Abstract
Most hypertensive patients require more than one drug for adequate blood pressure (BP) control. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends starting treatment with a thiazide diuretic or, when BP is >20/10 mm Hg above goal or in patients with diabetes, using two different antihypertensive agents. Searches of MEDLINE, EMBASE, and BIOSIS databases identified four similarly designed, randomized, factorial studies comparing various doses of angiotensin II receptor blockers with hydrochlorothiazide as monotherapy and in combination. The methodology and results of these studies were compared. The primary efficacy end point in these studies was a decrease from baseline in mean diastolic BP after 8 weeks of therapy. All currently available angiotensin I receptor blocker/hydrochlorothiazide combinations evaluated (irbesartan, olmesartan medoxomil, telmisartan, and valsartan plus hydrochlorothiazide) produced significant systolic BP and diastolic BP reductions. Olmesartan medoxomil/hydrochlorothiazide 40 mg/25 mg provided the largest mean reduction in absolute and placebo‐corrected systolic BP/diastolic BP. For all angiotensin II receptor blocker/hydrochlorothiazide combinations evaluated, ≥63% of patients achieved a diastolic BP response (diastolic BP <90 mm Hg or ≥10‐mm Hg reduction). In conclusion, the combination of an angiotensin II receptor blocker and hydrochlorothiazide produces more substantial BP responses than monotherapy with either component.
The prevalence of hypertension is increasing in the United States, with almost 29% of the adult population (58.4 million individuals) estimated to have been hypertensive in 1999–2000. 1 Adequate control of high blood pressure (BP) is therefore of paramount importance because as BP increases, so does the risk of developing myocardial infarction, heart failure, stroke, and kidney disease. 2
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends a goal BP of <140/90 mm Hg for the general hypertensive population and <130/80 mm Hg for patients with diabetes or chronic kidney disease. 2 Although BP control rates are improving, they are still far from ideal. In 1999–2000, only an estimated 58% of known hypertensive patients were receiving BP medication. Of those receiving treatment, 53% achieved BP control consistent with JNC 7 definitions at that time. 1 Hence, there is a clear need for more aggressive treatment of hypertensive patients.
For stage 2 hypertensives (systolic BP [SBP] ≥160 mm Hg or diastolic BP [DBP] ≥100 mm Hg), JNC 7 guidelines recommend treatment that includes a thiazide diuretic either alone or in combination with another class of drug having a complementary mode of action as initial therapy. 2 Initial combination therapy for these patients has included a diuretic in combination with an angiotensin‐converting enzyme (ACE) inhibitor, β blocker, or angiotensin receptor blocker (ARB). 3 , 4
Studies have established that the addition of a thiazide‐type diuretic, such as hydrochlorothiazide (HCTZ), to ARB therapy enhances BP lowering ability and increases the proportion of patients who achieve goal BP. 5 , 6 , 7 , 8 However, despite the proven efficacy and increased utilization of ARB and HCTZ combinations, there are few direct head‐to‐head comparisons of the different formulations in the published literature. 9 , 10 , 11 , 12 Those that have been conducted provide incomplete information regarding comparative dose‐related BP lowering efficacy of such combinations in clinical practice.
Factorial studies (sometimes referred to as matrix studies) are designed to evaluate whether a specific combination therapy is more effective than either of the individual agents used as monotherapy. These studies are commonly used in regulatory submissions to the Food and Drug Administration because they provide data that would otherwise require multiple trials. 13 The factorial study design is not intended to evaluate a dose response relationship between different combinations, but rather is statistically powered to evaluate safe and effective doses of combination therapy. In such studies, every level of any variable or intervention is paired with each level of every other variable or intervention. These studies have been used with a number of agents, such as ACE inhibitors and β blockers, with HCTZ and have demonstrated greater efficacy with the combinations in comparison with their monotherapy components. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
To date, factorial studies have been published for four of the ARBs currently available on the market—irbesartan, olmesartan medoxomil (olmesartan), telmisartan, and valsartan. 22 , 23 , 24 , 25 All four studies used a similar design and patient population. Recent evidence from published meta‐analyses suggests that indirect comparison of agents may be valid when the data come from studies of similar design in comparable patient populations and with the same comparator (e.g., placebo). 26 Given the similarities of these four factorial studies, we conducted a comparative review to derive information about the relative efficacy of these ARB/HCTZ combinations and their constituent components. This review may provide clinicians with pertinent information in the absence of direct comparisons.
DESIGN AND METHODOLOGY
A search was undertaken on the MEDLINE (National Library of Medicine), EMBASE, and BIOSIS databases to identify factorial‐design, randomized, controlled trials with available ARBs and HCTZ. Four studies were identified, involving the following ARBs: irbesartan, 22 olmesartan, 23 telmisartan, 24 and valsartan. 25 The results presented in this review from the above mentioned studies were previously published in their entirety. 22 , 23 , 24 , 25 All were multicenter, randomized, double‐blind, placebo‐controlled studies of 8 weeks' duration. Study design and methodology of the four studies are summarized in Table I. Inclusion criteria were similar across the four studies, with only slight variations in the BP cutoff points used (Table I).
Table I.
