Abstract
Evaluation of early hypertension‐related alterations in retinal microcirculation has been subjective and poorly reproducible. The authors recently described a semiautomatic computerized system for evaluation of the calibre of retinal blood vessels that has shown very good reproducibility. In the study, this system was used to measure the calibres of retinal arterioles and veins, and their ratio, in a group of 51 hypertensive outpatients before and after 6 months of treatment with losartan or, if required for satisfactory blood pressure control, losartan plus hydrochlorothiazide. Mean retinal arteriole diameter increased from 0.0842±0.003 mm to 0.0847±0.003 mm (p=0.001). Arteriovenous ratio increased from 0.753±0.03 to 0.756±0.03 (p=0.005). This observation suggests regression of early hypertension‐related alterations in retinal microcirculation after 6 months of antihypertensive treatment.
According to hypertension management guidelines, hypertensive retinopathy is considered target organ damage constituting a cardiovascular risk factor. 1 , 2 Unfortunately, evaluation of the early alterations in retinal microcirculation that are observed in most hypertensive patients seen in daily practice (i.e., retinal arteriole narrowing or arteriovenous nicking without exudates or hemorrhage causing damage to the retina itself) has been subjective, imprecise, and poorly reproducible. This is largely the reason why European guidelines, citing a prevalence of grade 1 or grade 2 hypertensive retinopathy of almost 80% among newly referred hypertension patients, 3 explicitly exclude early alterations from use for cardiovascular risk stratification.
We recently described a semiautomatic computerized system for evaluation of the calibre of retinal blood vessels that has shown very good reproducibility. 4 Here, we report the results of hypertensive treatment on retinal arteriole narrowing in a group of 51 hypertensive patients during 6 months of treatment for hypertension.
MATERIAL AND METHODS
From among the hypertensive outpatients attending the internal medicine service of our hospital (defined as blood pressure [BP] ≥140/90 mm Hg in two or more opportunities in past months), who had clear eyes with no refractive defect greater than three diopters and who did not have significant hepatic or renal dysfunction (creatinine >2 mg/dL), congestive heart failure of New York Heart Association class III or IV, or other disorders that might complicate the results of the study, we studied 51 consecutive patients (48% men). Before and after 6 months of treatment with losartan or losartan plus hydrochlorothiazide, these patients were evaluated by detailed anamnesis, BP measurement, chest radiography, electrocardiography, and echocardiography. Relevant urine and blood analyses were also performed in some cases.
Clinic systolic BP (SBP) and diastolic BP (DBP), respectively, were determined following the recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). 1 Echocardiography was performed using an Esaote Biomedica SIM 7000 CFM Challenge apparatus (Esaote Biomedica, Florence, Italy). Two‐dimensional images were used to identify the most suitable M‐mode images. Left ventricular mass in grams was calculated using the anatomically validated formula of Ganau et al. 5 and divided by body surface area in m2 to obtain the left ventricular mass index (LVMI). Two categories of left ventricular hypertrophy (LVH) were defined: the criteria used for major LVH (LVH1) were LVMI >134 g/m2 for men and >110 g/m2 for women, 6 and the criteria for all‐degree LVH (LVH2) were LVMI >111 g/m2 for men and >106 g/m2 for women. 5 Other parameters extracted from the echocardiographic recordings included interventricular septum thickness and E/A ratio.
Following the above evaluations, 24‐hour ambulatory blood pressure monitoring (ABPM) was performed using a SpaceLabs 90207 apparatus (SpaceLabs Medical, Issaquah, WA) programmed to acquire pressure data every 20 minutes during the day (7 a.m. to 11 p.m.) and every 30 minutes during the night (11 p.m. to 7 a.m.). Recordings with ≥90% of the readings valid were deemed acceptable. From each acceptable recording the 24‐hour, daytime, and nighttime mean SBPs, DBPs, and heart rates were extracted. Patients were classified as nocturnal dippers if nighttime mean SBP and DBP were <90% of the corresponding daytime mean values.
Albumin in 24‐hour urine was determined to detect microalbuminuria, and serum analyses included fibrinogen, lipid profile components, and fasting glucose.
