Abstract
Hyperuricemia is commonly associated with traditional risk factors such as abnormalities in glucose metabolism, dyslipidemia, and hypertension. Recent studies have revived the controversy over the role of serum uric acid as an independent prognostic factor for cardiovascular mortality. The authors review clinical and experimental evidence concerning the role of serum uric acid in the development of cardiovascular and renal damage. Results of trials suggesting that serum uric acid variations over time may have a prognostic impact are also discussed.
Considerable experimental evidence suggests a causal role for serum uric acid (SUA) in the pathogenesis of hypertension. 1 , 2 , 3 , 4 Furthermore, the link between SUA levels and traditional metabolic risk factors is well known, and several large clinical studies have shown that asymptomatic hyperuricemia is associated with cardiovascular (CV) and renal complications. Whether SUA is just an innocent bystander in proximity to unfavorable events or whether it plays a mechanistic role in the development of CV damage is still under debate.
SUA AND CV EVENTS
Several reports indicate that SUA is independently associated with adverse events, especially in women (Table I). This finding has been confirmed by most, although not all, 11 studies among subjects at higher CV risk, such as those with hypertension 8 and diabetes. 6 Gueyffier et al. 21 analyzed the Individual Data Analysis of Antihypertensive Intervention Trials (INDANA) database and found that the prevalence of CV events associated with increased SUA levels is similar to what is attributable to blood pressure (BP) and total cholesterol. The association of SUA with cerebrocardiovascular disease is even stronger in well‐treated hypertensive patients and endures after successful BP control. 8
Table I.
Studies on the Association Between Serum Uric Acid (SUA) and Cardiovascular (CV) Events
| Study | Population (M/W) | Age (yr)* | Follow‐Up (yr)** | Events | Comparison | Adjusted Risk Estimate† | Independent Role of SUA |
|---|---|---|---|---|---|---|---|
| Wannamethee et al. 5 | 7688 men | 40–59 | 16.8 | Fatal or nonfatal CHD | Highest vs. lowest quintile | 1.55 | No |
| Lehto et al. 6 | 1017 patients with T2DM (551/466) | 58 | 7.2 | Fatal or nonfatal stroke | SUA >5.0 mg/dL | 1.91 | Yes |
| Culleton et al. 7 | 6763 subjects from general population (3075/3688) | 47 | 4(c) | 1) Overall mortality 2) CV diseases 3) CHD | Highest vs. lowest quintile | 1) ns (M); 3.63 (W) 2) ns (M); 5.69 (W) 3) ns(M); 4.11 (W) | No |
| Liese et al. Epidemiology. 1999 | 1044 men from general population | 45–64 | 8(c) | 1) All‐cause mortality 2) CV mortality 3) Myocardial infarction | SUA >6.3 mg/dL vs. <5.4 mg/dL | 1) 2.8 2) 2.2 (ns) 3) 1.7 (ns) | 1)Yes 2) No 3) No |
| Alderman et al. 8 | 7978 mild‐to‐moderate hypertensives (4883/3095) | 53 | 6.6 | CV events | SUA ≥7.6 mg/dL (M); ≥6.3 mg/dL (W) vs. lowest quartile | 1.48 | Yes |
| Moriarity et al. Ann Epidemiol. 2000 | 13,504 healthy subjects (5904/76 | 45–64 | 8(c) | CHD | SUA ≥7.6 mg/dL (M); ≥6.3 mg/dL (W) vs. lowest quartile | 1.02 (ns) (M) 1.18 (ns) (W) | No |
| Fang and Alderman. JAMA. 2000 | 5926 subjects from general population | 48 | 16.4 | CV mortality | SUA ≥7 mg/dL (M); ≥5.6 mg/dL (W) vs. lowest quartile | 1.77 (M) 3.0 (W) | Yes |
| Franse et al. 9 | 4327 patients with hypertension (1860/2467) | 71 | 5(c) | 1) CV mortality 2) CHD 3) Stroke 4) All‐cause mortality | SUA ≥6.7 mg/dL (M); ≥5.8 mg/dL (W) vs. lowest quartile | 1) 1.32 2) 1.43 3) 0.85 (ns) 4) 1.05 (ns) | 1)Yes 2) Yes 3) No 4) No |
| Verdecchia et al. Hypertension. 2000 | 1720 patients with primary hypertension (920/800) | 51 | 4 | 1) CV events 2) Fatal CV events 3) All‐cause mortality | SUA ≥6.2 mg/dL (M) ≥4.6 mg/dL (W) vs. second quartile | 1) 1.73 2) 1.96 3) 1.63 | Yes |
| Sakata et al. 10 | 8172 subjects from general population (3596/4576) | 49 | 14 (c) | All‐cause mortality | SUA ≥6.6 mg/dL (M) ≥5.0 mg/dL (W) vs. lowest quartile | NS (M) 2.25 (W) | No |
| Mazza et al. Eur J Epidemiol. 2001 | 3282 elderly subjects from genera population (1281/2001) | 74 | 14 (c) | Stroke | SUA >6.5 mg/dL | 1.61 | Yes |
| Wang et al. Hypertension. 2001 | 1873 elderly Chinese patients wit ISH (1207/666) | 66 | 3 | 1) CV mortality 2) Fatal stroke | Per additional SUA 0.8 mg/dL | 1) 1.14 2) 1.34 | Yes |
