Abstract
The AMAZE (A Multicenter Trial Using Atacand and Zestril vs. Zestril to Evaluate the Effects on Lowering Blood Pressure) program included two identical studies sponsored by AstraZeneca LP. The oral form of candesartan is cilexetil; for simplicity, the term “candesartan” is used throughout this manuscript. Two identical multicenter, randomized, double‐blind studies were performed to determine if addition of the angiotensin receptor blocker candesartan was more effective in lowering blood pressure than up‐titration of lisinopril. Hypertensive patients (N=1096) who were uncontrolled on lisinopril 20 mg daily were randomized (1:1) to receive either 8 weeks of high‐dose lisinopril (40 mg) or the addition of candesartan (16 mg) for 2 weeks followed by 32 mg for 6 weeks. Study 1 (n=538) demonstrated decreases in trough sitting systolic/diastolic blood pressures at Week 8 by 6.2/5.9 mm Hg, respectively, for the lisinopril up‐titration treatment group and by 11.6/8.3 mm Hg, respectively, for the lisinopril plus candesartan treatment group (p<0.01 in comparing both blood pressures reductions between the two treatment groups). Corresponding results for Study 2 (n=558) are reductions of 8.7/6.2 mm Hg and 9.5/7.4 mm Hg, respectively, for each of the two treatment groups. For Study 2, comparisons of systolic/diastolic blood pressures between the two treatment groups were not statistically significantly different (p=0.51/p=0.08, respectively). Post hoc pooled analysis (N=1096) demonstrated a slightly greater blood pressure reduction with lisinopril plus candesartan compared with lisinopril (3.1/1.7 mm Hg). A 95% confidence interval limit for the difference in least squares mean change from baseline in systolic blood pressure between the two treatment groups is ‐4.8 to ‐1.5 and is ‐2.8 to ‐0.7 in mm Hg for diastolic blood pressure. The blood pressure control rates (<140/<90 mm Hg) were 42.7% and 36.9%, respectively. Both treatment regimens were well tolerated in all groups. In conclusion, for hypertensive patients not controlled by lisinopril 20 mg once daily, addition of candesartan (32 mg once daily) or doubling the dose of lisinopril provides safe, additional reduction of blood pressure.
Combination therapy is now recommended for most people with hypertension, in part because the control rates for hypertension (>140 mm Hg systolic and >90 mm Hg diastolic) are still only 34% in hypertensive adults aged 18 to 74 years. 1 Among the most popular antihypertensive drugs are agents that block the renin‐angiotensin‐aldosterone system (RAAS), including angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). 2 , 3 , 4 , 5 ACE inhibitors block the main pathway for conversion of angiotensin I to the potent vasoconstrictor and pressor agent, angiotensin II, but the discovery of species‐specific alternate pathways for angiotensin II generation raises the possibility that ACE inhibitors do not fully block the RAAS under all circumstances. 6 , 7 ACE inhibitors also block the degradation and inactivation of kinins and other biologically active peptides, 3 and cause cough and other side effects. 8 , 9 In contrast, ARBs selectively block the AT1 receptor subtype, thereby inhibiting the effects of angiotensin II regardless of the pathway leading to its generation. Adverse event rates for ARBs are low, and their tolerability profiles are similar to that of placebo. 10 Some investigators have postulated that the addition of an ARB to an ACE inhibitor may allow more complete blockade of the RAAS pathway than either drug alone, 7 potentially leading to improved blood pressure (BP) control. 11 , 12 , 13 , 14 , 15 Alternatively, it may be possible to improve the effectiveness of ACE inhibition by simple dose titration.
This report summarizes the results of the AMAZE (A Multicenter Trial Using Atacand and Zestril vs. Zestril to Evaluate the Effects on Lowering Blood Pressure) program, which included two identically designed multicenter, randomized, double‐blind studies conducted with two separate cohorts of investigators, according to guidelines and recommendations from the US Food and Drug Administration. To reduce the risk of a false‐positive study, we elected to conduct two independent studies, each at a 0.05 alpha level. AMAZE sought to determine whether addition of the ARB candesartan (16 mg titrated to 32 mg daily) to lisinopril therapy (20 mg daily) is more effective in lowering BP than titration of lisinopril to 40 mg daily in hypertensive patients uncontrolled by lisinopril 20 mg daily.
METHODS
The design and methodology for both studies were identical and are presented separately and combined, in accordance with guidelines suggested by the US Food and Drug Administration.
Patients
Eligible patients consisted of men and women ≥18 years of age with essential hypertension, characterized by a mean sitting diastolic blood pressure (DBP) of 90 mm Hg–114 mm Hg, inclusive, despite receiving treatment with lisinopril 20 mg daily for ≥4 weeks. Women of childbearing potential were required to use an effective method of birth control throughout both studies. Patients were excluded if they had secondary hypertension; mean sitting DBP ≥115 mm Hg or systolic blood pressure (SBP) ≥200 mm Hg; angina pectoris requiring more than short‐acting nitrates; hemodynamically significant valvular heart disease; coronary angioplasty within the previous 3 months; myocardial infarction; coronary bypass surgery, stroke or transient ischemic attack within the previous 6 months; history of drug or alcohol abuse within the previous 2 years; significant renal impairment (serum creatinine level >2.0 mg/dL or serum potassium level >5.0 mEq/L); significant hepatic impairment; known hypersensitivity to ARBs or ACE inhibitors; current treatment with a dosage of lisinopril >20 mg daily; or current or prior treatment with candesartan, whether alone or in combination with other medications. All patients provided written informed consent before participating in the study.
