Abstract
Proteinuria is a sign of abnormal excretion of protein by the kidney but is a nonspecific term including any or all proteins excreted. In contrast, albuminuria specifically refers to an abnormal excretion rate of albumin. Microalbuminuria refers to an abnormally increased excretion rate of albumin in the urine in the range of 30–299 mg/g creatinine. It is a marker of endothelial dysfunction and increased risk for cardiovascular morbidity and mortality especially, but not exclusively, in high‐risk populations such as diabetics and hypertensives. Testing for microalbuminuria is now made easy by in‐office dipstick tests (semi‐quantitative) and widely available laboratory testing (quantitative). Physicians should screen all diabetics for albuminuria and strongly consider screening hypertensives to identify those at higher risk for cardiovascular disease. Appropriate intervention, including use of drugs that block the renin‐angiotensin‐aldosterone system, may be appropriate in such cases as suggested by the American Diabetes Association and the Seventh Report of Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Cardiovascular disease (CVD) is the major cause of morbidity and mortality among adults in the United States. Traditional risk factors for coronary heart disease that are identified in the Framingham Study 1 include hypertension, dyslipidemia, glucose intolerance, male gender, cigarette smoking, left ventricular hypertrophy, and age. Hypertension and dyslipidemia are the two most common risk factors; their effects on risk are additive. Both pharmacologic and nonpharmacologic strategies have been used for decades to successfully modify these factors and, in turn, decrease stroke and cardiac mortality rates, however, studies in hypertensive and diabetic populations have identified additional factors independently associated with increased risk for CVD and death. The most important nontraditional risk factor for CVD is proteinuria. 1 The purpose of this article is to define microalbuminuria, describe how to detect and monitor it, and in whom it should be measured. In addition, its importance as a marker of increased CVD and mortality risk in type 1 and type 2 diabetics and hypertensive nondiabetics will be highlighted. Finally, we will discuss how drugs that inhibit the renin‐angiotensin‐aldosterone system can reduce risk for the onset of diabetic nephropathy.
DEFINITIONS
Proteinuria is defined as an excessive excretion of any protein or proteins into the urine, however, the most abundant protein found in urine in those with proteinuria is albumin. Albumin is normally filtered by the glomerulus and reabsorbed in the proximal tubule of the kidney; therefore, only minute quantities of albumin are normally present in the urine. When urine albumin is persistently present in excessive amounts, it is a sign of potentially serious CVD.
PROTEINURIA
Proteinuria refers to any excessive excretion of protein in the urine. Under normal circumstances, urinary excretion of all proteins combined is <150 mg/d. The most abundant protein in normal urine is Tamm‐Horsfall protein, an α‐2 globulin synthesized by the renal tubules in the loop of Henle. Proteinuria results from three basic mechanisms: 1) glomerular injury resulting in excess filtration of protein; 2) tubular injury resulting in excessive production and excretion of tubular proteins (e.g., Tamm‐Horsfall protein); and 3) overfiltration of plasma proteins due to high plasma concentration, e.g., in multiple myeloma. Various disease states can cause excessive excretion of protein in the urine, including glomerular and tubular diseases.
MICROALBUMINURIA
Under normal conditions, daily albumin excretion is in the range of 5–10 mg and the urine albumin:creatinine ratio is in the range of 0–29 mg albumin/g creatinine. Forty years ago, technology for measuring small amounts of urine albumin was described to determine abnormal amounts of albumin excretion or albuminuria. 2 , 3 Microalbuminuria is defined as an abnormal increase in albumin excretion rate within the specific range of 30–299 mg of albumin/g of creatinine. It is important to recognize that the term microalbuminuria specifically refers to an abnormal albumin excretion rate and not the presence of an abnormal (small) albumin molecule. The term was coined in the early 1980s, 4 when technical advances made it possible to identify small, but abnormal, increases in albumin in the urine of patients with diabetes and other diseases—hence the term “microalbuminuria.”
Expressing albumin as a ratio to creatinine is desirable as it allows one to use a routine (“spot”) urine sample to detect an abnormal amount of albumin in the urine obviating a 24‐hour urine collection. The National Kidney Foundation, the American Diabetes Association, and the National Institutes of Health recommend the measurement of albumin in the urine using the technique of the albumin:creatinine ratio. 5 , 6 , 7 The presence of persistent microalbuminuria is a marker of increased vascular permeability associated with a variety of cardiovascular risk factors. It alone is not definite evidence of nephropathy, however, some type 1 and type 2 diabetics with microalbuminuria develop nephropathy over time. 8 , 9 Table I illustrates the salient points that differentiate proteinuria and microalbuminuria.
