Abstract
This multicenter, prospective, open‐label, single‐arm study determined the efficacy and safety of irbesartan/hydrochlorothiazide (HCTZ) fixed combinations in patients (n=1005), aged 18 years and older, with uncontrolled systolic blood pressure (SBP) of 140–159 mm Hg (130–159 mm Hg for type 2 diabetes mellitus) after at least 4 weeks of antihypertensive monotherapy. Treatment was sequential: placebo (4–5 weeks), HCTZ 12.5 mg (2 weeks), irbesartan/HCTZ 150/12.5 mg (8 weeks), and irbesartan/HCTZ 300/25 mg (8 weeks). Enrolled patients (n=844) were aged 57.3±11.2 years; 52% were women, 23% were African American, and 14% were Hispanic. Thirty percent had type 2 diabetes mellitus, 46% had metabolic syndrome, and baseline blood pressure was 154.0±10.3/91.3±8.8 mm Hg. The mean change in SBP from placebo end to the primary end point, Week 18 (intent‐to‐treat population, n=736) was −21.5±14.3 mm Hg (p<0.001). The mean change in diastolic blood pressure (DBP) was −10.4 ±8.7 mm Hg (p<0.001). The mean Week 18 SBP/DBP was 132.9±13.8/81.1±9.7 mm Hg. Overall, 77% (95% confidence interval, 74%–80%) of patients achieved SBP goal (<140 mm Hg; <130 mm Hg for type 2 diabetes mellitus); 83% (95% confidence interval, 80%–86%) achieved DBP goal (<90 mm Hg; <80 mm Hg for type 2 diabetes mellitus); and 69% (95% confidence interval, 66%–72%) achieved dual SBP/DBP goal. Treatments were well tolerated. This irbesartan/HCTZ treatment regimen achieved SBP goals in more than 75% of patients uncontrolled on monotherapy.
Hypertension is a highly prevalent, modifiable cardiovascular disease risk factor affecting approximately 65 million adults in the United States (total prevalence of 31.3%) 1 and one billion people worldwide. 2 , 3 Since blood pressure (BP) remains uncontrolled in two thirds of hypertensive adults in the United States, 2 the public health returns from its successful treatment on a population‐wide scale will be substantial.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), 2 the consensus statement of the International Society on Hypertension in Blacks (ISHIB), 4 and the European Society of Hypertension‐European Society of Cardiology (ESH‐ESC) guidelines 5 recommend a goal of <140 mm Hg for systolic BP (SBP) and <90 mm Hg for diastolic BP (DBP) for the general population with uncomplicated hypertension. The goals are even lower (<130/80 mm Hg) for patients with diabetes or chronic renal disease. 2 , 4 , 5 , 6 , 7
Hypertension guidelines, based on experience from clinical trials, 8 , 9 , 10 , 11 , 12 , 13 recognize that many individuals will require two or more antihypertensive drugs at appropriate doses, either in fixed combination or as separate prescriptions, to achieve their BP goal. 2 , 4 , 5 , 6 , 7 Fixed combinations of antihypertensive agents may also have the potential to offer economic benefit to the patient, since only a single copayment is necessary for a drug combination product.
Combinations of an angiotensin receptor blocker (ARB) and hydrochlorothiazide (HCTZ) provide additive BP reductions through different mechanisms of action. The efficacy of HCTZ at high doses may be limited by a reactive rise in angiotensin II levels in response to reduced plasma volume, which may counterbalance the diuretic effect to some extent and result in a trend toward sodium and water retention and vasoconstriction. Although the resulting possible counterbalancing effect on BP may limit the full effects of the diuretic, on balance, high‐dose diuretics significantly lower BP. Simultaneously blocking the renin‐angiotensin‐aldosterone system with an ARB (or β blocker or angiotensin‐converting enzyme [ACE] inhibitor) may increase the effectiveness of the diuretic. ARB/HCTZ as well as ACE inhibitor/HCTZ and β blocker/HCTZ combinations are well tolerated; these agents tend to blunt changes in serum potassium and uric acid levels associated with higher doses of HCTZ. 14 , 15 Controlled clinical trials have demonstrated the antihypertensive efficacy and tolerability of treatment with several ARBs as well as ACE inhibitors and β blockers combined with HCTZ, including irbesartan, losartan, valsartan, eprosartan, telmisartan, candesartan cilexetil, olmesartan, enalapril, lisinopril, captopril, and bisoprolol. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
The ARB/HCTZ combination used in the current study, irbesartan/HCTZ, has been investigated in previous clinical trials. 14 , 25 , 26 , 27 , 28 In one of these trials, add‐on irbesartan produced further BP reductions in patients with hypertension uncontrolled on short‐term HCTZ 25‐mg 25 therapy, and in a factorial study investigating the dose–response effect of irbesartan 0, 37.5, 100, and 300 mg plus HCTZ 0, 6.25, 12.5, and 25 mg, the greatest mean reduction in SBP from baseline (23.1 mm Hg) was achieved with irbesartan/HCTZ 300/25 mg. 14
The Irbesartan/HCTZ Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial extends these findings by evaluating the antihypertensive efficacy and safety of a fixed combination of irbesartan/HCTZ at low dose (irbesartan/HCTZ 150/12.5 mg) and high dose (irbesartan/HCTZ 300/25 mg) in patients with SBP uncontrolled on a minimum of 4 weeks of antihypertensive monotherapy. The study population included, but was not limited to, patient groups in which goal attainment is particularly challenging, such as the elderly, African Americans, Hispanics/Latinos, patients with type 2 diabetes mellitus, and people with metabolic syndrome. INCLUSIVE followed the emphasis that JNC 7 places on the importance of SBP control, and on the wider use of combination therapy, by exploring the efficacy of a thiazide diuretic plus an ARB in lowering BP.