Study Design and Methodology of the ARB/HCTZ Factorial Studies
| Drug Combination | Study Design | Entry Criteria | Treatment Arms/Drug Doses | Primary Efficacy Endpoint |
|---|---|---|---|---|
| Irbesartan/HCTZ 22 | Multicenter, randomized, double‐blind, placebo‐controlled, 4 × 4 factorial design | SeDBP 95–110 mm Hg at Weeks 3 and 4 of placebo run‐in period | Placebo Irbesartan 37.5, 100, or 300 mg HCTZ 6.25, 12.5, or 25 mg | Δ from baseline in mean SeDBP at Week 8 |
| Olmesartan/HCTZ 23 | Multicenter, randomized, double‐blind, placebo‐controlled, 4 × 3 factorial design | SeDBP 100–115 mm Hg at Weeks 3 and 4 of placebo run‐in period | Placebo Olmesartan 10, 20, or 40 mg HCTZ 12.5 or 25 mg | Δ from baseline in mean SeDBP at Week 8 |
| Telmisartan/HCTZ 24 | Multicenter, randomized, double‐blind, placebo‐controlled, 4 × 5 factorial design* | SuDBP 95–114 mm Hg during last 2 weeks of 4‐week placebo run‐in period | Placebo Telmisartan 20, 40, 60, or 80 mg HCTZ 6.25, 12.5, or 25 mg | Δ from baseline in mean SuDBP at Week 8 |
| Valsartan/HCTZ 25 | Multicenter, randomized, double‐blind, placebo‐controlled, 3 × 3 factorial design | SeDBP 95–115 mm Hg after a 2‐ to 4‐week placebo run‐in period | Placebo Valsartan 80 or 160 mg HCTZ 12.5 or 25 mg | Δ from baseline in mean SeDBP at Week 8 |
| ARB=angiotensin receptor blocker; HCTZ=hydrochlorothiazide; SeDBP=seated diastolic blood pressure; Δ=change; SuDBP=supine diastolic blood pressure; *although the factorial study had 20 treatment groups, the published paper reports results from only six treatment groups (placebo, HCTZ 12.5 mg, telmisartan 40 mg, telmisartan 80 mg, telmisartan 40 mg/HCTZ 12.5 mg, telmisartan 80 mg/HCTZ 12.5 mg) | ||||
Patients were randomized to receive placebo, ARB or HCTZ monotherapy (doses of ARB and HCTZ monotherapy used in each study are shown in Table I) or ARB/HCTZ combination therapy. Patients received all possible combinations of dosages used in the monotherapy groups. These protocols allowed ARB/HCTZ combination therapy to be compared with monotherapy with the ARB and HCTZ components and also with placebo.
The primary efficacy end point in all studies was the change from baseline in mean DBP. Other efficacy end points reported in these studies included change from baseline in mean SBP 23 , 24 , 25 and the proportion of patients responding to treatment (defined as DBP <90 mm Hg or a ≥10‐mm Hg reduction in DBP). 22 , 23 , 24 , 25
Seated BP measurements were used in three of the four factorial studies, while one study (the telmisartan study 24 ) used supine readings. In all studies, BP measurements were performed approximately 24 hours after the last dose of the investigational drug was taken (trough values).
RESULTS
Patients
The number of patients randomized to treatment ranged from 502 to 871 across the four factorial studies. In general, the demographic and baseline characteristics of the study populations were similar (Table II). The mean age of participants across all studies was 52–55 years, and the percentage of male patients was 56%–65%. The percentage of white participants was 74%–85% for three of the studies, and in one study (the telmisartan study 24 ) 27% of participants were black and the remainder were non‐black (this category included white, Hispanic, and other racial groups). Mean baseline BP was similar across the studies and ranged from 100–104.4 mm Hg for DBP and 151–156.6 mm Hg for SBP.
Table II.
Demographic and Baseline Characteristics of Patients in the ARB/HCTZ Factorial Studies
| Drug Combination | No. of Patients Randomized to Treatment | Mean Age (Years) | Gender | Ethnicity | Mean Baseline Blood Pressure (mm Hg) |
|---|---|---|---|---|---|
| Irbesartan/HCTZ 22 | 683 | 55 | 65% Male | 85% White | SBP/DBP=151/100 |
| Olmesartan/HCTZ 23 | 502 | 53 | 56% Male | 74% White | SBP=151.9–156.6 |
| DBP=102.6–104.4 | |||||
| Telmisartan/HCTZ 24 | 818 | 53 | 60% Male | 27% Black 73% Non‐black* | SDP/SBP=154/101 |
| Valsartan/HCTZ 25 | 871 | 52 | 58% Male | 75% White | SBP=152.0–155.9 |
| DBP=100.4–101.5 | |||||
| ARB=angiotensin receptor blocker; HCTZ=hydrochlorothiazide; SBP=systolic blood pressure; DBP=diastolic blood pressure; *patients included those of white, Hispanic, and other racial groups | |||||
Efficacy
Three of the four studies presented data for the intent‐to‐ treat populations 23 , 24 , 25 using the last observation carried forward for patients who either discontinued or were lost to follow‐up. The study by Kochar et al. 22 (irbesartan) presented data for the population of patients who completed the study or who discontinued treatment but had BP readings taken at Week 8.
In these four studies, placebo was generally associated with reductions in mean DBP of approximately 3.5–4.1 mm Hg compared with baseline after 8 weeks (Table III). 22 , 24 , 25 The exception is the olmesartan study, in which the mean reduction in DBP at 8 weeks in the placebo group was 8.2 mm Hg. 23 The impact of placebo on mean SBP was more consistent, with reductions of 1.9–3.3 mm Hg recorded in all four studies.
Table III.