MACROALBUMINURIA (PROTEINURIA)
Macroalbuminuria is defined as an abnormal increase in albumin excretion rate in the range ≥300 mg albumin/g creatinine. This level of albuminuria is widely accepted as evidence of established nephropathy in both diabetic and nondiabetic renal diseases. The level distinguishing microalbuminuria from macroalbuminuria is primarily based on clinical findings and not renal biopsy evidence of irreversible renal damage. Macroalbuminuria in both diabetic and nondiabetic individuals is a marker of accelerated decline in the glomerular filtration rate. 10 , 11 , 12 , 13 , 14 Macroalbuminuria indicates intrinsic renal disease and is also a marker for increased cardiovascular morbidity and mortality (Table II).
Table II.
Definition and Clinical Significance of Abnormal Albumin Excretion
| Microalbuminuria | Macroalbuminuria | |
|---|---|---|
| Definition | Urine albumin:creatinine ratio 30–299 mg/g | c =300 mg/g |
| Marker of established renal disease | No | Yes |
| Marker of endothelial dysfunction | Yes | Yes |
| Cardiovascular risk factor | Yes | Yes |
DETECTION AND MEASUREMENT OF ALBUMINURIA
It is most important to perform a screening test on any patient at risk for microalbuminuria in whom knowledge of the test will provide information on cardiovascular and renal risk stratification and/or have therapeutic implications. Confirmation of the presence of microalbuminuria is important because some conditions cause transient excess albumin in the urine that is neither a sign of CVD nor a reason to treat microalbuminuria (see below).
How to Detect (Screen) and Quantify Albuminuria
Albuminuria is not detected by routine urinalysis. Two methods can be used to screen for albuminuria in a random urine sample: 1) dipstick test strips designed to detect microalbuminuria (e.g., Micral II [Roche Diagnostics, Lewes, England] and ImmunoDip [Diagnostic Chemicals Limited, Prince Edward Island, Canada]); and 2) quantitative measurement of albumin and creatinine (Table III). Advantages of the microalbumin dipstick test strips include high sensitivity and specificity, semi‐quantitative estimate of albumin (below the level of routine dipsticks), office use, and cost ($5‐$ 10 per test sample). Disadvantages include lack of quantitative measurement, time required to perform the test in the office, and the need for confirmation of the specific amount of albumin with another test. The test strips provide an estimate of the concentration of albumin in the urine in mg/dL or mg/L but not true quantitation. As a consequence, diluted or concentrated urine samples can give rise to both false positive and false negative tests. The urine albumin:creatinine ratio, the recommended method, avoids this pitfall because it is a direct and quantitative measurement of albumin and creatinine in a random urine sample. Most clinical laboratories offer the test and report the ratio in mg/g albumin. Calculating the ratio improves the sensitivity of the test and is not confounded by a dilute or concentrated urine sample. Ordering a urine albumin and creatinine test on a random urine sample is simple to do and sample collection is easy, i.e., a random voided specimen obtained in the office or brought in from home will suffice. There is no need to collect a 24‐hour urine sample to screen for or confirm albuminuria; this method is not recommended. The urine albumin:creatine ratio is currently the recommended test of choice for accurately quantifying albuminuria. 5 , 15
Laboratory Methods of Albumin Detection in Urine
The quantitative measurement of albumin is carried out by a variety of methods, including immunoturbidimetric, radioimmunoassay, and high‐performance liquid chromatography. The latter method may be more accurate for detecting low levels of intact albumin molecules, whereas other methods not only detect intact albumin but also detect albumin fragments (break down products of albumin) in the urine. The significance of detection of albumin fragments is not yet known.
Cost
The cost of microalbuminuria dipstick tests is $4‐$8 per test, whereas the albuminxreatinine ratio is about $12 per test. The dipstick is immediately available, whereas the urine albumin:creatinine ratio takes 1–2 days, depending on the laboratory. Regardless of which method is used to screen for microalbuminuria, a urine albumin:creatinine ratio is recommended to confirm a screening test.
