Abstract
Severe hypertension is difficult to control. This prospective, randomized, double‐blind, active‐controlled, multicenter trial compared efficacy and safety of once‐daily irbesartan/hydrochlorothiazide (HCTZ) combination therapy with irbesartan monotherapy in severe hypertension. Patients who were untreated or uncontrolled on monotherapy (seated diastolic blood pressure [BP] ≥110 mm Hg) received fixed‐dose irbesartan 150 mg/HCTZ 12.5 mg combination therapy for 7 weeks, force‐titrated to irbesartan 300 mg/HCTZ 25 mg at week 1 (n=468); or irbesartan 150 mg monotherapy, force‐titrated to 300 mg at week 1 (n=269). Significantly more patients on combination therapy achieved seated diastolic BP <90 mm Hg at week 5 (primary end point) compared with monotherapy recipients (47.2% vs 33.2%; P=.0005). Likewise, significantly more patients attained goals per the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) (<140/90 mm Hg) at week 5 (34.6% vs 19.2%, respectively; P<.0001), while the mean difference between combination and monotherapy in seated diastolic BP and seated systolic BP was 4.7 mm Hg and 9.7 mm Hg (P<.0001). Greater and more rapid BP reduction with irbesartan/HCTZ was achieved without additional side effects.
Patients continually exposed to severe blood pressure (BP) levels (seated diastolic BP [SeDBP] ≥110 mm Hg) are at risk of developing cardiovascular complications including hypertensive crises. 1 , 2 In recognition of the urgency of treating severe hypertension effectively, guidelines of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure have consistently recommended faster and more aggressive treatment. 3 , 4 , 5 , 6 Guidelines support initial combination therapy for severe hypertension based on the need to lower BP within weeks rather than months, an approach supported by data from the Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) 7 and the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT). 8 In fact, morbidity and mortality trials in severe hypertension carried out over 40 years ago provided some of the earliest evidence of the benefits of combination antihypertensive therapy in this difficult‐to‐treat population. 9 , 10
Clinical studies in which irbesartan has been administered with HCTZ show that this combination is both safe and effective, producing significantly greater dose‐dependent reductions in BP compared with single‐agent therapy alone. 11 , 12 , 13 , 14 Fixed‐dose irbesartan 150 mg/HCTZ 12.5 mg combination therapy, as a single tablet, is indicated for patients who are unable to achieve BP control on antihypertensive monotherapy.
The present study was designed to test the hypothesis that the combination of irbesartan/HCTZ as initial therapy would achieve BP control to SeDBP <90 mm Hg over 5 weeks in a greater proportion of patients with severe hypertension (SeDBP >110 mm Hg) than treatment with irbesartan alone. Secondary objectives included assessment of safety and tolerability in the 2 treatment groups over the 7‐week study period, focusing in particular on the frequency of hypotension, dizziness, syncope, headache, and hypokalemia/hyperkalemia, as well as discontinuations due to adverse events.
METHODS
Eligible patients were adult men and women 18 years and older with uncontrolled hypertension, defined as either currently untreated with an SeDBP ≥110 mm Hg or currently receiving anti‐hypertensive monotherapy with an SeDBP ≥100 mm Hg (stage 2 hypertension). Monotherapy was defined as treatment with 1 antihypertensive medication for at least 4 weeks; fixed combination therapy did not represent monotherapy.
Women of childbearing potential were eligible to participate in this study providing they had a negative pregnancy test within 72 hours of the start of study medication and used an adequate method of contraception throughout the study and for up to 1 week after study completion. Women who were pregnant or lactating were not eligible to participate. Patients were also excluded if they had a seated systolic BP (SeSBP) of ≥220 mm Hg, SeDBP of ≥120 mm Hg, known or suspected secondary hypertension, or any condition that required more immediate BP lowering. Other exclusion criteria included concomitant cardiovascular or cerebrovascular disease, significant chronic renal impairment or renovascular disease, hepatic disease, systemic lupus erythematosus, malignancy, gastrointestinal disease, or gastrointestinal surgery that might interfere with drug absorption. Evidence of drug or alcohol abuse in the previous 5 years and any medical or psychiatric condition that might confound assessment of study medication or jeopardize the subject's safety were also criteria for exclusion.