| De Leeuwet al. 11 | 4556 elderly patients with ISH (1500/3056) | 70 | 2 | 1) Overall mortality 2) CV mortality 3) CV events | Per additional SUA 0.8 mg/dL | 1) ns 2) ns 3) 1.09 | No |
| Bickel et al. Am J Cardiol. 2002 | 1017 patients with angiographically defined coronary artery disease (747/270) | 62 | 2.2 | Overall mortality | SUA >7.1 mg/dL vs. lowest quartile | 2.71 | Yes |
| Wong et al. 12 | 354 stroke survivors | 69 | 2.8 | 1) CV mortality 2) All‐cause mortality | SUA >5.4 mg/dL | 1) 3.1 2) ns | 1) Yes 2) No |
| Weir et al. 13 | 2498 stroke survivors (1199/1299) | 72 | 2.7 | Major vascular events | Per additional SUA 1.68 mg/dL | 1.27 | Yes |
| Anker et al. 14 | 1) 112 patients with chronic HF (101/11) 2) 182 patients with chronic HF (149/33) | 59 | 5(c) | Overall mortality (in 12 months) | SUA ≥9.5 mg/dL | 1) 3.9 2) 7.14 | Yes |
| Niskanen et al. Arch Intern Med. 2004 | 1423 healthy Finnish men | 52 | 11.9 | CV mortality | SUA ≥5.9 mg/dL | 4.77 | Yes |
| Hoieggen et al. 15 | 9193 hypertensive patients with LVH (4230/4963) | 67 | 4.8 (c) | Major vascular events | Per additional SUA 0.17 mg/dL | 1.006 (ns) (all) 1.006 (ns) (M) 1.013 | Yes(W) |
| Athyros et al. 16 | 1600 patients with established CHD (1256/344) | 59 | 3 | CV events | Per additional SUA 1 mg/dL | 1.29 | Yes |
| Hsu et al. 17 | 146 hemodialysis patients (68/78) | 60 | 1 | All‐cause mortality | ≥80th percentile vs. lower levels | 5.67 | Yes |
| Hakoda et al. J Rheumatol. 2005 | 10615 Japanese atomic bomb survivors (3860/6755) | 49 | 24.9 | 1) All‐cause mortality 2) CV mortality | SUA ≥8.0 mg/dL (M) ≥7.0 mg/dL (W) vs. lower levels | 1) 1.22 (M); 1.63 (W) 2) ns (M); 1.79 (W) | 1) Yes 2) Yes (W) |
| Simon et al. 18 | 2763 postmenopausal women | 66 | 4.1 (c) | CHD | Per additional SUA 1.3 mg/dL | 1.05 | No |
| Madsen et al. 19 | 1596 patients with angiographically defined coronary artery disease (1245/351) | 65 | 2.6 | All‐cause mortality | Highest vs. lowest quintile | 1.5 | Yes: patients not using diuretics No: diuretics users |
| Kojima et al. 20 | 1124 consecutive patients hospitalized within 48 hours of onset of symptoms of AMI (800/324) | 67 | 30 days | All‐cause mortality | SUA ≥6.8 mg/dL vs. lowest quartile | 3.7 | Yes |
| M=men; W=women; CHD=coronary heart disease; T2DM=type 2 diabetes mellitus; ISH=isolated systolic hypertension; HF=heart failure; LVH=left ventricle hypertrophy; AMI=acute myocardial infarction; *expressed as mean or range; **expressed as mean except where expressed as cumulative (c) years; †each report is statistically significant except when noted as nonsignificant (ns) | |||||||
A relationship between SUA and events has been observed in patients with overt CV disease. Anker et al. 14 reported that high SUA levels predict unfavorable outcome in patients with moderate‐to‐severe chronic heart failure, a finding that has also been confirmed in patients with angiographically proven coronary artery disease 19 , 22 or with previous acute myocardial infarction 20 or stroke. 12 , 13 Although an increase in SUA could be at least partly due to a subtle impairment of renal function, the association between SUA and CV events seems to be independent of serum creatinine. 8 , 12 , 13 , 14 , 21 Preliminary observations suggest that, even in patients on hemodialysis therapy, increased CV risk has been observed in patients with higher SUA levels. 17
On the other hand, several studies, especially those performed on the general population, have failed to prove the independent nature of these associations, further highlighting the complex relationship between SUA levels and CV outcome. 5 , 7 , 10
SUA AND RENAL EVENTS
Elevated SUA is a frequent finding in patients with kidney disease and may be the direct consequence of decreased renal clearance. However, hyperuricemia per se may be involved in the induction or aggravation of renal dysfunction. 23 Acute renal failure caused by marked hyperuricosuria, as observed in patients with tumor lysis syndrome, is a well recognized clinical entity. Moreover, subjects with recurrent gout attacks may develop chronic kidney disease, but the coexistence of vascular disease and age are better predictors of reduction in renal function. 24 A causal role for uric acid in renal disease is still under debate (Table II).
