Abstract
Hypertension guidelines recommend a steppedcare approach that starts with titration of the initial agent followed by the addition of other agents, as necessary, to achieve goal blood pressure. This study assessed the effectiveness of an antihypertensive treatment algorithm with olmesartan medoxomil as the initial agent. This was a 24‐week, open‐label trial in patients (N=201) with mean seated diastolic blood pressure of 90–109 mm Hg. Following placebo run‐in, all patients received olmesartan medoxomil 20 mg/d for 4 weeks. At subsequent 4‐week intervals, the regimen was modified in patients with blood pressure >130/85 mm Hg: up‐titration of olmesartan medoxomil to 40 mg/d; addition of hydrochlo‐rothiazide 12.5 mg/d; up‐titration of hydrochlo‐rothiazide to 25 mg/d; addition of amlodipine besylate 5 mg/d; and up‐titration of amlodipine besylate to 10 mg/d. Patients who achieved blood pressure ≤130/85 mm Hg at any point exited the study with no further follow‐up. At Week 24, reductions in blood pressure from baseline were 33.7/18.2 mm Hg. Altogether, 87.7% of patients reached the goal blood pressure of ≤130/85 mm Hg and 93.3% achieved a blood pressure of ≤140/90 mm Hg. Thus, an antihypertensive algorithm with olmesartan medoxomil as the initial agent controlled blood pressure in the majority of patients, but with >60% of patients also requiring the use of a thiazide diuretic or a thiazide and a calcium channel blocker.
Reducing blood pressure (BP) decreases the risks of morbidity and mortality in patients with hypertension. 1 , 2 , 3 However, in practice, achieving the recommended BP goals of <140/90 mm Hg for patients with uncomplicated hypertension and <130/80 mm Hg for high‐risk hypertensive patients such as those with concomitant diabetes, occurs infrequently. Only about 34% of people with hypertension in the United States have their BP controlled to <140/90 mm Hg, indicating a need for improved treatment. Although published guidelines recommend using a stepped‐care antihypertensive treatment algorithm to reach these goals, 3 the effectiveness of this approach in clinical practice and the optimal selection of agents for each step remain undefined.
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 3 defines normal BP as <120/80 mm Hg and prehy‐pertensive BP as 120–139/80–89 mm Hg. An analysis of 6859 normotensive participants in the Framingham Heart Study showed that persons with prehypertension had a significantly increased risk of developing both hypertension and cardiovascular (CV) disease compared with those whose entry BP was lower. 4
The Hypertension Optimal Treatment (HOT) study1 and the United Kingdom Prospective Diabetes Study (UKPDS 38) 2 both demonstrated that in hypertensive patients with diabetes, a more aggressive target BP was associated with improved cardioprotection. The HOT study also showed that self‐reported well‐being in all patients was highest in the treatment group assigned to the lowest diastolic blood pressure (DBP) goal (≤80 mm Hg). Importantly, at the end of the study, 77% of patients randomized to achieve a DBP of ≤80 mm Hg were taking two or more antihypertensive agents. In the UKPDS 38 trial, 2 many subjects required three or more medications to sustain the goal DBP of <85 mm Hg after 9 years of treatment. There is a need to demonstrate the ability of anti‐hypertensive treatment protocols to lower BP to these aggressive goals in clinical practice.
Clinical studies indicate that angiotensin II receptor blocker (ARB) therapy provides antihypertensive efficacy comparable to that of other antihypertensive classes, and a tolerability profile similar to that of placebo. 5 , 6 , 7 Therapy based on an ARB has been shown to slow renal disease progression in hypertensive patients with diabetic renal disease, and to reduce CV morbidity and mortality in patients with hypertension with left ventricular hypertrophy and in patients with congestive heart failure. 8 , 9 , 10 , 11 , 12 , 13
The ARB olmesartan medoxomil, the newest addition to the ARB class, is an efficacious antihypertensive agent. 14 , 15 , 16 , 17 This study was undertaken to assess in a clinical setting the percentage of patients with mild‐to‐moderate hypertension who would reach a goal BP of ≤140/90 mm Hg as well as a more aggressive goal BP of ≤130/85 mm Hg when physicians are provided with a specific BP goal and an algorithm designed to achieve that goal, using olmesartan medoxomil as the initial agent.
