Abstract
Once considered part of the normal aging process, the development of isolated systolic hypertension represents a late manifestation of increased arterial stiffness in older people. Furthermore, isolated systolic hypertension is the single most frequent subtype of hypertension in the US adult population. Indeed, central arterial stiffness rather than peripheral vascular resistance becomes the dominant hemodynamic factor in both normotensive and hypertensive individuals after the age of 50–60 years. Stiffening disease, an age‐related degeneration of the elastic elements of the thoracic aorta, is associated with a widening of brachial pulse pressure. Brachial pulse pressure predicts future cardiovascular disease events. However, pressure wave amplification produces higher brachial than aortic pressures and, therefore, central rather than peripheral blood pressure indices are more reliable measures of cardiovascular risk. Stiffening disease of aging is accompanied by early wave reflection, which results in a significant augmentation of central systolic pressure in late systole and further adds to increased cardiac afterload—so‐called ventricular‐vascular uncoupling. Diabetes, impaired renal function, and untreated or poorly treated hypertension may lead to premature arterial stiffening; its consequences are stiffening and hypertrophy of the left ventricle and predisposition to coronary heart disease, heart failure, stroke, vascular dementia, and chronic kidney disease.
With the aging of our population and the advent of effective antihypertensive therapy, there has been a shift toward a more slowly evolving form of hypertension that is predominately systolic in nature and affects middle‐aged and older persons. An age‐associated rise in systolic blood pressure (SBP), occurring as a consequence of increased arterial stiffness, was once considered an inconsequential part of the aging process; more recently, we recognize that it represents a major public health problem in the Western world, not only because it is extremely common, but also because it results in extensive cardiovascular disease. Hypertension was largely defined using only the criterion of elevated diastolic blood pressure (DBP) until the 1990s. This slowly rising SBP with aging, out of proportion to the rise in DBP, is referred to as isolated systolic hypertension (ISH). With the recognition of its true cardiovascular risk, ISH is defined as a SBP ≥140 and DBP <90 mm Hg.
EPIDEMIOLOGY
The National Health and Nutrition Examination Survey (NHANES III, 1988–1991) showed that three of four adults with hypertension were 50 years of age or older. Moreover, about 80% of untreated (or inadequately treated) individuals with hypertension from age 50 onward had ISH, which, by definition, represents wide pulse pressure hypertension (Figure 1). A recent Framingham study showed that persons who reach the age of 65 have a 90% lifetime risk of developing hypertension, almost exclusively of the ISH type. Noteworthy is the fact that since 1988–1991, the prevalence of hypertension has increased significantly in women and in all age groups, although most dramatically in those aged 60 years and older, who experienced an increase in prevalence from 57.9% in 1988–1991 to 65.4% in 1999–2000. Moreover, in the United States, prevalence of hypertension in the elderly is greatest among non‐Hispanic blacks and least among Mexican Americans.
Figure 1.
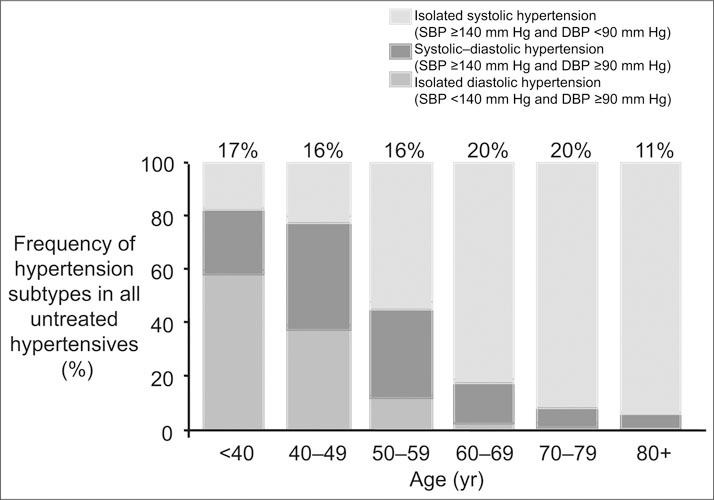
Frequency distribution of untreated hypertensive individuals by age and hypertension subtype. Numbers at the top of the bars represent the overall percentage distribution of all subtypes of untreated hypertension in the age group (National Health and Nutrition Examination Survey III, 1988–1994). SBP=systolic blood pressure; DBP=diastolic blood pressure. Reproduced with permission from Hypertension. 2001;37:869–874.