For determination of the calibres of retinal arterioles and veins and the ratio between the two (the arteriovenous ratio [AVR]), digital fundus photographs of both eyes (each centering on the papilla and covering an angle of 50 degrees), were taken 20–30 minutes after dilation of the pupil with tropicamide (two drops) and phenylephrine (two drops). The fundus camera was a Topcon TRC‐50 IA equipped with a 540‐nm filter connected to an Imagenet 1024 system (Topcon Instruments, Paramus, NJ), and retinal vessel calibres and AVR values were extracted from the photographs as previously described. 7 All photographic analyses were performed by a single ophthalmologist who, in evaluating post‐treatment photographs, was blind to the corresponding pre‐treatment values. Cardiovascular risk was calculated using the Framingham and Score tables. 2 , 8
Following initial evaluation, all patients were advised as to appropriate changes in lifestyle and diet and were prescribed losartan at a dosage of 50 mg/d, with subsequent modifications to 100 mg/d (in one morning dose) if BP was uncontrolled (≥140/90 mm Hg) after 1 month of treatment, to 50 mg/d of losartan plus 12.5 mg/d of hydrochlorothiazide if BP was still uncontrolled at the end of the second month, and to 100 mg of losartan plus 25 mg of hydrochlorothiazide if control had not been achieved by the end of the third month. These pharmacologic regimens were maintained until 6 months after initial patient evaluation, when evaluation was repeated.
Interruption of treatment in patients taking anti‐hypertensive drugs before inclusion in the study for 4 weeks before initial evaluation was required to eliminate the short‐term effects of these drugs on vasomotor tone and to prevent any interaction with the treatment given during the study. Patients with high serum cholesterol (>300 mg/dL), or high cardiovascular risk (>20% according to the Framingham equations) and low‐density lipoprotein cholesterol >130 mg/dL, were prescribed simvastatin (10 mg/d, increased to 20 mg/d if adequate control of lipid profile had not been achieved after 3 months).
Data Presentation and Statistical Analyses
Results below are presented as mean ± SD. The statistical significance of differences was judged using two‐sided tests at the p=0.05 level for paired or unpaired data, as appropriate. Univariate correlations between the mean AVR of the two eyes and other variables were calculated, as was the influence of the extent of nocturnal dipping on changes in AVR and cardiovascular risk. Multiple linear regressions were performed to identify the variables best explaining baseline and post‐treatment AVRs and the difference between them. All statistical analyses were performed using SPSS for Windows 6.0 (SPSS Inc., Chicago, IL).
RESULTS
Baseline Characteristics of the Sample
Initial clinic SBP and DBP were 157.7±17.2 mm Hg and 94.5±10.3 mm Hg, respectively. A total of 28 patients (55%) satisfied the European Hypertension Society criteria for grade I arterial hypertension (SBP 140–159 mm Hg and/or DBP 90–99 mm Hg), 16 (31%) were grade II (SBP 160–179 mm Hg and/or DBP 100–109 mm Hg), and seven (13.7%) were grade III (SBP ≥180 mm Hg and/or DBP ≥110 mm Hg). Arterial hypertension was diagnosed in 27 (53%) patients more than 1 year previously and in 24 (47%) more recently.
The mean age of the 51 patients was 51 years (range, 23/79 years). Forty‐five (88%) were non‐smokers. No patients were diabetic, but four had carbohydrate intolerance as diagnosed by oral glucose tolerance tests. 9 Thirteen (26%) had a body mass index (weight/height2) of 20–25 kg/m2, 23 (45%) had a body mass index of 25–30 kg/m2, and the remaining 15 (29%) had a body mass index >30 kg/m2. Four patients (8%) had a personal history of cardiovascular disease and 10 (20%) patients had a family history of early‐onset cardiovascular disease (defined as cardiovascular disease beginning before age 55 for men or before age 65 for women). Twenty‐five patients had been receiving pharmacologic antihypertensive treatment (in all cases without achieving adequate control of BP), and the other 26 had never been treated for arterial hypertension.