Transient Albuminuria
It is important to note that albuminuria can result from a transient increase in glomerular capillary permeability. Conditions associated with transient albuminuria include decompensated heart failure, vigorous exercise, fever, urinary tract infection, postural changes, or sleep apnea. The urine albumin:creatinine ratio should not be measured if these conditions are present. Also, urinary albumin values vary within the normal population with an intra‐individual day‐to‐day coefficient of variation ≤50%. Therefore, confirmation of persistent microalbuminuria within 3 months of initial detection is recommended by the American Diabetes Association and the National Kidney Foundation (Figure 1). Albumin is stable in urine at room temperature, so the storage process and time do not increase variation of current assays; therefore, it is not necessary to freeze urine samples. Assays for albumin and creatinine in urine are widely available. A number of assay techniques for albuminuria include StatLIA Assay Report (RIA), enzyme‐linked immunoabsorbent assay (ELISA), and high‐performance liquid chromatography (HPLC). The inter‐assay coefficient of variation is in the range of 5%–8%.
Figure 1.
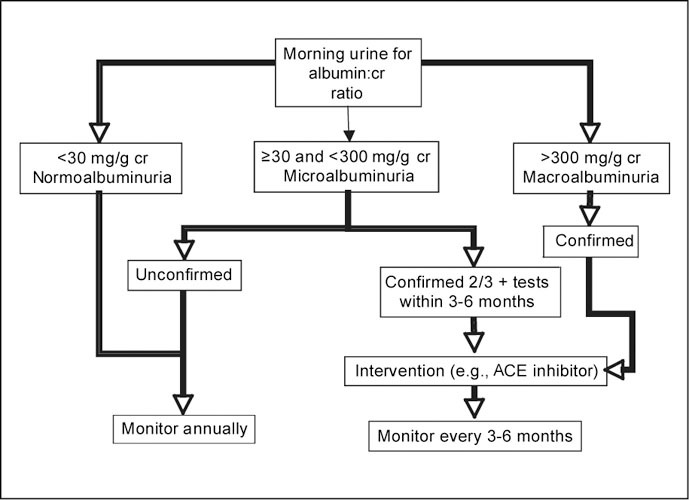
Detection and monitoring of microalbuminuria. cr=creatinine; ACE=angiotensin‐converting enzyme
CLINICAL SIGNIFICANCE OF MICROALBUMINURIA
Microalbuminuria is a widely identified marker of endothelial dysfunction (Figure 2). As depicted in Figure 2, microalbuminuria is associated with other cardiovascular risk factors, including markers of inflammation and markers of endothelial dysfunction. 16 The presence of microalbuminuria markedly increases the risk for cardiovascular morbidity and mortality among diabetics and nondiabetics. 17 , 18 It has been estimated that 6% of men and 10% of women in the United States have microalbuminuria; its prevalence is 16% among hypertensives and as high as 28% among diabetics. 19 The prevalence is higher among Hispanics and African Americans compared with non‐Hispanics. Observational studies in the general population have shown an increase in risk for total and cardiovascular mortality. In type 1 and type 2 diabetics, the presence of microalbuminuria is associated with increased cardiovascular and all‐cause mortality and ischemic heart disease events. 9 , 20 , 21 , 22 In addition, microalbuminuria is independently associated with angiographic evidence of coronary artery disease. 23 Most studies indicate that the presence of persistent microalbuminuria imposes a two‐fold increase in risk for cardiovascular mortality. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Cross‐sectional data indicate that microalbuminuria among hypertensive nondiabetics is associated with an increase in all‐cause mortality among hypertensives. 30 , 31 In general, most studies in nondiabetic hypertensives indicate a two‐ to three‐fold increase in cardiovascular risk. Furthermore, among hypertensives without diabetes followed prospectively, microalbuminuria is associated with a four‐fold increased risk for ischemic heart disease. 32 , 33
Figure 2.
