Abstract
Blood pressure is a major risk factor for cardiovascular events, although the role of pulse pressure, an independent predictor of arterial stiffness, has recently been emphasized. This study examines the baseline relationship between body mass index (BMI) and blood pressure indexes in 215 obese African Americans enrolled in a diet—exercise program. The subject population was 77% female, with a mean ± SD age of 46.7±10.7 years and a mean BMI of 42.5±7.5 kg/m2. In addition, the authors prospectively examined the effect of weight loss on cardiovascular parameters in a subset of 25 participants. The results show a closer significant correlation between pulse pressure and BMI (β=1.97 kgm−1; p=0.001) than between systolic blood pressure and BMI (β=1.58 kgm1; p=0.020). After 3 months of diet and exercise, average reductions were as follows: BMI, 4.2 kg/m2 (p<0.01); systolic blood pressure, 7.2 mm Hg (p<0.01); pulse pressure, 4.8 mm Hg (p<0.01); and cardiac output, 975 mL/min (p<0.01). Compliance index increased by 0.1 mL/mm Hg/m2 (p=0.03). The results highlight the potential value to cardiovascular health of a modest reduction in body weight in obese individuals.
The prevalence of obesity in the United States is on the rise among both blacks and whites, and is particularly high among blacks: 40% of black adults and 29% of white adults are clinically obese, classified as having a body mass index (BMI) ≥30 kg/m2. 1 , 2 , 3 Obesity increases the risk for cardiovascular (CV) disease through multiple mechanisms—including hypertension, diabetes, and dyslipidemia—disorders collectively referred to as the metabolic syndrome. 4 , 5 , 6 The importance of excess weight gain as a cause of high blood pressure (BP) has been demonstrated in experimental as well as clinical studies. 5 , 7 , 8
While increased BP has been established as a major risk factor for the development of CV events, 9 , 10 the role of pulse pressure (PP) in CV risk assessment has recently gained attention. 11 , 12 , 13 , 14 Domanski and colleagues 14 demonstrated that PP was an independent risk factor for stroke and all‐cause mortality in patients included in the Systolic Hypertension in the Elderly Program (SHEP). 15 Furthermore, a meta‐analysis 16 of three major systolic hypertension clinical trials (the European Working Party on High Blood Pressure in the Elderly, the Systolic Hypertension in European Trial [Syst‐Eur], and the Systolic Hypertension in China Trial), showed that a 10 mm Hg increase in PP increased the risk of CV mortality by nearly 20%. PP, defined as the difference between systolic BP (SBP) and diastolic BP (DBP), has also been associated with carotid stenosis, 17 coronary artery disease, congestive heart failure, 18 and left ventricular hypertrophy, 19 and has been implicated in the development and progression of large vessel arteriolosclerosis and small vessel disease. 20 , 21
An increased PP may be due to decreased arterial compliance and/or conditions associated with an increased stroke volume (SV).22–24 Causes of an increased SV may include aortic regurgitation, thyrotoxicosis, severe anemia, and arteriovenous shunts. 25 Decreased arterial compliance has been shown to increase PP by increasing SBP and decreasing DBP. 26 , 27
Several studies support the prognostic significance of 24‐hour ambulatory BP (ABP) monitoring. 28 , 29 , 30 This offers a convenient means of obtaining a large number of repeated BP measurements on each subject and thereby increases the statistical power to detect significant hemodynamic changes. The diurnal variation of ABP provides a measure of the percentage of BP readings that are elevated, the extent of BP decrease during sleep, and thus the overall BP load. Both cross‐sectional and longitudinal studies alike have shown that target organ damage accompanying hypertension is closely related to 24‐hour BP. 29 , 30 From a clinical perspective, interest may lie in the circadian BP profile over a 24‐hour period and differences in the profiles between groups or due to the impact of an intervention. While there are many studies on 24‐hour ABP, limited data exists for 24‐hour ambulatory PP.
The purposes of the present study were: 1) to examine and compare the effect of BMI on BP indexes; and 2) to examine the effect of a low‐saturated fat, low‐salt diet in conjunction with regular treadmill exercise for weight reduction on 24‐hour PP profile and other CV functions in a cohort of obese African Americans.