The Table (column 2) lists mean values and SDs of clinic BPs, ambulatory BPs and heart rate, albumin in urine, echocardiographic and ophthalmologic results, and the Framingham cardiovascular risk score. Eight patients were classified as nocturnal dippers. Baseline retinal arteriole calibre was negatively correlated with pulse pressure (p=0.01) and cardiovascular risk (p=0.045), and baseline AVR was negatively correlated with pulse pressure (p=0.006) and clinic SBP (p=0.038). After exclusion of patients with incomplete data and outliers, multivariate linear regression analysis showed some 77% of the variance in baseline AVR to be accounted for by fibrinogen (−2.558; p=0.018), alteration of diastolic function (E/A ratio <1) (−4.070; p=0.001), triglycerides (−1.925; p=0.067), and the SDs of nocturnal SBPs (−4.175; p=0.000) and DBPs (3.651; p=0.001).
Table.
Data on Retinal Microcirculation, Blood Pressures, Cardiac Hypertrophy, Microalbuminuria, and Cardiovascular Risk Before and After 6 Months of Hypertension Treatment
| Variable | Baseline | Final |
|---|---|---|
| Clinic SBP (mm Hg) | 157.7±17.2 | 132.4±7.8* |
| Clinic DBP (mm Hg) | 94.5±10.3 | 81.4±6.5* |
| Pulse pressure (mm Hg) | 62.8±14.9 | 50.9±7.1* |
| Weight (kg) | 79.0±13.2 | 79.0±13.5 |
| Body mass index (kg/m2) | 28.9±3.6 | 28.9±3.8 |
| 24‐h SBP (mm Hg) | 136.5±13.4 | 123.5±9.9* |
| 24‐h DBP (mm Hg) | 84.5±9.0 | 75.9±7.3* |
| 24‐h heart rate (bpm) | 73.0±8.0 | 73.5±8.9 |
| Daytime SBP (mm Hg) | 140.8±14.1 | 127.7±10.8* |
| Daytime DBP (mm Hg) | 88.3±9.0 | 79.4±8.1* |
| Nighttime SBP (mm Hg) | 129.6±14.2 | 115.9±10.4* |
| Nighttime DBP (mm Hg) | 77.7±10.4 | 69.8±7.8* |
| LVMI (g/m2) | 113.7±28.5 | 114.2±27.3 |
| LVH1 (%)** | 32 | 28.1 |
| LVH2 (%)† | 46.3 | 42.1 |
| E/A ratio <1 | 23/44 | 22/35 |
| Microalbuminuria (mg/24 h) | 55.6±124.7 | 24.6±29.2 |
| Retinal arteriole diameter (mm) | 0.0842±0.003 | 0.0847±0.003* |
| Retinal venule diameter (mm) | 0.1118±0.001 | 0.1119±0.001 |
| Arteriovenous ratio | 0.7530±0.03 | 0.7563±0.03†† |
| Cardiovascular risk‡ | 13.6±9.5 | 9.8±8.1* |
| Total cholesterol (mg/dL) | 210.7±39.2 | 184.0±32.2* |
| Data are mean ± SD except as indicated. SBP=systolic blood pressure; DBP=diastolic blood pressure; LVH=left ventricular hypertrophy; *p=0.00l; **left ventricular mass index (LVMI) >134 g/m2 for men and >110 g/m2 for women; ††LVMI >111 g/m2 for men and >106 g/m2 for women; ††p<0.005; ‡calculated using the Framingham tables | ||
Final Characteristics and Correlations
The Table (column 3) lists characteristics of the sample after 6 months, by which time 35 of 41 (85%) patients had achieved adequate control of clinic BP and 17 of 38 (45%) had achieved adequate dynamic control of BP (clinic values <140/90 mm Hg and 24‐hour mean <125/80 mm Hg, daytime mean <135/85 mm Hg, and nighttime mean <120/75 mm Hg 2 , 10 ). The remainder of patients were responders but did not reach the objective control of BP, although they were included in the subsequent analysis. Twenty‐eight patients (55%) had been prescribed simvastatin.