Reductions in Mean SBP and DBP Observed After 8 Weeks of Treatment With HCTZ Monotherapy in the Factorial Studies*
| Mean Absolute Change inBP (mm Hg) | Mean Placebo‐Corrected Change in BP (mm Hg) | ||||
|---|---|---|---|---|---|
| Factorial Studies | Hctz Doses | DBP | SBP | DBP | SBP |
| Irbesartan 22 | Placebo | −3.5 | −2.3 | … | … |
| 6.25 mg | −5.1 | −4.6 | −1.6 | −2.3 | |
| 12.5 mg | −6.2 | −8.9 | −2.7 | −6.6 | |
| 25 mg | −8.3 | −11.5 | −4.8 | −9.2 | |
| Olmesartan 23 | Placebo | −8.2 | −3.3 | … | … |
| 12.5 mg | −10.2 | −9.6 | −2.0 | −6.3 | |
| 25 mg | −12.9 | −17.1 | −4.7 | −13.8 | |
| Telmisartan** 24 | Placebo | −3.8 | −2.9 | … | … |
| 12.5 mg | −7.3 | −6.9 | −3.5 | −4.0 | |
| Valsartan 25 , 41 | Placebo | −4.1 | −1.9 | … | … |
| 12.5 mg | −7.1 | −7.3 | −3.0 | −5.4 | |
| 25 mg | −9.3 | −12.7 | −5.2 | −10.8 | |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; HCTZ=hydrochlorothiazide; BP=blood pressure; *data are mean changes in DBP and SBP at end point (or last observation carried forward) in the intent‐to‐treat population for studies involving olmesartan, telmisartan, and valsartan; the irbesartan study provided data on the population of patients who completed the study and/or had Week 8 BP readings; **the telmisartan study used supine BP measurements and the other studies used sitting BP measurements. | |||||
For monotherapy with HCTZ 12.5 mg, the mean absolute DBP reduction ranged from 6.2 mm Hg 22 to 10.2 mm Hg, 23 and the mean placebo‐corrected DBP reduction ranged from 2.0 mm Hg 23 to 3.5 mm Hg 24 (Table III). The magnitude of SBP changes from baseline was somewhat higher, with a range of absolute decreases of 6.9 mm Hg 24 to 9.6 mm Hg 23 and placebo‐adjusted decreases of 4.0 mm Hg 24 to 6.6 mm Hg 22 (Table III). Across the three studies that provided data on HCTZ 25 mg, the mean absolute decrease in DBP was 10 mm Hg (placebo‐corrected 5.0 mm Hg) and the mean absolute decrease in SBP was 13.5 mm Hg (placebo‐corrected 11.2 mm Hg). 22 , 23 , 25
In all studies, combination therapy with an ARB and HCTZ produced more marked lowering of DBP than did monotherapy with the component agents (Table IV). For most combinations, the absolute reduction in DBP exceeded 10 mm Hg (the exception was irbesartan 37.5 mg with either HCTZ 6.25 mg or 12.5 mg, which resulted in absolute DBP reductions of 8.1 mm Hg and 9.0 mm Hg, respectively). The placebo‐corrected DBP decrease exceeded 5 mm Hg for all combinations except irbesartan 37.5 mg/HCTZ 6.25 mg. Although not a primary end point, the reductions in SBP were also greater with the combination of ARB/HCTZ than with either agent given alone. Three of the studies (involving irbesartan, olmesartan, and telmisartan) used the global average test 27 to determine whether at least one combination was more effective than each of its components, and this was proven to be the case in all three of these studies. 22 , 23 , 24 The other study used two‐sided t tests to compare the combination of valsartan/HCTZ with every monotherapy component, and these showed statistically significant superiority with each combination (p<0.01). 25
Table IV.
Reductions in Mean SBP and DBP Observed After 8 Weeks of Treatment: Results From the Factorial Studies for the Fixed‐Dose Combinations of ARBs With HCTZ*
| Mean Absolute Change in BP (mm Hg) | Mean Placebo‐Corrected Change in BP (mm Hg) | ||||
|---|---|---|---|---|---|
| Drug Combination | Doses | DBP | SBP | DBP | SBP |
| Irbesartan/HCTZ 22 | 37.5 mg/6.25 mg | −8.1 | −10.2 | −4.6 | −7.9 |
| 100 mg/6.25 mg | −10.0 | −11.9 | −6.5 | −9.6 | |
| 300 mg/6.25 mg | −13.2 | −17.2 | −9.7 | −14.9 | |
| 37.5 mg/12.5 mg | −9.0 | −14.7 | −5.5 | −12.4 | |
| 100 mg/12.5 mg**†† | −11.9 | −14.9 | −8.4 | −12.6 | |
| 300 mg/12.5 mg†† | −15.0 | −15.9 | −11.5 | −13.6 | |
| 37.5 mg/25 mg | −11.7 | −16.8 | −8.2 | −14.5 | |
| 100 mg/25 mg | −13.8 | −21.5 | −10.3 | −19.2 | |
| 300 mg/25 mg†† | −14.4 | −23.1 | −10.9 | −20.8 | |
| Olmesartan/HCTZ 23 | 10 mg/12.5 mg | −13.5 | −17.4 | −5.3 | −14.1 |
| 20 mg/12.5 mg†† | −16.4 | −20.1 | −8.2 | −16.8 | |
| 40 mg/12.5 mg†† | −17.3 | −20.6 | −9.1 | −17.3 | |
| 10 mg/25 mg | −17.1 | −23.0 | −8.9 | −19.7 | |
| 20 mg/25 mg | −20.0 | −27.1 | −11.8 | −23.8 | |
| 40 mg/25 mg†† | −21.9 | −26.8 | −13.7 | −23.5 | |
| Telmisartan/HCTZ†24 | 40 mg/12.5 mg†† | −12.6 | −18.8 | −8.8 | −15.9 |
| 80 mg/12.5 mg†† | −14.9 | −23.9 | −11.1 | −21.0 | |
| Valsartan/HCTZ 25 , 41 | 80 mg/12.5 mg†† | −11.8 | −16.5 | −7.7 | −14.6 |
| 160 mg/12.5 mg†† | −13.5 | −17.7 | −9.4 | −15.8 | |
| 80 mg/25 mg | −15.3 | −21.1 | −11.2 | −19.2 | |
| 160 mg/25 mg†† | −15.3 | −22.4 | −11.2 | −20.5 | |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; ARB=angiotensin receptor blocker; HCTZ=hydrochlorothiazide; BP=blood pressure; *data are mean changes in DBP and SBP at end point (or last observation carried forward) in the intent‐to‐treat population for studies involving olmesartan, telmisartan, and valsartan; the irbesartan study provided data on the population of patients who completed the study and/or had Week 8 BP readings; **the dose combination evaluated in the irbesartan factorial study was 100 mg/12.5 mg; however, the marketed combination is 150 mg/12.5 mg; †the telmisartan study used supine BP measurements and the other studies used sitting BP measurements; ††approved US dosages | |||||
Although the telmisartan study included 20 separate treatment groups, the published report on telmisartan focused on only two dose levels of telmisartan (40 mg and 80 mg) and on one dose level of HCTZ (12.5 mg), 24 these doses being the commercially available ones. The other studies reported on a wider range of doses, including some that are not marketed. Figures 1 and Figures 2 present the change from baseline to Week 8 in mean absolute BP and placebo‐corrected BP, respectively, for the maximal marketed doses of the ARB/HCTZ combinations to provide a clinically relevant comparison. The greatest absolute reduction in both mean DBP and SBP was seen with maximal doses of olmesartan and HCTZ (40 mg and 25 mg, respectively) (Figures 1 and Figures 2). This also held true when the absolute data values were placebo corrected (Figures 3 and Figures 4).