METHODS
Study Population
This was a 24‐week, multicenter, open‐label trial including patients with mild‐to‐moderate hypertension. The study was conducted in accordance with central and local institutional review board committees at each of 21 investigation sites and conformed to the principles of the Declaration of Helsinki and its amendments. The study population included male and female patients with essential hypertension who were aged 18 years or older, were not institutionalized, and who gave written informed consent. Women with childbear‐ing potential were enrolled only if they had a negative serum pregnancy test at screening, were not breast‐feeding, and did not have plans to become pregnant while participating in the study.
Enrollment required patients to have a seated diastolic BP (SeDBP) ≤90 mm Hg and ≤109 mm Hg, and a seated systolic BP (SeSBP) <200 mm Hg, measured at two separate visits during the placebo run‐in period. The difference between the BP measurements of the two visits had to be slO mm Hg.
Patients were excluded if they had serious medical or psychiatric disorders; diabetes; a history of a CV event within 6 months before the study; secondary hypertension of any etiology, such as renal disease, pheochromocytoma, or Cushing's syndrome; a history of drug or alcohol abuse within 2 years before the study; a history of an allergic response to any ARB, calcium channel blocker (CCB), or diuretic; or a history of angioedema.
Study Design
The study consisted of six treatment periods lasting 4 weeks each. Following screening, all antihypertensive medications were discontinued and patients entered a single‐blind placebo run‐in period of up to 4 weeks. Patients who met entry criteria were initiated on therapy with olmesartan medoxomil 20 mg once daily (Figure 1). The end‐of‐study BP goal for all patients was ≤130/85 mm Hg. If the goal BP was not achieved, antihypertensive therapy was titrated at 4‐week intervals according to the following stepwise algorithm until the goal BP was attained: up‐titration of olmesartan medoxomil to 40 mg/d, addition of hydrochlorothiazide (HCTZ) 12.5 mg/d to the regimen, up‐titration of HCTZ to 25 mg/d, addition of amlodipine besylate 5 mg/d, then up‐titration of amlodipine besylate to 10 mg/d.
Figure 1.
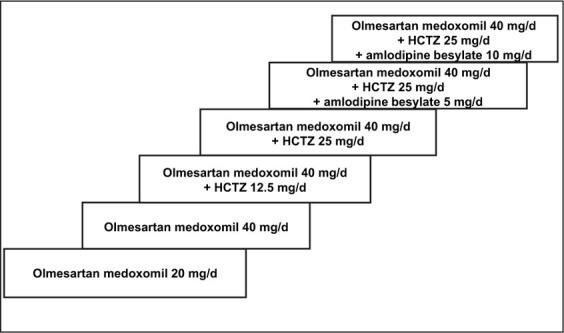
Olmesartan medoxomil‐based treatment algorithm. Treatment was initiated with olmesartan medoxomil 20 mg/d. If the target blood pressure (BP) of ≤130/85 mm Hg was not achieved at 4 weeks, olmesartan medoxomil was titrated to 40 mg/d. Hydrochlorothiazide (HCTZ) or amlodipine besylate could be added as needed, beginning at 8 weeks and then every 4 weeks thereafter, until goal BP was achieved.
Patients were seen every 2 weeks ± 3 days for study visits. SeBP values were obtained at each study visit at trough and calculated as the mean of three SeBP readings measured 2 minutes apart. To avoid observer bias, the Dinamap (model 117208, Critikon, Tampa, FL) automated oscillometric BP monitoring device was used for all cuff BP measurements. The device was preprogrammed to obtain and print three sitting cuff BP (and heart rate) measurements at 2‐minute intervals. The same cuff size was used for all cuff measurements on individual patients throughout the study. Patients who achieved the SeSBP target of ≤130 mm Hg and the SeDBP target of ≤85 mm Hg at any point exited the study.
Safety was monitored throughout the trial. Laboratory tests (including a complete blood cell count and a chemistry panel) and a physical examination were performed at screening and at the final visit, and an electrocardiogram was performed before study entry. In addition, serum potassium levels were measured at Week 8 (before adding HCTZ to the treatment regimen), Week 10 (2 weeks after adding HCTZ 12.5 mg/d), and Week 14 (2 weeks after up‐titrating to HCTZ 25 mg/d).
