Abstract
Recent studies have indicated a higher prevalence of primary aldosteronism (PA) than reported historically. Aldosterone excess induces sodium and fluid retention with consequential increases in blood pressure. Patients with PA are at an increased risk of developing left ventricular hypertrophy, chronic kidney disease, and endothelial dysfunction. Measurement of the plasma aldosterone/plasma renin activity ratio is an effective screening test for PA. The majority of patients with PA do not have a discernable aldosterone‐producing adenoma (APA), and the aldosterone excess is considered idiopathic in etiology and/or attributed to adrenal hyperplasia. Treatment of PA includes medical therapy with mineralocorticoid receptor antagonists and adrenalectomy for patients with a unilateral APA. A reasonable treatment strategy is to attempt medical therapy in all patients with a high plasma aldosterone/PRA ratio and reserve the extensive workup needed to identify an APA for those patients whose hypertension or hypokalemia cannot be controlled medically.
Primary aldosteronism (PA) was first described by Jerome Conn in 1955. The index case was a young woman presenting with resistant hypertension, hypokalemia, and a metabolic alkalosis. She was subsequently found to have an aldosterone‐producing adenoma (APA), which was surgically resected, effectively curing her of the syndrome. Screenings done during Dr. Conn's lifetime indicated that PA was an uncommon cause of hypertension, with an estimated prevalence of 1%–2% of hypertensive patients. In the early 1990s, however, investigators in Brisbane, Australia reported a surprisingly high prevalence of PA of approximately 12% among 52 hypertensive subjects responding to a newspaper advertisement for participation in an antihypertensive drug trial. A follow‐up study of 199 subjects referred to the hypertension clinic in Brisbane confirmed the high occurrence of PA, with an estimated prevalence of at least 9.5% and perhaps as high as 13%.
Since these reports, multiple studies have confirmed that PA is much more common than had been demonstrated historically, with a prevalence among general hypertensive patients of approximately 5%–10%. In one of the more compelling and clinically informative studies, Mosso et al screened more than 600 hypertensive patients for PA. The severity of the untreated hypertension based on the stages delineated in the sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) (stage 1, 140–159/90–99 mm Hg; stage 2, 160–179/100–109 mm Hg; stage 3, ≥180/110 mm Hg) was known for each subject. The investigators were therefore able to relate the prevalence of PA to the severity of the underlying hypertension. The overall prevalence of PA was 6.1%. The prevalence, however, increased progressively with the severity of hypertension. In subjects with stage 1 hypertension, the PA prevalence was only 2%, which was not different from normotensive controls. In subjects with stage 2 hypertension the PA prevalence was 8%, and in subjects with stage 3 hypertension the prevalence was 13% (Figure 1). The results are clinically relevant in demonstrating that the likelihood of PA increases with increasing severity of hypertension, ie, patients with mild hypertension are at low risk while patients with severe hypertension are at high risk of having PA.
Figure 1.
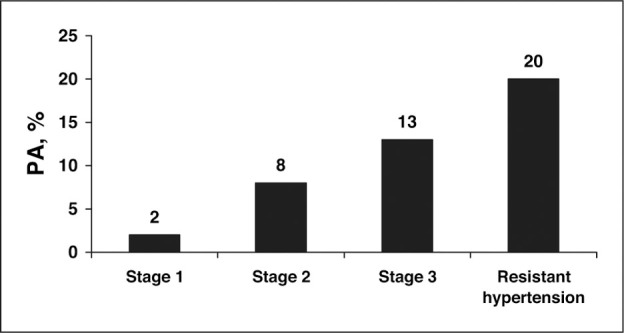
Prevalence of primary aldosteronism (PA) in subjects classified according to the sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) stages of severity of hypertension, and in patients with resistant hypertension. Adapted from Mosso et al. Hypertension. 2003;42:161–165 and Calhoun et al. Hypertension. 2002;40:892–896.