AVR increased from 0.753±0.03 to 0.756±0.03 (p=0.005) (Figure), and cardiovascular risk fell from 13.6±9.5 to 9.8±8.1 (p<0.001). The coefficient of variation of AVR between baseline and final measurements was 41.6%. Final AVR correlated in univariate analysis with modifications of pulse pressure (0.350; p=0.027) and the variations of some parameters obtained in ABPM recording.
Figure.
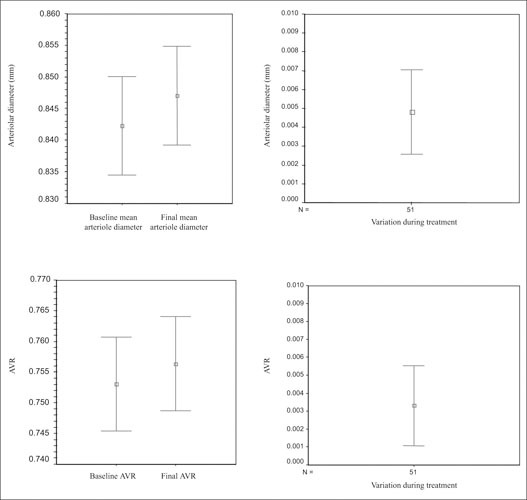
Mean diameters of retinal arterioles, and the arteriovenous ratio (AVR), before and after 6 months of antihypertensive treatment (left). Bars indicate 95% confidence intervals [CIs]. The differences in arteriole diameter and AVR (right) are statistically significant (p<0.001 and p=0.005, respectively). The correlation between baseline and final AVR is 0.957 (p=0.000). The 95% CIs of the variations of arteriole diameter and AVR ratio include no 0.000, confirming the difference between groups.
After exclusion of patients with incomplete data and outliers, multivariate linear regression analysis showed some 95% of the variance in final AVR to be accounted for by baseline AVR (26.224; p=0.000), baseline LVMI (−2.716; p=0.010) and the SD of diurnal DBP in the baseline ABPM recording (−2.433; p=0.020).
Changes and Effects of Nocturnal Dipping
There was no significant correlation between change in the ratios between nocturnal and diurnal mean SBPs and DBPs and change in cardiovascular risk in regard to either absolute change or percentage change. Of the eight nocturnal dippers identified at the beginning of the study, four had become nondippers by the end of the study and, of the 27 patients initially classified as nocturnal nondippers who provided valid ABPM recordings at the end of the study, nine had become dippers. Dippers who became nondippers did not differ significantly from nondippers who became dippers in regard to either change or percentage change in cardiovascular risk or AVR. Nor were there any significant correlations between changes in AVR and the baseline, final, and changes in the ratios between nocturnal and diurnal mean SBP or DBP, either in absolute or percentage terms.
DISCUSSION
Hypertension‐induced alterations in retinal microcirculation were first described in the 19th century by Marcus Gunn. 11 Since then, it has been generally accepted that such alterations are relevant to prognosis and, hence, to the choice of therapy, and fundus examination is recommended accordingly in current guidelines for management of the hypertensive patient. However, hypertensive retinopathy covers a wide range of alterations, from narrowing of the retinal arterioles to lesions compromising the parenchyma. 12 It is only quite recently that some studies of very large groups of subjects 13 , 14 , 15 —although not others 16 —have provided evidence that retinal arteriole narrowing may predict adverse cardiovascular events independently of other risk factors. Because of this, and in view of the limitations of qualitative fundoscopy for evaluation of patients with grade I and II hypertension, 17 the interpretation of the results of funduscopy recommended by the guidelines is based largely on studies carried out several decades ago in which, for various reasons, interpretation of early hypertensive retinopathy was less relevant than it is today. 17 , 18 , 19 Therefore, there is clearly a need for the development of methods of evaluation of early hypertensive retinopathy, retinal arteriole narrowing in particular, that are precise and reliable enough to allow effective use of this source of information on the hypertensive process. In this study, using a previously described computerized method for measurement of retinal blood vessel calibres, 4 we examined the changes in these parameters that accompanied 6 months of treatment in a group of patients with grade I and II hypertension. Our main finding is that mean retinal arteriole diameter and the AVR increased during this period, i.e., treatment was accompanied by the regression of early hypertensive retinopathy. The increment of AVR cannot be explained by the variability of our method, since the coefficient of variation between baseline and final measurements (41.6%) was much higher than the within‐operator, between‐operator, and within‐eye variability, all of them better than 3%. 4 As far as we know, this is the first time it has been possible to demonstrate the beneficial effect of treatment on this kind of target organ damage (and to corroborate the hypertensive origin of retinal arteriole narrowing in this way) by examination of a small group of patients.