ANATOMY AND PATHOLOGY
The elastic behavior of the arterial wall depends primarily on the composition and arrangement of the materials that make up the media of the arterial tree. In the media of the thoracic aorta and its immediate branches are large attachments of elastic lamellae to smooth muscle cells, constituting the contractile‐elastic unit, and arranged in an alternating oblique pattern that exert maximum force in a circumferential direction. The medial fibrous elements of the thoracic aorta contain a predominance of elastin over collagen, but as one proceeds distally along the arterial tree, there is a rapid reversal favoring collagen over elastin in the peripheral, largely muscular arteries. During each systole, the elastic thoracic aorta experiences the highest SBP of any blood vessel in the body and, by its cushioning function, converts peak SBP to lower mean arterial pressure values in the smaller and more vulnerable downstream peripheral arteries.
Elastin, with a half‐life of 40 years, is one of the most stable proteins in the body. Despite this stability, fatigue of elastin fibers and lamellae can occur by the sixth decade of life from the accumulated cyclic stress of more than 2 billion aorta expansions during ventricular contraction; this leads to eventual fracturing of elastin along with structural changes of the extracellular matrix that include proliferation of collagen and deposition of calcium. This diffuse degenerative process, termed arteriosclerosis, results in increased central elastic arterial stiffness with widening of pulse pressure.
HEMODYNAMICS OF AGING
Pulse pressure is determined not only by arterial stiffness, but also by stroke volume and, to a lesser extent, by the ejection rate of the left ventricle. In contrast, mean arterial pressure is determined by cardiac output and total peripheral resistance. By definition, ISH is characterized by an increase in pulse pressure, but not necessarily by an increase in mean arterial pressure, stroke volume, or ejection rate. Thus, in older subjects, brachial pulse pressure is regarded as a surrogate measure of arterial stiffness.
Both cross‐sectional and longitudinal population studies show that SBP rises from adolescence, whereas DBP, although initially increasing with age, levels off at about age 50 and decreases after age 60. Thus, pulse pressure begins to increase after age 50. The rise in SBP and DBP up to age 50 can best be explained by the dominance of peripheral vascular resistance (Table I). The transition age of 50–60 years, when DBP levels off, constitutes a near balancing of increased resistance and increased thoracic aortic stiffness. By contrast, after age 60, the fall in DBP and the rapid widening of pulse pressure become surrogate indicators of central elastic arterial stiffening. Indeed, after age 60, central arterial stiffness, rather than peripheral vascular resistance, becomes the dominant hemodynamic factor in both normotensive and hypertensive individuals. Arterial stiffness is also accompanied by the phenomenon of early wave reflection.
Table I.