Efficacy Variables
The primary objective of the study was to determine the percentages of patients who attained SeBP goals of ≤140/90 mm Hg and ≤130/85 mm Hg using the study algorithm. Other efficacy variables included the percentages of patients who achieved SeDBP goals of ≤90 mm Hg or ≤85 mm Hg; SeSBP goals of ≤140 mm Hg or ≤130 mm Hg; the percentage of diastolic responders, defined as SeDBP of <90 mm Hg or a reduction in SeDBP from baseline of ≥10 mm Hg; and the change from study baseline in mean SeDBP and mean SeSBP.
Statistical Methods
Sample size determination was based on the assumption that 175 patients entering the active drug treatment phase would provide sufficient data to determine the effectiveness of this treatment algorithm. Summary statistics were tabulated for all baseline demographic and clinical variables. Mean, median, and standard deviation were calculated for all BP measurements. BP change from baseline for patients who either reached the BP target or discontinued during the active treatment period was computed using the last observation carried forward. Safety data were summarized.
RESULTS
Patient Disposition and Baseline Characteristics
A total of 455 patients was screened for participation in this study. Of these, 76 patients failed the screening process. Three patients had a placebo run‐in visit but did not receive any placebo run‐in study medication. Thus, 376 patients were enrolled in the study and received at least one dose of placebo run‐in medication. Of these 376 patients, 175 were withdrawn during the placebo run‐in period. Reasons for withdrawal included patient request, average SeDBP <90 mm Hg or >109 mm Hg, adverse event, lost to follow‐up, and uncontrolled BP.
The safety population (n=201) included all patients who entered the active treatment period and received at least one dose of olmesartan medoxomil. The efficacy cohort (n=197) included all patients who received at least one dose of olmesartan medoxomil, had at least one postbaseline BP measurement, and received a dose of study drug 1 day before the postbaseline BP measurement. BP reductions from baseline were calculated for the efficacy cohort using the last observation carried forward. The eligible cohort (n=179) excluded 18 patients from the efficacy cohort who were not given the opportunity to reach target BP due to investigator error (the investigator incorrectly believed the patient had achieved target BP and thus completed the study), patient request, non‐compliance or protocol violations, or those who were lost to follow‐up. Goal rate assessments were performed on the eligible cohort.
For the efficacy cohort, the mean age was 52.9 years, 65.0% were male, 73.6% were white, and 16.2% were African American. The mean baseline BP was 161.2/96.6 mm Hg. Patient disposition by treatment group and baseline BP values of all patients entering each treatment phase are shown in Table I. Thirty‐eight percent of patients were not further titrated after receiving high‐dose ARB monotherapy; 62% required the addition of HCTZ to achieve goal BP ≤130/85 mm Hg. Most patients received two antihypertensive agents; amlodipine besylate was added to the regimen in approximately 25% of patients.
Table I.
Patient Disposition for the Efficacy Cohort
| Algorithm Step | n | % | Mean SeSBP/SeDBP at Study Baseline (mm Hg) |
|---|---|---|---|
| Olmesartan medoxomil 20 mg/d | 197 | 100 | 161.2/96.6 |
| Olmesartan medoxomil 40 mg/d | 156 | 79.2 | 163.3/97.2 |
| Olmesartan medoxomil 40 mg/d + HCTZ 12.5 mg/d | 123 | 62.4 | 164.6/97.6 |
| Olmesartan medoxomil 40 mg/d + HCTZ 25 mg/d | 78 | 39.6 | 166.6/98.2 |
| Olmesartan medoxomil 40 mg/d + HCTZ 25 mg/d + amlodipine besylate 5 mg/d | 49 | 24.9 | 168.1/98.5 |
| Olmesartan medoxomil 40 mg/d + HCTZ 25 mg/d + amlodipine besylate 10 mg/d | 22 | 11.2 | 170.7/100.9 |
| SeSBP=seated systolic blood pressure; SeDBP=seated diastolic blood pressure; HCTZ=hydrochlorothiazide | |||
Efficacy
BP Control Rates.