Like previous authors, 20 , 21 , 22 , 23 , 24 , 25 we found a close relationship between diffuse arteriolar narrowing and baseline hypertension. In this study, arteriole calibre and AVR both decreased with increasing pulse pressure, and AVR also decreased with increasing clinic SBP. Pulse pressure and clinic SBP are both valuable markers of vascular disease that are used for stratification of cardiovascular risk. In keeping with this, arteriole calibre was negatively correlated with total cardiovascular risk (both at the beginning and at the end of the study), which supports guideline recommendations to minimize cardiovascular risk of all kinds as soon as possible to prevent target organ damage.
Final AVRs were correlated with multiple parameters extracted from ABPM recordings. The best correlation (p<0.01) was with the final values, or the changes over the course of the study, of the variability of DBP over 24 hours as measured by its SD, and with the final SD of DBP during the nocturnal period.
Dahlof et al. 26 reported the regression of qualitatively evaluated early hypertensive retinopathy during treatment with enalapril, a drug known to affect vascular morphology and function. In this study, a similar effect of treatment with losartan, or losartan plus hydrochlorothiazide, was observed quantitatively by measurement of retinal blood vessel calibres. Note that the precision of the measurement system allowed observation of a significant effect in spite of the very small differences between the initial and final mean values of AVR and arteriole calibre (Table); larger differences would most likely result from more prolonged treatment. 27 These findings, and the accompanying significant reduction in cardiovascular risk, suggest that even though retinal arteriole narrowing may not be an independent predictor of cardiovascular risk, it may serve as a very useful noninvasively obtained summary of total risk and, hence, of the physiologic efficiency of BP control, at least for research purposes.
The genesis of retinopathy has been related to the absence of nocturnal dipping, hyperglycemia, 28 and endothelial inflammation or dysfunction, 29 inter alia. In this study, however, we found no significant relationship between nocturnal dipping and either AVR or change in AVR. In addition, no relationship was found between any of the metabolic parameters considered (lipids, glycemia) and either baseline or final AVR or arteriole calibre or the corresponding changes (final minus baseline values) or percentage changes, except that serum triglyceride level was one of the five predictors of baseline AVR identified by multivariate analysis. The absence of correlation between baseline or final values or changes in arteriole calibre or AVR and baseline or final values or changes in cholesterol or cholesterol fractions suggests that simvastatin cannot have affected retinal vessel calibre in the 55% of patients who took this drug during the study period, at least by any cholesterol‐mediated mechanism.
We found no relationship between AVR and baseline LVMI. A relationship between hypertensive retinopathy and LVH has been reported previously, but only on the basis of subjective direct ophthalmoscopy. 30
Somewhat surprising, however, was that there was no significant correlation between AVR and either 24‐hour urinary albumin or the change in 24‐hour urinary albumin. This seems likely to have been due to low statistical power, with 24‐hour urinary albumin excretion showing, as usual, 31 wide variation.
In conclusion, our method of evaluating early hypertension‐related alterations in retinal microcirculation has allowed objective, quantitative observation of regression associated with 6 months of hypertensive treatment. Further work remains to be done before retinal arteriole measurement can be recommended for routine monitoring of the individual patient.