The recent studies documenting a high occurrence of PA have been consistent in demonstrating that patients diagnosed with PA generally have not had a history of hypokalemia. Although originally described as an essential characteristic of the syndrome, Conn later recognized that hypokalemia was more often a late manifestation of the syndrome that was preceded by the development of hypertension. The recent prospective assessments determining the prevalence of PA seem to confirm such a progression in finding that hypertensive patients diagnosed with PA usually have normal serum potassium levels. While patients who present with hypokalemia (especially spontaneously occurring hypokalemia, but also including hypokalemia that develops with thiazide diuretic use) are at increased risk of having PA, the absence of hypokalemia does not exclude the presence of PA.
PA is particularly common in subjects with resistant hypertension, with a prevalence of approximately 20% (Figure 1). In an evaluation of patients referred to a hypertension specialty clinic, investigators at the University of Alabama at Birmingham found that 18 of 88 (20%) consecutively evaluated patients with resistant hypertension were diagnosed with PA based on suppressed renin activity and a high 24‐hour urinary aldosterone excretion in the course of a high dietary sodium intake. The prevalence of PA was similar in African American and white patients. A prevalence of PA of approximately 20% in patients with resistant hypertension has been a consistent observation. In a study conducted in Seattle, PA was diagnosed in 17% of patients with resistant hypertension. Similarly, investigators in Oslo have reported confirming PA in 23% of patients with resistant hypertension.
ETIOLOGY
Aldosterone is a steroid hormone produced in the adrenal cortex that contributes importantly to the maintenance of sodium and fluid balance. Aldosterone binds to epithelial mineralocorticoid receptors (MR) in the kidney, inducing sodium and fluid retention and potassium excretion. Angiotensin II and high serum potassium levels are the major stimulants of aldosterone release. Conversely, aldosterone release is suppressed by low serum potassium and low renin‐angiotensin activity, as occurs with intravascular volume expansion secondary to salt loading.
PA is characterized by inappropriate aldosterone secretion leading to excessive fluid retention and consequential increases in blood pressure. Aldosterone excess secondary to an APA, or true Conn's syndrome, was historically reported to cause 50%–70% of cases of PA. The more recent assessments of PA, however, have found a lower prevalence of APA, with an adenoma being present in approximately 30%–50% of patients with PA. In the absence of an APA, PA is referred to as idiopathic hyperaldosteronism and is attributed presumptively to unilateral or bilateral hyperplasia.
PROGNOSIS
In animal studies, aldosterone excess in combination with high dietary salt intake has been shown to promote target organ deterioration independent of increases in blood pressure. This target organ decline is characterized by perivascular inflammation and necrosis progressing to diffuse fibrosis. These proinflammatory and profibrotic effects of aldosterone observed experimentally are consistent with observational studies of patients with PA, indicating an increased likelihood of left ventricular hypertrophy, chronic kidney disease, and endothelial dysfunction, each of which independently predicts increased cardiovascular risk.
Cross‐sectional comparisons of patients with PA and hypertensive control patients indicate that the former are at increased risk of having cardiovascular disease. In a recent analysis, compared with control patients matched for severity and duration of hypertension, patients confirmed to have PA were more than 4 times as likely to have had a stroke, 6.5 times as likely to have had a prior myocardial infarction, and more than 12 times as likely to have developed atrial fibrillation. Although prospective evaluations are lacking, observational studies suggest that patients with evidence of aldosterone excess are at increased risk of cardiovascular complications compared with patients with primary hypertension.