The study was conducted at 250 study centers in the United States, Canada, Germany, France, the Netherlands, Belgium, Russia, and Israel, all of which received prior ethics committee and/or institutional review board approval. All patients gave written informed consent before study enrollment. The study was conducted in accordance with the ethical principles of the current Declaration of Helsinki and consistent with the International Conference on Harmonization Good Clinical Practice (ICH GCP).
Study Design
This was a randomized, double‐blind, active‐controlled, multicenter trial. Patients meeting eligibility criteria entered a 7‐day single‐blind, placebo lead‐in period, after which all those with an SeDBP of ≥110 mm Hg at 2 consecutive visits off medication were randomized 2:1 to 7‐week treatment with either irbesartan/HCTZ combination therapy or irbesartan monotherapy. Patients in the combination therapy arm received fixed‐dose irbesartan 150 mg/HCTZ 12.5 mg, which was force‐titrated to fixed‐dose irbesartan 300 mg/HCTZ 25 mg at the end of the first week. Patients in the monotherapy arm started on irbesartan 150 mg with forced titration to irbesartan 300 mg at the end of week 1. All patients continued taking the titrated dose for the remaining 6 weeks of the study. All study medication was taken once daily between 6 AM and 11 AM except on the morning of a study visit, so that BP could be measured at trough, 24±3 hours following the last dose of study medication.
Concomitant administration of vasoactive drugs, any drug or herbal preparation likely to affect BP, as well as those likely to interact with study medication, were not permitted for the duration of the study.
Efficacy Assessments
At study entry, all patients gave a detailed medical history and underwent a complete physical examination, including 12‐lead electrocardiogram. BP and heart rate were measured using a sponsor‐provided automatic BP monitor (OMRON automatic BP monitor, Omron Healthcare Inc, Vernon Hills, IL). At baseline visit, an average of 3 replicate measurements were obtained at least 1 minute apart with subjects resting for a minimum of 10 minutes in a seated position before any measurements were taken. BP was measured in both arms and the arm with the higher reading was used in those cases where BP readings differed between arms. The same arm was used at all subsequent visits.
BP and heart rate were measured again during the single‐blind placebo lead‐in period and at weeks 1, 3, 5, and 7 of the double‐blind active treatment period. During active treatment, an average seated and standing trough (24±3 hours postdose) BP and heart rate were measured, with seated measurements taken first.
The primary efficacy end point was the proportion of subjects with SeDBP of <90 mm Hg at week 5 in the 2 treatment groups. Other secondary efficacy end points included: the proportion of subjects who achieved seated BP <140/90 mm Hg at all visits; the proportion with a change from baseline SeSBP and SeDBP at all visits, and the proportion with SeDBP <90 mm Hg at weeks 1, 3, and 7.
Safety Assessments
All randomized patients who took at least 1 dose of double‐blind study medication were included in the safety analysis. Blood and urine samples for laboratory analyses were collected at baseline, during the single‐blind, placebo lead‐in period, and during weeks 1, 3, 5, and 7 of double‐blind active treatment.
Safety was assessed with respect to the nature, frequency, and severity of adverse events and their relationship to study medication, as well as the incidence of clinical laboratory test abnormalities during double‐blind treatment. The frequency of hypotension, dizziness, syncope, headache, and hypokalemia/hyperkalemia—prespecified adverse events of relevance to antihypertensive therapy with diuretics and angiotensin receptor blockers—were also analyzed, along with the frequency of treatment discontinuations due to adverse events.
Statistical Analyses
This study was designed to test the hypothesis that after 5 weeks of double‐blind treatment, a greater proportion of subjects in the combination treatment arm would achieve BP control (SeDBP <90 mm Hg) compared with those on irbesartan monotherapy.
