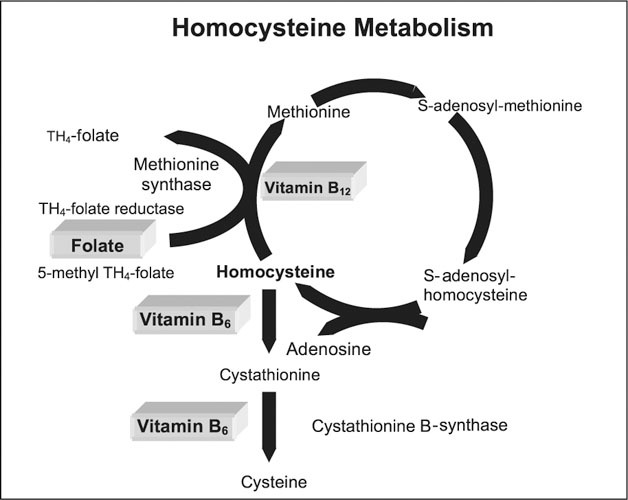
Depicted in the figure are the pathways for homocysteine metabolism
High performance liquid chromatography is the most widespread and accurate method for measuring plasma levels of Hcy. Enzyme conversion immunoassay has also been used. 4 The levels are usually measured in the fasting state and can also be measured after methionine loading. Methionine loading can be used to distinguish deficiencies in enzymes and/or cofactors in the two separate metabolic pathways. 5 Approximately 70% of Hcy is protein bound in the plasma, 25% circulates bound to itself as a dimer, and the remainder <5% circulates in combination with other thiols to form disulfide or circulates as a free‐thiol compound. 5 Hyperhomocysteinemia may be classified as mild or severe, based on plasma concentration. Normal Hcy levels are 5–15 μmol/L. Mild hyperhomocysteinemia is defined as 16–100 μmol/L and as severe as >100 μmol/L. 6 , 7
ROLE OF HOMOCYSTEINE IN CARDIOVASCULAR DISEASE: EPIDEMIOLOGY
Data gathered from population studies suggest that there is an association of higher plasma [Hcy] and risk of CVD. Using meta‐analysis, Boushey et al. 8 examined 27 reports of studies on the relationship between Hcy and CVD. Overall, the population data indicate that plasma [Hcy] higher than the 90th percentile were associated with an increased risk of fatal and nonfatal atherosclerotic disease in the coronary, cerebral, and peripheral circulation. An increase of 5 μmol/L was associated with a 60% increase in risk of coronary artery disease in men and an 80% increase in risk in women. There was also a reported 50% increase in cerebrovascular disease. This magnitude of increase in plasma [Hcy] was thought to be equivalent to the CVD risk of a 19‐mg/dL increase in cholesterol. The European Concerted Action Project 9 also confirmed that an elevated plasma [Hcy] was an independent risk factor for CVD, and calculated that an increase of 5 μmol/L was associated with an increase in relative risk for CVD of 1.35.
Prospective clinical studies that examine the effect of plasma [Hcy] on vascular events remain nonconclusive. A prospective cohort study from Norway showed a relative risk of 1.41 for each 4 μmol/L increase in serum Hcy. 10 Fallon et al. 11 reported an increasing risk of cerebral infarction with increasing Hcy in a Finnish cohort. The Physicians Health Study 12 found an adjusted relative risk for fatal or nonfatal myocardial infarctions of 3.4 for persons in whom plasma [Hcy] were in the top 5% of the entire cohort. 12 The British United Provident Association study 13 reported a relative risk for coronary heart disease of 2.9 among men in the highest Hcy quartile compared to men in the lowest quartile. Other studies reported a graded, positive association between plasma [Hcy] and the risk for stroke 14 and coronary artery disease. 15 Despite these positive reports, a recent report from the Physicians Health Study 16 on a more prolonged follow‐up of 7.5 years failed to demonstrate a significant association between plasma [Hcy] and risk for myocardial infarction or death. Sharabi et al. 17 also could not detect an effect of Hcy on the occurrence of atherothrombotic events.