Table II.
Studies on the Association Between Serum Uric Acid (SUA) and Renal Events
| Study | Population (M/W) | Age (yr)* | Follow‐Up (yr)** | Definition of Renal Events | Comparison | Adjusted Risk Estimate † | Independent Role of SUA |
|---|---|---|---|---|---|---|---|
| Tomita et al. 25 | 49,413 Japanese workers (M) | 25–60 | 5.4 | Renal failure | SUA ≥8.5 mg/dL vs. >5.0–6.4 mg/dL | 8.52 | No |
| Syrjanen et al. 26 | 1) 223 patients with IgAN (141/82) 2) Subgroup: 181 patients with IgAN with normal renal function | 41 | 10 (c) | SCr ≥1.4 mg/dL (M); ≥1.2 mg/ dL (W) at follow‐up; >20% elevation from baseline | SUA >7.5 mg/dL (M); >5.7 mg/dL (W) vs. lower | 1) 2.2 (ns) 2) 4.6 | 1) No 2) Yes |
| Iseki et al. 27 | 6210 Japanese from general population with normal SCr levels (4047/2163) | 48 | 2(c) | SCr ≥1.4 mg/dL (M); ≥1.2 mg/ dL (W) at follow‐up | SUA ≥8 mg/dL vs. <5 mg/dL | 2.91 (M) 10.39 (W) | Yes |
| Iseki et al. 28 | 48,117 Japanese from general population (22,949/25,228) | 52 | 7(c) | End‐stage renal disease | SUA ≥7 mg/dL (M); ≥6 mg/dL (W) vs. lower | 2.0 (M) (ns) 5.8 (W) | Yes (W) |
| Domrongkitchaiporn et al. 29 | 3499 Thai subjects | 35–55 | 12 (c) | 1) GFR <60 mL/min at follow‐up 2) SCr ≥1.49 mg/dL (M); ≥1.13 mg/dL (W) at follow‐up | SUA >6.3 mg/dL vs. <4.5 mg/dL | 1) 1.82 2) 1.75 | Yes |
| M=men; W=women; IgAN=IgA nephropathy;SCr=serum creatinine; GFR=glomerular filtration rate; *expressed as mean or range; **expressed as mean or cumulative (c) years; xach report is statistically significant except when noted as nonsignificant (ns) | |||||||
Recent epidemiologic studies have demonstrated that uric acid is an independent risk factor for the deterioration of glomerular filtration rate in the general population 27 , 28 , 29 as well as in patients with glomerulonephritis. 26 , 27 SUA levels proved to be a predictor of end‐stage renal disease in a prospective cohort of 48,117 Japanese subjects, after adjusting for common confounding variables such as proteinuria, high BP, and dyslipidemia. 28 On the basis of multivariate analysis, Domrongkitchaiporn et al. 29 identified systolic hypertension, hyperuricemia (>6.3 mg/dL), and high body mass index as promoters of the development of chronic kidney disease over a 12‐year follow‐up period. 29 Increased uric acid levels are also believed to contribute to the deterioration of glomerular filtration rate in IgA nephropathy; hyperuricemia increased the risk of progression of kidney disease 4.6‐fold after adjusting for proteinuria, hypertension, diabetes, dyslipidemia, age, gender, and body mass index. 26 At variance with these results, hyperuricemia was not found to predict renal deterioration in the Modification of Diet in Renal Disease (MDRD) study 30 or in other more recent studies in Japanese cohorts. 25 , 31
Altogether these findings suggest, but do not offer definitive proof of, the role of SUA as an independent renal risk factor, especially in women and in patients who initially have normal renal function. 27 , 28
IS SUA A PROMOTER OF CV AND RENAL DAMAGE?
The relationship between SUA and the development of subclinical CV and renal damage has been under investigation for several years. In several cross‐sectional and prospective reports, SUA levels were found to be associated with carotid intima‐media thickening and/or carotid plaque (Table III), but in some studies an independent correlation was observed only in a subset of patients 34 , 35 or could not be confirmed at all. 39
Table III.