Study Design
Local Institutional Review Boards approved the protocols for both studies. After a screening period in which patients received open‐label lisinopril, patients whose BP remained uncontrolled (sitting DBP ≥90 mm Hg) and who satisfied the eligibility criteria entered an 8‐week, double‐blind phase during which they were randomized (1:1) to receive lisinopril (40 mg) alone or candesartan (16 mg increased to 32 mg) plus lisinopril (20 mg) (Figure 1). No other antihypertensive medications were permitted. In the monotherapy arm, patients received lisinopril 40 mg daily for the entire 8 weeks. Doses of lisinopril above 40 mg daily provide little additional antihypertensive activity. 16 In the combination arm, patients received candesartan 16 mg daily plus lisinopril 20 mg daily for the first 2 weeks and candesartan 32 mg daily plus lisinopril 20 mg daily for the remaining 6 weeks. The 6 weeks of treatment with candesartan 32 mg daily was expected to be sufficient to achieve the full antihypertensive effect of this agent. Doses of candesartan above 32 mg daily provide little additional antihypertensive effect. 17 , 18
Figure 1.
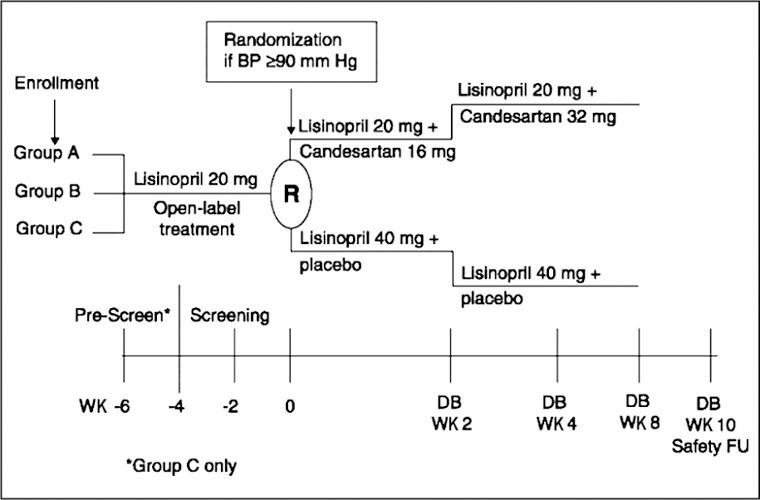
Study schematic for Studies 1 and 2. Group A patients were receiving monotherapy with lisinopril 20 mg once daily for ≥2 weeks before study entry; Group B patients had newly diagnosed hypertension or hypertension untreated for ≥30 days before study entry; Group C patients were uncontrolled or intolerant to their antihypertensive treatment before study entry. BP=blood pressure; DB=double‐blind; FU=follow‐up; WK=week
Three groups of patients entered the initial open‐label screening period (Figure 1):
-
1
• Group A. Patients receiving monotherapy with lisinopril 20 mg daily for ≥2 weeks and who met the BP eligibility criteria. These patients entered the open‐label screening period and continued to receive lisinopril 20 mg daily for 2 weeks.
-
2
• Group B. Patients with newly diagnosed hypertension or hypertension untreated for ≥30 days. These patients received 4 weeks of lisinopril 20 mg daily during the open‐label screening period.
-
3
• Group C. Patients uncontrolled or intolerant to their current antihypertensive treatment (including lisinopril combination therapies). After discontinuing all current antihypertensive medications, these patients entered a 2‐week prescreening period during which they were permitted to receive lisinopril ≤20 mg daily Thereafter, Group C patients entered the open‐label screening peri od and received lisinopril 20 mg daily for 4 weeks.
Patients visited the clinic for clinical evaluations every 2 weeks during the open‐label screening period and at Weeks 1,2,4, and 8 (or premature withdrawal) during the double‐blind phase. In addition, patients were contacted by telephone 2 weeks after the last dose of study medication for safety follow‐up information. The double‐blind study drug supplies were packaged in high‐density polyethylene bottles using a double‐dummy design. Placebo tablets were used to maintain blinding. Drug supplies were packaged to appear indistinguishable between study groups.
Lisinopril is an orally active, nonsulfhydryl ACE inhibitor that is widely used for hypertension. 16 Candesartan is a selective ARB devoid of agonist activity. 17 Insurmountable AT1 receptor blockade and long duration of activity result from distinctive AT1 receptor binding properties, such as high affinity and slow receptor dissociation rate.
Evaluations
At each clinic visit, trough (24±2 hours postdose) sitting BP measurements were performed 3 times at 2‐minute intervals using standard office mercury sphygmomanometers. The mean of three sequential BP readings (≤5‐mm Hg difference between the highest and lowest value) served as the BP determination for the visit. Patients were instructed not to take their study medication on the day of the clinic visit until after trough sitting BP measurements were obtained.
The primary efficacy measure of the antihypertensive effect was the mean change in trough sitting DBP from baseline to Week 8 of double‐blind therapy. Secondary efficacy measures of the antihypertensive effect included mean change in trough sitting SBP, the proportion of responders at Week 8 (trough sitting DBP <90 mm Hg or reduced by ≥10 mm Hg), and the proportion of controlled patients at Week 8 (trough sitting DBP <90 mm Hg and trough sitting SBP <140 mm Hg). Safety was evaluated by monitoring of adverse events, standard laboratory tests (serum chemistry, hematology, and urinalysis), physical examinations, and heart rate.