METHODS
Study Participants
Data were obtained from a cohort of African‐American participants who enrolled in a diet and exercise program for weight reduction conducted by Howard University's General Clinical Research Center. Each participant signed an informed consent approved by the University's Institutional Review Board. Details of the weight reduction program have been described previously. 31 , 32 Participants who enrolled in the weight reduction program were recruited from physicians' offices and clinics between August 1998 and July 2000. Inclusion criteria included a BMI ≥30 kg/m2. Exclusion criteria, determined by history, physical examination, and laboratory studies, included known metabolic and hormonal diseases that cause obesity and clinical conditions that would not permit moderate to vigorous exercise. Complete CV evaluations were performed by a cardiologist. Subjects with known coronary artery disease, ischemic coronary disease provoked by diagnostic treadmill exercise tests, a history or symptoms of heart failure, or electrocardiographic evidence of arrhythmia were excluded. Subjects with seizure disorders and/or musculoskeletal conditions precluding exercise and subjects unable to consume fat‐modified diets were also excluded. Height and weight were recorded, and BMI was calculated.
Diagnosis of Dyslipidemia, Hypertension, and Diabetes Mellitus
Participants were classified as having dyslipidemia if: 1) they were receiving treatment for dyslipidemia; 2) their total cholesterol was above 200 mg/dL; 3) their low‐density lipoprotein cholesterol was above 130 mg/dL; 4) their ratio of total cholesterol to high‐density lipoprotein cholesterol was greater than 4.5; or 5) their ratio of low‐density lipoprotein cholesterol to high‐density lipoprotein cholesterol was greater than 3.5. Participants on antihypertensive medications or those having office BPs greater than 140/90 mm Hg on three or more visits separated by at least 1 week were classified as having hypertension. Participants with fasting blood sugar levels ≥126 mg/dL, nonfasting blood sugar levels ≥200 mg/dL, or a history of diabetes mellitus requiring treatment were classified as having diabetes mellitus.
Hemodynamic Measurements
The arm circumference of each participant was measured and fitted with the appropriate size BP cuff, which was then appropriately positioned and connected to a SpaceLabs model 90207 ABP monitoring unit (SpaceLabs Medical, Inc., Issaqua, WA). The systems were programmed to obtain BP and heart rate (HR) readings every hour for 24 hours. Each subject was informed of the purpose and use of the 24‐hour ABP monitoring by an investigator who had demonstrated proficiency with the device. Participants were advised to continue their normal daily activities while they wore the ABP monitor and return in 24 hours. No changes were made to the participants' antihypertensive treatments during the 3‐month interval between baseline and repeat 24‐hour ABP evaluations. PP was calculated as the difference between the hourly ambulatory SBP and DBP for each subject over a 24‐hour period.
Within 5 days of acquiring the ABP measurements, SV and cardiac output were obtained by Doppler echocardiography. A two‐dimensional echocardiogram was used to determine the flow area of the aorta, and pulsed Doppler flow signal was used to determine velocity. Measurements were carried out in a controlled environment by the same experienced Doppler echocardiographer. Three to five steady‐state beats were averaged to determine SV, calculated as the product of the velocity‐time integral and flow area at the aortic root. 33 Cardiac output was derived as the product of average SV and the average 24‐hour HR. Arterial compliance was derived as the ratio of SV to the average 24‐hour PP. SV, cardiac output, and arterial compliance were normalized for body surface area to obtain stroke index, cardiac index, and compliance index, respectively.
Diet Composition and Exercise Routine
Participants were provided with meals specially formulated for the study, with a daily dietary composition of 1800–2000 calories, consisting of 58.7% carbohydrate, 25.3% total fat, 7% total saturated fat, 16% protein, and 2 g sodium. 32 These meals were prepared in 50 different menus, from which participants were allowed to select according to their preferences. Participants picked up their meals from the study center when they came in for exercise. They were instructed not to eat other food during the study period. Participants exercised on a treadmill for 45–60 minutes for at least three sessions per week. To ensure at least 80% compliance with the program, participants signed an “honor code” advising the investigators if they had not complied fully with the specially prepared diet. A sign‐in log at the research center was used to monitor exercise compliance.
Statistical Analysis
Pearson correlation coefficients were used to determine the relationship between selected variables. We examined and compared the effect of BMI on BP indexes (PP, SBP, DBP, and mean BP) in separate multiple regression analyses that included age, sex, and HR as covariates. Daytime BP was defined as the average reading from 6 a.m. to 10 p.m., and nighttime BP was defined as the average reading from 10 p.m. to 6 a.m. Individuals in whom the average daytime to nighttime reduction was less than 10% were classified as nondippers. The effect of BMI on the rate of nondipping was examined using logistic regression models that controlled for age, sex, HR, and the CV disease risk factors hypertension, diabetes mellitus, and dyslipidemia.
