Abstract
Calcium channel blockers (CCBs) comprise a heterogeneous group of compounds with unique structures and pharmacologic characteristics. These agents are employed in the treatment of hypertension, coronary ischemia, and/or supraventricular arrhythmias. CCBs are both substrates for, and in the instance of verapamil and diltiazem inhibitors of, cytochrome P450 3A4. In the case of verapamil and diltiazem, this inhibitory effect increases the likelihood of drug‐drug interactions with other compounds similarly metabolized by cytochrome P450 3A4. Much of the debate with reference to a cardiovascular risk for CCBs has been quieted with the advent of sustained‐release delivery systems that offer a more gradual rate of drug delivery. The most common side effects with CCBs are vasodilatory in nature and include peripheral edema, flushing, and headache. Despite the potential for side effects with CCBs, their potent blood pressure‐lowering effect makes them a prerequisite for blood pressure control in many patients.
Ten calcium channel blockers (CCBs) are currently marketed in the United States. These agents are employed in the treatment of hypertension, angina, and/or supraventricular arrhythmias. Nimodipine is approved only for short‐term use in patients having experienced a subarachnoid hemorrhage. Diltiazem, nicardipine, and verapamil are the only CCBs currently available in IV formulations. Long‐term treatment with CCBs is typically by the oral route and long‐acting CCBs are the preferred mode of therapy in the treatment of hypertension and/or angina when a CCB is felt to be indicated (Table I). 1
Table I.
Dosing Information for Calcium Channel Blockers Currently Marketed in the United States
| Drug | Proprietary Name | Indications Approved | Form; Strength; Dose | Time to Peak Effect (h) | Elimination Half‐Life (h) | Comments |
|---|---|---|---|---|---|---|
| Amlodipine | Norvasc (Pfizer Inc., New York, NY) | Hypertension; chronic, stable, and vasospastic angina | Tablet; 2.5, 5, 10 mg; once daily | 6–12 | 30–50 | No effect of grapefruit juice on pharmacokinetics; available in fixed‐dose combinations with benazepril (Lotrel; Novartis Pharmaceutical Corporation, East Hanover, NJ) or atorvastatin (Caduet; Pfizer Inc., New York, NY). |
| Bepridil | Vascor (Ortho‐McNeil Pharmaceutical, Inc., Raritan, NJ) | Refractory angina | Tablet; 200, 300, 400 mg; once daily | 2–3 | 26–64 | Can cause torsade de pointes‐type ventricular tachycardia. Does not lower blood pressure. |
| Diltiazem | Tiazac (Forest Laboratories Inc., New York, NY); Cardizem CD, LA (Biovail Pharmaceuticals, Inc., Morrisville, NC); Cartia XT (Andrx Laboratories, Hackensack, NJ) Dilacor XR (Watson Laboratories, Inc., Corona, CA) | Hypertension; chronic, stable, and vasospastic angina; atrial fibrillation or flutter; paroxysmal supraventricular tachycardia | Immediate‐release (IR), controlled release (CR) and IV forms; 180–540 mg; once daily | 0.5–1.5 (IR); 6–11 (SR) | 2–5 (IR); 2.5 (SR) | Contraindicated in patients with sick‐sinus syndrome and 2nd or 3rd degree atrioventricular block. Cardizem LA can be given at bedtime. Known inhibitor of CYP3A4; as such it slows metabolism of drugs such as simvastatin, cyclosporine, and erythromycin. |
| Felodipine | Plendil (AstraZeneca Pharmaceuticals LP, Wilmington, DE) | Hypertension | CR;2.5, 5, 10 mg; once daily | 2.5–5 | 11–16 | Grapefruit juice causes a two‐fold or more increase in felodipine bioavailability; grapefruit juice effect may last 24–72 hours; available as a fixed‐dose combination with enalapril (Lexxel; AstraZeneca Pharmaceuticals LP, Wilmington, DE). |