Figure 1.
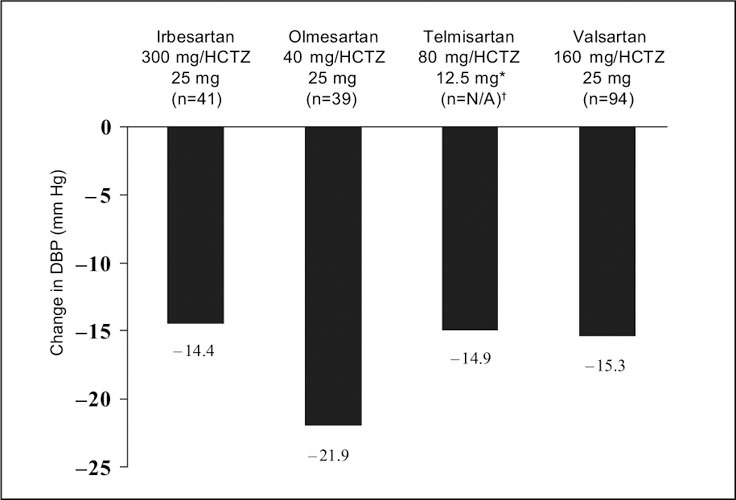
Mean absolute change from baseline in diastolic blood pressure (DBP) in subjects with hypertension treated at maximal marketed doses of an angiotensin receptor blocker plus hydrochlorothiazide (HCTZ) for 8 weeks. *highest dose reported in the published paper;†ITT population not reported in this study
Figure 2.
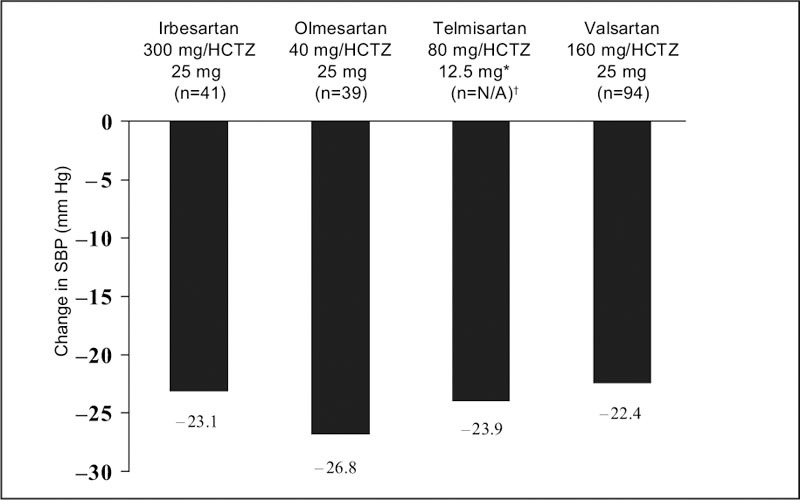
Mean absolute change from baseline in systolic blood pressure (SBP) in subjects with hypertension treated at maximal marketed doses of an angiotensin receptor blocker plus hydrochlorothiazide (HCTZ) for 8 weeks, *highest dose reported in the published paper; †ITT population not reported in this study
Figure 3.
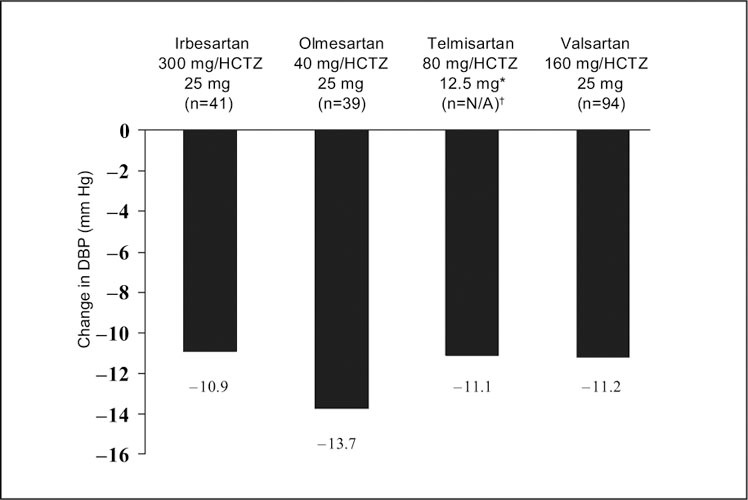
Mean placebo‐adjusted change from baseline in diastolic blood pressure (DBP) in subjects with hypertension treated at maximal marketed doses of an angiotensin receptor blocker plus hydrochlorothiazide (HCTZ) for 8 weeks. *highest dose reported in the published paper; †ITT population not reported in this study
Figure 4.