The paired t test was used for baseline and 3‐month comparisons. The 24‐hour PP and HR profiles were examined and described by fitting a Fourier series to the readings. 34 , 35 The Fourier curve is characterized by the average 24‐hour PP or HR value (the midline estimating statistic of rhythm), the rise above or below the average 24‐hour value (the amplitude), and the time‐to‐peak PP or HR (the acrophase), calculated in this study from 6:00 a.m. BP not only follows a circadian rhythm, but also varies from minute to minute, depending on levels of stress, physical activity, and other determinants of CV tone. In view of such individual variabilities, a random‐effect term was included to account for interindividual heterogeneity.
Based on baseline mean BMI of 42.6 and SD of 7.5 kg/m2, a sample size of 25 had adequate statistical power (at least 80%) to detect a 10% reduction in BMI over 6 months using methods based on the paired t test. All of the analyses were conducted using SAS software (version 8.1, SAS Institute, Inc., Cary, NC). All tests were two‐sided at the 5% level of significance. Data are generally expressed as mean ± SD.
RESULTS
The study population consisted of 215 African Americans with a mean age ± SD of 46.7±10.7 years and BMI of 42.5±7.5 kg/m2. All study participants were US born, aged 35–66 years. The participants were nondrinkers or social drinkers with an average of three to four drinks per month. Baseline demographic and clinical characteristics are shown in Table I. Hypertension was found in 66% of the participants, dyslipidemia in 36%, and diabetes mellitus in 24%. At baseline, the self‐reported daily dietary intake consisted of 2684.4±1733 kcal with 38.2% total fat. Table II compares the self‐reported baseline dietary nutrient information with what was provided during the intervention period.
Table I.
Demographic and Clinical Characteristics* of Participants (N=215)
| Age (years) | 46.7±10.7 |
| Female (n [%]) | 166 (77) |
| Body mass index (kg/m2) | 42.5±7.5 |
| Systolic blood pressure (BP) (mm Hg) | |
| 24‐Hour | 129.2±12.8 |
| Day | 131.1±12.7 |
| Night | 125.9±14.8 |
| Nondippers (n [%]) | 178 (82.8) |
| Diastolic BP (mm Hg) | |
| 24‐Hour | 78.8±9.6 |
| Day | 81.7±9.8 |
| Night | 73.8±11.3 |
| Nondippers (n [%]) | 99 (46.1) |
| Pulse pressure (mm Hg) | |
| 24‐Hour | 50.5±8.2 |
| Day | 49.3±8.6 |
| Night | 52.1±9.6 |
| Heart rate (bpm) | |
| 24‐Hour | 81.3±9.6 |
| Day | 83.6±10.4 |
| Night | 77.3±10.2 |
| Hypertension** (n [%]) | 141 (66) |
| Hyperlipidemia† (n [%]) | 78 (36) |
| Diabetes mellitus†† (n [%]) | 52 (24) |
| *Data are mean ± SD unless specified; **BP >140/90 mm Hg or on treatment; †total cholesterol ≥200 mg/dL or on treatment; ††fasting blood glucose ≥126 mg/dL or on treatment | |
Table II.
Dietary Nutrient Information* at Baseline (Self‐Reported) and During Intervention
| Daily Dietary Intake | Self‐Reported** | Intervention† |
|---|---|---|
| Calories (kcal) | 2684.4±1732.9 | 1978.3±193.0↓ |
| Portions of daily calories (%) | ||
| Protein | 12.3 | 15.9↑ |
| Carbohydrate | 69.4 | 58.7↓ |
| Total fat | 38.2 | 25.3↓ |
| Total saturated fat | 13.5 | 7.1↓ |
| Calcium (mg) | 766.3±489.8 | 804.6±225↑ |
| Sodium (mg) | 3487.9±2537 | 1738.0±561↓ |
| Potassium (mg) | 3319.9±1866 | 5113.4±317↑ |
| Arrows=decrease (↓) or increase (↑) compared to the self‐reported diet; *data are mean ± SD unless specified; **assessed with food questionnaires (Block 98.2; Berkeley Nutrition Services, Berkeley, CA); †analyzed with US Dept of Agriculture National Nutrient Database Software (Health Tech, Inc., Overland Park, KS). Adapted with permission from Atherosclerosis. 2004;172:155–160. 32 | ||
In univariate analysis, baseline BMI correlated positively with PP (r=0.28; p <0.001) and SBP (r=0.18; p=0.008), but not with DBP (r=0.01; p=0.922) or mean BP (r=0.08; p=0.232). There was a strong correlation between PP and SBP (r=0.67; p<0.001) and no correlation between PP and DBP (r=0.04; p=0.556). Eighty‐three percent of participants were SBP nondippers, and 46% were DBP nondippers. Consistent with other studies, 35 , 36 increasing BMI correlated positively with DBP nondipping. A 5 kg/ m2 rise in BMI increased the risk of DBP nondipping by 34% (relative risk, 1.34; 95% confidence interval, 1.06–2.01; p=0.02). There was no significant association between BMI and SBP nondipping.