| Isradipine | Dynacirc CR (Reliant Pharmaceuticals, Liberty Corner, NJ) | Hypertension | Tablet; 2.5, 5 mg; once daily | 1.5 | 8–12 | Edema rate is low compared with other calcium channel blockers. |
| Nicardipine | Cardene (Roche Pharmaceuticals, Nutley, NJ) | Hypertension, angina | IR tablet; 20, 30 mg; three times daily | 0.5–2.0 | 8 | Available in an IV form for hypertension treatment. As effective as nitroprusside for hypertensive emergencies. |
| Nifedipine | Adalat (Bayer Pharmaceuticals, Pittsburgh, PA); Procardia XL (Pfizer Inc., New York, NY) | Hypertension, angina | Dose varies based on indication; CR capsule; 30, 60, 90 mg; once daily | 0.5 | 2 | IR capsules are no longer used for the control of essential hypertension or for the acute reduction of blood pressure. |
| Nimodipine | Nimotop (Bayer Pharmaceuticals, Pittsburgh, PA) | Subarachnoid hemorrhage | Gelatin capsule; 60 mg; every 4 hours for 21 days | 1 | 1–2 | Crosses the blood‐brain barrier readily; oral therapy should commence within 96 hours of the subarachnoid hemorrhage. |
| Nisoldipine | Sular (First Horizon Pharmaceutical Corporation, Alpharetta, GA) | Hypertension | SR tablet; 10,20, 30, 40 mg; once daily | 6–12 | 7–12 | Grapefruit juice significantly increases nisoldipine bioavailability. |
| Verapamil | Calan, Calan SR (GD Searle LLC, New York, NY); Covera HS (GD Searle LLC, New York, NY); Verelan PM (Elan Biopharmaceuticals, San Diego, CA) | Hypertension; angina atrial fibrillation or flutter; paroxysmal supraventricular tachycardia | IR tablet: dose varies based on indication; SR products: 120–360 mg; once daily | 0.5–1.0 (IR); 4–6 (SR) | 4.5–12 (IR); 4.5–12 (CR) | Nocturnally indicated forms of verapamil available with early morning peak action. |
| CYP3A4=cytochrome P450 3A4 | ||||||
CLASS HETEROGENEITY
CCBs are a heterogeneous group of compounds, with distinctive structures and pharmacologic properties. There are three distinct subclasses of CCBs, which explain the differences observed with these agents. These subclasses are the phenylalkylamines (e.g., verapamil), the benzothiazepines (e.g., diltiazem) and the dihydropyridines (DHPs) (e.g., nife‐dipine, amlodipine, isradipine). All available CCBs are vasodilators—and therein lies their ability to reduce blood pressure (BP). The relative potency of CCBs as vasodilators varies, with DHP‐type compounds such as nifedipine viewed as the most potent subclass, with verapamil, diltiazem, and bepridil being comparably less potent.
In vitro, several calcium antagonists (e.g., nifedipine, nisoldipine, and isradipine) bind with some selectivity to the L‐type calcium channel present in blood vessels, whereas verapamil binds equally well to cardiac and vascular L‐type calcium channels. 2 , 3 The applicability of these in vitro findings to treatment response in humans remains ill defined. In vitro, all CCB subclasses both depress sinus‐node activity and slow atrioventricular (AV) conduction. Only verapamil and diltiazem delay AV conduction or cause sinus‐node depression at doses clinically in common use. 1 In this regard, diltiazem (and less so verapamil) are used intravenously and orally for acute and chronic rate control, respectively, in patients with atrial fibrillation and normal left ventricular function (Tables I and II). 4 , 5
Table II.