Statistical Methods
Each study planned to enroll 494 patients to ensure 370 patients completed the study, assuming an alpha of 0.05, power of 85%, and a desired detectable difference in DBP of 2.5 mm Hg with a standard deviation of 8 mm Hg (two‐tailed test). For the efficacy analyses, which are presented separately by study, the intent‐to‐treat (ITT) population included all randomized patients who received at least one dose of study medication and who had a baseline and at least one trough sitting DBP measurement during the double‐blind phase of the study. A last observation carried forward (LOCF) approach was used to impute the Week 8 values for patients who withdrew from the study before Week 8. In addition, the analyses were repeated using actual data (no carrying forward of observations). Changes in trough sitting DBP and SBP from baseline to Week 8 were analyzed by analysis of covariance (ANCOVA) with the baseline BP as the covariate, and the changes were compared using least squares means from the ANCOVA model. Ninety‐five percent confidence intervals (CIs) for the least squares mean changes from baseline and for the difference between the least squares mean changes from baseline were also calculated. Differences between the two treatment groups in rates and proportions (response and control rates) were compared by Fisher's exact test. Changes in trough sitting DBP and SBP were also analyzed by subgroups, based on race (black vs. non‐black), age (≥65 years vs. <65 years), gender, and diagnosis of diabetes using the ITT population.
The safety databases were pooled for presentation. All randomized patients who received at least one dose of study medication and who had at least one postbaseline contact with the investigational site were included in safety analyses. Although designed as two independent studies, the AMAZE program statistical analysis plans prespecified additional pooled analyses for patients with diabetes. A post hoc analysis was also conducted using BP data pooling from the two studies together using two‐way ANCOVA methods with factors treatment, study, and the interaction terms in the model.
RESULTS
Disposition
In Study 1, 74 investigational sites screened 945 patients, of whom 543 were randomized; 538 qualified for the ITT population (267 in the lisinopril group and 271 in the candesartan plus lisinopril group). One patient in the combination group was excluded from the ITT/LOCF analysis because all postbaseline values were missing, and therefore, there was not a valid observation to carry forward. Thus, the ITT/LOCF analysis included 267 patients in the monotherapy group and 270 in the combination group. A total of 240 patients (90%) in the lisinopril group and 238 (88%) in the candesartan plus lisinopril group completed the 8‐week, double‐blind period.
In Study 2, 69 investigational sites screened 852 patients, of whom 560 were randomized; 558 qualified for the ITT population (279 in the lisinopril group and 279 in the candesartan plus lisinopril group). Two patients in the monotherapy group and one patient in the combination group were excluded from the ITT/LOCF analysis because one patient had a DBP <90 mm Hg, one patient had a serum potassium value outside of previously defined parameters, and one patient had significant renal impairment. Thus, the ITT/LOCF analysis included 277 patients in the monotherapy group and 278 in the combination group. A total of 253 patients (91%) in the lisinopril group and 247 (89%) in the candesartan plus lisinopril group completed the 8‐week, double‐blind period.
Baseline Characteristics
The sample populations were very similar for the two AMAZE studies, and each study's randomization process produced treatment groups well balanced for all baseline characteristics (Table I). For Studies 1 and 2, mean ages were 54.4 years and 53.8 years; 17% and 15% were ≥65 years of age; mean weights were 205.8 lb and 206.1 lb; and mean body mass indices were 31.9 kg/m 2 and 31.8 kg/m2, respectively. In Study 1, 17% of patients were black, and in Study 2, 24% were black. The mean trough sitting SBP/DBP at baseline was also nearly identical for the two treatment groups across both studies: 148.7/96.6 mm Hg in Study 1 and 148.6/96.8 mm Hg in Study 2. There were 57 patients with diabetes in Study 1 and 47 in Study 2, all of whom had type II diabetes. For these studies, patients were classified as having diabetes if they cited a positive history for diabetes. Across both studies, the most common prior antihypertensive medications were ACE inhibitors and their combinations with diuretics (40%), calcium channel blockers and their combinations with diuretics (16%), diuretics (14%), ARBs (other than candesartan, which was an exclusion criterion) and their combinations with diuretics (11%), and β blockers and their diuretic combinations (8.6%). Compliance with the study medication was >96% in both treatment groups for both studies.
Table I.
Baseline Demographic Characteristics
| Study 1 | Study 2 | Pooled Data | ||||
|---|---|---|---|---|---|---|
| Candesartan | Candesartan | Candesartan | ||||
| Characteristic | Lisinopril (n=267) | + Lisinopril (n=271) | Lisinopril (n=279) | + Lisinopril (n=279) | Lisinopril (n=546) | + Lisinopril (n=550) |
| Age (yr) | 54.8 | 54.0 | 54.4 | 53.2 | 54.6 | 53.6 |
| Male (%) | 62 | 57 | 60 | 54 | 61 | 56 |
| Weight (lb) | 204.6 | 207.0 | 205.5 | 206.6 | 205.1 | 206.8 |
| BMI (kg/m2) | 31.6 | 32.1 | 31.8 | 31.8 | 31.7 | 32.0 |
| Race (%) | ||||||
| Black | 15 | 20 | 25 | 24 | 20 | 22 |
| Non‐black | 85 | 80 | 75 | 76 | 80 | 78 |
| Baseline SBP/DBP (mm Hg) | 148.0/96.7 | 149.3/96.5 | 148.9/97.0 | 148.3/96.5 | 148.5/96.9 | 148.8/96.5 |
| BMI=body mass index; DBP=diastolic blood pressure; SBP=systolic blood pressure | ||||||
Efficacy
In Study 1, DBP, the primary efficacy measure, declined with both treatments: 5.90 mm Hg (95% confidence interval [CI], ‐7.26 to ‐4.55 mm Hg) with lisinopril monotherapy and 8.29 mm Hg (95% CI, ‐9.63 to ‐6.95 mm Hg) with candesartan plus lisinopril. This was a significant difference of 2.39 mm Hg (95% CI, ‐3.86 to ‐0.92 mm Hg; p<0.01). In Study 2, DBP also declined with both treatments: 6.24 mm Hg (95% CI, ‐7.50 to ‐4.98 mm Hg) with lisinopril monotherapy and 7.44 mm Hg (95% CI, ‐8.67 to ‐6.22 mm Hg) with candesartan plus lisinopril. This was a difference of 1.21 mm Hg (95% CI, ‐2.57 to 0.16 mm Hg; p=0.08). Figure 2A and Table II present the least squares mean changes in trough sitting DBP from baseline to Week 8.