After 8 weeks of monotherapy with olmesartan medoxomil (20–40 mg/d), 58.7% of patients in the eligible cohort reached the BP goal of ≤140/90 mm Hg, and 35.2% achieved the more rigorous BP goal of ≤130/85 mm Hg. The percentage of diastolic responders (SeDBP <90 mm Hg or decreased by ≥10 mm Hg) was 83.8%. Secondary SeDBP goals of ≤90 mm Hg and ≤85 mm Hg were achieved by 81.6% and 65.9% of patients, respectively, and secondary SeSBP goals of ≤140 mm Hg and ≤130 mm Hg were achieved by 61.5% and 39.1% of patients, respectively.
Following an additional 8 weeks of therapy with olmesartan medoxomil + HCTZ, the percentages of patients achieving the BP control rates of ≤140/90 mm Hg and ≤130/85 mm Hg increased to 83.2% and 69.3%, respectively (Figure 2). The percentage of diastolic responders at Week 16 was 96.1%. The SeDBP goals of ≤90 mm Hg and ≤85 mm Hg were achieved by 96.1% and 87.2% of eligible patients, respectively, and the percentages of patients achieving the SeSBP goals of ≤140 mm Hg and ≤130 mm Hg were 86.0% and 72.6%, respectively (Table II).
Figure 2.
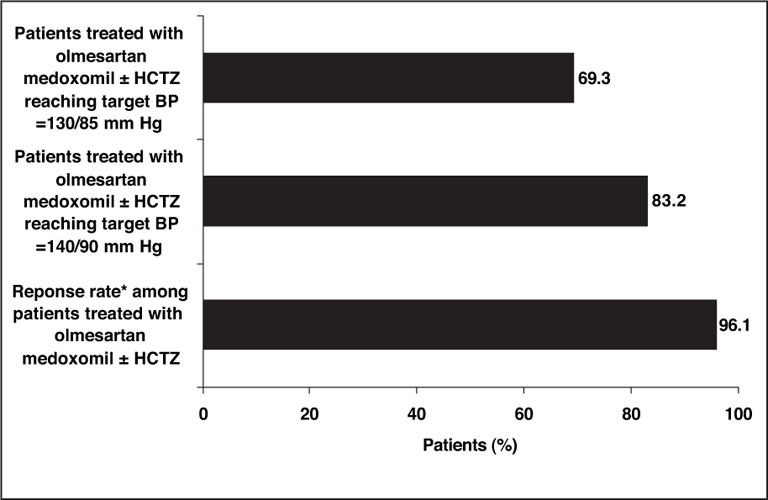
Primary efficacy goal rates at Week 16. Patients were treated with olmesartan medoxomil (20–40 mg/d) for up to 8 weeks, and if the target blood pressure (BP) of ≤130/85 mm Hg was not achieved, hydrochlorothiazide (HCTZ) (12.5–25 mg/d) was added to the treatment regimen. Using this two‐drug algorithm, 83.2% of patients achieved the BP goal of ≤140/90 mm Hg, and 69.3% of patients achieved the more stringent BP goal of ≤130/85 mm Hg. *The response rate, defined as seated diastolic BP <90 mm Hg or seated diastolic BP reduced by ≥10 mm Hg from baseline, was 96.1%.
Table II.
Cumulative Percentage of Patients Achieving Efficacy Goals With Olmesartan Medoxomil + Hydrochlorothiazide (HCTZ) at Week 16*
| Efficacy Goal | %of Patients |
|---|---|
| SeDBP | |
| ≥90 mm Hg | 96.1 |
| ≥85 mm Hg | 87.2 |
| SeSBP | |
| ≥140 mm Hg | 86.0 |
| ≥130 mm Hg | 72.6 |
| Responders** | 96.1 |
| SeDBP=seated diastolic blood pressure; SeSBP=seated systolic blood pressure; *based on the eligible patient population (n=179); **SeDBP <90 mm Hg or decreased by ≥10 mm Hg | |
At the end of the 24‐week treatment period, 93.3% of eligible patients (167/179) achieved the BP goal of ≤140/90 mm Hg and 87.7% (157/179) achieved the more aggressive goal BP of ≤130/85 mm Hg with the olmesartan medoxomil‐based treatment regimen (Figure 3). A total of 95.0% and 88.3% of patients achieved the SeSBP goals of ≤140 mm Hg and ≤130 mm Hg, respectively, and 97.2% and 95.0% of patients achieved the SeDBP goals of ≤90 mm Hg and ≤85 mm Hg, respectively. Overall, 97.2% of patients (174/179) were categorized as diastolic responders at the end of 24 weeks of treatment.