Studies on the Association Between Serum Uric Acid (SUA) and Target Organ Damage (TOD)
| Study | Population (M/W) | Age (yr)* | TOD | Correlation Between SUA and TOD | Independent Role of SUA |
|---|---|---|---|---|---|
| Persky et al. 32 | 24,997 subjects | 18–64 | ECG abnormalities | Yes | Yes (W) |
| Mazza et al. Metabolism. 2000 | 130 T2DM patients (60/70) | 53 | Carotid abnormalities | Yes | Yes (W) |
| Bo et al. Eur J Clin Invest. 2001 | 1186 T2DM patients (628/558) | 65 | 1) Microalbuminuria by UAE 2) Macroalbuminuria by UAE | Yes | 1) No 2) Yes |
| Tuttle et al. 33 | 277 patients admitted for elective coronary angiography (195/82) | 62 | Angiographically defined coronary artery disease severity | Yes (W) | No |
| Ishizaka et al. 34 | 8141 general population (5470/2671) | 57 | 1) Carotid plaque 2) Carotid IMT | Yes | 1) Yes (M without MS) 2) No |
| Kawamoto et al. Intern Med. 2005 | 919 elderly persons (398/521) | 75 | Carotid IMT | Yes | Yes |
| Tsioufis et al. 35 | 842 patients with HTN (406/436) | 53 | 1) LVH (echo) 2) Microalbuminuria by UAE | 1) No 2) Yes | 1) No 2) Yes |
| Viazzi et al. 36 | 425 patients with HTN (265/160) | 47 | 1) LVH (echo) 2) Carotid abnormalities 3) Microalbuminuria by ACR | 1) Yes (W) 2) Yes (W) 3) No | 1) Yes (W) 2) Yes (W) 3) No |
| Tseng Kidney Int. 2005 | 343 patients with T2DM (144/199) | 63 | Albuminuria by ACR | Yes | Yes (patients without HTN) |
| Wu et al. 37 | 1005 IgA nephropathy | 31 | Arterial‐arteriolar lesions (light microscopy semiquantitative scoring) | Yes | Yes |
| Myllymaki et al. 38 | 202 patients with IgA nephropathy | 41 | Tubular atrophy (light microscopy semiquantitative scoring) | Yes | Yes |
| Iribarren et al. 39 | 11,488 subjects free of cardiovascular disease (4966/6522) | 45–64 | Carotid abnormalities | Yes (W, white M) | No |
| M=men; W=women; ECG=electrocardiographic; T2DM=type 2 diabetes mellitus; UAE=urinary albumin excretion; IMT=intima‐media thickness;MS=metabolic syndrome; HTN=hypertension; LVH (echo)=left ventricular hypertrophy assessed by echocardiogram; ACR=albumin/creatinine ratio; *expressed as mean or range | |||||
As for cardiac end points, a link between SUA levels and electrocardiographic abnormalities, 32 left ventricular hypertrophy, 28 , 35 and coronary artery disease 33 has been reported. It is noteworthy that this association was found to be stronger in women than in men. 32 , 33 , 36 The lack of relationship between left ventricular hypertrophy and SUA reported by Tsioufis et al. 35 may have been due to the failure to stratify patients by gender.
SUA has also been described as an independent correlate of the urinary albumin excretion rate both in patients with type II diabetes (Table III) and with primary hypertension. 35 , 36 Moreover, in patients with IgA nephropathy, the severity of intrarenal arterial lesions 37 and of tubular atrophy, 38 as assessed at biopsy, were closely correlated with SUA levels, regardless of other well known markers of adverse renal outcome.
In a recent cross‐sectional study on untreated patients with primary hypertension, 36 we observed an independent association between the presence and degree of early signs of subclinical organ damage and SUA in women (Figure). These findings provide information relating to the association of SUA to CV events and renal progression. Subclinical organ damage represents an intermediate step between exposure to risk factors and occurrence of overt disease and has previously been shown to be a potent predictor of major CV and renal events. The correlation between SUA and target organ damage that we and others have observed in women compared with men (Table III) may account for the previously reported CV and renal predictive power of uric acid in women (Tables I and II).
Figure.