Figure 2.
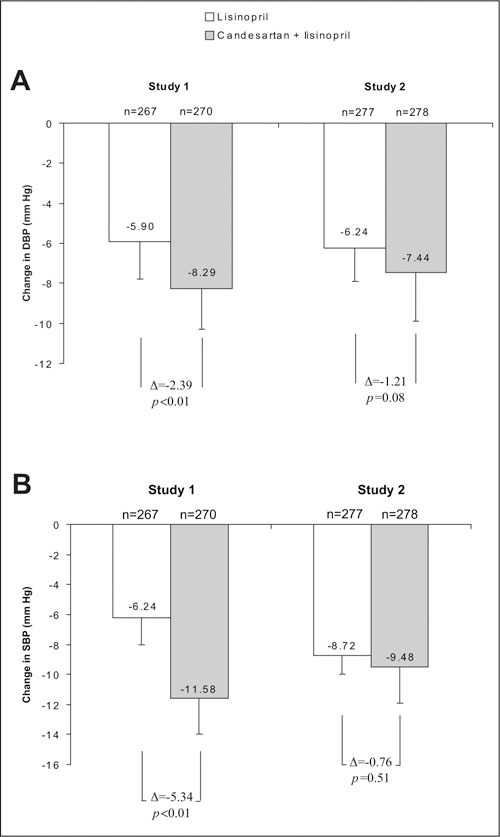
Least squares mean changes in trough sitting diastolic blood pressure (DBP) (A) and trough sitting systolic blood pressure (SBP) (B) from baseline to Week (WK) 8 in Studies 1 and 2. Results shown are for the intent‐to‐treat analysis (last observation carried forward). Bars represent standard error.
Table II.
Summary of Efficacy Results at Week 8
| Study 1 | Study 2 | Pooled Data | ||||
|---|---|---|---|---|---|---|
| Candesartan | Candesartan | Candesartan | ||||
| Characteristic | Lisinopril (n=267) | + Lisinopril (n=270) | Lisinopril (n=277) | + Lisinopril (n=278) | Lisinopril (n=544) | + Lisinopril (n=548) |
| Least squares mean change in SBP/DBP (mm Hg) | ‐6.24/‐5.90 | ‐11.58†/‐8.29† | ‐8.72/‐6.24 | ‐9.48/‐7.44 | ‐7.24/‐6.49 | ‐10.37†/‐8.21† |
| Responder (n [%])* | 134 (50.2) | 159 (58.9)ɛ | 158 (57.0) | 169 (60.8) | 292 (53.7) | 328 (59.9) |
| Controlled (n [%]) | ||||||
| DBP <90 mm Hg | 126 (47.2) | 144 (53.3) | 143 (51.6) | 158 (56.8) | 269 (49.4) | 302 (55.1) |
| SBP <140 mm Hg | 127 (47.6) | 148 (54.8) | 146 (52.7) | 154 (55.4) | 273 (50.2) | 302 (55.1) |
| SBP/DBP <140/<90 mm Hg | 90 (33.7) | 114 (42.2)ɛ | 111 (40.1) | 120 (43.2) | 201 (36.9) | 234 (42.7) |
| All blood pressure measurements were obtained at trough in the sitting position. *DBP <90 mm Hg or reduced by ≥10 mm Hg; † p<0.01 vs. lisinopril monotherapy; ɛ p=;0.05 vs. lisinopril monotherapy | ||||||
In Study 1, for SBP, the least squares mean change from baseline to Week 8 was ‐6.24 mm Hg (95% confidence interval [CI], ‐8.53 to ‐3.95 mm Hg) with lisinopril monotherapy and ‐11.58 mm Hg (95% CI, ‐13.84 to ‐9.32 mm Hg) with candesartan plus lisinopril, a statistically significant difference (‐5.34 mm Hg [95% CI, ‐7.82 to ‐2.85 mm Hg]; p<0.01). In Study 2, for SBP, the least squares mean change from baseline to Week 8 was ‐8.72 mm Hg (95% CI, ‐10.82 to ‐6.62 mm Hg) with lisinopril monotherapy and ‐9.48 mm Hg (95% CI, ‐11.51 to ‐7.45 mm Hg) with candesartan plus lisinopril, a difference of ‐0.76 mm Hg (95% CI, ‐3.03 to 1.50 mm Hg; p=0.51). Figure 2B and Table II present the least squares mean changes in trough sitting SBP from baseline to Week 8.
Figure 3 illustrates mean DBP levels over the 8‐week, double‐blind period. Most of the decline in DBP occurred in the first 1–2 weeks, and the difference in BP reduction in the direction favoring candesartan plus lisinopril was evident throughout the treatment periods. A similar pattern was observed for SBP (data not shown).
Figure 3.