METHODS
Study Design
This multicenter, prospective, open‐label, single‐arm, four‐phase study was conducted at 119 sites across the United States between July 2003 and August 2004. Entry into each phase was conditional upon BP qualification criteria. Patients not meeting these criteria at the start of each phase—either because they had achieved their BP goal or their BP was higher than allowed by the protocol—were withdrawn.
Qualifying patients discontinued previous antihypertensive monotherapy and entered the sequential four‐phase treatment period as follows: 1) placebo for 4–5 weeks; 2) HCTZ 12.5 mg for 2 weeks (one tablet once daily); 3) irbesartan/HCTZ 150/12.5 mg for 8 weeks (one fixed‐combination tablet once daily); and 4) irbesartan/HCTZ 300/25 mg for 8 weeks (two irbesartan/HCTZ 150/12.5‐mg fixed‐combination tablets once daily). To enter the placebo, HCTZ 12.5‐mg, and irbesartan/HCTZ 150/12.5‐mg treatment periods, SBP was required to be 140–179 mm Hg, or 130–179 mm Hg for patients with type 2 diabetes mellitus. Per protocol, patients were titrated to irbesartan/HCTZ 300/25 mg at Week 10 if their SBP was 120–179 mm Hg. Patients with SBP below 120 mm Hg were discontinued from the study because of preset criteria. A lower SBP limit for this treatment period was set as a safety measure for this titration step; additionally, JNC 7 suggests that SBP risk starts at 115 mm Hg. 2 Patients were seen at Week 12 to ensure the safety of the higher dose. DBP was required to be 70–109 mm Hg throughout the study.
Patients were instructed to take their medication at 8 a.m. (±2 hours) except on the morning of a clinic visit, when medication was administered after BP had been measured. Seated BP was measured at trough (8 a.m. [±2 hours]) at each clinic visit using a validated, automatic Omron measurement device (Model HEM 705CP; Omron Healthcare Inc., Bannockburn, IL). This device fulfills the criteria for accuracy of the revised standard of the Association for the Advancement of Medical Instrumentation 29 and has an error rate of ±4 mm Hg. BP was calculated from the mean of three readings obtained 2 minutes apart.
The Institutional Review Board/Ethics Committee of each participating site approved the study. All patients gave written informed consent before enrollment.
Patients
Participants were men and women, at least 18 years of age, of self‐identified race/ethnic group, with hypertension and uncontrolled SBP on antihyper‐tensive monotherapy (140–159 mm Hg; 130–159 mm Hg for patients with type 2 diabetes mellitus). Monotherapy was defined as treatment with one antihypertensive medication for at least 4 weeks. Data were not available for patients on therapy for longer than this period. Fixed‐dose diuretic–diuretic combinations of HCTZ and triamterene were considered monotherapy.
Patients were excluded from the study if they had SBP >180 mm Hg, DBP >110 mm Hg, or secondary hypertension; hypertensive encephalopathy, stroke, or transient ischemic attack in the preceding year; myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, or unstable angina pectoris in the preceding 6 months; symptomatic resting bradycardia; other significant cardiac, hepatic, renal, or gastrointestinal disease; systemic lupus erythematosus; malignancy in the previous 5 years (except localized skin carcinoma); drug or alcohol abuse in the preceding 5 years; hypersensitivity to irbesartan, other ARBs, HCTZ, or other thiazide diuretics; or an arm circumference >17 inches. Also excluded were patients receiving insulin or drugs that might affect the efficacy or safety evaluations and women who were pregnant, lactating, or of childbearing potential and not using an approved method of contraception.
Efforts were made to recruit at least 100 patients in each of the following subpopulations: the elderly (at least 65 years of age), African Americans, Hispanics/Latinos, patients with type 2 diabetes mellitus (fasting plasma glucose ≥126 mg/dL and/or taking antidiabetic medication), and patients with the metabolic syndrome (three or more National Cholesterol Education Program [NCEP] 30 criteria, including hypertension [BP ≥130/85 mm Hg]).