In multivariable linear regression models, BMI correlated significantly with increasing PP (β=1.97; p=0.001) and SBP (β=1.58; p=0.020). There were no significant correlates with DBP (β=−0.35; p=0497) or mean BP (β=0.29; p=0.588). Because PP has a high correlation with SBP, examining the independent association between BMI and PP requires adjustment for SBP. After adjustment for SBP, a direct and consistent relationship persisted between BMI and PP (β=1.43; p=0.0001).
The average percent changes in BMI and the hemodynamic parameters for 25 participants who completed echocardiography and had 24 hourly ABP recordings at the same time at baseline and 3 months are shown in Table III. BMI reductions averaged 4.2 kg/m2 (9.8%; p<0.01). The BP reductions averaged 7.2 mm Hg (5.5%; p<0.01) for SBP, 3.3 mm Hg (3.9%; p=0.09) DBP, and 4.8 mm Hg (7.9%; p<0.01) for PP after 3 months on the program. Thus, the decrease in the magnitude of PP was a consequence of a greater decrease in SBP than in DBP. The correlation between percent reduction in BMI and that of SBP was 0.43 (p=0.03). The correlation was weaker between the reduction in BMI and the reduction in DBP (r=0.32; p=0.12). These observations are consistent with other studies. 37 In addition, there was a 5.9 bpm (5.7%; p<0.01) decrease in HR, a 975 mL/min (16.2%; p<0.01) decrease in cardiac output, and a 319 mL/min/m 2 (11.4%; p<0.01) decrease in cardiac index following weight loss. Though arterial compliance did not increase significantly, normalized for body size, arterial compliance index increased significantly (11.1%; p=0.03). SV and SV index did not change significantly.
Table III.
Percent Changes in Body Mass Index and Noninvasive Hemodynamic Measurements at 3 Months (n=25)
| Parameter | Baseline* | 3 Months* | Change (%) | p Value |
|---|---|---|---|---|
| Body mass index (kg/m2) | 42.8±6.4 | 38.6±5.9 | −9.8 | <0.01 |
| Body surface area (m2) | 2.2±0.2 | 2.1±0.2 | −5.2 | <0.01 |
| Systolic BP (mm Hg) | 131.9±12.9 | 124.7±10.8 | −5.5 | <0.01 |
| Diastolic BP (mm Hg) | 79.6±8.9 | 76.5±7.3 | −3.9 | 0.09 |
| Pulse pressure (mm Hg) | 52.7±8.8 | 48.0±7.3 | −7.9 | <0.01 |
| Heart rate (bpm) | 82.8±10.6 | 77.9±10.7 | −5.7 | <0.01 |
| Cardiac output (mL/min) | 5841±895 | 4866±807 | −16.2 | <0.01 |
| Cardiac index (mL/min/m2) | 2685.3±416.7 | 2366.2±414.1 | −11.4 | <0.01 |
| Stroke volume (mL) | 78.3±13.1 | 75.2±12.3 | −3.1 | 0.23 |
| Stroke volume index (mL/m2) | 36.7±7.1 | 36.2±7.0 | −2.5 | 0.68 |
| Arterial compliance (mL/mm Hg) | 1.3±0.4 | 1.4±0.4 | 0.9 | 0.56 |
| Arterial compliance index (mL/mm Hg/m2) | 0.7±0.2 | 0.8±0.2 | 11.1 | 0.03 |
| BP=blood pressure; *data are mean ± SD | ||||
Figure 1 shows the relationship between PP and heartbeat period (1/HR) at baseline and at 3 months. Each plotted point is an hourly average of 25 participants. As expected, there were significant positive correlations (r=0.47; p=0.02 and r=0.64; p<0.01 at baseline and at 3 months, respectively). Figure 1 further illustrates the role of the autonomic nervous system, in that at any given heartbeat period, the PP is smaller in the same patients after weight loss. The time course profile of the average PP is displayed in Figure 2. The graph shows no change in the pattern of PP profile at 3 months when compared with baseline.