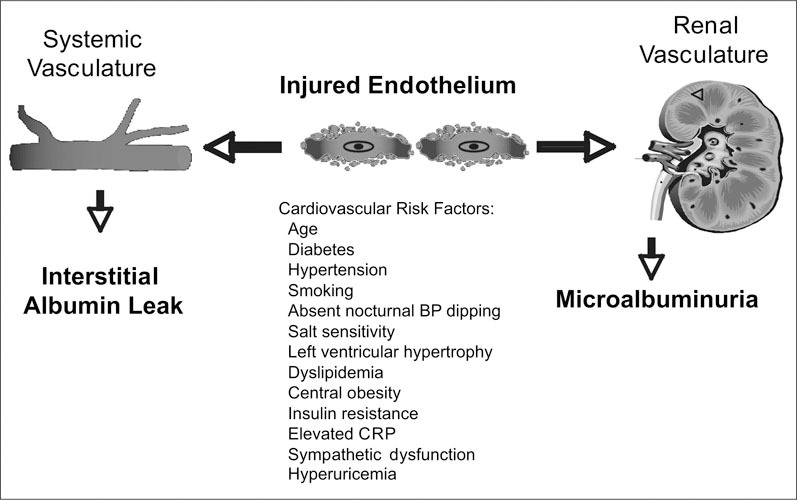
Microalbuminuria: manifestation of diffuse endothelial cell injury. BF=blood pressure; CRF=C‐reactive protein
Clinical trials have also demonstrated an increased risk for cardiovascular events in individuals with microalbuminuria at baseline. In addition, these trials have demonstrated improved outcomes among both diabetics and nondiabetics treated with agents that block the renin‐angiotensin system. For example, in the Heart Outcomes Prevention Evaluation (HOPE) study, 34 , 35 type 2 diabetics with microalbuminuria had a two‐fold higher rate of cardiovascular events, including myocardial infarction, stroke, and cardiovascular death, as compared with patients without microalbuminuria. 18 In this trial, ramipril treatment was associated with improved cardiovascular outcomes in both diabetics and nondiabetics. 34 , 35 In the Losartan Intervention For Endpoints 36 (LIFE) trial, patients with microalbuminuria also had increased stroke and myocardial infarction rates as compared with those without microalbuminuria. Patients treated with a losartan‐based regimen, including microalbuminurics, had improved cardiovascular morbidity and mortality as compared with those treated with a treatment program based on atenolol.
It is important to emphasize that microalbuminuria does not cause CVD. Instead, its presence signals or identifies people who need more intensive therapy and closer follow‐up exams for known cardiovascular risk factors. Meta‐analysis of observational and clinical trial data indicate that microalbuminuria is associated with increased cardiovascular and total mortality. 37 Why microalbuminuria is associated with an increase in cardiovascular mortality is still unknown, however, it would appear that it is a marker of inflammation and/or endothelial damage. From a clinical management standpoint, patients with microalbuminuria should be considered at increased risk for CVD, which should result in prompt attention to the management of modifiable risk factors including diabetes, hypertension, smoking, dyslipidemia, obesity, and left ventricular hypertrophy. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 38 now recognizes microalbuminuria as a cardiovascular risk factor and recommends testing for microalbuminuria as one way to help identify high‐risk individuals. Figure 1 illustrates a detection and management pathway for individuals with persistent microalbuminuria as recommended by the American Diabetes Association, the National Kidney Foundation, and the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
CONCLUSIONS
Microalbuminuria indicates the presence of an abnormal urinary excretion of albumin, signifying endothelial dysfunction and an increased risk for cardiovascular morbidity and mortality. Reducing albuminuria with pharmacologic therapy has been associated with improved outcomes in studies employing angiotensin‐converting enzyme inhibitors and angiotensin type‐1 receptor antagonists as part of a therapeutic regimen. Future studies are needed to determine whether reducing albuminuria in the general population will reduce cardiovascular morbidity and mortality.