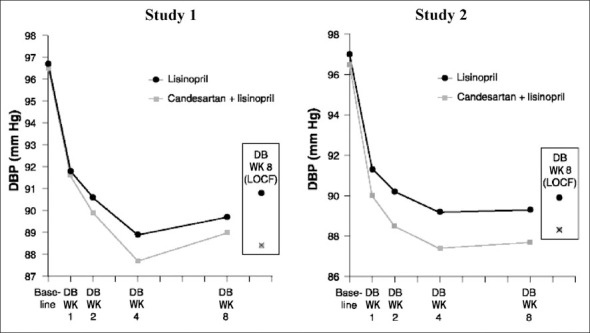
Mean trough sitting diastolic blood pressure (DBP) over time in Studies 1 and 2. Results shown at baseline and Weeks 1, 2, and 4 are for the intent‐to‐treat analysis (without carrying forward observations). At Week (WK) 8, results are shown for both intent‐to‐treat analyses (without carrying forward observations and last observation carried forward [LOCF]). DB=double‐blind
The response and control rates in both studies reflected the findings for mean change in BP, i.e., the rates were slightly greater for the combination treatment and these were statistically significant in Study 1 for the percentage of responders (DBP <90 mm Hg or reduced by ≥10 mm Hg) and the percentage of patients whose BP was controlled at <140/<90 mm Hg. Table II presents the response and control rates for both studies.
As shown in Figure 4, analysis of antihypertensive efficacy by subpopulations suggests that response to the treatments was in the same direction as the overall population, i.e., in the direction favoring combination treatment. For DBP, the only exception included black patients who exhibited greater BP reduction with lisinopril monotherapy in Study 1. Results were similar when SBP was analyzed by the same subgroups. Of note is the observation that, as a group, black patients tended to exhibit a somewhat lesser degree of BP lowering than non‐black patients, while patients with diabetes tended to exhibit greater BP reductions than patients without diabetes.
Figure 4.
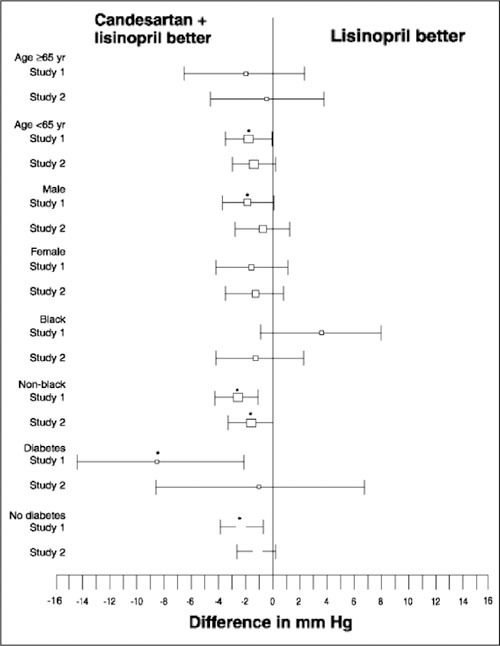
Subpopulation analyses. Differences in least squares mean changes in trough sitting diastolic blood pressure from baseline to Week 8 in Studies 1 and 2. Results shown are for the intent‐to‐treat analysis (without carrying forward observations). Point estimate boxes are approximately proportional to sample sizes; bars represent 95% confidence intervals; *p≤0.05, condensation and lisinopril vs. lisinopril monotherapy
For both studies, repeated primary analyses based on the ITT population with no imputations on missing values and a per‐protocol population (ITT population minus patients with significant protocol violations and poor compliance) were consistent with the primary analyses based on the ITT/LOCF population. Analyses based on patient screening group were also consistent with the overall results, except for Group B (newly diagnosed hypertension or patients off treatment for ≥30 days). For Group B patients in Study 1, lisinopril monotherapy was more effective than the combination by 1.43 mm Hg for DBP (p=041), while in Study 2 the combination was substantially better (DBP difference of 4.22 mm Hg, p=0.01).
Pooled Efficacy in Patients With Diabetes
For the 103 diabetic patients included in the ITT/LOCF analysis in the two studies (one patient had no post‐baseline BP measurements), BP declined by 6.68/6.37 mm Hg with lisinopril monotherapy and 11.88/8.94 mm Hg with candesartan plus lisinopril. The difference of 5.20/2.57 mm Hg in favor of combination treatment was not statistically significant (p=0.07/p=0.15) in this small sample size. Microalbuminuria was not assessed in these studies; however, semiquantitative urinary protein levels were recorded by study sites (dipstick method). Only 18 patients with diabetes had demonstrable urinary protein at the baseline visit, and there was no apparent change in the urinary protein distributions in the small diabetic patient group.
Pooled Safety
The safety findings in the two studies were similar, as were the safety findings in the two treatment groups. The most commonly reported adverse events were respiratory infection (6.2% lisinopril, 8.5% combination), headache (6.2%, 4.4%), cough (4.4%, 5.3%), and dizziness (4.2%, 5.6%) (Table III). Serious adverse events occurred in eight patients in the monotherapy group (1.5%) and in six patients in the combination group (1.1%). Only one serious adverse event, angioedema after 11 days of double‐blind lisinopril monotherapy, was considered possibly related to study medication. One death occurred during double‐blind therapy with candesartan plus lisinopril, which the investigator attributed to acute cocaine toxicity. Adverse events resulted in the discontinuation of 20 patients (3.7%) in the lisinopril group and 33 patients (6.0%) in the candesartan plus lisinopril group. Cough contributed to the discontinuation of five patients in the monotherapy group and six patients in the combination group, dizziness in one and six patients, respectively, and hyperkalemia in zero and four patients, respectively. Eleven patients in the lisinopril group and nine in the candesartan plus lisinopril group experienced hyperkalemia (defined as a serum potassium level of ≥6.0 mEq/L), had hyperkalemia reported as an adverse event, or were withdrawn from the study because of hyperkalemia. No patient experienced any apparent adverse consequences of hyperkalemia.
Table III.