Since the primary analysis involved a comparison of proportions between the 2 groups, calculation of sample size and power of the test was based on the Fisher exact test performed at a 2‐sided 5% level of significance. The proportion of subjects normalized under irbesartan monotherapy was assumed to be 0.1 (ie, 10% of patients) for this calculation. Using a 2:1 randomization scheme with 430 patients treated with combination therapy and 215 patients treated with irbesartan monotherapy, the study had at least 90% power to detect a 10% difference in the proportion of normalized patients for combination therapy with respect to monotherapy, if such a difference truly existed. Comparison of proportions of patients in each treatment group with simultaneous systolic and diastolic BP control at weeks 1, 3, 5, and 7 and with SeDBP <90 mm Hg at weeks 1, 3, and 7 was analyzed in the same way as the primary efficacy variable. Change from baseline in mean systolic BP and mean diastolic BP at weeks 1, 3, 5, and 7 in the 2 treatment groups was analyzed using analysis of covariance, with treatment as the main effect and the baseline value as covariate. The covariate‐adjusted mean difference between treatment groups was tested at the 2‐sided 5% significance level.
RESULTS
Patient Disposition
A total of 697 patients were randomized to study medication, of whom 468 received irbesartan/HCTZ combination therapy and 227 received irbesartan monotherapy. Of these 697 patients, only 2 did not receive double‐blind treatment; 1 withdrew consent and the second had a positive pregnancy test. Of the remaining 695 patients, 621 (>90%) completed double‐blind therapy. Lack of efficacy (severe hypertension unresponsive to therapy) was the most common reason for premature discontinuation from study in both groups, followed equally by adverse events and consent withdrawal. In the irbesartan/HCTZ combination and irbesartan monotherapy groups respectively, 15 (3.2%) and 12 (5.2%) patients withdrew for lack of efficacy, while 9 (1.9%) and 5 (2.2%), respectively, withdrew prematurely because of adverse events.
Baseline Characteristics
Duration of hypertension, baseline BP readings, and demographic characteristics were similar for the 2 treatment groups (Table II). The majority of patients were men (57.5%), Caucasian (84.2%) and aged between 40 and 64 years (87%); 13% were 65 years and older. With 1 exception, patients had some evidence of cardiovascular risk factors, in addition to hypertension, with hyperlipidemia (33.9%) and diabetes (11.8%) the most common. About 3% of patients had stable angina pectoris, 1% previous myocardial infarction, and 2% a history of transient ischemic attack or stroke.
Table I.
Patient Characteristics at Baseline (All Randomized Patients)
| Characteristic | Irbesartan/HCTZ (n=468) | Irbesartan Monotherapy (n=229) |
|---|---|---|
| Demographics | ||
| Men/women, No. (%) | 277/191 (59.2/40.8) | 124/105 (54.1/45.9) |
| Race, No. (%) | ||
| Caucasian | 395 (84.4) | 192 (83.8) |
| Black/African American | 67 (14.3) | 34 (14.8) |
| Asian | 3 (0.6) | 2 (0.9) |
| Native Hawaiian/other Pacific Islander | 1 (0.2) | 0 |
| Age, mean (range), y | 52.2 (23–81) | 52.9 (25–83) |
| Weight, mean (range), kg | 89.7 (48.0–164.3) | 91.8 (44.0–151.5) |
| Cardiovascular history | ||