Hemodynamic Effects of Calcium Channel Blockers
| Effect | Phenylalkylamines (Verapamil) | Benzothiazepines (Diltiazem) | Dihydropyridines |
|---|---|---|---|
| Peripheral vasodilation | ↓ | ↔↓ | ↓↓ |
| Coronary vascular resistance | ↓ | ↓ | ↓↓ |
| Myocardial contractility | ↓↓ | ↓ | ↔↓ |
| Cardiac output | variable | variable | ↔↓ |
| Heart rate | ↑ acute;↓ chronic | ↓ | ↓ acute; ↔ chronic |
| Atrioventricular conduction | ↓ | ↓ | ↔ |
| ↓=Decrease; ↔=neutral effect; ↑=increase |
Similarly, all CCB subclasses exhibit a concentration‐dependent negative inotropic effect in vitro, but only verapamil and diltiazem do so in vivo. The disparities between the in vitro and in vivo effects may relate, in part, to the sympathetic activation triggered by DHP‐induced vasodilation, which blunts any direct negative chronotropic and inotropic effects; however, there is active debate as to whether DHP CCBs activate the sympathetic nervous system in all instances.
In this regard, in animal studies, direct central administration of DHPs such as nifedipine or amlodipine lowers sympathetic nerve activity and thereby BP. At low rates of peripheral administration nifedipine or amlodipine gradually accumulate in the central nervous system, lower sympathetic nerve activity, and thereby reduce BP by a complementary mechanism to direct arterial vasodilation. In addition, hypertensive subjects treated with long‐acting DHPs, wherein minimal arterial baroreflex activation occurs, have demon‐strably lower sympathetic activity (as assessed by plasma norepinephrine), but in other instances have increased sympathetic activity (as assessed by plasma norepinephrine or microneurography). This sympathoexcitatory response may be due to activation of the renin‐angiotensin system, particularly at higher doses. 6
PHARMACOKINETIC, DRUG‐DRUG, AND NUTRITIONAL INTERACTIONS
As a class, the CCBs share a number of common pharmacokinetic characteristics. In general, the volume of distribution, protein binding, and plasma half‐life of CCBs are comparable in normal renal function subjects, chronic kidney disease patients, and end‐stage renal disease subjects, with a few notable exceptions (Figure I). 7 One such exception is nicardipine—where hepatic metabolism and therein plasma clearance—is decreased in chronic kidney disease patients when compared with normal subjects. 8 , 9 Of note, this decrease in plasma clearance is corrected by hemodialysis. 8 Aging is associated with a significant reduction in the clearance of many CCBs and is at least one of the reasons why drugs in this class are started at lower doses in elderly hypertensive patients. 7 , 10
Figure 1.
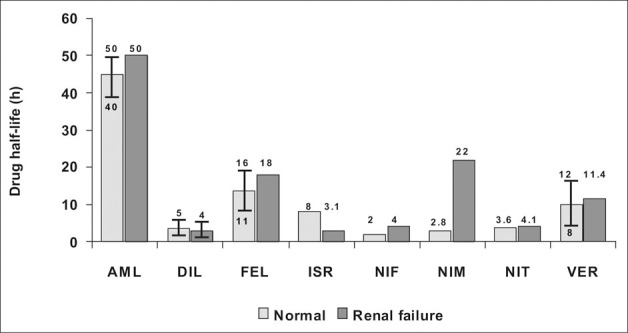
Drug half‐life for calcium channel blockers in the presence of renal failure. AML=amlodipine; DlL=diltiazem; FEL=felodipine; lSR=isradipine; NIF=nifedipine; NIM=nimodipine; NIT=nitrendipine; VER=verapamil
The cytochrome P450 system dictates both the presystemic and systemic clearance of CCBs, with cytochrome P450 3A4 (CYP3A4) being most prominently involved in this process. 11 All CCBs are substrates for CYP3A4, with only verapamil and diltiazem inhibiting the activity of this P450 isozyme as well. This ability of verapamil and diltiazem to inhibit CYP3A4 is of the greatest importance for compounds that share this metabolic pathway. The drug‐drug interactions that arise from the co‐administration of verapamil or diltiazem with other known substrates for CYP3A4 are predominantly pharmacokinetic, with plasma concentrations of the alternative CYP3A4 substrate typically rising.