Efficacy End Points
The primary efficacy end point was the mean change in SBP from the end of the placebo run‐in period (baseline) to the end of the irbesartan/HCTZ 300/25‐mg treatment period (Week 18).
Secondary end points included the mean change in DBP from baseline to Week 18 and the mean changes in SBP and DBP from baseline to irbesartan/HCTZ 150/12.5‐mg treatment end (Week 10). Mean change in SBP and DBP from baseline to HCTZ 12.5‐mg treatment end (Week 2) was also assessed, although it was not a predefined end point. BP goal attainment rates were assessed at Weeks 2, 10, and 18 according to the following definitions:
SBP goal: <140 mm Hg (<130 mm Hg for patients with type 2 diabetes mellitus);
DBP goal: <90 mm Hg (<80 mm Hg for patients with type 2 diabetes mellitus).
These definitions of BP goal are consistent with current hypertension management guidelines, including JNC 7 2 and those issued by the ISHIB, 4 the American Diabetes Association, 6 the National Kidney Foundation, 7 and the ESH‐ESC. 5
Safety Evaluations
Symptoms, severity, causal relationship to the study drugs, intervention, and outcome were documented for all adverse events. Clinical laboratory safety evaluations were conducted on blood and urine samples. Any clinically significant changes in physical examination or laboratory findings were recorded as adverse events.
Statistics
Allowing for a 20% dropout rate, a sample of 1042 patients was calculated to provide an estimate of the overall mean change in SBP from baseline to Week 18 to within 0.8 mm Hg of its true value, with a 95% confidence interval (CI). The SD for the mean change in SBP was estimated at 12 mm Hg.
Efficacy data were analyzed for the intent‐to‐treat (ITT) population, comprising patients who had at least one valid SBP measurement after taking one or more doses of irbesartan/HCTZ 150/12.5 mg. This allowed for an assessment of irbesartan/HCTZ (i.e., the ITT population excluded patients who only received HCTZ), as the trial was designed to assess the response to combination therapy in patients who had already failed a minimum of 4 weeks of antihypertensive monotherapy. Efficacy end points were calculated from the last observation carried forward for ITT patients who withdrew from the study before Week 18. The safety population was comprised of all patients taking at least one dose of study medication, including placebo.
Mean, SD, median, minimum, and maximum values were calculated for continuous variables. Approximate 95% CIs were calculated for point estimates of mean change scores in continuous variables. Mean changes in BP from baseline were tested using a paired t test for a normally distributed population. If sample data appeared to be selected from a population that was not normally distributed, the Wilcoxon signed rank test was employed. Goal attainment rates were calculated as frequency counts and percentages with 95% CIs. If a point estimate was <0.1 or >0.9, the upper and lower limits of the interval were calculated using alternative formulae. 31
RESULTS
Patient Characteristics
A total of 1568 patients were screened. Of these, 1005 entered the placebo phase (safety population), 844 completed the placebo phase and were enrolled into the HCTZ 12.5‐mg treatment phase, and 614 completed the 18‐week study period (Figure 1). A total of 391 patients were withdrawn or discontinued during the course of the study. Discontinuation was mainly because patients did not meet BP qualification criteria at the start of the next treatment period (n=219); this included patients who experienced a rise in BP above the predefined safety limits (n=45) and patients whose SBP or DBP was below the BP qualification limits (n=174). Adverse events accounted for 14.6% (n=57) of discontinuations.
Figure 1.