Figure 3.
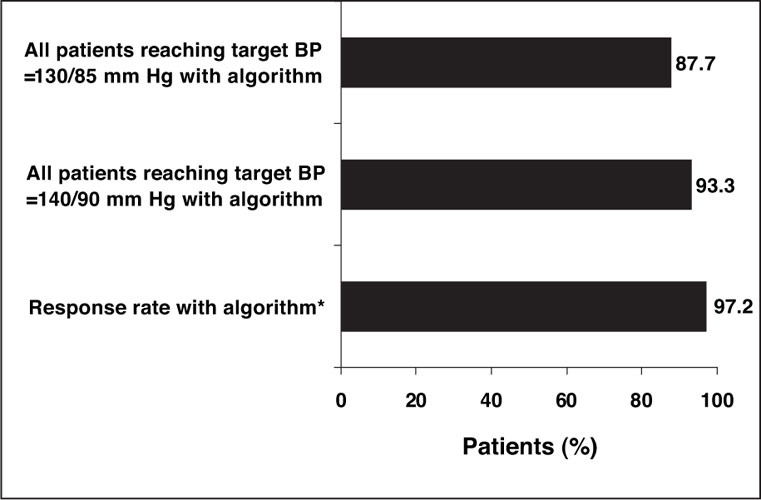
Summary of results of all patients. With stepped‐care antihypertensive therapy with olmesartan medoxomil as the initial agent, 93.3% of patients achieved the blood pressure (BP) goal of ≤140/90 mm Hg, and 87.7% of patients achieved the more stringent BP goal of ≤130/85 mm Hg. *The response rate, defined as seated diastolic BP <90 mm Hg or seated diastolic BP reduced by ≥10 mm Hg from baseline, was 97.2%.
BP Reductions.
Reductions in BP among patients treated with olmesartan medoxomil, olmesartan medoxomil + HCTZ, and olmesartan medoxomil + HCTZ + amlodipine, are presented in Figure 4. Patients who were treated for 8 weeks with olmesartan medoxomil alone (n=198) had a mean reduction in SeDBP from 96.6 mm Hg (baseline) to 85.9 mm Hg (−10.7 mm Hg), and a mean reduction in SeSBP from 161.2 mm Hg (baseline) to 143.5 mm Hg (−17.7 mm Hg). At Week 16, after an additional 8 weeks of therapy with olmesartan medoxomil + HCTZ (n=123), mean SeDBP decreased from 97.6 mm Hg (baseline) to 81.5 mm Hg (−16.1 mm Hg) and mean SeSBP decreased from 164.6 mm Hg (baseline) to 135.3 mm Hg (−29.3 mm Hg), an additional increment of −11.6/−5.4 mm Hg, relative to monotherapy. The mean BP reduction from baseline among patients who received olmesartan medoxomil + HCTZ + amlodipine (n=49) was −33.7/−18.2 mm Hg.
Figure 4.