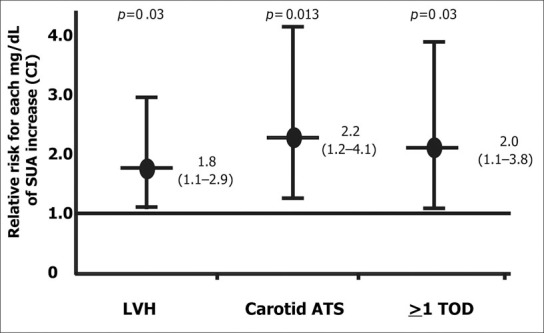
Relative risk in women (with 95% confidence interval [CI]) for left ventricular hypertrophy (LVH), carotid atherosclerosis (ATS), and early signs of sub clinical target organ damage (TOD) in at least one organ, for each mg/dL rise in serum uric acid (SUA)
ROLE OF SUA IN THE PATHOGENESIS OF VASCULAR DAMAGE
Although experimental evidence supports a role for SUA in the pathogenesis of vascular damage, 23 the issue remains controversial. SUA may exert its detrimental effects by entering vascular smooth muscle cells via an organic anion transport system, 40 and thus activating mitogen‐activated protein kinases 3 , 41 , 42 and nuclear transcription factors. 3 Subsequently, cyclooxygenase‐2, 37 platelet‐derived growth factor, 3 , 43 and various inflammatory mediators, including C‐reactive protein 42 and monocyte chemoattractant protein‐1 44 are stimulated. The complex of these events may lead to vascular smooth muscle cell hypertrophy. Uric acid may also be implicated in the development of endothelial dysfunction and atherosclerosis by inactivating NO and arresting the proliferation of endothelial cells. 42 , 45 Hyperuricemic rats have been reported to show a decrease in serum nitrites, a reflection of NO production. The combination of a proliferative effect on vascular smooth muscle cells and an inhibitory effect on endothelial cells may account for the ability of SUA to induce small‐vessel disease in experimental models.
Some human studies have investigated the relationship between SUA levels and endothelial damage. In high‐risk patients with 46 or without 47 CV disease, some studies have shown a relationship between hyperuricemia and impaired flow‐mediated vasodilation, a measurement of in vivo vascular NO activity. These findings, however, have not been confirmed in a recent study, which failed to show any effect of acute IV infusion of uric acid on endothelial function. 48 Thus, there is also some debate regarding the exact role of elevated SUA in the pathogenesis of renal and cardiac disease. 49
Although renin is increased in the kidney of the hyperuricemic rat and angiotensin‐converting enzyme inhibition ameliorates renal injury, 2 the mechanism by which uric acid increases renin‐angiotensin system activity is not clear. It should be noted that uric acid may also act as an antioxidant, 50 due to its ability to preferentially react with peroxynitrite, thus leading to stabilization of endothelial NO synthase activity. 51 Uric acid has also been shown to stimulate the expression of extracellular superoxide dismutase, thereby conferring antioxidant activity. 52
In summary, SUA plays several pathophysiologic roles both at the cellular and tissue level. The net balance of these contrasting mechanisms may result in adverse vascular effects.
SUA AS A POTENTIAL TARGET FOR TREATMENT
Available data on the prognostic impact of the pharmacologic lowering of SUA are scanty, and to date, only a few randomized studies have described any CV benefits related to SUA changes (Table IV).
Table IV.
Drugs With Proven or Potential Serum Uric Acid‐Lowering Effect
| Drug(s) | Mechanism (s) of Action | Reference (s) | Effect* |
|---|---|---|---|
| Allopurinol | Xanthine oxidase inhibition | Johnson et al. 53 | Yes |
| Rashid and William‐Olsson 54 | |||
| Tabayashi et al. 55 | |||
| Gavin and Struthers 56 | No | ||
| Losartan | Uricosuric | Hoieggen et al. 15 | Yes |
| Insulin‐sensitizing | |||
| Estrogen + progestin | Uricosuric | Simon et al. 18 | No |
| Atorvastatin | Uricosuric | Athyros et al. 16 | Yes |
| Insulin‐sensitizing | Milionis et al. 58 | ||
| Sulfinpyrazone (antiplatelet) | Uricosuric | The Anturane Reinfarction Trial. N Engl J Med. 1978 | Yes |
| Cilostazol (antiplatelet) | Uricosuric | Mitsuhashi et al. Endocr J. 2004 | Yes |
| Fenoflbrate (PPAR‐α) | Uricosuric | Elisaf et al. J Cardiovasc Pharmacol. 1999 | ? |
| PPAR agonist | Seber et al. Diabetes Res Clin Pract. 2005 | ||
| Insulin‐sensitizing | |||
| Rosiglitazone (PPAR‐γ) | PPAR agonist | Tsunoda et al. Am J Hypertens. 2002 | ? |
| Insulin‐sensitizing | |||
| Metformin | Insulin‐sensitizing | Gokcel et al. Diabetes Obes Metab. 2002 | ? |
| Sibutramine‐orlistat (weight loss) | Insulin‐sensitizing | Gokcel et al. Diabetes Obes Metab. 2002 | ? |
| Low‐energy diet | Insulin‐sensitizing | Tsunoda et al. Am J Hypertens. 2002 | ? |
| Amlodipine | Uricosuric | Sennesael et al. Am J Kidney Dis. 1996 | ? |
| Chanard et al. Nephrol Dial Transplant. 2003 | |||
| PPAR=peroxisome proliferator‐activated receptors; ?=potential, but still unproven; *attributed effect on renal and cardiovascular disease | |||
Allopurinol
Small studies have suggested that allopurinol, a widely used uric acid production blocker that inhibits xanthine oxidase activity, can improve endothelial function in patients with heart failure. 59 , 60 There are data suggesting that lowering uric acid levels did not result in improvement in outcome in patients with heart failure. 53 , 54 , 55 , 56 , 57
Losartan
This angiotensin II receptor blocker lowers SUA by interfering with urate reabsorption in the renal proximal tubule. 61 This effect has been credited with at least some of the benefit in stroke outcome in the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, 15 which compared an angiotensin receptor blocker with an atenolol‐based treatment program.