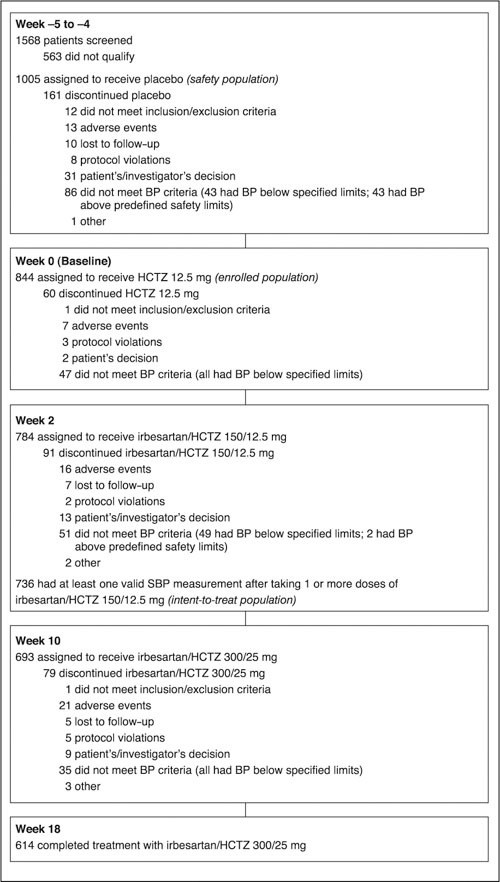
Patient disposition. BP=blood pressure; HCTZ=hydrochlorothiazide; SBP=systolic BP
Table I shows the demographic characteristics of the 844 patients enrolled. Mean age was 57.3 years, 436 (52%) were women, and mean BP was 154.0/91.3 mm Hg. Previous monotherapy included ACE inhibitors (34%), calcium channel blockers (20%), ARBs (20%), diuretics (14%), 0 blockers (11%), α blockers (1%), and other categories of antihypertensive drugs (1%). The most common concomitant medications for the ITT population (n=736) were hydroxymethylglutaryl‐coenzyme A reductase inhibitors (statins; n=151; 21%), platelet aggregation inhibitors (aspirin, thienopyridines; n=125; 17%), nonsteroidal anti‐inflammatory drugs (n=98; 10%), multivitamins (n=93; 13%), hypoglycemic agents (metformin; n=72; 10%), sulfonylureas (n=55; 7%), thiazolidinediones (n=24; 3%), and cyclooxygenase‐2 inhibitors (n=68; 9%).
Table 1.
Baseline Characteristics of the Enrolled Study Population
| Characteristic | Total population (n=844) |
|---|---|
| Age (yr) | |
| Mean (SD) | 57.3 (11.2) |
| Minimum, maximum | 24, 90 |
| <65 (n [%]) | 632 (75) |
| ≥65 (n [%]) | 212 (25) |
| Women (n [%]) | 436 (52) |
| Race/ethnic group (n [%])* | |
| Caucasian | 515 (61) |
| African American | 191 (23) |
| Hispanic/Latino | 119 (14) |
| Other | 21 (2) |
| Previous antihypertensive monotherapy (n [%])** | |
| β Blockers | 97 (11) |
| α Blockers | 11 (1) |
| Calcium channel blockers | 168 (20) |
| Angiotensin receptor blockers | 166 (20) |
| Angiotensin‐converting enzyme inhibitors | 283 (34) |
| Diuretics | 114 (14) |
| Other | 10 (1) |
| Medical history (n [%]) | |
| Hyperlipidemia | 406 (48) |
| Coronary artery disease | 63 (7) |
| Post‐myocardial infarction | 20 (2) |
| Other cardiac conditions | 147 (17) |
| Vascular disease | 14 (2) |
| Type 2 diabetes mellitus (n [%])† | 254 (30) |
| Metabolic syndrome (n [%])† | 386 (46) |
| Systolic blood pressure (mm Hg) | |
| Mean (SD) | 154.0 (10.3) |
| Minimum, maximum | 130, 179 |
| Diastolic blood pressure (mm Hg) | |
| Mean (SD) | 91.3 (8.8) |
| Minimum, maximum | 63, 109 |
| *Two patients self‐identified into more than one race/ethnic group. **Eight patients were categorized into more than one antihypertensive drug class (data missing for three patients). †Includes 177 patients with both metabolic syndrome and type 2 diabetes mellitus | |
Mean Changes in SBP and DBP From Baseline
For the ITT population, which excluded patients treated with HCTZ alone, mean SBP was 154.4±10.2 mm Hg at baseline and 132.9±13.8 mm Hg at the end of the irbesartan/HCTZ 300/25‐mg treatment period (Week 18). The mean change in SBP from baseline to Week 18 was −21.5±14.3 mm Hg (95% CI, −22.5 to −20.5 mm Hg; p<0.001) (Figure 2).
Figure 2.
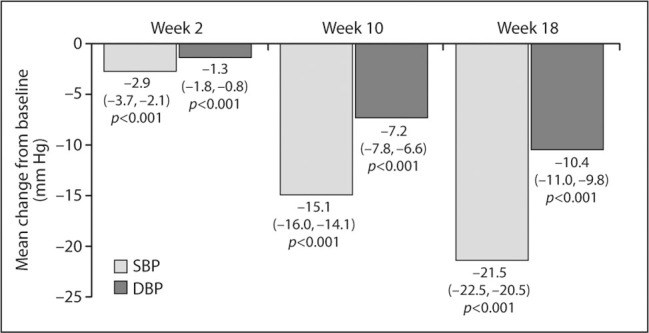
Mean change from baseline in systolic blood pressure (SBP) and dtastolic blood pressure (DBP) at the end of each treatment period. Week 2, end of hydrochlorothiazide (HCTZ) 12.5‐mg treatment; Week 10, end of irbesartan/HCTZ 150/12.5‐mg treatment; Week IS, end of irbesartan/HCTZ 300125‐mg treatment. Data are mean (95% confidence interval) changes from baseline for the intent‐to‐treat population (n=736); the last observation carried forward was used for Week 18 data.