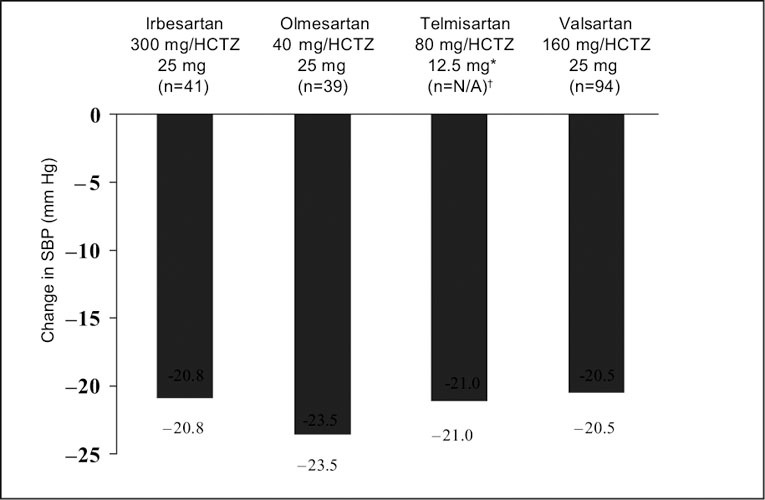
Mean placebo‐adjusted change from baseline in systolic blood pressure (SBP) in subjects with hypertension treated at maximal marketed doses of an angiotensin receptor blocker plus hydrochlorothiazide (HCTZ) for 8 weeks. *highest dose reported in the published paper; †ITT population not reported in this study
In clinical practice, a second antihypertensive agent is usually added when the response to initial monotherapy is inadequate. In the factorial studies under assessment, the combination of HCTZ and ARB produced incremental increases in the magnitude of mean BP reduction compared with ARB monotherapy (Table V). In addition, the combination of HCTZ 25 mg plus ARB was almost invariably associated with a greater change in mean BP than the combination of HCTZ 12.5 mg plus ARB. For example, the mean absolute (and placebo‐corrected) DBP decrease at 8 weeks was 9.4 (5.3) to 14.6 (6.4) mm Hg with maximal doses of ARB monotherapy, 13.5 (9.4) to 17.3 (9.1) mm Hg with the same dose of ARB + HCTZ 12.5 mg, and 14.4 (10.9) to 21.9 (13.7) mm Hg with the same ARB dose + HCTZ 25 mg. However, an exception was the change in mean DBP between irbesartan 300 mg/HCTZ 12.5 mg and irbesartan 300 mg/HCTZ 25 mg; no incremental increase was seen with the higher dose of HCTZ in these combinations. In fact, the mean placebo‐corrected change in DBP with irbesartan 300 mg/HCTZ 25 mg was slightly lower than the change with irbesartan/HCTZ 300 mg/12.5 mg (−10.9 mm Hg with HCTZ 25 mg and −11.5 mm Hg with HCTZ 12.5 mg).
Table V.
Mean Reduction in SBP and DBP With the Maximal Tested Dose of Each ARB, as Monotherapy and in Combination With HCTZ 12.5 mg and 25 mg
| Mean Change in BP (mm Hg) | |||
|---|---|---|---|
| ARB Alone | ARB + HCTZ 12.5 mg | ARB + HCTZ 25 mg | |
| Irbesartan 300 mg 22 | |||
| SBP: absolute (placebo corrected) | −14.9 (‐12.6) | −15.9 (‐13.6) | −23.1 (‐20.8) |
| DBP: absolute (placebo corrected) | −10.2 (‐6.7) | −15.0 (‐11.5) | −14.4 (‐10.9) |
| Olmesartan 40 mg 23 | |||
| SBP: absolute (placebo corrected) | −16.0 (‐12.7) | −20.6 (‐17.3) | −26.8 (‐23.5) |
| DBP: absolute (placebo corrected) | −14.6 (‐6.4) | −17.3 (‐9.1) | −21.9 (‐13.7) |
| Telmisartan 80 mg*24 | |||
| SBP: absolute (placebo corrected) | −15.4 (‐12.5) | −23.9 (‐21.0) | N/A |
| DBP: absolute (placebo corrected) | −11.5 (‐7.7) | −14.9 (‐11.1) | N/A |
| Valsartan 160 mg 25 , 41 | |||
| SBP: absolute (placebo corrected) | −12.1 (‐10.2) | −17.7 (‐15.8) | −22.4 (‐20.5) |
| DBP: absolute (placebo corrected) | −9.4 (‐5.3) | −13.5 (‐9.4) | −15.3 (‐11.2) |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; ARB=angiotensin receptor blocker; HCTZ=hydrochlorothiazide; BP=blood pressure; /N/A=not analyzed or presented in the published report; *the telmisartan study used supine BP measurements and the other studies used sitting BP measurements | |||
Three of the factorial studies reported the proportion of patients in each treatment group who were considered responsive to therapy (DBP <90 mm Hg or a ≥10‐mm Hg reduction from baseline). 23 , 24 , 25 Placebo response rates ranged from 24% 22 to 38.1%. 23 For combination therapy, the lowest marketed doses produced absolute (and placebo‐corrected) response rates of 63% (34%) to 77% (39%), and the highest marketed doses produced absolute (and placebo‐corrected) response rates of 79% (50%) to 92% (54%). The highest absolute and placebo‐corrected response rate was seen with olmesartan/HCTZ 40 mg/25 mg (92% and 54%, respectively). 23
Safety
The combination of ARB/HCTZ was generally well tolerated in the factorial studies evaluated in this review, with the incidence of adverse events being similar to ARB monotherapy. The rate of withdrawal due to the occurrence of adverse events in the ARB/HCTZ combination groups was ≤5% across all studies. Most adverse events were mild to moderate and transient. The most common adverse events reported in these studies included dizziness, headache, and upper respiratory tract infection. The combination of an ARB with HCTZ appeared to attenuate the potassium loss normally associated with HCTZ therapy.