References
- 1. Seventh Report of the National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears in JAMA. 2003; 290:197]. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 3. Cuspidi C, Macca G, Salerno M, et al. Evaluation of target organ damage in arterial hypertension: which role for qualitative funduscopic examination? Ital Heart J. 2001;2:702–706. [PubMed] [Google Scholar]
- 4. Pose‐Reino A, Gómez‐Ulla F, Hayik B, et al. Computerized measurement of retinal blood vessel calibre: description, validation and use to determine the influence of ageing and hypertension. J Hypertens. 2005;23:843–850. [DOI] [PubMed] [Google Scholar]
- 5. Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. [DOI] [PubMed] [Google Scholar]
- 6. Devereux RB. Detection of left ventricular hypertrophy by M‐mode echocardiography. Anatomic validation, standardization and comparison to other methods. Hypertension. 1987;9(2 pt 2):II19–II26. [DOI] [PubMed] [Google Scholar]
- 7. Mosquera A, Dosil R, Leborán V, et al. ART‐VENA: retinal vascular calibre measurement. In: Perales FJ, Campilho JC, Perez de la Blanca N, et al., eds. LNCS 2652: Pattern Recognition and Image Analysis. Berlin, Germany: Springer‐Verlag; 2003:598–605. [Google Scholar]
- 8. Anderson KM, Wilson PW, Odell PM, et al. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. [DOI] [PubMed] [Google Scholar]
- 9. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 10. O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. [DOI] [PubMed] [Google Scholar]
- 11. Gunn RM. Ophthalmoscopic evidence of (1) arterial changes associated with chronic renal diseases and (2) increased arterial tension. Trans Ophthalmol Soc U K. 1892;12:124–125. [Google Scholar]
- 12. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. [DOI] [PubMed] [Google Scholar]
- 13. Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. [DOI] [PubMed] [Google Scholar]
- 14. Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong TY, Knudtson MD, Klein R, et al. A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol. 2004;159:819–825. [DOI] [PubMed] [Google Scholar]
- 16. Cuspidi C, Meani S, Salerno M, et al. Retinal microvascular changes and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:2095–2102. [DOI] [PubMed] [Google Scholar]
- 17. Dimmitt SB, West JN, Eames SM, et al. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103–1106. [DOI] [PubMed] [Google Scholar]
- 18. Keith NM, Wagener HP, Barker NW Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1939;197:332–343. [DOI] [PubMed] [Google Scholar]
- 19. Breslin DJ, Gifford RW Jr, Fairbairn JF II, et al. Prognostic importance of ophthalmoscopic findings in essential hypertension. JAMA. 1966;195:335–338. [PubMed] [Google Scholar]
- 20. Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology. 2003;110:658–666. [DOI] [PubMed] [Google Scholar]
- 21. Van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and non‐diabetic population: the Hoorn Study. Arch Ophthalmol. 2003;121:245–251. [DOI] [PubMed] [Google Scholar]
- 22. Wang JJ, Mitchell P, Leung H, et al. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension. 2003;42:534–541. [DOI] [PubMed] [Google Scholar]
- 23. Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 24. Wong TY, Klein R, Klein BE, et al. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. [DOI] [PubMed] [Google Scholar]
- 25. Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. [DOI] [PubMed] [Google Scholar]
- 26. Dahlof B, Stenkula S, Hansson L. Hypertensive retinal vascular changes: relation to left ventricular hypertrophy and arteriolar changes before and after treatment. Blood Press. 1992;1:35–44. [DOI] [PubMed] [Google Scholar]
- 27. Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17(12 pt 1):1192–1200. [DOI] [PubMed] [Google Scholar]
- 28. Wong TY, Klein R, Klein BE, et al. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. [DOI] [PubMed] [Google Scholar]
- 29. Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vase Biol. 2000;20:1644–1650. [DOI] [PubMed] [Google Scholar]
- 30. Pose‐Reino A, Gonzalez‐Juanatey JR, Castroviejo M, et al. Relation between left ventricular hypertrophy and retinal vascular changes in mild hypertension [in Spanish], Med Clin (Bare). 1997;108:281–285. [PubMed] [Google Scholar]
- 31. American Diabetes Association. Diabetic nephropathy. Diabetes Care. 2002;25:S85–S89. [Google Scholar]