At the end of HCTZ 12.5‐mg treatment (Week 2), mean SBP was 151.5±10.4 mm Hg; the mean change from baseline was −2.9±10.5 mm Hg (95% CI, −3.7 to −2.1 mm Hg; p<0.001). Mean SBP at irbesartan/HCTZ 150/12.5‐mg treatment end (Week 10) was 139.6±12.4 mm Hg; the mean change from baseline was −15.1±12.5 mm Hg (95% CI, −16.0 to −14.1 mm Hg; p<0.001).
DBP also decreased throughout the study. Mean DBP for the ITT population was 91.5±8.8 mm Hg at baseline, 90.1±9.1 mm Hg at Week 2, 84.6±8.8 mm Hg at Week 10, and 81.1±9.7 mm Hg at Week 18. The mean change in DBP from baseline was −1.3±6.8 mm Hg (95% CI, −1.8 to −0.8 mm Hg; p<0.001) to Week 2, −7.2±8.0 mm Hg (95% CI, −7.8 to −6.6 mm Hg; p<0.001) to Week 10, and −10.4±8.7 mm Hg (95% CI, −11.0 to −9.8 mm Hg; p<0.001) to Week 18 (Figure 2).
BP Goal Attainment Rates
Overall, 77% (95% CI, 74%–80%) of patients reached SBP goal, 83% (95% CI, 80%–86%) reached DBP goal, and 69% achieved both their SBP and DBP goals (95% CI, 66%–72%) by Week 18 (Table II). Forty‐eight percent of patients achieved the dual SBP/DBP goal by Week 10.
Table 2.
Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) Goal Attainment Rates (Intent‐to‐Treat Population)*
| Baseline to Week 2 (n=736) | Baseline to Week 10 (n=736) | Baseline to Week 18 (n=736) | Baseline to Week 18 95% CI | |
|---|---|---|---|---|
| SBP goal (n [%]) | 19 (3) | 411 (56) | 569 (77) | 74%, 80% |
| DBP goal (n [%]) | 264 (36) | 518 (70) | 610 (83) | 80%, 86% |
| SBP and DBP goal (n [%]) | 13 (2) | 353 (48) | 507 (69) | 66%, 72% |
| CI=confidence interval; *percentage of patients achieving SBP or DBP goal at the end of hydrochlorothiazide (HCTZ) 12.5‐mg (Week 2); irbesartan/HCTZ 150/12.5‐mg (Week 10); and irbesartan/HCTZ 300/25‐mg treatment (Week 18). SBP goal: <140 mm Hg (<130 mm Hg for patients with type 2 diabetes mellitus); DBP goal: <90 mm Hg (<80 mm Hg for patients with type 2 diabetes mellitus) | ||||
Safety and Tolerability
All 1005 patients entering the placebo run‐in period were evaluated for safety. Study medications were well tolerated, with the majority of adverse events being mild or moderate, transient, and considered unrelated to the study medication. Overall, 551 patients (55%) experienced an adverse event: 240 (24%) during placebo treatment, 144 (17%) during HCTZ 12.5‐mg treatment, 212 (27%) during irbesartan/HCTZ 150/12.5‐mg treatment, and 178 (26%) during irbesartan/HCTZ 300/25‐mg treatment. Table III lists adverse events reported in ≥1% of the safety population. Three patients experienced hyperkalemia: one during treatment with HCTZ 12.5 mg (most likely data artifact or laboratory error), one during treatment with irbesartan/HCTZ 150/12.5 mg, and one during treatment with irbesartan/HCTZ 300/25 mg. In addition, four patients experienced hypokalemia: two during irbesartan/HCTZ 150/12.5‐mg treatment and two during irbesartan/HCTZ 300/25‐mg treatment. None were discontinued from the study.
Table 3.