Most Common Adverse Events Across Both Studies
| Adverse Event | Lisinopril (n=546) | Candesartan+ Lisinopril (n=550) |
|---|---|---|
| Respiratory infection | 35 (6.4) | 47 (8.5) |
| Headache | 36 (6.6) | 25 (4.5) |
| Cough | 26 (4.8) | 32 (5.8) |
| Dizziness | 23 (4.2) | 31 (5.6) |
| Peripheral edema | 15 (2.7) | 18 (3.3) |
| Rhinitis | 17 (3.1) | 13 (2.4) |
| Values presented are number (%) of patients reporting the adverse event | ||
DISCUSSION
The large AMAZE program sought to determine whether adding the ARB candesartan to lisinopril provided better BP control than up‐titration of lisinopril in hypertensive patients inadequately controlled with lisinopril 20 mg daily. Both treatments induced an additional decline in trough BP. In Studies 1 and 2, the combination of candesartan plus lisinopril reduced BP by 11.6/8.3 mm Hg and 9.5/7.4 mm Hg, respectively, compared with lisinopril 40 mg daily, which lowered BP by 6.2/5.9 mm Hg and 8.7/6.2 mm Hg, respectively. When all data from both studies were pooled, post hoc analysis indicated a somewhat greater BP reduction with the candesartan/lisinopril combination (3.1/1.7 mm Hg; 95% CI, ‐4.8 to ‐1.5 systolic and ‐2.8 to ‐0.7 mm Hg diastolic). A major finding of the AMAZE program was the safety and tolerability of both regimens. The frequency of adverse events was also similar with lisinopril or candesartan, but it is important to emphasize that the study design eliminated patients unable to tolerate lisinopril 20 mg daily. Hyperkalemia, a potential concern for the combination treatment arm, was highly unusual, and probably due to the fact that the study population was essentially free of significant renal impairment.
The greater BP reduction with combination treatment in the AMAZE program is consistent with previous observations with ACE inhibitor/ARB combination therapy in smaller, open‐label studies, 7 , 11 , 12 but none of these studies has proven definitively that the ACE inhibitor/ARB combination is superior for BP reduction to either drug used alone. Ultimately, proof of the potential superiority of any combination over its individual components is critically dependent on the use of maximal doses of each component. Such studies (often 10‐fold above maximum recommended doses) have only been done in animals. Thus, higher doses of lisinopril or candesartan may have achieved a greater degree of RAAS blockade. The maximum approved daily doses of lisinopril and candesartan are 80 mg and 32 mg, respectively. The 40 mg lisinopril dose was based in part on the observation that clinicians do not usually prescribe lisinopril in doses higher than 40 mg daily.
A potential rationale for the combination of ACE inhibitor and ARB is the belief that plasma angiotensin II levels return toward baseline values during long‐term ACE inhibitor therapy, yet there is very little evidence of “ACE escape” in the literature. One very small study in hypertension found that plasma angiotensin II levels returned toward baseline several months after initiation of 20 mg enalapril twice daily in nine subjects, five of whom were on diuretic therapy, 19 but used an assay that had significant cross‐reactivity with angiotensinogen and angiotensin I (which increase substantially during chronic ACE inhibition). In contrast, a recent small study in heart failure found persistent suppression of plasma angiotensin II during chronic ACE inhibition. 20 With respect to ischemic heart disease, addition of the ARB valsartan to the ACE inhibitor benazepril did not improve postinfarction outcomes. 21 In contrast, AMAZE results are in agreement with those of several recent studies in patients with diabetic and nondiabetic renal disease, where dual RAAS blockade with moderate doses of ACE inhibitors and ARBs provided superior BP control and greater antiproteinuric effects than either agent alone. 13 , 14 , 15 , 22 , 23 , 24 Such results should be anticipated in studies using submaximal doses of RAAS blockers, however. Although the presence of non‐ACE pathways for angiotensin II production has been established in some tissues, 6 , 25 , 26 the clinical significance of these pathways remains unclear. It also remains possible that different racial groups may respond differently to these drugs used alone or in combination due to potential differences in phenotypic expression of various “non‐ACE” or “non‐AT1” pathways.
Other considerations also deserve mention. All patients had prerandomization experience with lisinopril and demonstrated both tolerance and a degree of resistance to its BP lowering effects; there was no similar pretrial exposure to candesartan. The double‐blind lisinopril monotherapy dose of 40 mg was administered for the entire 8 weeks, whereas the full dose of candesartan (32 mg) was administered for only 6 weeks. Another limitation was the lack of a placebo arm, which would have allowed the magnitude of any drug‐specific effect to be expressed as a fraction of the total BP decline. Regression to the mean remains a consideration in all such trials, but the magnitude of the observed BP reductions in these studies was similar to the magnitude of BP reductions with ARBs in placebo‐controlled trials that were not restricted to patients “resistant” to ACE‐inhibitor treatment. 27
Clinical application of the AMAZE results must be tempered by several caveats. First, as stated in JNC 7, usual clinical practice should include addition of a thiazide diuretic to either agent before consideration of the lisinopril‐candesartan combination. 1 Nevertheless, the AMAZE results indicate clearly that when trough BP is not well controlled on lisinopril 20 mg daily, increased RAAS blockade is clinically useful because small reductions in mean BP usually represent a reduction in overall population risk. Vigorous BP reduction is also of particular importance in individuals with BP elevations >20/10 mm Hg above ideal target values (>160/100 mm Hg in uncomplicated hypertension or >150/90 mm Hg in patients with diabetes or chronic kidney disease as per JNC 7 guidelines). 1 In AMAZE, the 84 individuals whose BP values exceeded 160/100 mm Hg achieved a BP decline at 8 weeks of 13.7/‐6.1 mm Hg with combination therapy and 13.5/‐6.1 mm Hg with lisinopril up‐titration. In the diabetic subpopulation, the BP reductions at 8 weeks in the combination therapy group (11.9/8.9 mm Hg) were substantial and tended to be greater than the corresponding reductions in the lisinopril group (6.7/6.4 mm Hg).