| Duration of hypertension, mean (range), y | 7.2 (0–48.9) | 7.1 (0–35.8) |
| SeDBP, mean (range), mm Hg | 113.4 (92.8–131.6) | 113.3 (103.0–135.0) |
| SeSBP, mean (range), mm Hg | 171.5 (132.3–221.7) | 171.6 (134.7–220.7) |
| SeHR, mean (range), bpm | 77.4 (53.0–111.0) | 76.6 (56.0–117.0) |
| Diabetes mellitus, No. (%) | 52 (11.1) | 30 (13.1) |
| Hyperlipidemia, No. (%) | 158 (33.8) | 78 (34.1) |
| Stable angina pectoris, No. (%) | 12 (2.6) | 12 (5.2) |
| Stroke or TIA, No. (%) | 6 (1.3) | 7 (3.1) |
| Myocardial infarction, No. (%) | 6 (1.3) | 3 (1.3) |
| HCTZ indicates hydrochlorothiazide; SeDBP, seated diastolic blood pressure (BP); SeSBP, seated systolic BP; SeHR, seated heart rate; and TIA, transient ischemic attack. | ||
Primary and Secondary Outcomes: Efficacy
At week 5 of the double‐blind treatment period, 33.2% of patients on monotherapy reached the primary end point of trough SeDBP <90 mm Hg, whereas 47.2% of patients in the combination therapy arm reached this goal (P=.0005; Figure 1). Even after only 1 week of treatment, more patients receiving combination therapy had achieved SeDBP <90 mm Hg than those on irbesartan monotherapy (15.2% vs 9.2%; P=.03) (Figure 1). By week 7 (end of the study), 51.9% of patients on combination therapy had trough SeDBP of <90 mm Hg compared with 32.8% of irbesartan‐treated patients (P<.0001). During weeks 3–7, during which patients were on maximum fixed doses of study medication, the percentage of patients who achieved SeDBP <90 mm Hg on combination therapy was 14%–19% higher than with monotherapy (P<.0001 at each time point).
Figure 1.
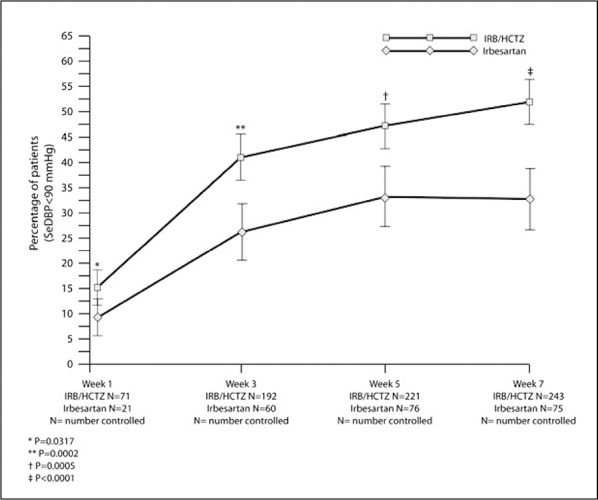
Percentage of patients achieving seated diastolic blood pressure (SeDBP) <90 mm Hg during 7 weeks' double‐blind treatment. The primary efficacy end point was SeDBP <90 mm Hg at 5 weeks. IRB indicates irbesartan; HCTZ, hydrochlorothiazide; and NC, number controlled.
Secondary end points demonstrated similar efficacy. The proportion of patients with controlled SeSBP (<140 mm Hg) and SeDBP (<90 mm Hg) at each week of double‐blind therapy was significantly greater for irbesartan/HCTZ combination therapy than for irbesartan monotherapy (Table II). The proportion of patients controlled at week 5 according to the guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) (BP <140/90 mm Hg) also differed significantly between the groups (34.6% of patients on combination therapy compared with 19.2% of patients on monotherapy; P<.0001). By week 7, this had increased to 37.8% and 21.4% of patients, respectively (P<.0001).
During the double‐blind treatment period, the reduction from baseline in both SeDBP and SeSBP was statistically significantly greater with irbesartan/HCTZ combination therapy than with irbesartan monotherapy at all time points (Figure 2). Combination therapy reduced mean SeDBP by >20 mm Hg by week 3, whereas monotherapy did not achieve a similar decrease until week 7. Similarly, combination therapy reduced mean SeSBP by 27 mm Hg by week 3, whereas monotherapy did not achieve a similar decrease until week 7. In both treatment groups, mean BP was approximately 172/113 mm Hg at baseline. Within 7 weeks of combination therapy, SeDBP had fallen by an average of 24.5 mm Hg and SeSBP by an average of 31.7 mm Hg.