Adverse Events Reported by >1% of Patients During any Treatment Phase (Safety Population)
| Treatment Period | |||||
|---|---|---|---|---|---|
| Placebo | HCTZ 12.5 mg | Irbesartan/HCTZ 150/12.5 mg | Irbesartan/HCTZ 300/25 mg | Total | |
| Patients in safety population (n) | 1005 | 844 | 782 | 693 | 1005 |
| Adverse event (n [%])* | |||||
| Headache | 54 (5) | 6 (<1) | 12 (2) | 11 (2) | 76 (8) |
| Dizziness | 10 (1) | 9 (1) | 16 (2) | 18 (3) | 50 (5) |
| Upper respiratory tract infection | 20 (2) | 3 (<1) | 14 (2) | 7 (1) | 43 (4) |
| Nasopharyngitis | 18 (2) | 8 (<1) | 6 (<1) | 10 (1) | 39 (4) |
| Sinusitis | 17 (2) | 6 (<1) | 9 (1) | 7 (1) | 37 (4) |
| Influenza | 5 (<1) | 4 (<1) | 9 (1) | 3 (<1) | 22 (2) |
| Arthralgia | 3 (<1) | 4 (<1) | 3 (<1) | 9 (1) | 20 (2) |
| Fatigue | 2 (<1) | 5 (<1) | 3 (<1) | 8 (1) | 18 (2) |
| Hypotension | 0 | 0 | 1 (<1) | 8 (1) | 10 (1) |
| HCTZ=hydrochlorothiazide; *some patients may have experienced more than one adverse event | |||||
Twenty‐seven patients (3%) experienced a serious adverse event during the study period, including two deaths (described below). Of the 25 serious adverse events, excluding the deaths, three occurred during placebo treatment, four during HCTZ 12.5‐mg treatment, eight during irbesartan/HCTZ 150/12.5‐mg treatment, and seven during irbesartan/HCTZ 300/25‐mg treatment. For the three remaining adverse events, the particular treatment phase of occurrence was not captured because adverse events were attributed to treatment periods based on the last recorded date of study medication, even if further study drug had been dispensed. The relationship of one serious adverse event (hypotension) to study medication (irbesartan 150 mg/HCTZ 12.5 mg) was considered probable; all other serious adverse events were judged unrelated to the study medication.
There were three deaths. One patient died from a myocardial infarction while on placebo, and a second patient was involved in a fatal motor vehicle accident while on irbesartan/HCTZ 150/12.5 mg. In addition, one patient had a cardiac arrest from natural causes; since this occurred following enrollment but before commencement of any study treatment, the patient was not included in the safety population. The deaths were not considered related to the study medications.
DISCUSSION
The INCLUSIVE trial has shown that a treatment regimen with irbesartan/HCTZ combination therapy leads to substantial reductions in SBP that allow SBP goals to be attained in the majority of patients previously uncontrolled on a minimum of 4 weeks of antihypertensive monotherapy. A statistically significant mean change in SBP of −21.5 mm Hg was observed between baseline and Week 18; final SBP at Week 18 was 132.9 mm Hg.
INCLUSIVE enrolled a large population of patients typical of those seen in clinical practice. One third of the study population had been previously uncontrolled on ACE inhibitors, reflecting the relatively poor control rates achieved with ACE inhibitor monotherapy observed in the general population. 32 Interestingly, only 14% had previously been treated with diuretic monotherapy, the recommended first‐line agent for hypertension. A filter study design allowed selection of patients with uncontrolled SBP after at least 4 weeks of antihypertensive monotherapy and after 2 weeks of treatment with HCTZ 12.5 mg. This enabled direct evaluation of the add‐on effect of irbesartan 150 mg and up‐titration to irbesartan/HCTZ 300/25 mg. The study design and BP goals were also consistent with current hypertension management recommendations, including JNC 7, which advocates the wider use of combination therapy, 2 , 4 , 5 , 6 , 7 and with current labeling for the drug. 33
The SBP goal attainment rate following HCTZ 12.5‐mg monotherapy was only 3%, which may be due, at least in part, to the short (2‐week) treatment period. The degree of BP decline with HCTZ alone in this study was also considerably less than reported in other studies. As noted, evidence suggests that many patients require more than one drug to attain BP control. 8 , 9 , 10 , 11 , 12 , 13 The addition of irbesartan 150 mg, a drug with a complementary mechanism of action, and titration to irbesartan/HCTZ 300/25 mg, if necessary, allowed the majority (77%) of patients to achieve their SBP goal. This is consistent with previous studies showing that diuretics enhance the efficacy of other anti‐hypertensive drug classes, including ARBs, ACE inhibitors, and β blockers. The effects on SBP are clinically important, given the emphasis that recent guidelines place on SBP goal attainment and the projected increase in the prevalence of systolic hypertension as the US population ages. Also of clinical relevance is that the SBP goal attainment rate was observed within a diverse study population, including a substantial number of patients recognized as being more challenging in terms of achieving BP goal. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 8 reported almost equivalent BP‐lowering effects and cardiovascular outcomes with a diuretic (chlorthalidone), a calcium channel blocker (amlodipine), and an ACE inhibitor (lisinopril) across high‐risk hypertensive subgroups, including older patients, African Americans, and patients with diabetes. 34