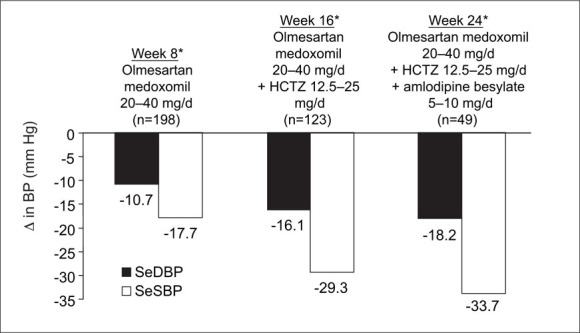
Reductions in blood pressure (BP). Patients who were treated for 8 weeks with olmesartan medoxomil alone (n=198) had a mean reduction in seated diastolic BP (SeDBP) from 96.6 mm Hg (baseline) to 85.9 mm Hg (−10.7 mm Hg), and a mean reduction in seated systolic BP (SeSBP) from 161.2 mm Hg (baseline) to 143.5 mm Hg (−17.7 mm Hg). At Week 16, after an additional 8 weeks of combination therapy for patients who did not achieve BP goal at Week 8 (n=123), mean BP was decreased an additional increment of −11.61–5.4 mm Hg relative to monotherapy. The mean BP reduction among patients who received all three antihypertensive medications (olmesartan medoxomil + hydrochlorothiazide [HCTZ] + amlodipine) (n=49) was −33.7/−18.2 mm Hg, an additional −4.4/−2.1 mm Hg, compared with two‐drug combination therapy.
Safety
Regimens that included olmesartan medoxomil as initial therapy were well tolerated. The overall incidence of treatment‐emergent clinical adverse events during the active treatment period was approximately 39%. Most of these adverse events were judged by the investigators to be remotely or definitely not drug related, and mild or moderate in severity.
A summary of the most commonly reported treatment‐emergent adverse events is provided in Table III. There were five reported treatment‐emergent serious adverse events in three patients; all of the events were assessed by the investigators as remotely or definitely not related to study drug treatment. Eight patients discontinued the study due to treatment‐emergent adverse events; of these, two were serious and/or severe (myocardial infarction, viral bronchitis). There were no trends suggestive of an adverse event on laboratory test values related to the treatment algorithm.
DISCUSSION
Olmesartan medoxomil has demonstrated an excellent safety and tolerability profile, with efficacy that appeared to be similar to that of other classes of antihypertensive agents. 14 , 15 , 16 , 17 Previous investigators have documented the importance of initial agent choice for long‐term compliance with antihypertensive therapy. 18 Our results suggest that the use of an ARB such as olmesartan medoxomil, followed by the addition of a thiazide diuretic, if necessary, and a dihydropyridine CCB, represents an effective antihypertensive stepped‐care regimen. Given that approximately 70% of the enrolled patients had stage II hypertension, based on baseline SeSBP ≥160 mm Hg, sizable SeSBP reductions were needed to achieve both BP goals. Because patients were discontinued from the study as they reached the BP goal of ≤130/85 mm Hg, the BP reductions observed may have been even greater had all the patients been allowed to progress through the entire algorithm. On the other hand, this study did not provide information that goal BP would be maintained over time. The multidrug regimen was well tolerated throughout, with no increases in adverse effects noted with the addition of HCTZ and/or amlodipine.
Published data suggest that only 50%–60% of patients achieve BP control (<140/90 mm Hg) with monotherapy with a drug from any antihypertensive class, including diuretics, β blockers, CCBs, and angiotensin‐converting enzyme inhibitors. 19 In designing a three‐drug algorithm for this study, the complementary mechanisms of action of the drugs to be used were considered. Using ARBs as first‐line agents for the management of hypertension is a reasonable approach given their antihypertensive efficacy and safety profile and their ability to provide end‐organ protection. 8 , 9 , 10 , 11 , 12 , 13 Diuretics lower BP but may affect intrarenal mechanisms by stimulating the renin‐angiotensin‐aldosterone system, making BP more dependent on angiotensin II and thereby potentiating the antihypertensive effect of an ARB. 5 An ARB/HCTZ combination has been shown to be highly effective in controlled clinical trials. 20 Therefore, the addition of HCTZ to the ARB used in this study was a rational choice. The stepwise algorithm of olmesartan medoxomil (20–40 mg/d) with add‐on HCTZ (12.5–25 mg/d) allowed 83.2% of hypertensive patients to reach the BP goal of ≤140/90 mm Hg, and 69.3% of patients to reach the more aggressive BP goal of ≤130/85 mm Hg, indicating that the majority of patients can be controlled with these two drugs.