DISCUSSION
Randomized, controlled, direct comparisons of ARB/HCTZ combinations are currently lacking in the published literature. Most studies of these agents employ a design in which HCTZ is added to the treatment regimen only after there is an inadequate response to ARB monotherapy and consequently provide clinicians with little information about the magnitude of BP reduction that can be expected from ARB/HCTZ compared with either agent alone.
In contrast, factorial‐design studies provide a unique opportunity to compare the efficacy of a combination therapy with the efficacy of each component of the combination in a single study. The factorial studies identified for this analysis used a similar methodology, design, and primary efficacy variable, and all end‐of‐treatment BP measurements were taken 24 hours after the last dose (at trough). Given these similarities, it seemed reasonable to undertake a cross‐study comparison of these trials to evaluate the efficacy results with different ARB/HCTZ combinations. This approach is supported by recent evidence from published meta‐analyses showing that the results of adjusted indirect comparisons from comparable studies are similar to the results of direct randomized comparisons. 26
This review of published factorial studies of ARB/HCTZ combinations has shown that such combinations are more effective than the corresponding dose of either HCTZ or ARB when given as monotherapy. All of the currently available combinations of ARB and HCTZ evaluated in these factorial studies produced significant lowering of both DBP and SBP vs. baseline. The effects on BP lowering were reported to be additive for all doses of ARB/HCTZ combinations when compared with monotherapy with either the constituent ARB or HCTZ. At maximal marketed doses, olmesartan 40 mg plus HCTZ 25 mg provided the largest absolute reductions in both mean DBP and SBP (−21.9 mm Hg and −26.8 mm Hg, respectively) compared with baseline, as well as the largest placebo‐corrected reductions (−13.7 mm Hg and −23.5 mm Hg, respectively). In addition to measuring the mean reduction in DBP and SBP, some of the factorial studies in the current analysis assessed the proportion of patients achieving a DBP response (defined as DBP <90‐mm Hg or a ≥10‐mm Hg reduction in DBP). For the studies in which this end point was reported, the ARB/HCTZ combinations at commercially available doses were associated with absolute DBP response rates of 63% (with telmisartan 40 mg/HCTZ 12.5 mg) to 92% (with olmesartan 40 mg/HCTZ 12.5 mg). This is consistent with data from a meta‐analysis of ARB/HCTZ randomized controlled trials showing that 68% of patients achieve a BP response across all combinations. 6 At maximal marketed doses, placebo‐adjusted response rates were ≥50% for all ARB/HCTZ combinations. Hence, available data suggest that the majority of hypertensive patients achieve a DBP response with ARB/HCTZ combination therapy.
An important finding of this review is the consistency of the mean reductions in BP associated with placebo and HCTZ monotherapy. For example, the difference between the lowest and highest mean change in SBP with placebo was only 1.5 mm Hg across these four studies. The mean placebo‐corrected reductions in DBP associated with 12.5 or 25 mg of HCTZ in the ARB factorial studies in this analysis were similar in magnitude to those achieved in similar factorial studies with ACE inhibitors and HCTZ. 14 , 15 , 16 , 17 The consistency of the reductions in BP with HCTZ and placebo in the four factorial studies and in other factorial studies supports the validity of comparing results from separate but similar studies.
In addition to describing the effects of ARB/HCTZ combination therapy, this comparison also provided information on the comparative efficacy of ARB monotherapies. The findings for the antihypertensive effect of ARB monotherapy in this analysis are comparable to those reported in published meta‐analyses. For example, in a meta‐analysis by Conlin et al., 6 the mean absolute DBP reduction with valsartan 80 mg was −8.8 mm Hg (data derived from 1455 patients in randomized controlled trials). In the factorial study, the mean reduction was −8.6 mm Hg. 25 Similarly, in a pooled analysis, the weighted average reduction in DBP (placebo‐corrected) in four valsartan studies was −4.2 mm Hg with valsartan 80 mg and −5.2 mm Hg with valsartan 160 mg. 28 This compares with corresponding placebo‐corrected reductions of −4.5 mm Hg and −5.3 mm Hg, respectively, in the factorial study. 25
At the maximal tested doses, ARB monotherapy produced similar reductions in mean BP across all four factorial studies. However, the JNC 7 guidelines note that most patients with hypertension will require more than one drug to achieve BP goals. 2 In these circumstances, it is recommended that the patients receive an additional agent from a separate class. 2 Administering a combination of agents that have complementary modes of action can produce clinical benefits not associated with merely increasing the dose of a single agent. These benefits may include better compliance rates and quicker BP control. 29 , 30 Thiazide diuretics, recommended as initial therapy for most patients with uncomplicated hypertension, promote salt elimination and stimulate the renin‐angiotensin‐aldosterone system via intrarenal mechanisms, thereby making BP more dependent on angiotensin II. 31 Thus, the antihypertensive effects of ARBs, β‐blockers, and ACE inhibitors are enhanced in the presence of thiazide diuretics. 3 , 31 , 32 , 33
For patients who fail to respond adequately to ARB monotherapy, the addition of HCTZ produces additional decrements in BP 8 , 34 , 35 , 36 The factorial studies in this review support this approach, showing that the combination of ARB plus HCTZ produces greater mean decreases in BP than does ARB monotherapy. The effect is generally greater with 25 mg of HCTZ than with 12.5 mg of HCTZ, suggesting that clinicians can try increasing the dose of HCTZ if 12.5 mg fails to achieve the target BP for ARB/HCTZ combinations when both the 12.5‐mg and 25‐mg dose forms are available. Notwithstanding, the dose‐response effect was not seen in all the studies, and there were differences in the magnitude of BP reductions achieved with the different ARB/HCTZ combinations. For example, irbesartan 300 mg/HCTZ 25 mg produced a mean placebo‐corrected decrease in DBP that was no greater than that seen with irbesartan 300 mg/HCTZ 12.5 mg. In addition, the magnitude of BP reductions with the olmesartan/HCTZ combinations was greater than with the other ARB/HCTZ combinations.