Sixty‐nine percent of patients enrolled in the INCLUSIVE trial achieved both their SBP and DBP goals. This is similar to findings in the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) 11 and ALLHAT 8 studies; more than one medication was necessary in most patients in these trials to achieve goal BPs. In comparison, an analysis of the US National Health and Nutrition Examination Survey (NHANES) 1999–2000 revealed that only 31.0% (53.1% of those on antihypertensive treatment) achieved the dual BP goal. 35 The use of irbesartan/HCTZ combination therapy might therefore be a convenient treatment option to help more patients achieve their BP goals and to simplify the treatment of hypertension across a broad patient base. Although INCLUSIVE enrolled only patients uncontrolled on monotherapy, it is recognized that a substantial proportion of patients are uncontrolled even on multiple therapies, especially those at high cardiovascular risk. 36 , 37 , 38 , 39
All treatments were well tolerated in this heterogeneous population of patients with hypertension, with most adverse events being of mild or moderate intensity and transient in duration. In general, there was no evidence of an increase in side effects as patients were titrated from one treatment level to the next; exceptions were dizziness and hypotension, although their overall incidence was low. This is in line with the proven tolerability profiles of irbesartan and other ARBs when administered alone or in combination with HCTZ. 40
Several limitations of the current study should be noted. The active treatment period was relatively short (a total of 18 weeks, with 8 weeks for each of the irbesartan/HCTZ combinations), allowing only short‐term evaluation of efficacy and tolerability. The long‐term (1 year) safety and efficacy of irbesartan/HCTZ, 27 however, as well as other ARB/diuretic combination therapies, has been reported previously. It is also conceivable that the 2‐week HCTZ monotherapy treatment period may have been insufficient for the maximal antihypertensive effects of the drug to be expressed. This may account for the observation that the BP reductions achieved with HCTZ 12.5‐mg monotherapy in INCLUSIVE were lower than previously reported. 41 The goal attainment rates observed in the current study might be higher than can be achieved in general practice. This is because patients volunteering for clinical trials are likely to be more adherent with clinic visits and treatment regimens than the general population of hypertensive patients. In addition, there was no control group, an open study design was used, and patients were not randomized to treatment. Although there was not a randomized control group in INCLUSIVE, however, the initial placebo and HCTZ phases, and the measurement of BP with a validated automatic manometer, should have minimized potential sources of bias and regression to the mean. In addition, the BP reductions observed in INCLUSIVE were similar to previous randomized controlled studies with combinations of ARBs and HCTZ.
CONCLUSION
INCLUSIVE demonstrated that physicians can achieve goal BP among a diverse population of patients (including the elderly, African Americans, Hispanics/Latinos, patients with type 2 diabetes mellitus, and those with metabolic syndrome) whose SBP is uncontrolled on a minimum of 4 weeks of antihypertensive monotherapy by following a treatment algorithm with ARB/diuretic combination therapy. The use of irbesartan/HCTZ therapy in fixed combination at low (150/12.5 mg) or high (300/25 mg) dose is likely to produce SBP control in more than three quarters of patients previously uncontrolled on at least 4 weeks of anti‐hypertensive monotherapy.
Acknowledgments and disclosures:
The authors thank Elaine Griffin, DPhil, who provided medical writing services on behalf of the Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership. The clinical study considered by this publication was sponsored by the Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership. The sponsor provided funding and in‐kind support for the clinical trial and for the publication. The sponsor participated in discussions with the INCLUSIVE Trial Steering Committee regarding study design and protocol development and provided logistic support during the study. Integrium, a contract research organization under contract with the sponsor, monitored the study and site management. PAREXEL, a contract research organization under contract with the sponsor, conducted data entry and performed statistical analysis of the study data. Dr. Neutel and Dr. Weber have an interest in and provide consulting services to Integrium, the clinical research organization that provided clinical management for this trial.
APPENDIX
In addition to the authors, the INCLUSIVE Investigators are as follows: L. Altschul, Z. Ansari, P. Arcuri, H. Bays, H. Biermann, N. Bittar, J. Bloom, K. Bordenave, D. Brautigam, P. Buchanan, A. Carr, J. Champlin, D. Cheung, S. Chrysant, J. Clower III, G. Collins, M. DeBruin, E. Denoia, V. Desai, M. Dewan, M. El Shahawy, A. Elkind, R.D. Ferrera, J. Fidelholtz, N. Fraser, L. Gidday, L. Gilderman, R. Ginsburg, E. Hare, D. Hassman, M. Heitbrink, M. Henriquez, J. Herron, G. Hilliard, P. Hughes, H. Ibrahim, T. Isakov, W. Jacobs, J. Jernigan, L. Judy, D. Kereiakes, B. Kerzner, L. Koehler, M. Koren, M. Kozinn, E. Kunst, G. Lankin, A. Lewin, T. Littlejohn III, B. Lubin, R. Madder, R. Marple, J. Mersey, F. Messerli, N. Messina III, M. Mollen, P. Narayan, S. Nesbitt, S. Ong, S. Oparil, R. Owens, C. Patel, M. Peshimam, F. Pettyjohn, A. Phillips, I. Plisco, M. Pohl, J. Quesada, G. Raad, B. Rankin, D. Riff, S. Rosansky, E. Roth, G. Ruoff, J. Sandoval, J. Saponaro, S. Shahzad, K. Sheehan, T. Sherraden, D. Sica, J. Siemienczuk, N. Singh, G. Smith, W. Smith, J. Soufar, W. Starling, K. Stone, C. Strout, R. Strzinek, D. Sugimoto, G. Szenkiel, R. Tidman, M. Tonkon, M.S. Touger, H. Underwood, C. Uy, J. Wadleigh, V. Wassily, J. Wayne, V. Weber, R. Weiss, N. Winer, D. Young, J. Zebrack.