Hormones
Estrogens have been reported to lower SUA levels in hyperuricemic patients. 62 Although the exact mechanism is unclear, it has been suggested that they might increase uric acid renal clearance. 63 Recent studies with estrogen and progestin reported lowered SUA (−0.2 mg/dL) regardless of baseline levels, but lowering did not affect overall risk for CV events. 18 Thus, this issue cannot be considered settled.
Atorvastatin
In one study, 16 treatment with atorvastatin was associated with a 24% reduction in coronary heart disease‐related events for each mg/dL decrease in SUA levels, regardless of changes in low‐ or high‐density lipoprotein cholesterol. 16 The pathophysiologic mechanisms underlying the hypouricemic effect of atorvastatin are a matter of speculation; whether this effect is specific for this molecule or whether it is a class effect remains unclear. It has been hypothesized that uric acid production may be reduced by effects on carbohydrate metabolism; improvements in insulin sensitivity have been observed with atorvastatin in elderly patients with dyslipidemia and non‐insulin‐dependent diabetes. 64 It has been reported that the hypouricemic action of atorvastatin is, at least in part, mediated by an increase in the fractional excretion of uric acid. 58
An interesting finding regarding the possible prognostic role of SUA comes from the Systolic Hypertension in the Elderly Program (SHEP). 9 After 1 year of treatment with 12.5–25 mg chlorthalidone, the benefits of the diuretic on coronary events were offset in patients who experienced an increase in SUA ≥1 mg/dL. 9 However, over a 5‐year follow‐up, diuretic treatment was associated with significantly fewer CV events compared with placebo. 65 To date, analyses of data on changes in SUA over the entire study period have not been published; the issue remains controversial. Furthermore, in the context of a decline in age‐adjusted CV mortality, recent speculations about the effects of diuretic use and end‐stage renal disease incidence are unproven. 66 Recent data 67 from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) indicate that CV events in diuretic‐treated subjects were adversely affected in patients with varying degrees of renal impairment. Based on these data, there appears to be little reason to withhold the use of these agents in hypertensive patients if they are required to lower BP.
CONCLUSIONS
At present, there is insufficient evidence to recommend the routine use of SUA‐lowering therapies in patients at high CV risk with asymptomatic hyperuricemia. Large‐scale, prospective intervention trials are warranted, however, to ascertain the exact role that reducing SUA levels will play in reducing CV risk.