3‐hydroxy‐3‐methylglutaryl A reductase inhibitors or statins are widely used drugs for the treatment of hyperlipidemia. Many of the drugs in this class share the CYP3A4 metabolic pathway with CCBs, and adverse drug‐drug interactions have occurred with co‐administration of these drugs with either verapamil or diltiazem. Verapamil and diltiazem inhibit the metabolism of both lovas‐tatin 12 and simvastatin, 13 , 14 which increases the risk of myotoxicity (including rhabdomyolysis), a concentration‐dependent side effect with statins. 15 , 16 Atorvastatin is also metabolized by CYP3A4, and a similar drug‐drug myotoxic interaction has been described when it has been combined with diltiazem. 17 Pravastatin does not undergo P450‐dependent metabolism, and fluvastatin is metabolized by CYP2D6; thus, verapamil and diltiazem would not be expected to have a significant pharmacokinetic interaction with these two statins, making them safer alternatives. 18
Verapamil and diltiazem also inhibit the metabolism of cyclosporine or tacrolimus, which can increase the concentration of these drugs and lead to nephrotoxicity. 19 Verapamil and diltiazem also inhibit the metabolism of a variety of other drugs—including carbamazepine, midazolam, tri‐azolam, buspirone and quinidine. 20 Since many of these drugs have a low therapeutic index, even modest increases in the blood levels of these compounds can lead to drug concentration‐dependent adverse reactions.
In general, DHP CCBs do not cause—but can be subject to—this type of adverse drug‐drug interaction. For example, diltiazem seems to inhibit the clearance of nifedipine in a dose‐dependent manner. 21 , 22 This interaction occurs quickly and is near maximal within 3 days of dosing. 22 Less well appreciated is the fact that nifedipine influences the pharmacokinetics of diltiazem. Early observations have found that pretreatment with nifedipine increased diltiazem concentrations, presumably secondary to both a decrease in its hepatic clearance as well as an increase in its bioavailability. 23 Thus, the interaction between verapamil and diltiazem and a DHP CCB can be exploited clinically to more effectively treat the hypertensive patient. 24
Data from epidemiologic studies reveal that approximately 1%–2% of the US population consumes at least one glass of regular‐strength grapefruit juice per day. This level of intake makes this a pertinent consideration in the hypertensive population in the United States—many of whom are receiving CCB therapy—since an interaction can occur with several CCBs and grapefruit juice or products which originate from grapefruit. This interaction is most prominent with felodipine, nisoldipine, nicardipine, and nitrendipine and derives from grapefruit products' increasing the bioavailability of these compounds. Patients receiving an established CCB dose who experience an unexpected BP response and/or vasodilator side effects should be questioned with regard to their intake of grapefruit juice. 25
DELIVERY SYSTEMS
Since the early 1980s, an increasing number of pharmaceutical products have been converted from immediate‐release to controlled‐release (CR) products by way of novel drug delivery systems. In a generic sense there are several advantages to CR preparations, including targeted blood concentrations; decreased administration frequency, dispensing costs, and adverse effects; and improved medication adherence. 26 Alternatively, disadvantages to CR dispensing systems include a delay in attaining the pharmacodynamic effect on initiation of therapy, sustained toxicity, and altered absorption with derangements in gastrointestinal motility. 26
CR delivery systems have found some of their greatest utility with CCBs. First, CR delivery systems have allowed a number of otherwise short‐acting CCBs to behave as once‐daily drugs (Figure 2). With this adjustment in how the drug is systemically presented, the peaks and valleys of drug concentration that might characterize a short‐acting drug such as nifedipine no longer exist. This change in the manner in which the drug is made available is of the utmost importance for short‐acting DHP CCBs such as nifedipine. 27 Second, delivery system technology has facilitated the development of preparations that are characterized by both delayed‐release and CR components. The delayed‐release element of these preparations allows for targeting of early morning post‐awakening BPs when drug administration occurs at bedtime—known as chronotherapy. While clinical trials have not yet shown outcome improvement with this approach, this approach does provide optimal drug concentrations when most cardiovascular events occur—which is in the morning hours—and minimizes the amount of drug delivered when BP is at its nadir during sleep. 26 , 28
Figure 2.