In conclusion, for the reduction of BP in hypertensive patients not controlled by lisinopril 20 mg daily, adding candesartan (16 mg daily, up‐titrated to 32 mg daily) is an alternative to increasing the lisinopril dose to 40 mg daily. Both antihypertensive regimens are effective and well tolerated, and the combination treatment appears to have no unique adverse safety findings relative to the individual drugs. Whether dual ACE inhibitor/ARB treatment is particularly useful in prevention of target organ damage or in antihypertensive therapy for subpopulations of patients remains to be tested.
Acknowledgments: The authors thank Rebecca Wang, MD, for her help in protocol development. AMAZE Invesigators:
Study 1
Abbott, Richard, Rochester, NY; Ahmad, Suhail, Seattle, WA; Allison, Richard, Columbia, SC; Altschuller, Alexander, North Dartmouth, MA; Azad, Habib, Orange, CA; Bakris, George, Chicago, IL; Barri, Yousri, Little Rock, AR; Bilazarian, Seth, Haverhill, MA; Bleser, Scott, Bellbrook, OH; Bloch, Michael, Sparks, NV; Brose, John, Athens, OH; Buell, James, San Antonio, TX; Cabezas‐Mijuste, Maritza, Carolina, PR; Cattan, Rogelio, Miami, FL; Corder, Clinton, Oklahoma City, OK; Corn, Lydia, Sarasota, FL; Cronin, Robert, Dallas, TX; Crouse, Linda, Kansas City, MO; Dachman, William, Mesa, AZ; Desai, Vikas, Natick, MA; Dworkin, Lance, Providence, RI; Fried, David, Warwick, RI; Garland, W. Thomas, Lawrenceville, NJ; Gillie, Edward, Fort Myers, FL; Gove, Ronald, Linwood, NJ; Greely, Thomas, Concord, CA; Hampsey, James, Clearwater, FL; Harris, H. Freeman, Lake Oswego, OR; Izzo, Joseph, Buffalo, NY; Jabro, Mark, San Diego, CA; Jackson, Bruce, Redondo Beach, CA; Kerwin, Edward, Medford, OR; Kopyt, Nelson, Allentown, PA; Kushner, Pamela, Long Beach, CA; LeLevier, Jon Anton, Vista, CA; Levinson, Lawrence, Altoona, PA; Lindenfeld, JoAnn, Denver, CO; Marcadis, Abe, Boyton Beach, FL; McCluskey, Dennis, Mogadore, OH; Mihalik, John, Cloverdale, CA; Miller, Michael, Baltimore, MD; Montoro, Rafael, Coral Gables, FL; Munger, Mark, Salt Lake City, UT; Narayan, Puneet, Springfield, VA; Ostrowsky, Avi, Las Vegas, NV; Ou, Kuang, Pittsburgh, PA; Papademetriou, Vasilios, Washington, DC; Patron, Andres, Hollywood, FL; Pettyjohn, Frank, Mobile, AL; Phillips, Robert, New York, NY; Preston, Richard, Miami, FL; Reisin, Efrain, New Orleans, LA; Rogers, Brenda, Kansas City, MO; Rosanwo, Ayodeji, Fort Wayne, IN; Roth, Eli, Cincinnati, OH; Rothschild, Henry, New Orleans, LA; Saponaro, Joseph, Jupiter, FL; Schultz, Roger, Houston, TX; Settipane, Russell, Providence, RI; Shane, Louis, White Plains, NY; Slabic, Stan, Erie, PA; Smith, Ronald, Winston‐Salem, NC; Timm, Nicholas, South Bend, IN; Truitt, Timothy, Melbourne, FL; Vidt, Donald, Cleveland, OH; Vijayaraghavan, Kris, Phoenix, AZ; Weisman, Gilbert, Warminster, PA; Weitzman, Simon, Needham, MA; White, William, Farmington, CT; Winer, Nathaniel, Brooklyn, NY; Zaharowitz, Herman, St. Petersburg, FL; Zeig, Steven, Pembroke Pines, FL; Zervos, Marcus, Royal Oak, MI.