References
- 1. Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal‐independent mechanism. Hypertension. 2001;38:1101–1106. [DOI] [PubMed] [Google Scholar]
- 2. Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary arteriolopathy in rats by a blood pressure‐independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution and the pathogenesis of salt‐sensitivity. Hypertension. 2002;40:355–360. [DOI] [PubMed] [Google Scholar]
- 4. Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–841. [DOI] [PubMed] [Google Scholar]
- 5. Wannamethee SG, Shaper AG, Whincup PH. Serum urate and the risk of major coronary heart disease events. Heart. 1997;78:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehto S, Niskanen L, Ronnemaa T, et al. Serum uric acid is a strong predictor of stroke in patients with non‐insulin‐dependent diabetes mellitus. Stroke. 1998;29:635–639. [DOI] [PubMed] [Google Scholar]
- 7. Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- 8. Alderman MH, Cohen H, Madhavan S, et al. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. [DOI] [PubMed] [Google Scholar]
- 9. Franse LV, Pahor M, Di Bari M, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens. 2000;18:1149–1154. [DOI] [PubMed] [Google Scholar]
- 10. Sakata K, Hashimoto T, Ueshima H, et al., for the NIPPON DATA 80 Research Group . Absence of an association between serum uric acid and mortality from cardiovascular disease: NIPPON DATA 80, 1980–1994. National Integrated Projects for Prospective Observation of Non‐communicable Diseases and its Trend in the Aged. Eur J Epidemiol. 2001;17:461–468. [DOI] [PubMed] [Google Scholar]
- 11. De Leeuw PW, Thijs L, Birkenhager WH, et al., for the Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators . Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst‐Eur trial. J Am Soc Nephrol. 2002;13:2213–2222. [DOI] [PubMed] [Google Scholar]
- 12. Wong KY, MacWalter RS, Fraser HW, et al. Urate predicts subsequent cardiac death in stroke survivors. Eur Heart J. 2002;23:788–793. [DOI] [PubMed] [Google Scholar]
- 13. Weir CJ, Muir SW, Walters MR, et al. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34:1951–1956. [DOI] [PubMed] [Google Scholar]
- 14. Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. [DOI] [PubMed] [Google Scholar]
- 15. Hoieggen A, Alderman MH, Kjeldsen SE, et al., for the LIFE Study Group . The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–1049. [DOI] [PubMed] [Google Scholar]
- 16. Athyros VG, Elisaf M, Papageorgiou AA, et al., for the GREACE Study Collaborative Group . Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study. Am J Kidney Dis. 2004;43:589–599. [DOI] [PubMed] [Google Scholar]
- 17. Hsu SP, Pai MF, Peng YS, et al. Serum uric acid levels show a ‘J‐shaped’ association with all‐cause mortality in haemodialysis patients. Nephrol Dial Transplant. 2004;19:457–462. [DOI] [PubMed] [Google Scholar]
- 18. Simon JA, Lin F, Vittinghoff E, et al., for the Heart and Estrogen‐Progestin Replacement Study (HERS) Research Group . The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen‐Progestin Replacement Study (HERS). Ann Epidemiol. 2006;16:138–145. [DOI] [PubMed] [Google Scholar]
- 19. Madsen TE, Muhlestein JB, Carlquist JF, et al. Serum uric acid independently predicts mortality in patients with significant, angiographically defined coronary disease. Am J Nephrol. 2005;25:45–49. [DOI] [PubMed] [Google Scholar]
- 20. Kojima S, Sakamoto T, Ishihara M, et al., for the Japanese Acute Coronary Syndrome Study (JACSS) Investigators . Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol. 2005;96:489–495. [DOI] [PubMed] [Google Scholar]
- 21. Gueyffier F, Boissel JP, Pocock S, et al. Identification of risk factors in hypertensive patients: contribution of randomized controlled trials through an individual patient database. Circulation. 1999;100:e88–e94. [DOI] [PubMed] [Google Scholar]
- 22. Bickel C, Rupprecht HJ, Blankenberg S, et al. Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am J Cardiol. 2002;89:12–17. [DOI] [PubMed] [Google Scholar]
- 23. Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. [DOI] [PubMed] [Google Scholar]
- 24. Yu TF, Berger L, Dorph DJ, et al. Renal function in gout. V. Factors influencing the renal hemodynamics. Am J Med. 1979;67:766–771. [DOI] [PubMed] [Google Scholar]
- 25. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–409. [DOI] [PubMed] [Google Scholar]
- 26. Syrjanen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000;15:34–42. [DOI] [PubMed] [Google Scholar]
- 27. Iseki K, Oshiro S, Tozawa M, et al. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–697. [DOI] [PubMed] [Google Scholar]
- 28. Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650. [PubMed] [Google Scholar]
- 29. Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12‐year cohort study. J Am Soc Nephrol. 2005;16:791–799. [DOI] [PubMed] [Google Scholar]
- 30. Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. [DOI] [PubMed] [Google Scholar]
- 31. Ito K, Nakashima J, Hanawa Y, et al. The prediction of renal function 6 years after unilateral nephrectomy using preoperative risk factors. J Urol. 2004;171:120–125. [DOI] [PubMed] [Google Scholar]
- 32. Persky VW, Dyer AR, Idris‐Soven E, et al. Uric acid: a risk factor for coronary heart disease? Circulation. 1979;59:969–977. [DOI] [PubMed] [Google Scholar]
- 33. Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. Am J Cardiol. 2001;87:1411–1414. [DOI] [PubMed] [Google Scholar]