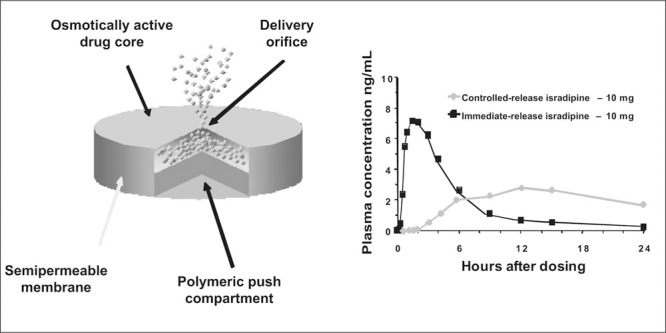
Gastrointestinal technology utilized to provide sustained‐release characteristics for drug delivery (left side). Concentration time curves for an immediate and controlled‐release form of isradipine. The less desirable peak‐and‐valley aspect of drug delivery is evident with the immediate‐release form of isradipine.
CONCLUSIONS
CCBs are widely used in the treatment of hypertension. Their pattern of use has evolved over time in concert with novel technologies that offer unique delivery characteristics for these drugs. The phar‐macokinetics of CCBs are marked by an important dependence on CYP3A4 for their metabolism, with verapamil and diltiazem also acting as inhibitors of CYP3A4. The latter is associated with the potential for a series of relevant drug‐drug interactions. Immediate‐release CCBs have been supplanted by SR delivery systems, which have improved toler‐ability and safety for these compounds. These technological advances are of no minor importance since CCBs are increasingly viewed as important components of many antihypertensive regimens.
References
- 1. Abernethy D, Schwartz JB. Calcium‐antagonist drugs. N Engl J Med. 1999;341:1447–1457. [DOI] [PubMed] [Google Scholar]
- 2. Morel N, Buryi V, Feron O, et al. The action of calcium channel blockers on recombinant L‐type calcium channel alphal‐subunits. Br J Pharmacol. 1998;125:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soldatov NM, Bouron A, Reuter H. Different voltage‐dependent inhibition by dihydropyridines of human Ca2+ channel splice variants. J Biol Chem. 1995;270:10540–10543. [DOI] [PubMed] [Google Scholar]
- 4. Ellenbogen KA, Dias VC, Cardello FP, et al. Safety and efficacy of IV diltiazem in atrial fibrillation or atrial flutter. Am J Cardiol. 1995;75:45–49. [DOI] [PubMed] [Google Scholar]
- 5. McNamara RL, Tamariz LJ, Segal JB, et al. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echo‐cardiography. Ann Intern Med. 2003;139:1018–1033. [DOI] [PubMed] [Google Scholar]
- 6. Leenen FH, Ruzicka M, Huang BS. Central sympathoinhibitory effects of calcium channel blockers. Curr Hypertens Rep. 2001;3:314–321. [DOI] [PubMed] [Google Scholar]
- 7. Sica DA, Gehr TW. Calcium‐channel blockers and end‐stage renal disease: pharmacokinetic and pharmacodynamic considerations. Curr Opin Nephrol Hypertens. 2003;12:123–131. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed JH, Grant AC, Rodger RS, et al. Inhibitory effect of uraemia on the hepatic clearance and metabolism of nicardipine. Br J Clin Pharmacol. 1991;32:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clair F, Belief M, Guerret M, et al. Hypotensive effect and pharmacokinetics of nicardipine in patients with severe renal failure. Curr Ther Res. 1985;38:74–81. [Google Scholar]
- 10. Schwartz JB. Calcium antagonists in the elderly. A risk‐benefit analysis. Drugs Aging. 1996;9:24–36. [DOI] [PubMed] [Google Scholar]