SCREENING
As aldosterone excess causes inappropriate fluid retention with subsequent suppression of the renin‐angiotensin pathway, PA manifests as high aldosterone levels in the setting of suppressed renin activity. As opposed to assessing plasma aldosterone levels or plasma renin activity (PRA) independently, measurement of the plasma aldosterone/ PRA ratio (ARR) has been shown to have sufficient sensitivity to serve as an effective screening test for PA. Although the exact test characteristics of the ARR have varied widely among studies, its negative predictive value has been generally good; a low ARR (<20 when plasma aldosterone is measured in ng/dL and PRA is measured in ng/mL/min reliably excludes PA. The specificity of the ARR is less consistent; a high ratio (>20–30) is suggestive but not diagnostic of PA. The lower specificity of the ARR likely reflects a high prevalence of low‐renin hypertension, particularly among patients with resistant hypertension. Accordingly, a high ARR is suspicious for PA, but the diagnosis must be confirmed with suppression testing.
Use of the ARR to screen for PA is best done after withdrawal of antihypertensive medications. Angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and diuretics tend to increase PRA, while β‐blockers have the opposite effect, tending to suppress PRA. ACE inhibitors and ARBs may suppress plasma aldosterone levels, particularly with initial use. As the effects of these medications on the ARR would tend to result in a falsely low ratio, however, a high ratio in the context of ongoing medication is even more sensitive for aldosterone excess. (The exception to this rule is that potassium‐sparing diuretics, particularly MR antagonists such as spironolactone or eplerenone, must be withdrawn for 4–6 weeks before screening, as they falsely elevate both PRA and plasma aldosterone.) Therefore, it is more pragmatic to first check the ARR without withdrawing medications, because a high ratio in this setting is suspicious for PA. A low ratio in the setting of ongoing medication use loses sensitivity and therefore is less reliable in excluding PA. If there remains a high level of suspicion for PA, the ratio should be repeated after withdrawal of antihypertensive medications for 4–6 weeks. If it is deemed unsafe to totally withdraw antihypertensive treatment, α‐antagonists and nondihydropyridine calcium channel blockers likely have the least effect on the ARR.
The higher the ARR, the more likely the patient has PA (Table). The ARR is very dependent on the PRA, however, so that if the PRA is extremely low, it can result in a falsely positive ratio. This risk can be reduced by using a minimum PRA value of 0.5 ng/mL/h to calculate the ARR, so that a plasma aldosterone level of at least 10 ng/dL is needed to have a ratio of ≥20. Risk of a falsely positive ratio can be further reduced by requiring a minimum plasma aldosterone level of 15 ng/dL, but this will reduce the sensitivity of the ratio, resulting in more false‐negative screenings (Table).
Table.
Test Characteristics of Various Levels of Plasma Aldosterone/Plasma Renin Activity Ratio (ARR) to Identify Primary Aldosteronism in Patients Being Treated for Resistant Hypertension
| Cut Point | Sensitivity, % | Specificity, % | +PV, % | −PV, % |
|---|---|---|---|---|
| ARR >20 | 78 | 83 | 56 | 93 |
| ARR >50 | 10 | 99 | 86 | 80 |
| ARR >20 and PAC >15 | 57 | 88 | 57 | 88 |
| +PV indicates positive predictive value; −PV, negative predictive value; and PAC, plasma aldosterone concentration. Adapted from Nishizaka et al. Am J Hypertens. 2003;18:805–812. | ||||
To avoid the confounding effects of circadian‐related fluctuations, the ARR should be checked in ambulatory patients in the early morning. Low serum potassium levels will suppress aldosterone secretion; therefore, the ARR should be checked after correction of hypokalemia. To avoid the potential aldosterone stimulatory effect of acute potassium loading, the ARR should not be checked until the serum potassium level is corrected and the dose of potassium supplementation has been stable for 4–6 weeks.
DIAGNOSIS
Confirmation of PA requires demonstration of lack of suppression of aldosterone secretion with volume expansion (intravenous saline infusion or dietary salt loading) or with blockade of the renin‐angiotensin‐aldosterone system with ACE inhibition. Historically, the gold standard has been failure to suppress upright plasma aldosterone to <5–6 ng/dL after 4 days of oral dietary salt loading with concomitant administration of fludrocortisone (0.1 mg PO every 6 hours). Such an approach produces extreme fluid retention and potassium wasting such that hospitalization is required to monitor for excessive fluid overload and to avoid severe hypokalemia.