Figure 1.
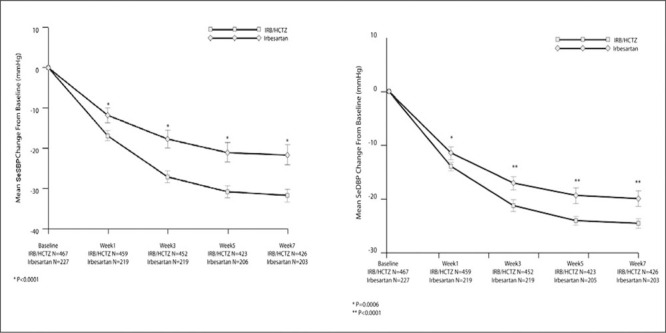
Mean change from baseline in seated systolic blood pressure (SeSBP) and seated diastolic blood pressure (SeDBP) during 7 weeks' double‐blind treatment (secondary efficacy end points). IRB indicates irbesartan; HCTZ, hydrochlorothiazide.
As Figure 3 illustrates, the more rapid and sustained BP reduction achieved with irbesartan/HCTZ combination therapy in comparison with irbesartan monotherapy significantly reduced exposure to severe elevations of SeDBP. Analyses of the impact of this treatment effect show that, overall, patients treated with combination therapy had significantly less exposure to severe levels of DBP (P=.004) compared with monotherapy, corresponding to a difference of 26 patient‐weeks for every 100 patients treated.
Figure 3.
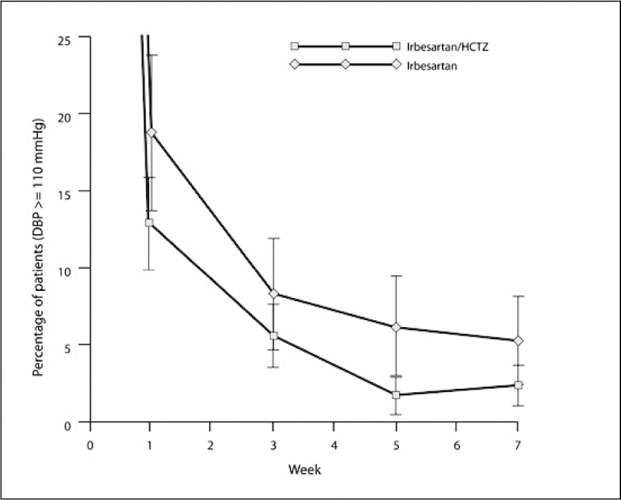
Percentage of patients with persistent severe hypertension (seated diastolic blood pressure [SeDBP] ≥110 mm Hg) during 7 weeks' double‐blind treatment.
Secondary Outcomes: Safety and Tolerability
The overall frequency of adverse events during the 7‐week, double‐blind treatment period was lower in the irbesartan/HCTZ combination therapy group than in the monotherapy group (Table III). The majority of adverse events were of mild‐to‐moderate intensity. Serious adverse events were reported in 1 patient in each treatment group: colitis secondary to irritable bowel syndrome and pyelonephritis of moderate intensity in the irbesartan/HCTZ combination arm, and mild renal artery stenosis in the monotherapy arm. All were unrelated to study therapy, as determined by the investigator, and neither subject discontinued treatment. The case of renal artery stenosis was almost certainly present at study enrollment and should have led to patient exclusion.
Although the number of elderly patients (older than 65 years) in the study was relatively small (n=92), there was no evidence of any increased risk of adverse events among older patients. There were fewer adverse events reported by elderly patients in both the irbesartan/HCTZ combination therapy group (26.4%) and monotherapy group (34.2%) compared with those younger than 65 years (30.4% and 36.5% respectively). No deaths were recorded during the study. Fewer patients in the irbesartan/HCTZ treatment group experienced prespecified adverse events, which included dizziness, syncope, hypotension, headache, hyperkalemia, and hypokalemia, compared with the monotherapy group (8.8% vs 11.5%, respectively). There was no reported syncope in either group.