For some patients, the use of a combination product may be preferable to increasing the dose of their monotherapy. A combination may allow the use of lower doses of component drugs, which may decrease the risk of adverse events and potentially improve patient adherence. 29 , 30 , 37 , 38 In fact, a meta‐analysis of combination antihypertensive therapy has shown that, although the BP‐lowering efficacy of two agents is additive, the prevalence of adverse events is less than additive. 30 The combination of an ARB with HCTZ was well tolerated in all of the factorial studies in this review, with most adverse events being mild to moderate in intensity and transient in nature. Consistent with other studies, 30 the incidence of adverse events with ARB/HCTZ combination therapy in the factorial studies was similar to that with ARB monotherapy.
Combining an ARB with HCTZ may also have the additional benefit of blunting, or even preventing, the adverse metabolic effects associated with diuretic stimulation of the renin‐angiotensin‐aldosterone system, such as hypokalemia and hyperuricemia. 22 , 39 , 40 Indeed, the combination of an ARB with HCTZ was reported to attenuate the potassium loss normally associated with HCTZ therapy in most of the factorial studies in this review. 22 , 24 , 25
While the data in the factorial studies evaluated in this review appear to be comparable with those in the published literature, it is important to acknowledge the limitations inherent in this analysis. First, despite the marked similarities between the factorial studies, there were some differences. For example, the telmisartan study used supine BP measurements, whereas the other studies used seated BP measurements. This study also included a higher percentage of black patients compared with the other trials.
The irbesartan study reported data on the population of patients who completed the study and/or had BP readings at Week 8; however, the other studies presented data from the intent‐to‐treat populations, with the last observation carried forward. This may result in a slight overestimation of irbesartan efficacy relative to the other ARBs in this review. In addition, the differences between treatments in terms of BP‐lowering ability were numeric only, which had been identified from independent studies of different patient populations. Results were not subject to statistical analysis and are not reflective of direct comparisons via clinical trials. Therefore, the findings of this comparative review should ideally be substantiated in a well designed head‐to‐head comparison. Such a comparison would also provide evidence to support the validity of indirect comparisons of factorial studies.
CONCLUSION
The results of this review support the rational use of ARB/HCTZ combination therapy for the treatment of patients with hypertension. All of the ARB/HCTZ formulations evaluated resulted in significant lowering of both DBP and SBP compared with baseline. Comparison of the currently available marketed combinations of an ARB plus HCTZ evaluated in these factorial studies revealed that olmesartan plus HCTZ at the maximal dosage of 40 mg/d and 25 mg/d, respectively, provided the greatest placebo‐corrected reduction in mean DBP and SBP. The findings from this review should be substantiated in a carefully designed comparative study.
Acknowledgment: This manuscript was supported through a grant provided by Sankyo Pharma Inc. and Forest Pharmaceuticals, Inc.
References
- 1. Hajjar I, Kotchen T. Trends in the prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199 – 206. [DOI] [PubMed] [Google Scholar]
- 2. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560 – 2572. [DOI] [PubMed] [Google Scholar]
- 3. Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103:904 – 912. [DOI] [PubMed] [Google Scholar]
- 4. Puchler K, Laeis P, Stumpe KO. Blood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonist. J Hypertens. 2001;19(suppl 1):S41 – S48. [DOI] [PubMed] [Google Scholar]
- 5. Ruilope L, Simpson R, Toh J, et al. Controlled trial of losartan given concomitantly with different doses of hydrochlorothiazide in hypertensive patients. Blood Press. 1996;5:32 – 40. [DOI] [PubMed] [Google Scholar]
- 6. Conlin P, Spence J, Williams B, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens. 2000;13(4 part 1):418 – 426. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez‐Hernandez R, Sosa‐Canache B, Velasco M, et al. Angiotensin II receptor antagonists role in arterial hypertension. J Hum Hypertens. 2002;16(suppl 1):93 – 99. [DOI] [PubMed] [Google Scholar]
- 8. Lacourciere Y, Martin K. Comparison of a fixed‐dose combination of 40 mg telmisartan plus 12.5 mg hydrochlorothiazide with 40 mg telmisartan in the control of mild to moderate hypertension. Am J Ther. 2002;9:111 – 117. [DOI] [PubMed] [Google Scholar]
- 9. Oparil S, Guthrie R, Lewin AJ, et al. An elective‐titration study of the comparative effectiveness of two angiotensin II‐receptor blockers, irbesartan and losartan. Clin Ther. 1998;20:398 – 409. [DOI] [PubMed] [Google Scholar]
- 10. Manolis AJ, Grossman E, Jelakovic B, et al. Effects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertension. Clin Ther. 2000;22:1186 – 1203. [DOI] [PubMed] [Google Scholar]
- 11. Ohma KP, Milon H, Valnes K. Efficacy and tolerability of a combination tablet of candesartan cilexetil and hydrochlorothiazide in insufficiently controlled primary hypertension—comparison with a combination of losartan and hydrochlorothiazide. Blood Press. 2000;9:214 – 220. [DOI] [PubMed] [Google Scholar]