Detection of an independent effect of Hcy on the risk of CVD in clinical studies remains difficult due to the many factors that can affect plasma [Hcy]. Aging and lifestyle behaviors can contribute to mild elevations in Hcy without associated hyperhomocysteinemia. Included in this list are older age, male sex, postmenopausal status, smoking, sedentary lifestyle, and dietary factors, including increased intake of animal proteins, alcohol use, coffee/tea consumption, decreased intake of folic acid, and vitamins B6 and B12. 3 , 18 It is estimated that 40%–50% of elderly North American people do not consume adequate amounts of folic acid and vitamin B6; this may contribute to some elevation in plasma [Hcy]. 18 Given all of these variables, it is difficult to determine if there is an independent effect of Hcy on CVD or blood pressure (BP).
GENETICS AND PATHOPHYSIOLOGY
A genetic predisposition for hyperhomocysteinemia has been investigated. A highly prevalent C677T point mutation has been associated with a thermolabile MTHFR variant. It is estimated that between 5%–12% of the Caucasian population may be homozygous for a genotype which results in a reduction of MTHFR activity and increased levels of plasma [Hcy]. 19 , 20 Even the heterozygotic state may be associated with slightly increased plasma [Hcy]. Although it has been suggested that the genotype (VV) is an independent risk factor for CVD, most studies have failed to confirm this association. 21 In addition, Pullin et al. 22 found that healthy subjects, who were homozygous for the MTHFR–VV genotype and also had higher plasma [Hcy], did not have evidence of endothelial dysfunction. A meta‐analysis by Brattstrom et al. 6 reported that although mutant homozygotes had Hcy 25% higher than those with the wild genotype, they had no increased risk of CVD. Alternatively, a study that examined the MTHFR genotype in patients undergoing coronary artery stenting reported that the homozygous mutant genotype may increase the risk of restenosis. 23 However, a recent study on a much larger sample of 800 patients could detect no effect of either plasma [Hcy] or MTHFR genotype on restenosis after stenting. 24 Thus, the exact independent effects of genotype status are not yet known.
There are several possible mechanisms by which Hcy could contribute to vascular injury. In experimental models, Hcy administration has caused endothelial cell injury, both in vitro and in vivo. Hcy induces oxidative stress to the endothelium and reduces available nitric oxide. Hcy may also generate free radicals and inhibit the production of other antioxidants. 25 Endothelial injury is followed by platelet aggregation and thrombus formation. Toxic endothelial damage is also related to the stimulation of smooth muscle cell proliferation and susceptibility to oxidation of low‐density lipoproteins. Another mechanism by which Hcy can induce vascular injury is the increased thrombogenicity mediated by increased platelet adherence and the release of platelet‐derived growth factors, activated Factor V, X, and XII, inhibition of protein C activation, inhibition of cell surface expression of thrombomodulin, and decreased tissue plasminogen activator activity. 21 , 26 , 27 , 28 Hcy has also been thought to increase arterial stiffness by damaging elastin fibers, increasing collagen production and stimulating smooth muscle activity. 3
HOMOCYSTEINE AND BLOOD PRESSURE
Some investigators have reported an effect of plasma [Hcy] on BP, and there is some suggestion that Hcy could increase the risk of hypertension. Lam and Cassani 25 analyzed data from the National Health and Nutrition Examination Survey (NHANES) to determine if there is a relationship between plasma [Hcy] and BP. After adjusting for cardiovascular risk factors, Hcy was shown to have an independent, positive association with BP. The association of Hcy with BP was stronger in women than in men. A one‐standard deviation increase in Hcy was associated with an increase in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of 0.7 and 0.5 mm Hg in men, respectively, and in women, the increases in SBP and DBP were 1.2 and 0.7 mm Hg, respectively. 25 Plasma [Hcy] in hypertensive patients were compared to normotensives by Sutton‐Tyrrell et al. 29 who reported that the concentration of plasma Hcy rose from 9.7 μmol/L at a SBP <140 mm Hg to 13 μmol/L at SBP >180 mm Hg. 29 Hcy also was reported to be higher in hypertensive children in a study by Glowinska et al. 30 who examined obese and nonobese adolescents. Although the mean plasma [Hcy] concentration was less than 10 μmol/L, they found that both obese hypertensive and nonobese hypertensive adolescents had higher mean plasma [Hcy] compared to the normotensive groups. 30 A significant and independent relationship of plasma [Hcy] with SBP was also reported by Malinow et al. 31 In the Hordaland study, 15 however, the association of Hcy with BP was positive, but weak. Individuals with a DBP of more than 100 mm Hg had a plasma [Hcy] that was 0.5 to 0.8 μmol higher than did those with DBP <70 mm Hg. Another study reported that hypertensive patients had mean plasma [Hcy] that was 3.8 μmol/L higher compared to normotensive subjects. 17