References
- 1. Kannel W. The prognostic significance of proteinuria: the Framingham study. Am Heart J. 1984;108:1347–1352. [DOI] [PubMed] [Google Scholar]
- 2. Keen H, Chlouverakis C. An immunoassay method for urinary albumin at low concentrations. Lancet. 1963;186:913–914. [DOI] [PubMed] [Google Scholar]
- 3. Keen H, Chlouverakis C. Urinary albumin excretion and diabetes mellitus. Lancet. 1964;14:1155–1156. [DOI] [PubMed] [Google Scholar]
- 4. Viberti GC, Keen H. Microalbuminuria and diabetes. Lancet.1983;1:352. [DOI] [PubMed] [Google Scholar]
- 5. American Dibetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26: S33–S50. [DOI] [PubMed] [Google Scholar]
- 6. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. [DOI] [PubMed] [Google Scholar]
- 7. Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kidney Dis. 2003;42:617–622. [DOI] [PubMed] [Google Scholar]
- 8. Viberti GC, Jarrett RJ, Keen H. Microalbuminuria as prediction of nephropathy in diabetics. Lancet. 1982;2:611. [DOI] [PubMed] [Google Scholar]
- 9. Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity‐onset diabetes. N Engl J Med. 1984;310:356–360. [DOI] [PubMed] [Google Scholar]
- 10. Nelson RG, Meyer TW, Myers BD, et al. Clinical and pathological course of renal disease in non‐insulin‐dependent diabetes mellitus: the Pima Indian experience. Semin Nephrol. 1997;17:124–131. [PubMed] [Google Scholar]
- 11. Nelson RG, Meyer TW, Myers BD, et al. Course of renal disease in Pima Indians with non‐insulin‐dependent diabetes mellitus. Kidney Int Suppl. 1997;63:S45–S48. [PubMed] [Google Scholar]
- 12. Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non‐diabetic renal disease. Kidney Int. 2001;60:1131–1140. [DOI] [PubMed] [Google Scholar]
- 13. Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. [DOI] [PubMed] [Google Scholar]
- 14. Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. [DOI] [PubMed] [Google Scholar]
- 15. Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis. 2003;42:617–622. [DOI] [PubMed] [Google Scholar]
- 16. Stehouwer CDA, Nauta JJ, Zeldenrust GC, et al. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non‐insulin‐dependent diabetes mellitus. Lancet. 1992;340:319–323. [DOI] [PubMed] [Google Scholar]
- 17. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 18. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 19. Jones CA, Francis ME, Eberhardt MS, et al. Micro‐albuminuria in the US population: third national health and nutrition examination survey. Am J Kidney Dis. 2002;39:445–459. [DOI] [PubMed] [Google Scholar]
- 20. Gall MA, Borch‐Johnsen K, Hougaard P, et al. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303–1309. [DOI] [PubMed] [Google Scholar]
- 21. Borch‐Johnsen K. Incidence of nephropathy in IDDM as related to mortality. Costs and benefits of early intervention. In: Mogensen CE, ed. The Kidney and Hypertension in Diabetes Mellitus. Boston , MA : Kluwer Academic Publishers; 2001:169–178. [Google Scholar]
- 22. Allen KV, Walker JD. Microalbuminuria and mortality in long‐duration type 1 diabetes. Diabetes Care. 2003;26:2389–2391. [DOI] [PubMed] [Google Scholar]
- 23. Tuttle KR, Puhlman ME, Cooney SK, et al. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis. 1999;34:918–925. [DOI] [PubMed] [Google Scholar]
- 24. Jarrett RJ, Viberti GC, Argyropoulos A, et al. Micro‐albuminuria predicts mortality in non‐insulin‐dependent diabetics. Diabet Med. 1984;1:17–19. [DOI] [PubMed] [Google Scholar]
- 25. Morgensen CE, Damsgaard EM, Froland A, et al. Microalbuminuria in non‐insulin‐dependent diabetes. Clin Nephrol. 1992;38:528–538. [PubMed] [Google Scholar]
- 26. Mattock MB, Barnes DJ, Viberti G, et al. Microalbuminuria and coronary heart disease in NIDDM: an incidence study. Diabetes. 1998;47:1786–1792. [DOI] [PubMed] [Google Scholar]
- 27. Damsgaard EM, Froland S, Jorgensen OD, et al. Microalbuminuria as predictor of increased mortality in elderly people. BMJ. 1990;300:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossing P, Hougaard P, Borch‐Johnsen K, et al. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agewall S, Wikstrand J, Ljungman S, et al. Does microalbuminuria predict cardiovascular events in nondiabetic men with treated hypertension? Am J Hypertens. 1995;8:337–342. [DOI] [PubMed] [Google Scholar]
- 31. Agewall S, Wikstrand J, Ljungman S, et al. Urinary albumin excretion is associated with the intima‐media thickness of the carotid artery in hypertensive males with non‐insulin‐dependent diabetes mellitus. J Hypertens. 1995;13:463–469. [PubMed] [Google Scholar]
- 32. Jensen T, Borch‐Johnsen K, Kofoed‐Enevoldsen A, et al. Coronary heart disease in young type 1 (insulin‐dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987;30:144–148. [DOI] [PubMed] [Google Scholar]
- 33. Jensen JS, Feldt‐Rasmussen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898–903. [DOI] [PubMed] [Google Scholar]
- 34. The Hope Trial Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICROHOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 35. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 36. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. [DOI] [PubMed] [Google Scholar]
- 37. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 38. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]