Figure 1.
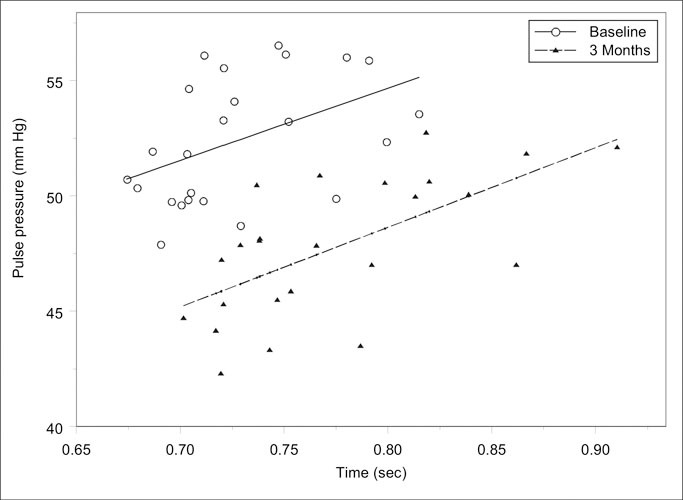
Relationship between pulse pressure and heartbeat period (1/heart rate x 60 sec) at baseline (r=0.47; p=0.022) and after 3 months (r=0.64; p<0.001) of intervention. Plotted points are hourly averages of 25 participants.
Figure 2.
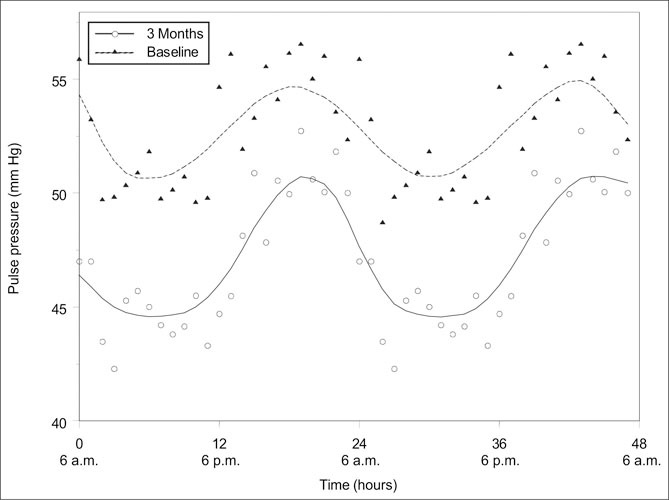
The effect of diet and exercise on pulse pressure profile after a 3‐month diet and exercise intervention. Note that there was no significant change in the pattern.
Analysis by Fourier series showed significant differences in the average 24‐hour PP value. The mean time‐to‐peak PP was slightly longer at 3 months than at baseline (14.2 hours vs. 13.6 hours; p=0.33), though not statistically significant. There was no significant difference in the PP amplitude. Similarly, there was a significant difference in the average 24‐hour HR value, but no statistically significant differences in either the mean time‐to‐peak or amplitude. Thus, there was little or no evidence that either the PP or the HR profiles differed in shape except for a downward shift from baseline. This observation is consistent with recent studies. 38
DISCUSSION
The present study was undertaken to examine and compare the effects of BMI on BP components as well as to investigate the impact of an exercise program in conjunction with a low‐saturated fat, low‐salt diet on PP and other cardiac functions in obese African Americans. The results indicate that BMI correlated directly with PP and SBP but not with DBP or mean BP. There was, however, a closer significant correlation of BMI with PP than with SBP. In these obese individuals, the rate of nondipping was about two times higher in SBP than DBP. In addition, BMI correlated significantly with DBP nondipping, but not with SBP. The underlying mechanisms for dipping are complex and are determined in part by changes in sympathetic nervous system activity. Though not apparent from the present study, the autonomic nervous system regulation of hemodynamic variables—including HR, resistance, SV, and arterial compliance, which influence systolic phase CV function and BP—is minimally affected during sleep. Its effect on DBP, in the passive phase of the cardiac cycle, is diminished and likely results in the greater rate of nondipping in SBP than DBP.