Dietary salt supplementation for 3 or 4 days, sufficient to increase urinary sodium to >200 mEq/24 h, has been shown to compare favorably to fludrocortisone suppression testing in terms of specificity if the urinary aldosterone excretion fails to reach <12–14 μg/24 h. Such an approach can be done as an outpatient, although blood pressure should be monitored daily to detect excessive increases in blood pressure. Neither approach should be attempted in patients with poorly controlled hypertension and/or a history of congestive heart failure. Patients with a history of chronic kidney disease will be at increased risk of excessive fluid retention. In patients with resistant hypertension in whom salt loading may be hazardous, we will first obtain the 24‐hour urine for aldosterone and sodium while they are on a normal diet. (Measuring aldosterone and sodium from the same urine collection requires the use of a nonsalt preservative such as acetic acid.) If the aldosterone is high (>12–14 μg/24 h) and the sodium is high (>200 mEq/24 h), indicative of chronic high salt intake, we have found that is not necessary to do additional salt loading to confirm PA (Figure 2). If the aldosterone is high but the sodium level is <200 mEq/24 h in the first collection, we will repeat the collection after salt supplementation sufficient to increase the sodium to >200 mEq/24 h.
Figure 2.

Flow chart for the diagnostic evaluation for primary aldosteronism (PA). PRA indicates plasma renin activity; PAC, plasma aldosterone concentration. Adapted from Nishizaka et al. Am J Hypertens. 2003;18:805–812.
As an alternate to dietary salt loading, failure to suppress plasma aldosterone to <5–10 ng/dL after infusion of 2 L of normal saline over 4 hours confirms the diagnosis of PA. Lastly, failure of captopril 25 mg to suppress plasma aldosterone to <12 ng/dL after 2 hours has been suggested to reliably indicate PA, but there has been much less experience with this method of confirmation.
Computed Tomographic Imaging
After confirmation of biochemical PA, thin‐cut abdominal computed tomographic (CT) imaging is recommended in an attempt to identify adrenal tumors that are potentially APAs. The specificity of CT imaging to identify APAs is poor; therefore, screening for APAs with CT imaging without having confirmed biochemical PA is not recommended. Presence of an adrenal tumor(s) suggestive of an APA increases the likelihood that the patient will benefit from adrenalectomy. The absence of a visible tumor on CT imaging does not exclude the possibility of a microadenoma as a possible source of the excess aldosterone, but if a tumor is not seen we will generally attempt medical therapy before proceeding to adrenal vein sampling (AVS).
Adrenal Vein Sampling
Even in the setting of confirmed biochemical PA, CT imaging has a poor specificity for identifying APAs. In a recent retrospective analysis of cases of confirmed PA, concordance between CT imaging and AVS was observed in only 54% of patients, so 45% of patients would have received inappropriate therapy (either incorrectly excluded from having surgery, having nonindicated surgery, or having the wrong adrenal gland removed) if CT imaging alone had been used to guide therapy.
AVS confirms or excludes lateralization of aldosterone excretion consistent with a unilateral APA. Although generally safe, it is technically difficult, particularly in terms of reliably sampling the right adrenal vein. It is sometimes suggested that patients younger than 40 years with confirmed PA and a unilateral tumor on CT imaging can be reliably referred for surgery without confirmation of lateralization (ie, AVS). Likewise, because of increased cancer potential, resection of very large tumors (>2–3 cm) is also often recommended regardless of AVS results. These recommendations, however, are based largely on anecdote. Opinions as to when AVS is needed or not needed before surgery can vary widely between experts. In equivocal cases, referral to an institution experienced with AVS and adrenalectomy is recommended.