Study medications were well tolerated by patients in this study, as reflected by the overall low rate of treatment discontinuations for adverse events: 9 patients (1.9%) in the combination group and 5 patients (2.2%) in the irbesartan monotherapy group. Among patients who discontinued therapy in the irbesartan/HCTZ combination therapy group, 3 were considered treatment‐related and due to dizziness, fatigue, and hypertension. All other adverse events leading to discontinuation could not be definitively linked to study medication.
The incidences of hyperkalemia and hypokalemia were low and comparable between treatment groups. Six patients had serum potassium levels of >6.0 mmol/L: 3 patients (0.6%) in the combination group and 3 (1.3%) in the monotherapy group. No patients had potassium levels <3.0 mmol/L.
DISCUSSION
This randomized, double‐blind, active‐controlled, multicenter trial demonstrated that combination therapy with irbesartan/HCTZ was superior to irbesartan monotherapy in reducing BP in severely hypertensive patients.
The primary end point was the proportion of patients achieving SeDBP <90 mm Hg after 5 weeks' therapy. While irbesartan 300 mg achieved this level of BP control in 33% of patients, 47% of patients reached this target on initial irbesartan/HCTZ combination therapy force‐titrated to a dose of 300 mg and 25 mg respectively.
Combination therapy lowered BP more rapidly and to a greater extent than monotherapy. Within 7 weeks of treatment, approximately one third of patients on combination therapy achieved the current JNC 7 goal of <140/90 mm Hg compared with one fifth of those on monotherapy. Initial use of combination therapy reduced BP by approximately 10/5 mm Hg (systolic/diastolic) more than monotherapy in the short term; benefit was seen in both treatment‐naïve patients and patients inadequately controlled on previous monotherapy. This difference may persist long term, as studies have shown that greater short‐term efficacy in BP lowering is associated with greater long‐term efficacy. 15 , 16 Poor compliance is an especially common problem with severe hypertension. 17 By providing more rapid control, fewer titrations, lower drug burden, and fewer side effects, combination therapy should contribute toward greater long‐term treatment compliance.
There were fewer withdrawals for lack of efficacy in the irbesartan/HCTZ combination treatment arm compared with those on irbesartan monotherapy in this study. Combination therapy also reduced the risk of adverse events such as potassium abnormalities and headache. Drug tolerability is essential to ensure patient adherence to lifelong therapy. The forced titration design of this study addressed the primary safety concern of hypertension management—the potential that reducing BP too quickly may lead to hypotension, dizziness, and syncope. Alternatively, it is also important not to reduce BP too slowly, as this could lead to an increased incidence of headache. 18 Regardless of BP measurement at the time of titration, patients in both treatment arms were titrated rapidly from starting dose to final fixed dose. Even though irbesartan/HCTZ combination therapy reduced BP more rapidly and to a greater extent than irbesartan monotherapy, adverse events occurred less frequently in the combination treatment group. There was no increased incidence of dizziness and syncope with combination therapy, or of abnormalities in serum potassium. More patients with severe hypertension, who are among the most difficult to control, reached the current treatment goal of SeSBP <140 mm Hg/SeDBP <90 mm Hg with combination therapy. Over 11% of patients in this study were 65 years or older. Adverse events occurred with no greater frequency in older patients receiving either combination therapy (26.4%) or monotherapy (34.2%) than younger patients and, with the exception of 2 cases of dizziness, the elderly subgroup receiving combination therapy was free of hypotension or symptoms of hypotension, a particular concern in this population.
Results from the present study are consistent with other trials and support current treatment guidelines that recommend combination antihypertensive therapy as initial treatment in patients with severe hypertension. 6 , 19 The results are consistent with findings from other studies in which irbesartan plus HCTZ 20 and other combination therapies have been used in difficult‐to‐treat hypertensive patients.