Our findings demonstrated that aerobic exercise and a special dietary program can result in a 9.8% decrease in BMI, which favorably altered PP and HR by decreasing their magnitudes while preserving their 24‐hour profiles. The identical 24‐hour PP profiles at baseline and after intervention are consistent with a recent study 38 and support the autonomic nervous system's regulation of this hemodynamic variable and the notion that BP follows a circadian pattern. The concomitant reduction in both PP and HR levels after 3 months of intervention suggests an alteration in the SV or CV biophysical properties or both. The slight delay in the time‐to‐peak PP is indicative of a decrease in the rate of increase of PP, which is consistent with an improvement in CV conditioning. The analyses further showed a significant decrease in cardiac output and increase in arterial compliance index. There was a minimal increase in arterial compliance and a decrease in SV.
The minimal increase in arterial compliance may be related to the fact that the pressure‐volume relationship of the aorta and arteries is inherently curvilinear even during the increase in pressure from minimum DBP to maximum SBP. At a decreased pressure, the corresponding increase in compliance is likely to result from working on a flat portion of the pressure‐volume curve, 39 , 40 and decreased pressure has also been shown to alter the composition of coronary vessels over time. 21 The small decrease in SV together with the small increase in compliance was, however, sufficient to significantly decrease PP. Changes in cardiac output during aerobic exercise are strongly influenced by HR more than SV. 39 In a given steady state, HR and SV, which define cardiac output, are expected to change in opposite directions, to maintain a constant rate of tissue perfusion, while BMI and SV are expected to change in the same direction. The opposing effect of BMI and HR on SV possibly accounted for the expected insignificant change in SV observed in our study. In the absence of a significant decrease in SV, the observed decrease in cardiac output relates to the decreased HR. The reduction in HR after undergoing the aerobic exercise routine was an expected outcome and may be related to a decrease in sympathetic nervous system activity. The observed decrease in PP in this study may be attributed to the improvement in arterial vascular properties. 39 , 41 , 42
The improvement in arterial properties represents the autonomic nervous system's adjustment of vasculartone. The role of the autonomic nervous system is illustrated in Figure 2, where for any given heartbeat period, the PP is smaller in the subject population after the 3‐month intervention.
The goal of hypertensive CV research is to identify and prevent or reduce factors that are predictive of CV morbidity and mortality. The present study did not assess the risk for an adverse CV outcome over time through an evaluation of conventional hemodynamic variables such as SBP, mean arterial pressure, total peripheral resistance, or mean 24‐hour ABP. Recent evidence has suggested a closer evaluation of PP as a reliable predictor of CV disease risk. 16 , 17 , 18 , 29 , 30
PP has been identified as an important marker of CV physiopathologic status. In all probability, this is because it reflects the changes from normal to abnormal vascular biology at all levels of the arterial system. It appears that to better understand the development of vascular abnormalities related to hypertension, acquisition and analysis of PP data will be superior to studying either SBP or DBP alone. Several studies have noted an association between PP and the risk of CV morbid events, and this association was independent of SBP and DBP. 43 , 44 , 45 Perhaps the observed higher PP during nighttime than daytime (Figure 2) may be related to such associations. Future studies should evaluate the ability of a 24‐hour PP load and variability to predict CV outcome.
The findings of the present study are limited by certain factors. There was a selection bias in that the participants were limited to a group of obese African‐American individuals motivated and able to participate in an exercise and weight‐reduction program. The prevalence of obesity is disproportionately higher among African Americans. 1 , 31 Furthermore, the major CV disease risk factors—including diabetes mellitus, hypertension, and dyslipidemia—are very common in these obese individuals. 46 The high prevalence of these comorbidities emphasizes the need to focus on obesity reduction in this population. Other limitations include confounding by factors not controlled for during the 3‐month intervention. Whether our findings would apply to individuals in general would therefore require further studies.
The underlying physiologic mechanism by which diet and exercise result in favorable changes in 24‐hour ABP is not clear from this study. Nevertheless, our prospective study shows that in these obese individuals, significant improvements in CV parameters were observed after weight loss. These observed improvements are consistent with a reduction in sympathetic nervous system activity and hyperperfusion as a means of decreasing the risk of developing CV disease. Other studies have shown improvement in left ventricular function and other cardiac functions after weight loss in obesity through dieting and exercise. 47 , 48 , 49 One study attributed adverse changes in hemodynamic variables to the activation of the autonomic nervous system. 50 The increased adrenergic activity was induced by the presence of excessive adipose tissue.
CONCLUSIONS
Our findings emphasize that changes in factors predictive of morbidity and mortality associated with obesity can be achieved through modest reduction in body weight with adherence to diet and regular exercise, which decrease PP—an indication of improvement in the biophysical properties of the arterial system.
Disclosure: This research was supported by the National Center for Research Resources/National Institutes of Health, Howard University General Clinical Research Center, No. MO1 RR10284.