Hemodynamic Patterns of Age‐Related Changes in Blood Pressure (BP)
| Age (yr) | Diastolic BP | Systolic BP | MAP | PP | Hemodynamics |
|---|---|---|---|---|---|
| 30–49 | ↑ | ↑ | ↑ | → or ↑ | R>S |
| 50–59 | → | ↑ | → | ↑↑ | R=S |
| ≥60 | ↓ | ↑ | → or ↓ | ↑↑↑↑ | S>R |
| MAP=mean arterial pressure; PP=pulse pressure; ↑=increase; →=no change; R=small‐vessel resistance; S=large‐vessel stiffness; ↓=decrease. Adapted with permission from Circulation. 1997;96:308–315. | |||||
The central pressure waveform is produced by two major components: a forward travelling or incident wave generated by ventricular ejection and a backward wave reflecting off of distal arteries at the branching origins of arterioles. In young subjects, the reflected pressure waves return to the ascending aorta in diastole and serve to elevate mean DBP, thus boosting coronary artery perfusion. The summation of the incident pressure wave with the reflected wave in young adults produces a normal phenomenon of pressure amplification of pulse pressure and SBP, whereby brachial artery values are 15–25 mm Hg higher than that recorded in the ascending aorta. Between the ages of 20 and 70 years, as arteries stiffen, the pulse wave velocity doubles. In older individuals, the reflected pressure wave returns to the ascending aorta earlier—during late systole rather than in diastole—and, therefore, increases or “augments” the central SBP, lowers DBP, and hence increases pulse pressure. Thus, central pulse pressure increases directly from the intrinsic increase in large artery stiffness and indirectly from the effect of early wave reflection. This process of shifting the reflected wave from diastole (where it boosts coronary blood flow) to systole (where it adds to cardiac load) is called ventricular‐vascular uncoupling. Ventricular‐vascular uncoupling is a normal result of aging, but diseases such as diabetes, chronic renal failure, and untreated or poorly treated hypertension can accelerate the process.
PATHOPHYSIOLOGY OF INCREASED PULSE PRESSURE
The pathophysiologic correlates of increased elastic artery stiffness, wide pulse pressure, and ventricular‐vascular uncoupling are many, including decreased coronary perfusion, increased oxygen demand, and potential for subendocardial ischemia (Table II). In addition to arterial stiffening, the left ventricle itself becomes stiff. This is particularly notable in hearts that develop left ventricular hypertrophy, a common occurrence in the elderly and particularly in those individuals with ISH. A stiffer left ventricle coupled with a stiffer arterial system can contribute to increased cardiovascular risk in several ways: First, there is increased late‐systolic wall stress and increased cardiac energy costs imposed on the heart. Second, the imposition of a markedly increased late‐systolic load slows the cardiac relaxation rate, potentially leading to incomplete diastolic relaxation, compromised cardiac reserve and, eventually, heart failure with normal ejection fraction. Third, loss of arterial distensibility appears to alter vascular mechanosignaling so that the normal augmentation of nitric oxide release from a stiff ascending aorta with each contraction of the heart is diminished, leading to compromised vasoprotection. Lastly, increased pulsatile stress, secondary to the loss of the conduit artery cushioning function, can contribute to endothelial dysfunction, increased coronary atherosclerosis, rupture of unstable atherosclerotic plaques, and acute coronary heart syndromes. Many of these disturbances in cardiovascular function characterize the elderly person with long‐standing ISH and markedly elevated pulse pressure.
Table II.
Pathophysiology of Wide Pulse Pressure in the Elderly
| ↑ Large artery stiffness |
| ↑ Early wave reflection, ↑ systolic blood pressure in late systole |
| ↓ Coronary perfusion pressure |
| ↑ Oxygen demand and subendocardial ischemia |
| ↑ Ventricular‐vascular uncoupling |
| ↑ Vascular afterload leading to ↑ left ventricular hypertrophy and ↑ left ventricular stiffness |
| ↑ Late systolic wall load leading to ↓ left ventricular relaxation |
| ↑ Defective mechanical signaling: ↓ nitric oxide production |
| ↓ Cardiac reserve, diastolic dysfunction, congestive heart failure |
| ↑ Flow turbulence, endothelial dysfunction, and atherosclerosis |
| ↑ Pulsatile strain, plaque rupture, acute coronary syndrome |
| ↑ =increased; ↓ =decreased |
In summary, stiffness of both the heart and conduit arteries interact to produce diastolic dysfunction and heart failure; this results from the combination of an elevated cardiac afterload presented to a compromised left ventricle, which is unable to handle the load. Thus, cardiovascular risk of an increased pulse pressure is defined by: 1) increased SBP, a marker of cardiac afterload; and 2) discordant decreased DBP in association with an increased SBP, a marker of increased stiffness of the left ventricle and the proximal aorta.