The poor BP control rates documented in the general population are unacceptable. Only 34% of persons with hypertension in the general population are controlled to a BP of <140/90 mm Hg3; however, physicians in clinical trials and in some practices have been more successful in achieving prespecified BP targets. In clinical trials such as the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) study (n=16,602), 84.8% of patients treated with a forced titration regimen attained the BP target of 140/90 mm Hg, and 67%–69% were able to maintain a BP of <140/90 mm Hg over 2 years of treatment.21 In the blinded Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), the percentage of patients (n=33,357) at the BP goal of <140/90 mm Hg at the initial visit was 27%. After a mean of 4.9 years of follow‐up, the percentage of subjects with controlled BP had improved to 66%.22 Thus, if physicians or other health care providers are provided with a BP goal and a simple, easy‐to‐use algorithm to achieve that goal, then goals are more easily attainable.
The results of the current study, in which the goal BP of ≤140/90 mm Hg was achieved by 83.2% of patients treated with two drugs, and 93.3% of patients treated with three drugs, support previous findings of the efficacy of antihypertensive treatment algorithms. However, until this study, the effectiveness of such a treatment algorithm had not been documented in a real‐world, clinical practice setting. The findings of this study are limited by the lack of follow‐up with patients after they reached the BP goal of ≤130/85 mm Hg. Determining whether BP control can be sustained over a long term using a treatment algorithm similar to the one applied in this study is a worthwhile objective for future research.
CONCLUSION
In this clinical, practice‐based trial, when a step‐wise antihypertensive drug algorithm utilizing an ARB, diuretic, and CCB in patients with mild‐to‐moderate hypertension (mean 161.3/96.6 mm Hg), 93.3% of patients achieved the BP goal of ≤140/90 mm Hg and 87.7% of patients achieved the more stringent BP goal of ≤130/85 mm Hg. This suggests that this approach is an effective option for initial antihypertensive drug therapy. These BP reductions were obtained without a significant increase in adverse events. Future research should focus on similar studies utilizing alternative drug algorithms, especially for special patient groups.
Disclosure:
This study was supported by an unrestricted grant from Sankyo Pharma Inc., Parsippany, NJ.
References
- 1. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1296. [DOI] [PubMed] [Google Scholar]
- 5. Burnier M. Angiotensin II type I receptor blockers. Circulation. 2001;103:904–912. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan NM. Angiotensin II‐receptor blockers: will they replace angiotensin‐converting enzyme inhibitors in the treatment of hypertension? J Hum Hypertens. 2000;14(suppl l):S87–S90. [DOI] [PubMed] [Google Scholar]
- 7. Piichler K, Laeis P, Stumpe KO. Blood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonist. J Hypertens Suppl. 2001;19(suppl 1):S41–S48. [DOI] [PubMed] [Google Scholar]
- 8. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 9. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. [DOI] [PubMed] [Google Scholar]
- 10. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 11. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 12. Cohn JN, Tognoni G. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 13. Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus. Circulation. 2002;106:672–678. [DOI] [PubMed] [Google Scholar]
- 14. Ball KJ, Williams PA, Stumpe KO. Relative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensives. J Hypertens Suppl. 2001;19(suppl 1):S49–S56. [DOI] [PubMed] [Google Scholar]
- 15. Neutel JM. Clinical studies of CS‐866, the newest angiotensin II receptor antagonist. Am J Cardiol. 2001;87(suppl):37C–43C. [DOI] [PubMed] [Google Scholar]
- 16. Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich). 2001;3(5):283–291, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stumpe KO, Ludwig M. Antihypertensive efficacy of olmesartan compared with other antihypertensive drugs. J Hum Hypertens. 2002;16(suppl 2):S24–S28. [DOI] [PubMed] [Google Scholar]
- 18. Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999;160:41—46. [PMC free article] [PubMed] [Google Scholar]
- 19. Materson BJ, Reda DJ, Cushman WC, et al. Single‐drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. N Engl J Med. 1993;328:914–921. [DOI] [PubMed] [Google Scholar]
- 20. Conlin PR, Spence JD, Williams B, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens. 2000;13(pt 4):418–426. [DOI] [PubMed] [Google Scholar]
- 21. Black HR, Elliott WJ, Neaton JD, et al. Baseline characteristics and early blood pressure control in the CONVINCE trial. Hypertension. 2001;37:12–18. [DOI] [PubMed] [Google Scholar]
- 22. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]