- 34. Ishizaka N, Ishizaka Y, Toda E, et al. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–1044. [DOI] [PubMed] [Google Scholar]
- 35. Tsioufis C, Chatzis D, Vezali E, et al. The controversial role of serum uric acid in essential hypertension: relationships with indices of target organ damage. J Hum Hypertens. 2005;19:211–217. [DOI] [PubMed] [Google Scholar]
- 36. Viazzi F, Parodi D, Leoncini G, et al. Serum uric acid and target organ damage in primary hypertension. Hypertension. 2005;45:991–996. [DOI] [PubMed] [Google Scholar]
- 37. Wu J, Chen X, Xie Y, et al. Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant. 2005;20:719–727. [DOI] [PubMed] [Google Scholar]
- 38. Myllymaki J, Honkanen T, Syrjanen J, et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005;20:89–95. [DOI] [PubMed] [Google Scholar]
- 39. Iribarren C, Folsom AR, Eckfeldt JH, et al. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities . Ann Epidemiol. 1996;6:331–340. [DOI] [PubMed] [Google Scholar]
- 40. Kang DH, Han L, Ouyang X, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. [DOI] [PubMed] [Google Scholar]
- 41. Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. [DOI] [PubMed] [Google Scholar]
- 42. Kang D‐H, Park SK, Lee IK, et al. Uric acid induced C‐reactive protein (CRP) expression: implication on cell proliferation and nitric oxide production in human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. [DOI] [PubMed] [Google Scholar]
- 43. Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet‐derived growth factor A‐chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 44. Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein‐1 production in vascular smooth muscle cells via mitogen‐activated protein kinase and cyclooxygenase‐2. Hypertension. 2003;41:1287–1293. [DOI] [PubMed] [Google Scholar]
- 45. Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. [DOI] [PubMed] [Google Scholar]
- 46. Maxwell AJ, Bruinsma KA. Uric acid is closely linked to vascular nitric oxide activity. Evidence for mechanism of association with cardiovascular disease. J Am Coll Cardiol. 2001;38:1850–1858. [DOI] [PubMed] [Google Scholar]
- 47. Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:932–935. [DOI] [PubMed] [Google Scholar]
- 48. Waring WS, Adwani SH, Breukels O, et al. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakagawa T, Mazzali M, Kang DH, et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003;23:2–7. [DOI] [PubMed] [Google Scholar]
- 50. Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defence against oxidant and radical caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. [DOI] [PubMed] [Google Scholar]
- 52. Hink HU, Santanam N, Dikalov S, et al. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–1408. [DOI] [PubMed] [Google Scholar]
- 53. Johnson WD, Kayser KL, Brenowitz JB, et al. A randomized controlled trial of allopurinol in coronary bypass surgery. Am Heart J. 1991;121:20–24. [DOI] [PubMed] [Google Scholar]
- 54. Rashid MA, William‐Olsson G. Influence of allopurinol on cardiac complications in open heart operations. Ann Thorac Surg. 1991;52:127–130. [DOI] [PubMed] [Google Scholar]
- 55. Tabayashi K, Suzuki Y, Nagamine S, et al. A clinical trial of allopurinol (Zyloric) for myocardial protection. J Thorac Cardiovasc Surg. 1991;101:713–718. [PubMed] [Google Scholar]
- 56. Gavin AD, Struthers AD. Allopurinol reduces B‐type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oxypurinol fails to improve HF outcomes in phase 2 trials. Available at: http:www.theheart.orgarticle544687.do. Accessed August 18, 2005.
- 58. Milionis HJ, Kakafika AI, Tsouli SG, et al. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am Heart J. 2004;148:635–640. [DOI] [PubMed] [Google Scholar]
- 59. Farquharson CA, Struthers AD. Increasing plasma potassium with amiloride shortens the QT interval and reduces ventricular extrasystoles but does not change endothelial function or heart rate variability in chronic heart failure. Heart. 2002;88:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo‐controlled studies. Circulation. 2002;105:2619–2624. [DOI] [PubMed] [Google Scholar]
- 61. Burnier M, Roch‐Ramel F, Brunner HR. Renal effects of angiotensin II receptor blockade in normotensive subjects. Kidney Int. 1996;49:1787–1790. [DOI] [PubMed] [Google Scholar]
- 62. Sumino H, Ichikawa S, Kanda T, et al. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354:650. [DOI] [PubMed] [Google Scholar]
- 63. Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paolisso G, Barbagallo M, Petrella G, et al. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non‐insulin‐dependent diabetic patients. Atherosclerosis. 2000;150:121–127. [DOI] [PubMed] [Google Scholar]
- 65. Kostis JB, Wilson AC, Freudenberger RS, et al.; SHEP Collaborative Research Group . Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95:29–35. [DOI] [PubMed] [Google Scholar]
- 66. Hawkins RG, Houston MC. Is population‐wide diuretic use directly associated with the incidence of end‐stage renal disease in the United States? A hypothesis. Am J Hypertens. 2005;18:744–749. [DOI] [PubMed] [Google Scholar]
- 67. Rahman M, Pressel S, Davis BR, et al. ALLHAT Collaborative Research Group . Cardiovascular outcomes in high‐risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med. 2006;144:172–180. [DOI] [PubMed] [Google Scholar]