ADRENALECTOMY
Resection of unilateral APAs generally corrects the hyperaldosteronism, particularly the associated potassium wasting. The blood pressure response to adrenalectomy is variable, with young patients tending to respond better and older patients less so, particularly if there is a lengthy history of poorly controlled hypertension. Unless there is a contraindication, adrenalectomy should be done laparoscopically to minimize the recovery time.
MEDICAL THERAPY
Medical therapy with use of MR antagonists is indicated for treatment of PA in the absence of unilateral APAs (ie, idiopathic hyperaldosteronism or bilateral APAs), patients who would be at high surgical risk, or patients who simply desire to avoid surgery. Spironolactone in doses of 25–200 mg daily is used. Generally it is used in combination with a thiazide diuretic to maximize the antihypertensive benefit and to minimize the risk of hyperkalemia. In patients with good renal function the risk of hyperkalemia is generally low, but it can occur; close monitoring is indicated. The risk of hyperkalemia with spironolactone is increased in older patients and diabetics; if used in combination with ACE inhibitors, ARBs, or nonsteroidal anti‐inflammatory agents; and in particular, in patients with chronic kidney disease. In these higher‐risk patients, spironolactone can be started at 12.5 mg daily (this requires splitting a 25‐mg tablet). In beginning spironolactone therapy, patients should be advised against using salt substitutes or herbal preparations that may contain potassium.
The major limitation with using spironolactone is development of breast tenderness with or without breast enlargement. These effects are secondary to cross‐stimulation of progesterone and androgen receptors. It occurs mostly in men, but not exclusively. It is uncommon with doses of 12.5–25 mg, but there is a steep increase in occurrence with doses of 50 mg or more. The breast tenderness occurs most commonly during titration but can occur up to a year later on the same dose. Other adverse effects of spironolactone include sexual dysfunction, particularly erectile dysfunction in men, and menstrual irregularities in women.
Eplerenone is a selective MR antagonist with a low affinity for progesterone and androgen receptors and is therefore much less likely than spironolactone to cause breast tenderness, gynecomastia, sexual dysfunction, and menstrual irregularities. While its antihypertensive efficacy has been established in treating primary hypertension, it has not been specifically evaluated for treatment of PA. Given its better tolerability, eplerenone seems an appropriate alternate if the use of spironolactone is limited by adverse effects. Eplerenone is not as potent as spironolactone, and higher doses will generally be needed. As with spironolactone, hyperkalemia can occur with eplerenone, necessitating monitoring.
Amiloride, in blocking the epithelial sodium channel, acts as an indirect MR antagonist. It has been documented as effective in treating aldosterone‐related hypertension, particularly in patients with resistant hypertension, but there is less experience using it specifically to treat PA. It is well tolerated, without any of the sex‐hormone related adverse effects of spironolactone. As with direct MR antagonists, there is risk of hyperkalemia.
CONTROVERSY
Controversy exists as to whether or not to pursue confirmation of PA in patients with a high ARR, as opposed to simply beginning an MR antagonist. The argument for the latter approach is that even with confirmation of PA, the large majority of patients will not be surgical candidates and, therefore, will simply end up being treated medically. Confirmation of PA and possible adrenalectomy is reserved for patients who fail medical therapy either because of refractory hypertension or hypokalemia. Such an approach avoids for almost all patients the expense and risks of an extensive workup that may proceed as far as CT imaging and AVS. The counterargument is that in treating all patients with a high ARR without having excluded PA, those patients with APAs, although a small minority, will never be identified and, therefore, must endure lifelong medical therapy when they might have been more effectively treated or even cured of their hypertension with adrenalectomy.