PULSE PRESSURE AS A MARKER OF INCREASED CARDIOVASCULAR RISK
ISH is frequently associated with coronary heart disease, thrombotic and hemorrhagic stroke, dementia, peripheral arterial disease, and slowly progressive heart and renal failure. Systolic rather than diastolic hypertension is most often associated with a greater increased risk for all cardiovascular disease morbid events in middle‐aged and older persons. Recently, however, ISH in general, and increased pulse pressure in particular, have been identified as independent cardiovascular disease risk factors. Indeed, numerous studies over the past decade have shown that pulse pressure may be a more sensitive indicator of cardiovascular risk than SBP. In the presence of concordant elevations of SBP and DBP, such as systolic‐diastolic hypertension, there is no advantage of SBP over pulse pressure in predicting risk. However, when there is discordantly low DBP along with elevated SBP leading to the development of ISH, pulse pressure becomes dominant in predicting risk. This was shown in the original Framingham cohort (Figure 2). In normotensive and untreated subjects aged 50–79 years, using a model adjusted for age, sex, and other risk factors, coronary heart disease risk was inversely related to DBP at any given SBP level, suggesting that pulse pressure predicted coronary heart disease risk better than either SBP or DBP alone. This leads to the conclusion that at any given elevation in SBP, the elderly person with ISH will most often be at greater risk than the person with systolic‐diastolic hypertension. However, pulse pressure cannot replace SBP as a single measure of cardiovascular risk, since pulse pressure may significantly underestimate the extent of peripheral vascular resistance. Thus, the best strategy for assessing risk in the elderly begins with determining the level of SBP elevation and is followed by the adjustment of risk upward when wide pulse pressure, i.e., discordantly low DBP, is present.
Figure 2.
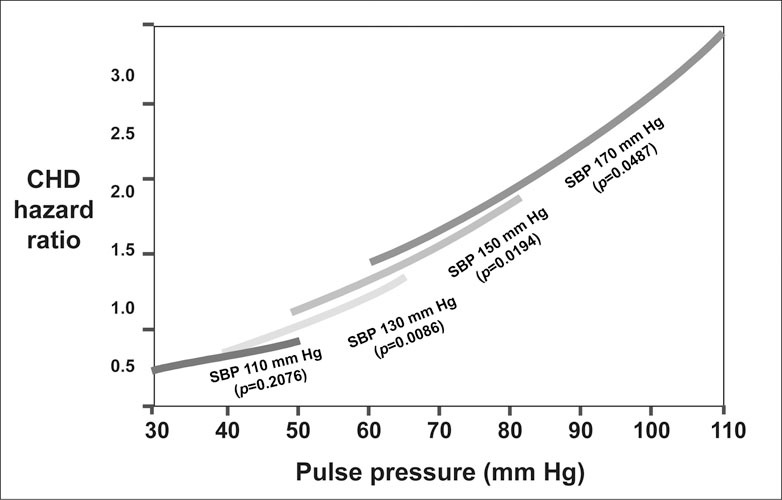
Joint influences of systolic blood pressure (SBP) and pulse pressure on coronary heart disease (CHD) risk, from the Framingham Heart Study. CHD hazard ratio was determined from level of pulse pressure within SBP groups. Hazard ratios were set to a reference value of 1.0 for SBP values of 110, 130, 150, and 170 mm Hg, respectively. All estimates were adjusted for age, sex, body mass index, cigarettes smoked per day, glucose intolerance, and total cholesterol/high‐density lipoprotein. Adapted from Circulation. 1999;100:354–360.