- 11. Davidson MH. Does differing metabolism by cytochrome P450 have clinical importance? Curr Atheroscler Rep. 2000;2:14–19. [DOI] [PubMed] [Google Scholar]
- 12. Azie NE, Brater DC, Becker PA, et al. The interaction of diltiazem with lovastatin and pravastatin. Clin Pharmacol Ther. 1998;64:369–377. [DOI] [PubMed] [Google Scholar]
- 13. Kantola T, Kivisto KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–182. [DOI] [PubMed] [Google Scholar]
- 14. Mousa O, Brater DC, Sunblad KJ, et al. The interaction of diltiazem with simvastatin. Clin Pharmacol Ther. 2000;67:267–274. [DOI] [PubMed] [Google Scholar]
- 15. Kusus M, Stapleton DD, Lertora JJ, et al. Rhabdomyolysis and acute renal failure in a cardiac transplant recipient due to multiple drug interactions. Am J Med Sci. 2000;320:394–397. [DOI] [PubMed] [Google Scholar]
- 16. Peces R, Pobes A. Rhabdomyolysis associated with concurrent use of simvastatin and diltiazem. Nephron. 2001;89:117–118. [DOI] [PubMed] [Google Scholar]
- 17. Lewin JJ III, Nappi JM, Taylor MH. Rhabdomyolysis with concurrent atorvastatin and diltiazem. Ann Pharmacother. 2002;36:1546–1549. [DOI] [PubMed] [Google Scholar]
- 18. Sica DA, Gehr TWB. Rhabdomyolysis and statin therapy: relevance to the elderly. Am J Geriatr Cardiol. 2002;11:48–55. [DOI] [PubMed] [Google Scholar]
- 19. Jones TE. The use of other drugs to allow a lower dosage of cyclosporine to be used. Therapeutic and pharmacoeconomic considerations. Clin Pharmacokinet. 1997;32:357–367. [DOI] [PubMed] [Google Scholar]
- 20. Sica DA, Gehr TWB. Calcium‐channel blockers and the cytochrome P450 system. In: Epstein, Murray , ed. Calcium Antagonists in Clinical Medicine. 3rd ed. Philadelphia, PA: Hanley and Belfus, Inc.; 2002:93–106. [Google Scholar]
- 21. Ohashi K, Tateishi T, Sudo T, et al. Effects of diltiazem on the pharmacokinetics of nifedipine. J Cardiovasc Pharmacol. 1990;15:96–101. [DOI] [PubMed] [Google Scholar]
- 22. Tateishi T, Ohashi K, Sudo T, et al. Dose dependent effect of diltiazem on the pharmacokinetics of nifedipine. J Clin Pharmacol. 1989;29:994–997. [DOI] [PubMed] [Google Scholar]
- 23. Tateishi T, Ohashi K, Sudo T, et al. The effect of nifedipine on the pharmacokinetics and dynamic of diltiazem: the preliminary study in normal volunteers. J Clin Pharmacol. 1993;33:738–740. [DOI] [PubMed] [Google Scholar]
- 24. Sica DA. Combination calcium channel blocker therapy in the treatment of hypertension. J Clin Hypertens (Greenwich). 2001;3:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sica DA. Grapefruit juice and calcium channel blocker interaction. Am J Hypertens. In Press. [DOI] [PubMed] [Google Scholar]
- 26. Prisant LM, Elliott WJ. Drug delivery systems for treatment of systemic hypertension. Clin Pharmacokinet. 2003;42:931–940. [DOI] [PubMed] [Google Scholar]
- 27. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergen‐cies? JAMA. 1996;276:1328–1331. [PubMed] [Google Scholar]
- 28. Smith DH. Pharmacology of cardiovascular chronothera‐peutic agents. Am J Hypertens. 2001;14:296S–301S. [DOI] [PubMed] [Google Scholar]