Kahleova et al. 32 recently reported higher levels of plasma [Hcy] in adolescents and young adults with hypertension compared to normotensive matched controls. Again, a significant association with SBP was demonstrated, and the investigators suggested that Hcy may play a role in the pathogenesis of hypertension. 32 Different results were reported by Dinavahi et al. 33 who investigated the relationship of plasma [Hcy] with BP in a young African‐American cohort. These investigators corroborated a significant, direct correlation of plasma [Hcy] with SBP and DBP in premenopausal women, but not in men. However, when adjustments were made for age and body mass index, no significant correlation was found. Nearly all patients in the cohort had plasma [Hcy] within the normal range, and there was a significant inverse relationship between plasma [Hcy] with the B vitamins and folate. Hypertensive women in the study had a lower dietary intake of folic acid, indicating that the association of higher BP with higher Hcy may be reflective of a dietary effect. 33 The Framingham Offspring cohort 34 also reported no relationship between plasma [Hcy] and BP. These conflicting reports make it difficult to determine if plasma [Hcy] has an independent effect on BP, or if plasma [Hcy] is a surrogate measure of some other factor, such as diet. 35 , 36 , 37
HOMOCYSTEINE AND RENAL DISEASE
Plasma [Hcy] is elevated in patients with end‐stage renal disease (ESRD), but the specific pathophysiologic basis for the elevation is unknown. Even modest decreases in glomerular filtration rate are associated with an increase in plasma [Hcy]. 38 Daily renal excretion accounts for only 0.1% of Hcy daily production. 39 The metabolism of Hcy in renal parenchymal cells is responsible for the major fraction of the total renal clearance of Hcy, and the loss of this capacity may lead to the increases in plasma [Hcy] that are seen in ESRD. 38
CVD is a major cause of morbidity and mortality in ESRD, which could make the possible contribution of Hcy to CVD relevant. Studies have shown that vitamin supplementation in ESRD patients decreases plasma [Hcy]. 40 , 41 Although decreased levels of plasma [Hcy] occur after supplementation, optimal plasma [Hcys] of <10 μmol/L are difficult to achieve in patients with renal disease. Even among renal transplant patients, folate supplementation results in a plasma [Hcy] of <10 μmol/L in only 21.4% of patients. 40 The reason that plasma [Hcy] is difficult to normalize in ESRD patients is uncertain, but may be due to the combined effects of suboptimal nutrition, loss of renal parenchymal cells, and possibly some other metabolic derangement due to uremia. From another investigation, it was found that any doses of folic acid above 1 mg/d had no additional benefit in prevention of cardiovascular mortality in maintenance hemodialysis patients. 41
Contrary to the relationship of plasma [Hcy] with outcome in the general population, plasma [Hcy] in patients with ESRD appear to have an inverse association with morbidity and mortality. Kalantar‐Zadeh et al. 42 showed a two‐fold increased risk of cardiovascular mortality among patients with Hcy in the lowest quartile in their study sample compared to the higher Hcy quartiles. Other investigators reported a higher mortality over a 9‐month period among maintenance hemodialysis patients with low Hcy. 43 This reverse relationship between plasma [Hcy] and mortality may be reflective of poor nutritional status. 41
SUMMARY
There are a considerable number of clinical studies that describe an association of plasma [Hcy] with hypertension, and with cardiovascular events. Dietary factors have been implicated in modulating plasma [Hcy]. Folic acid and vitamin B12 replacement have had some success in the lowering of plasma [Hcy]. Suggestions have been made that advising a higher intake of vitamin B and folic acid would be helpful in preventing CVD. Elevated Hcy has been linked to increased cardiovascular morbidity and mortality in patients without ESRD. However, the presence of ESRD seems to demonstrate a paradoxical relationship between plasma [Hcy] and cardiovascular mortality. Although there is compelling evidence indicating that plasma [Hcy] contributes to hypertension and heightens the risk for cardiovascular events, further investigation is needed to determine if Hcy is an independent risk factor or if it is linked with other modifiable risk factors for CVD, including hypertension. For the general population, a diet rich in multiple nutrients, including B vitamins, should be encouraged to achieve an optimal plasma [Hcy]. Patients who have multiple risk factors for CVD may benefit from Hcy screening and supplementation with B complex vitamins.