Study 2 Abdelghany, Amin, Panama City, FL; Alwine, Lawrence, Downingtown, PA; Angls, Luis, Mission, KS; Bordenave, Kristine, Albuquerque, NM; Borge, Aleyda, Cooper City, FL; Buchanan, Patricia, Eugene, OR; Calhoun, David, Birmingham, AL; Captain, Anthony, Marietta, GA; Chandler, Bronell, Upper Darby, PA; Cheung, Deanna, Long Beach, CA; Chrysant, Steven, Oklahoma City, OK; Conrad, James, Sellersville, PA; Corser, Bruce, Cincinnati, OH; Crock, Ronald, Canton, OH; Cushman, William, Memphis, TN; Denny, D. Marty, Jeffersonville, IN; Doig, David, Denver, CO; Eelani, Frood, Fort Worth, TX; Ellis, Jonathan, Abington, MA; Ferrer, Bonafacio, Parma, OH; Gilderman, Larry, Pembroke Pines, FL; Glasser, Stephen, Tampa, FL; Gradman, Alan, Pittsburgh, PA; Graff, Alan, Fort Lauderdale, FL; Hargrove, Joe, Little Rock, AR; Hejeebu, Srini, Toledo, OH; Jack, David, Sandy, UT; Katz, Lois Anne, New York, NY; Kipperman, Robert, Oklahoma City, OK; Kloner, Robert, Los Angeles, CA; Kozinn, Marc, Williamsville, NY; Ladson‐Wofford, Stephanie, Columbus, OH; Lasseter, Kenneth, Miami, FL; Lillestol, Michael, Fargo, ND; London, Diane, Natick, MA; Marshall, Edward, Encino, CA; Mroczek, William, Falls Church, VA; Myers, Edward, Warren, OH; Neutel, Joel, Orange, CA; Okusa, Mark, Charlottesville, VA; Owens, Douglas, Greer, SC; Passer, Jeffrey, Omaha, NE; Pitterman, Arthur, Las Vegas, NV; Plevin, Sanford, New Port Richey, FL; Pohl, Stephen, Lexington, KY; Pool, James, Houston, TX; Rankin, Bruce, DeLand, FL; Raval, Pramod, Oak Park, MI; Reif, Max, Cincinnati, OH; Rice, Lucian, Asheville, NC; Ripley, Elizabeth, Richmond, VA; Shanes, Jeffrey, Melrose Park, IL; Shettigar, Udipi, Bay Pines, FL; Stoukides, John, East Providence, RI; Tonkon, Melvin, Santa Ana, CA; Wagner, Margaret, Idaho Falls, ID; Waser, Gregory, Melbourne, FL; Weerasinghe, Mervyn, Rochester, NY; Weinberg, Marc, Providence, RI; Weinberger, Myron, Indianapolis, IN; Weiss, Daniel, Mentor, OH; Williams, Kenneth, Baltimore, MD; Wofford, Marion, Jackson, MS; Yarows, Steven, Chelsea, MI.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Borghi C, Ambrosioni E. Evidence‐based medicine and ACE inhibition. J Cardiovasc Pharmacol. 1998;32(suppl 2):S24–S35. [DOI] [PubMed] [Google Scholar]
- 3. Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. [DOI] [PubMed] [Google Scholar]
- 4. Bosch J, Yusuf S, Pogue J, et al. Heart outcomes prevention evaluation. Br Med J. 2002;324:699–702. 11909785 [Google Scholar]
- 5. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 6. Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–392. [DOI] [PubMed] [Google Scholar]
- 7. Weinberg MS, Weinberg AJ, Zappe DH. Effectively targeting the renin‐angiotensin‐aldosterone system in cardiovascular and renal disease: rationale for using angiotensin II receptor blockers in combination with angiotensin‐converting enzyme inhibitors. J Renin Angiotensin Aldosterone Syst. 2000;1:217–233. [DOI] [PubMed] [Google Scholar]
- 8. Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin‐converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–242. [DOI] [PubMed] [Google Scholar]
- 9. Linz W, Wiemer G, Gohlke P, et al. Contribution of kinins to the cardiovascular actions of angiotensin‐converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 10. Mazzolai L, Burnier M. Comparative safety and tolerability of angiotensin II receptor antagonists. Drug Saf. 1999;21:23–33. [DOI] [PubMed] [Google Scholar]
- 11. Schulte KL, Fischer M, Lenz T, et al. Efficacy and tolerability of candesartan cilexetil monotherapy or in combination with other antihypertensive drugs: results of the AURA study (ATACAND Under Real‐Life Aspects). Clin Drug Invest. 1999;18:453–460. [Google Scholar]
- 12. Weir MR, Weber MA, Neutel JM, et al., for the ACTION study investigators. Efficacy of candesartan cilexetil as addon‐therapy in hypertensive patients uncontrolled on background therapy: a clinical experience trial. Am J Hypertens. 2001;14:567–572. [DOI] [PubMed] [Google Scholar]
- 13. Berger ED, Bader BD, Ebert C, et al. Reduction of proteinuria; combined effects of receptor blockade and low dose angiotensin‐converting enzyme inhibition. J Hypertens. 2002;20:739–743. [DOI] [PubMed] [Google Scholar]
- 14. Kincaid‐Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant. 2002;17:597–601. [DOI] [PubMed] [Google Scholar]
- 15. Rossing K, Christensen PK, Jensen BR, et al. Dual blockade of the renin‐angiotensin system in diabetic nephropathy. Diabetes Care. 2002;25:95–100. [DOI] [PubMed] [Google Scholar]
- 16. Zestril (lisinopril) prescribing information. In:Physicians' Desk Reference. Montvale , NJ : Thomson PDR; 2003:691–695. [Google Scholar]
- 17. Atacand (candesartan cilexetil) prescribing information. In:Physicians' Desk Reference. Montvale , NJ : Thomson PDR;2003:594–596. [Google Scholar]
- 18. Weinberg MS, Weinberg AJ, Cord R, et al. The effect of high‐dose angiotensin II receptor blockade beyond maximal recommended doses in reducing urinary protein excretion. J Renin Angiotensin Aldosterone Syst. 2001;2(suppl 1):S196–S198. [DOI] [PubMed] [Google Scholar]
- 19. Biollaz J, Brunner HR, Gavras I, et al. Antihypertensive therapy with MK 421: angiotensin II‐renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol. 1982;4:966–972. [PubMed] [Google Scholar]
- 20. Grassi G, Cattaneo BM, Seravalle G, et al. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. 1997;96:1173–1179. [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 22. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin‐angiotensin system in patients with hypertension, microalbuminuria, and non‐insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. Br Med J. 2000;321:1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lun̈o J, Barrio V, Goicoechea MA, et al. Effects of dual blockade of the renin‐angiotensin system in primary proteinuric nephropathies. Kidney Int. 2002;62(suppl 82): S47–S52. [DOI] [PubMed] [Google Scholar]
- 24. Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117–124. [DOI] [PubMed] [Google Scholar]
- 25. Juillerat L, Nussberger J, Menard J, et al. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension. 1990;16:564–572. [DOI] [PubMed] [Google Scholar]
- 26. Urata H, Kinoshita A, Misono KS, et al. Identification of a highly specific chymase as the major angiotensin II‐forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 27. Conlin PR, Spence JD, Williams B, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens. 2000;13:418–426. [DOI] [PubMed] [Google Scholar]