- 12. Lacourciere Y, Gil‐Extremera B, Muller O, et al. Efficacy and tolerability of fixed‐dose combinations of telmisartan plus HCTZ with losartan plus HCTZ in patients with essential hypertension. Int J Clin Pract. 2003;57:273 – 279. [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services . Guidance for Industry. Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research; May 1998. [Google Scholar]
- 14. Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. J Hypertens. 1993;11:217 – 221. [DOI] [PubMed] [Google Scholar]
- 15. Canter D, Frank GJ, Knapp LE, et al. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. J Hum Hypertens. 1994;8:155 – 162. [PubMed] [Google Scholar]
- 16. Pordy RC. Cilazapril plus hydrochlorothiazide: improved efficacy without reduced safety in mild to moderate hypertension. A double‐blind, placebo‐controlled, multicenter study of factorial design. Cardiology. 1994;85:311 – 322. [DOI] [PubMed] [Google Scholar]
- 17. Pool JL, Cushman WC, Saini RK, et al. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens. 1997;10:117 – 123. [DOI] [PubMed] [Google Scholar]
- 18. Frishman WH, Bryzinski BS, Coulson LR, et al. A multifactorial trial design to assess combination therapy in hypertension. Treatment with bisoprolol and hydrochlorothiazide. Arch Intern Med. 1994;154:1461 – 1468. [PubMed] [Google Scholar]
- 19. Lacourciere Y, Arnott W. Placebo‐controlled comparison of the effects of nebivolol and low‐dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens. 1994;8:283 – 288. [PubMed] [Google Scholar]
- 20. Chalmers JP, Korner PI, Tiller DJ, et al. Double‐blind factorial trial of prindolol and hydrochlorothiazide in hypertension. Med J Aust. 1976;1:650 – 653. [PubMed] [Google Scholar]
- 21. Chalmers J, Tiller D, Horvath J, et al. Effects of timolol and hydrochlorothiazide on blood‐pressure and plasma renin activity. Double‐blind factorial trial. Lancet. 1976;2:328 – 331. [DOI] [PubMed] [Google Scholar]
- 22. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12(8 pt 1):797 – 805. [DOI] [PubMed] [Google Scholar]
- 23. Chrysant S, Weber M, Wang A, et al. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17:252 – 259. [DOI] [PubMed] [Google Scholar]
- 24. McGill J, Reilly P. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clin Ther. 2001;23:833 – 850. [DOI] [PubMed] [Google Scholar]
- 25. Benz J, Black H, Graff A, et al. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double‐blind placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens. 1998;12:861 – 866. [DOI] [PubMed] [Google Scholar]
- 26. Song F, Altman DG, Glenny A‐M, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ. 2003;326:472 – 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hung HMJ, Chi GYH, Lipicky RJ. Testing for the existence of a desirable dose combination. Biometrics. 1993;49:85 – 94. [PubMed] [Google Scholar]
- 28. Elmfeldt D, Olofsson B, Meredith P. The relationship between dose and antihypertensive effect of four AT1‐receptor blockers. Differences in potency and efficacy. Blood Press. 2002;11:293 – 301. [DOI] [PubMed] [Google Scholar]
- 29. Neutel J, Smith D, Weber MA. Low‐dose combination therapy: an important first‐line treatment in the management of hypertension. Am J Hypertens. 2001;14:286 – 292. [DOI] [PubMed] [Google Scholar]
- 30. Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427 – 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corvol P, Plouin P. Angiotensin II receptor blockers: current status and future prospects [in French]. Drugs. 2002;62:53 – 64. [PubMed] [Google Scholar]
- 32. Biagini G, Zoli M, Torri C, et al. Protective effects of delapril, indapamide and their combination chronically administered to stroke‐prone spontaneously hypertensive rats fed a high‐sodium diet. Clin Sci (Lond). 1997;93:401 – 411. [DOI] [PubMed] [Google Scholar]
- 33. Scalbert E, Abdon D, Devissaguet M, et al. Interaction between an angiotensin converting enzyme inhibitor, perindopril, and a thiazide diuretic in the spontaneously hypertensive rat. Can J Cardiol. 1992;8:381 – 386. [PubMed] [Google Scholar]
- 34. Lacourciere Y, Tytus R, O'Keefe D, et al. Efficacy and tolerability of a fixed‐dose combination of telmisartan plus hydrochlorothiazide in patients uncontrolled with telmisartan monotherapy. J Hum Hypertens. 2001;15:763 – 770. [DOI] [PubMed] [Google Scholar]
- 35. Sachse A, Verboom C, Jager B. Efficacy of eprosartan in combination with HCTZ in patients with essential hypertension. J Hum Hypertens. 2002;16:169 – 176. [DOI] [PubMed] [Google Scholar]
- 36. Flack JM, Saunders E, Gradman A, et al. Antihypertensive efficacy and safety of losartan alone and in combination with hydrochlorothiazide in African Americans with mild to moderate hypertension. Clin Ther. 2001;23:1193 – 1208. [DOI] [PubMed] [Google Scholar]
- 37. Chrysant SG, Wombolt DG, Feliciano N, et al. Long‐term efficacy, safety, and tolerability of valsartan and hydrochlorothiazide in patients with essential hypertension. Curr Ther Res. 1998;59:762 – 772. [Google Scholar]
- 38. Moser M, Black H. The role of combination therapy in the treatment of hypertension. Am J Hypertens. 1998;11 (6 pt 2): 73S – 78S. [DOI] [PubMed] [Google Scholar]
- 39. Weinberger M. Influence of an angiotensin converting‐enzyme inhibitor on diuretic‐induced metabolic effects in hypertension. Hypertension. 1983;5(5 part 2):III132 – III138. [DOI] [PubMed] [Google Scholar]
- 40. Ram CV. Angiotensin receptor blockers and diuretics as combination therapy: clinical implications. Am J Hypertens. 2004;17:277 – 280. [DOI] [PubMed] [Google Scholar]
- 41. Valsartan Study Protocol 301 . Taken from Valsartan New Drug Application. Medical Review. A multiple dose, randomised, double‐blind, placebo controlled, multifactorial, parallel trial comparing the combination therapy of valsartan (80 or 160 mg), HCTZ (12.5 or 25 mg) and placebo in hypertensive patients age 18–80 years. 2004;1.29–1.30.