References
- 1. Ford ES, Smith SJ, Stroup DF, et al. Homocyst(e)ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case‐control studies and nested case‐control studies. Inter J Epidemiol. 2002:31:59–70. [DOI] [PubMed] [Google Scholar]
- 2. Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157(suppl 2):S40–S44. [DOI] [PubMed] [Google Scholar]
- 3. van Guldener C, Nanayakkara PW, Stehouwer CD. Homocysteine and blood pressure. Curr Hypertens Rep. 2003;5:26–31. [DOI] [PubMed] [Google Scholar]
- 4. Frantzen F, Faaren AL, Alfheim I, et al. Enzyme conversion immunoassay for determining total homocysteine in plasma or serum. Clin Chem. 1998;44:311–316. [PubMed] [Google Scholar]
- 5. Ueland PM. Homocysteine species as components of plasma redox thiol status. Clin Chem. 1995;41:340–342. [PubMed] [Google Scholar]
- 6. Brattstrom L, Wilcken DE, Ohrvik J, et al. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of meta‐analysis. Circulation. 1998;98:2520–2526. [DOI] [PubMed] [Google Scholar]
- 7. McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. [DOI] [PubMed] [Google Scholar]
- 8. Boushey CJ, Beresford SAA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. [DOI] [PubMed] [Google Scholar]
- 9. Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277:1775–1781. [DOI] [PubMed] [Google Scholar]
- 10. Arnesen E, Refsum H, Bonaa KH, et al. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24:704–709. [DOI] [PubMed] [Google Scholar]
- 11. Fallon UB, Virtamo J, Young I, et al. Homocysteine and cerebral infarction in Finnish male smokers. Stroke. 2003;34:1359–1363. [DOI] [PubMed] [Google Scholar]
- 12. Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 13. Wald NJ, Watt HC, Law MR, et al. Homocysteine and ischemic heart disease: results of a prospective study with implications regarding prevention. Arch Intern Med. 1998;158:862–867. [DOI] [PubMed] [Google Scholar]
- 14. Perry IJ, Refsum H, Morris RW, et al. Prospective study of serum total homocysteine concentration and risk of stroke in middle‐aged British men. Lancet. 1995;346:1395–1398. [DOI] [PubMed] [Google Scholar]
- 15. Nygard O, Vollset SE, Refsum H, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533. [DOI] [PubMed] [Google Scholar]
- 16. Chasan‐Taber L, Selhub J, Rosenberg IH, et al. A prospective study of folate and vitamin B6 and risk of myocardial infarction in US physicians. J Am Coll Nutr. 1996;15:136–143. [DOI] [PubMed] [Google Scholar]
- 17. Sharabi Y, Doolman R, Rosenthal T, et al. Homocysteine levels in hypertensive patients with a history of cardiac or cerebral atherothrombotic events. Am J Hypertens. 1999;12:766–771. [DOI] [PubMed] [Google Scholar]
- 18. Chandalia M, Abate N, Cabo‐Chan AV Jr, et al. Hyperhomocysteinemia in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88:1089–1095. [DOI] [PubMed] [Google Scholar]
- 19. Wirta V, Saransaari P, Wirta O, et al. Methylenetetrahydrofolate reductase gene polymorphism, hyperhomocysteinemia and occlusive retinal vascular disease in type 2 diabetic and non‐diabetic subjects. Clin Nephrol. 2002;58:171–178. [DOI] [PubMed] [Google Scholar]
- 20. Goyette P, Sumner JS, Milos R, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA mapping and mutation identification. Nat Genet. 1994;7:195–200. [DOI] [PubMed] [Google Scholar]
- 21. Eikelboom JW, Lonn E, Genest J, Jr et al. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363–375. [DOI] [PubMed] [Google Scholar]
- 22. Pullin CH, Wilson JF, Ashfield‐Watt PA, et al. Influence of methylenetetrahydrofolate reductase genotype, exercise and other risk factors on endothelial function in healthy individuals. Clin Sci. 2002;102:45–50. [PubMed] [Google Scholar]
- 23. Kosokabe T, Okumura K, Sone T, et al. Relation of a common methylenetetrahydrofolate reductase mutation and plasma homocysteine with intimal hyperplasia after coronary stenting. Circulation. 2001;103:2048–2054. [DOI] [PubMed] [Google Scholar]