Although each patient is considered individually, our approach is somewhat in between the 2 extremes. We screen with an ARR all patients who are at increased risk of having PA: patients who present with a history of hypokalemia, resistant hypertension, or severe hypertension. A low ARR excludes PA. In patients with a high ARR, we will obtain a 24‐hour urine for aldosterone. Having documented the 24‐hour urinary aldosterone excretion, we will typically attempt medical therapy in most patients. The exceptions are patients at high risk of having an APA, which in our experience are patients with urinary aldosterone excretion >18–20 μg/24 h. Although anecdotal, patients with urinary aldosterone excretion <18 μg/24 h have rarely had an APA and generally respond well to low‐dose MR antagonism. Even having documented a high urinary aldosterone excretion, we will often attempt medical therapy if preferred by the patient. AVS and possible adrenalectomy are therefore reserved for patients with high levels of urinary aldosterone excretion who fail medical therapy or who prefer surgery without having tried medical therapy.
SUMMARY
For reasons that are unclear, PA is seemingly a much more common cause of hypertension than had been thought historically, with recent studies suggesting a prevalence of 5%–10% of general hypertensive populations. PA is particularly common in subjects with resistant hypertension, with a prevalence of approximately 20%. Although there are suggestions by some experts to screen all hypertensive patients for PA, given the number of persons with hypertension and the risk of false positive results, it seems prudent to reserve screening for patients at increased risk of having PA. This would include patients with a history of hypokalemia, resistant hypertension, or severe hypertension.
Screening for PA is best done by checking the ARR. A low ARR (<20 when aldosterone is measured in ng/dL and PRA in ng/mL/h) has a high negative predictive value and reliably excludes PA. A high ratio (>20–30) is suggestive of PA, but is not diagnostic. A high ratio has a relatively low positive predictive value; many subjects with a high ratio will not have PA. Accordingly, confirmatory testing is required.
PA is confirmed by demonstrating the lack of suppression of aldosterone secretion with volume expansion. This is perhaps most easily accomplished with measurement of 24‐hour urinary aldosterone excretion after 3–4 days of dietary salt supplementation. With confirmation of biochemical PA, identification and localization of an APA can be attempted with CT imaging of the adrenal gland followed by AVS. Unilateral adenomas can be removed by laparoscopic adrenalectomy. Medical therapy with use of MR antagonists is appropriate for treatment of PA not amenable to surgery; or alternatively, as initial treatment in all patients with a high ARR, while reserving further evaluation for APAs for patients whose hypertension or hypokalemia remains poorly controlled.
Suggested Reading
- •. Nishizaka MK, Calhoun DA. Primary aldosteronism: diagnostic and therapeutic considerations. Curr Cardiol Rep. 2005;7:412–417. [DOI] [PubMed] [Google Scholar]
- •. Mahmud A, Mahgoub M, Hall M, et al. Does aldosterone‐to‐renin ratio predict the antihypertensive effect of the aldosterone antagonist spironolactone? Am J Hypertens. 2005;18:1631–1635. [DOI] [PubMed] [Google Scholar]
- •. Grim CE. Evolution of diagnostic criteria for primary aldosteronism: why is it more common in “drug‐resistant” hypertension today? Curr Hypertens Rep. 2004;6:485–492. [DOI] [PubMed] [Google Scholar]
- •. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. [DOI] [PubMed] [Google Scholar]
- •. Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. [DOI] [PubMed] [Google Scholar]
- •. Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902. [DOI] [PubMed] [Google Scholar]
- •. Mosso L, Carvajal C, González A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–165. [DOI] [PubMed] [Google Scholar]
- •. Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism—effect of adrenal vein sampling on surgical outcome. Arch Surg. 2006;141:497–503. [DOI] [PubMed] [Google Scholar]
- •. Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. [DOI] [PubMed] [Google Scholar]
- •. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. [DOI] [PubMed] [Google Scholar]
- •. Doi SAR, Abalkhail S, Al‐Qudhaiby MM, et al. Optimal use and interpretation of the aldosterone renin ratio to detect aldosterone excess in hypertension. J Hum Hypertens. 2006;20:482–489. [DOI] [PubMed] [Google Scholar]
- •. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low‐dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. [DOI] [PubMed] [Google Scholar]