The benefits of rapidly achieving and then sustaining aggressive BP targets were recently illustrated in both the VALUE 7 and ASCOT 8 trials in hypertensive patients at high cardiovascular risk. In VALUE, early BP control, independent of drug type used, was associated with significant benefits in terms of combined cardiac events, stroke, myocardial infarction, or mortality. 21 BP response at 1 month also predicted events and survival. 21 The findings suggest that recommended BP goals need to be reached within a relatively short time, at least in patients with hypertension who have high cardiovascular risk.
CONCLUSIONS
In patients with severe hypertension, irbesartan/HCTZ combination therapy lowered BP more rapidly and to a greater extent than maximum‐dose irbesartan monotherapy. Combination therapy was well tolerated by patients of all ages, with a safety profile similar to monotherapy. Our data support the initial use of fixed‐dose irbesartan 300 mg/HCTZ 25 mg combination therapy in patients with severe hypertension, for whom early aggressive antihypertensive therapy is warranted.
Disclosure: This study was supported by an unrestricted grant from Bristol‐Myers Squibb.
References
- 1. Zampaglione B, Pascale C, Marchiso M, et al. Hypertensive urgencies and emergencies: prevalence and clinical presentation. Hypertension. 1996;27:144–147. [DOI] [PubMed] [Google Scholar]
- 2. Preston RA, Baltodano NM, Cienki J, et al. Clinical presentation and management of patients with uncontrolled, severe hypertension: results from a public teaching hospital. J Hum Hypertens. 1999;13:249–255. [DOI] [PubMed] [Google Scholar]
- 3. The 1988 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1988;148:1023–1038. [PubMed] [Google Scholar]
- 4. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 5. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI). Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 6. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Julius S, Kjeldsen E, Weber MA, et al. Outcomes in hypertensive patients at high cardiovascular risk treatment with regimens based on valsartan or amlopidine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 8. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled study. ASCOT Investigators . Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 9. Wolff FW, Linderman RD. Effects of treatment in hypertension: results of a controlled study. J Chronic Dis. 1966;19:227–240. [DOI] [PubMed] [Google Scholar]
- 10. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 11. Pool JL, Guthrie RM, Littlejohn TW, et al. Dose‐related antihypertensive effects of irbesartan in patients with mild‐to‐moderate hypertension. Am J Hypertens. 1998;11:462–470. [DOI] [PubMed] [Google Scholar]
- 12. Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. [DOI] [PubMed] [Google Scholar]
- 13. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 14. Franklin SS, Lapuerta P, Bhaumik A, et al. Early reduction of exposure to severe blood pressure levels with irbesartan/HCTZ as a first‐line treatment of severe hypertension. Poster presented at European Society of Hypertension; June 1215, 2006; Madrid, Spain. [Google Scholar]
- 15. Mourad J‐J, Waeber B, Zannad F, et al, on behalf of the investigators of the SRATHE trial . Comparison of different therapeutic strategies in hypertension: a low‐dose combination of perindopril/indapamide versus a sequential monotherapy or stepped‐care approach. J Hypertens. 2004;22:2379–2386. [DOI] [PubMed] [Google Scholar]
- 16. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker or diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 17. Payne KA, Esmonde‐White S. Observational studies of antihypertensive medication use and compliance: is drug choice a factor in treatment adherence? Curr Hypertens Rep. 2000;2:515–524. [DOI] [PubMed] [Google Scholar]
- 18. Hansson L, Smith DHG, Reeves R, et al. Headache in mild‐to‐moderate hypertension and its reduction by irbesartan. Arch Intern Med. 2000;160:1654–1658. [DOI] [PubMed] [Google Scholar]
- 19. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 20. Coca A, Calvo C, Sobrino J, et al. Once‐daily fixed‐combination irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003;25:2849–2864. [DOI] [PubMed] [Google Scholar]
- 21. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]