- 24. Koch W, Ndrepepa G, Mehilli J, et al. Homocysteine status and polymorphisms of methylenetetrahydrofolate reductase are not associated with restenosis after stenting in coronary arteries. Arterioscler Thromb Vasc Biol. 2003;23:2229–2234. [DOI] [PubMed] [Google Scholar]
- 25. Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002;156:1105–1113. [DOI] [PubMed] [Google Scholar]
- 26. Acevedo M, Pearce GL, Kottke‐Marchant K, et al. Elevated fibrinogen and homocysteine levels enhance the risk of mortality in patients from a high‐risk preventive cardiology clinic. Arterioscler Thromb Vasc Biol. 2002:22:1042–1045. [DOI] [PubMed] [Google Scholar]
- 27. Roeters van Lennep JE, Westerveld HT, Erkelens DW, et al. Risk factors for coronary heart disease: implications of gender. Cardiovasc Res. 2002;53:538–549. [DOI] [PubMed] [Google Scholar]
- 28. Fiorina P, Lanfredini M, Montanari A, et al. Plasma homocysteine and folate are related to arterial blood pressure in type 2 diabetes mellitus. Am J Hypertens. 1998;11:1100–1107. [DOI] [PubMed] [Google Scholar]
- 29. Sutton‐Tyrrell K, Bostom A, Selhub J, et al. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96:1745–1749. [DOI] [PubMed] [Google Scholar]
- 30. Glowinska B, Urban M, Koput A, et al. New atherosclerosis risk factors in obese, hypertensive and diabetic children and adolescents. Atherosclerosis. 2003;167:275–286. [DOI] [PubMed] [Google Scholar]
- 31. Malinow MR, Levenson J, Giral P, et al. Role of blood pressure, uric acid, and homorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis. 1995;114:175–183. [DOI] [PubMed] [Google Scholar]
- 32. Kahleova R, Palyzova D, Zvara K, et al. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens. 2002;15:857–864. [DOI] [PubMed] [Google Scholar]
- 33. Dinavahi R, Cossrow N, Kushner H, et al. Plasma homocysteine concentration and blood pressure in young adult African Americans. Am J Hypertens. 2003;16:767–770. [DOI] [PubMed] [Google Scholar]
- 34. Jacques PF, Bostom AG, Wilson PW, et al. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. [DOI] [PubMed] [Google Scholar]
- 35. Quinlivan EP, McPartlin J, McNulty H, et al. Importance of both folic acid and vitamin B12 in reduction of risk of vascular disease. Lancet. 2002;359:227–228. [DOI] [PubMed] [Google Scholar]
- 36. Falkner B, Sherif K, Michel S, et al. Dietary nutrients and blood pressure in urban minority adolescents at risk for hypertension. Arch Pediatr Adolesc Med. 2000;154:918–922. [DOI] [PubMed] [Google Scholar]
- 37. Mennen LI, De Courcy GP, Guilland JC, et al. Homocysteine, cardiovascular disease risk factors, and habitual diet in the French Supplementation with Antioxidant Vitamins and Minerals Study. Am J Clin Nutr. 2002;76:1279–1289. [DOI] [PubMed] [Google Scholar]
- 38. Neugebauer S, Tarnow L, Stehouwer C, et al. Total plasma homocysteine is associated with hypertension and Type I diabetic patients. Diabetologia. 2002;45:1315–1324. [DOI] [PubMed] [Google Scholar]
- 39. Bortolotto LA, Safar ME, Billaud E, et al. Plasma homocysteine, aortic stiffness, and renal function in hypertensive patients. Hypertension. 1999;34:837–842. [DOI] [PubMed] [Google Scholar]
- 40. Manrique J, Errasti P, Lavilla J, et al. Treatment of hyperhomocysteinemia after renal transplantation. Transplan Proc. 2003;35:1742–1744. [DOI] [PubMed] [Google Scholar]
- 41. Wrone EM, Hornberger JM, Zehnder JL, et al. Randomized trial of folic acid for prevention of cardiovascular events in endstage renal disease. J Am Soc Nephrol. 2004;15:420–426. [DOI] [PubMed] [Google Scholar]
- 42. Kalantar‐Zadeh K, Block G, Humphreys MH, et al. A low, rather than high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. J Am Soc Nephrol. 2004;15:442–453. [DOI] [PubMed] [Google Scholar]
- 43. Sirrs S, Duncan L, Djurdjev O, et al. Homocyst(e)ine and vascular access complications in haemodialysis patients: insights into a complex metabolic relationship. Nephrol Dial Transplant. 1999;14:738–743. [DOI] [PubMed] [Google Scholar]