References
- 1. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trend in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA. 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 3. Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States 1991–1998. JAMA. 1999;282:1519–1522. [DOI] [PubMed] [Google Scholar]
- 4. Wilson PW, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk‐the Framingham experience. Arch Intern Med. 2002;162:1867–1872. [DOI] [PubMed] [Google Scholar]
- 5. Hall JE, Crook ED, Jones DW, et al. Mechanisms of obesity‐ associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–137. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Krauss RM. for the AHA Nutrition Committee . American Heart Association call to action: obesity as a major risk factor for coronary heart disease. Circulation. 1998;97:2099–2100. [DOI] [PubMed] [Google Scholar]
- 7. Sundquist J, Winkeby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican‐American, and white women and men: analysis of NHANES III, 1988–1994. J Am Geriatr Soc. 2001;49:109–116. [DOI] [PubMed] [Google Scholar]
- 8. He Q, Ding YZ, Fong DY, et al. Blood pressure is associated with body mass index in both normal and obese children. Hypertension. 2000;36:165–170. [DOI] [PubMed] [Google Scholar]
- 9. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 10. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 11. Miwa Y, Tsushima M, Arima H, et al. Pulse pressure is an independent predictor for the progression of aortic wall calcification in patients with controlled hyperlipidemia. Hypertension. 2004;43:536–540. [DOI] [PubMed] [Google Scholar]
- 12. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: metaanalysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 13. Chae CU, Pfeffer MA, Glynn RJ, et al. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–643. [DOI] [PubMed] [Google Scholar]
- 14. Domanski MJ, Davis BR, Pfeffer MA. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. [DOI] [PubMed] [Google Scholar]
- 15. SHEP Cooperative Research Group. Prevention of stroke by antihypertension drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 16. Blacher J, Staessen JA, Girerd X. Pulse pressure not mean pressure determines cardiovascular risk in older hypertension patients. Arch Intern Med. 2000; 160:1085–1089. [DOI] [PubMed] [Google Scholar]
- 17. Franklin SS, Sutton‐Tyrell K, Belle S, et al. The importance of pulsatile components of hypertension in predicting carotid stenosis in older adults. J Hypertens. 1997;15:1143–1150. [DOI] [PubMed] [Google Scholar]
- 18. Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–136. [DOI] [PubMed] [Google Scholar]
- 19. Girerd X, Laurent S, Pannier B, et al. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am Heart J. 1991;122:1210–1214. [DOI] [PubMed] [Google Scholar]
- 20. Lyon RT, Runyon‐Hass A, Davis HR, et al. Protection from atherosclerotic lesion formation by reduction of artery wall motion. J Vasc Surg. 1987;5:59–67. [PubMed] [Google Scholar]
- 21. Baumbach GL, Siems JE, Heistad DD. Effects of local reduction in pressure on distensibility and composition of cerebral arterioles. Circ Res. 1991;68:338–351. [DOI] [PubMed] [Google Scholar]
- 22. Randall OS, Kwagyan J, Greene T, et al. The impact of pulse pressure on target organ function in African‐ Americans with non‐diabetic kidney diseases. Hypertension. 2004;44:497–564.Abstract P115. [Google Scholar]
- 23. Dart AM, Kingwell BA. Pulse pressure—a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. [DOI] [PubMed] [Google Scholar]
- 24. Randall OS. Effect of arterial compliance on systolic blood pressure and cardiac function. Clin Exp Hypertens A. 1982;4:1045–1057. [DOI] [PubMed] [Google Scholar]
- 25. O'Rourke RA, Shever JA, Salerni R, et al. The history, physical examination, and cardiac auscultation. In: Fuster V, Alexander WA, O'Rourke RA, eds. Hurst's The Heart. 10th ed. New York , NY :McGraw‐Hill;1998:221–343. [Google Scholar]
- 26. Randall OS, van den Bos GC, Westerhof. Systemic compliance: does it play a role in the genesis of essential hypertension? Cardiovasc Res. 1984;18:455–462. [DOI] [PubMed] [Google Scholar]