HYPERTENSION IN THE YOUNG VS. THE OLDER ADULT
Hypertension in young adults may initially be associated with high cardiac output/high stroke volume, which later transforms into a normal cardiac output/high peripheral vascular resistance hemodynamic pattern (Table III). In contrast, ISH in the elderly is usually associated with a normal or even low cardiac output and a combination of: 1) a small element of increased peripheral vascular resistance; and 2) markedly increased large artery stiffness. Hypertension can produce arterial stiffness by both functional and structural mechanisms. In young hypertensive individuals, there is increased distending pressure that stretches the load bearing elastic lamellae of the conduit arteries, whereby they become stiffer on a functional basis. In contrast, there are long‐standing intrinsic structural changes in the elderly with ISH that cause increased stiffness of the thoracic aorta and its branches and spare the more peripheral muscular arteries. As suggested by their age‐dependent divergent patterns of onset and differences in hemodynamics, diastolic hypertension (which is commonly referred to as essential hypertension) and ISH may be two distinct disorders with significant overlap. The conversion from diastolic hypertension to ISH in the older age group has been attributed to “burned‐out” diastolic hypertension. People with untreated or poorly treated diastolic hypertension at a younger age can develop ISH as they become older; however, data from the Framingham Heart Study suggest that only about 40% of patients acquire ISH in this manner. The majority of people who developed ISH (about 60%) do not go through a stage of diastolic hypertension, but evolve directly from high‐normal blood pressure. The bias toward DBP over SBP by earlier generations of physicians may be, in part, due to the emphasis on hypertension as a young person's condition. However, with the aging of the population during the past half century, hypertension has largely become a condition affecting older persons, i.e., those with the ISH subtype.
Table III.
Hypertension Differences Between the Young and the Older Patient
| Young Patients | Older Patients |
|---|---|
| Normal elastic tissue | Degenerated elastic tissue |
| Early ↑ and late normal cardiac output | Normal/↓ cardiac output |
| Early normal and late ↑ arteriolar vascular resistance | Normal/↑ arteriolar vascular resistance |
| ↑ Functional large artery stiffness | ↑↑ Anatomic large artery stiffness |
| ↑ In both systolic and diastolic blood pressure | ↑ Systolic and ↓ diastolic blood pressure |
| Isolated diastolic hypertension | Isolated systolic hypertension |
| Systolic‐diastolic hypertension | (↑ pulse pressure) |
| “Essential hypertension” | 40%“Burned‐out” essential hypertension; 60% de novo isolated systolic hypertension |
| ↑=increase(d); ↓=decrease(d) | |
References
- •. Nichols WW, O'Rourke ME McDonald's Blood Flow in Arteries. 5th ed. London, England: Hodder Arnold; 2005. [Google Scholar]
- •. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- •. Kass DA. Ventricular arterial stiffening. Hypertension. 2005;46:185–193. [DOI] [PubMed] [Google Scholar]
- •. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US Hypertensives ‐ Analysis based on NHANES III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- •. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- •. Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–360. [DOI] [PubMed] [Google Scholar]
- •. Franklin SS, Pio JR, Wong ND, et al. Predictors of new‐onset diastolic and systolic hypertension. The Framingham Heart Study. Circulation. 2005;111:1121–1127. [DOI] [PubMed] [Google Scholar]
- •. Staessen JA, Fagard R, Thijs L, et al. Subgroup and perprotocol analysis of randomized European Trial on Isolated Systolic Hypertension in the Elderly. Arch Intern Med. 1998;158:1681–1691. [DOI] [PubMed] [Google Scholar]
- •. Gueyffier F, Bulpitt C, Boissel JP, et al. Antihypertensive drugs in very old people: a subgroup meta‐analysis of randomized controlled trials. Lancet. 1999;353:793–796. [DOI] [PubMed] [Google Scholar]
- •. Blood Pressure Lowering Treatment Trialists' collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomized trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- •. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blockers vs diuretic. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]