- 27. Randall OS, Esler MD, Calfee RV, et al. Arterial compliance in hypertension. Aust N Z J Med. 1976;6 suppl 2:49–59. [DOI] [PubMed] [Google Scholar]
- 28. Staessen JA, Thijs L, Fagard R. Predicting cardiovascular risk using conventional versus ambulatory blood pressures in older patients with systolic hypertension. JAMA. 1999;282:539–546. [DOI] [PubMed] [Google Scholar]
- 29. Verdecchia P, Schillaci G, Borgioni C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988. [DOI] [PubMed] [Google Scholar]
- 30. Mancia G, Zanchetti A, Agabiti‐Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment‐induced regression of left ventricular hypertrophy. Circulation. 1997;95:1464–1470. [DOI] [PubMed] [Google Scholar]
- 31. Randall OS, Randall D. Menu for Life: African Americans Get Healthy, Eat Well, Lose Weight and Live Beautifully. New York , NY :Random House Inc.; 2003:22. [Google Scholar]
- 32. Randall OS, Feseha HB, Illoh K, et al. Response of lipoprotein(a) to therapeutic life‐style change in obese African‐Americans. Atherosclerosis. 2004;172:155–160. [DOI] [PubMed] [Google Scholar]
- 33. Calafiore P, Stewart WJ. Doppler echocardiographic quantitation of volumetric flow rate. Cardiol Clin. 1990;8:191–202. [PubMed] [Google Scholar]
- 34. Staessen J, Fagard R, Thijs L. Fourier analysis of blood pressure profiles. Am J Hypertens. 1993;6:184S–187S. [DOI] [PubMed] [Google Scholar]
- 35. Chau NP, Mallion JM, de Gaudemaris R, et al. Twentyfour‐ hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. [DOI] [PubMed] [Google Scholar]
- 36. Staessen JA, Bieniaszewski L, O'Brien E, et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The ‘Ad Hoc’ Working Group. Hypertension. 1997; 29:30–39. [DOI] [PubMed] [Google Scholar]
- 37. Hagberg JM, Park J, Brown MD. The role of exercise training in the treatment of hypertension. Sports Med. 2000; 30:193–206. [DOI] [PubMed] [Google Scholar]
- 38. Hermida RC, Ayala DA, Iglesia M. Differences in circadian pattern of ambulatory pulse pressure between healthy and complication pregnancies. Hypertension. 2004;44:316–321. [DOI] [PubMed] [Google Scholar]
- 39. Burton AC. Physiology and Biophysics of the Circulation. Chicago , IL : Year Book Medical Publishers; 1972:160–174. [Google Scholar]
- 40. Monahan MK, Dinenno FA, Seals DR, et al. Smaller age‐associated reductions in leg venous compliance in endurance exercise‐trained men. Am J Physiol Heart Circ Physiol. 2001;281:H1267–H1273. [DOI] [PubMed] [Google Scholar]
- 41. Randall OS, Westerhof N, van den Bos GC, et al. Reliability of stroke volume to pulse pressure ratio for estimating and detecting changes in arterial compliance. J Hypertens Suppl. 1986;4:S293–S296. [PubMed] [Google Scholar]
- 42. Ferguson JJ, Randall OS. Hemodynamic correlates of arterial compliance. Cathet Cardiovasc Diagn. 1986;12:376–380. [DOI] [PubMed] [Google Scholar]
- 43. Dyer AR, Stamler J, Shekelle RB, et al. Pulse pressure III: prognostic significance in the four Chicago epidemiological studies. J Chronic Dis. 1982;35:283–294. [DOI] [PubMed] [Google Scholar]
- 44. Madhavan S, Ooi WL, Cohen H, et al. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infaction. Hypertension. 1994;23:395–401. [DOI] [PubMed] [Google Scholar]
- 45. Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. [DOI] [PubMed] [Google Scholar]
- 46. Randall OS, Retta RM, Kwagyan J, et al. Obese African Americans: the prevalence of dyslipidemia, hypertension, and diabetes mellitus. Ethn Dis. 2004; 14:384–388. [PubMed] [Google Scholar]
- 47. Dasgupta P, Ramhanmdany E, Brigden G, et al. Improvement in left ventricular function after weight loss in obesity. Eur Heart J. 1992;8:1060–1066. [DOI] [PubMed] [Google Scholar]
- 48. Ramhamadany E, Dasgupta P, Brigden G, et al. Cardiovascular changes in obese subjects on very low calorie diet. Int J Obes. 1989;13 suppl 2:95–99. [PubMed] [Google Scholar]
- 49. Alpert MA, Terry BE, Kelly DL. Effect of weight loss on cardiac chamber size, wall thickness and left ventricular function in morbid obesity. Am J Cardiol. 1985;55:783–786. [DOI] [PubMed] [Google Scholar]
- 50. Ferrannini E. The hemodynamics of obesity: a theoretical analysis. J Hypertens. 1992;10:1417–1423. [DOI] [PubMed] [Google Scholar]