References
- 1. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 2. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 3. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 4. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. [DOI] [PubMed] [Google Scholar]
- 5. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 7. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 8. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 9. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 10. Wright JT Jr, Agodoa L, Contreras G, et al. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2002;162:1636–1643. [DOI] [PubMed] [Google Scholar]
- 11. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. [DOI] [PubMed] [Google Scholar]
- 12. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 13. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 14. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 15. McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clin Ther. 2001;23:833–850. [DOI] [PubMed] [Google Scholar]
- 16. Benedict CR. Safe and effective management of hypertension with fixed‐dose combination therapy: focus on losartan plus hydrochlorothiazide. Int J Clin Pract. 2000;54:48–54. [PubMed] [Google Scholar]
- 17. Chrysant SG. Fixed combination therapy of hypertension: focus on valsartan/hydrochlorothiazide combination (Diovan/HCT). Expert Rev Cardiovasc Ther. 2003;1:335–343. [DOI] [PubMed] [Google Scholar]
- 18. Croom KF, Curran MP, Goa KL, et al. Irbesartan: a review of its use in hypertension and in the management of diabetic nephropathy. Drugs. 2004;64:999–1028. [DOI] [PubMed] [Google Scholar]
- 19. Bohm M, Sachse A. Safety and tolerability of eprosartan in combination with hydrochlorothiazide. Drug Saf. 2002;25:599–611. [DOI] [PubMed] [Google Scholar]
- 20. Maillard M, Burnier M. Telmisartan/hydrochlorothiazide: a new fixed dose combination. Expert Rev Cardiovasc Ther. 2005;3:375–386. [DOI] [PubMed] [Google Scholar]
- 21. Melian EB, Jarvis B. Candesartan cilexetil plus hydrochlorothiazide combination: a review of its use in hypertension. Drugs. 2002;62:787–816. [DOI] [PubMed] [Google Scholar]
- 22. Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother. 2004;5:657–667. [DOI] [PubMed] [Google Scholar]
- 23. Waeber B. Combination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertension. Expert Rev Cardiovasc Ther. 2003;1:43–50. [DOI] [PubMed] [Google Scholar]
- 24. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–462. [DOI] [PubMed] [Google Scholar]
- 25. Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. [DOI] [PubMed] [Google Scholar]
- 26. Howe P, Phillips P, Saini R, et al., on behalf of the Irbesartan Multicenter Study Group . The antihypertensive efficacy of the combination of irbesartan and hydrochlorothiazide assessed by 24‐hour ambulatory blood pressure monitoring. Clin Exp Hypertens. 1999;21:1373–1396. [DOI] [PubMed] [Google Scholar]
- 27. Raskin P, Guthrie R, Flack J, et al. The long‐term antihypertensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens. 1999;13:683–687. [DOI] [PubMed] [Google Scholar]
- 28. Coca A, Calvo C, Sobrino J, et al. Once‐daily fixed‐combination irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003;25:2849–2864. [DOI] [PubMed] [Google Scholar]
- 29. O'Brien E, Mee F, Atkins N, et al. Evaluation of three devices for self‐measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM‐705CP, Philips HP5332, and Nissei DS‐175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- 30. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 31. Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed.. New York, NY: John Wiley & Sons; 1981. [Google Scholar]
- 32. Materson BJ, Reda DJ, Cushman WC, for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents . Department of Veterans Affairs single‐drug therapy of hypertension study. Revised figures and new data. Am J Hypertens. 1995;8:189–192. [DOI] [PubMed] [Google Scholar]
- 33. Bristol‐Myers Squibb Company. Sanofi‐Synthelabo. Avalide United States prescribing information. Available at: http:www.avalide.com. Accessed April 25, 2005.
- 34. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 35. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 36. Knight EL, Bohn RL, Wang PS, et al. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension. 2001;38:809–814. [DOI] [PubMed] [Google Scholar]
- 37. Mallion JM, Genes N, Vaur L, et al. Blood pressure levels, risk factors and antihypertensive treatments: lessons from the SHEAF study. J Hum Hypertens. 2001;15:841–848. [DOI] [PubMed] [Google Scholar]
- 38. Amar J, Chamontin B, Genes N, et al. Why is hypertension so frequently uncontrolled in secondary prevention? J Hypertens. 2003;21:1199–1205. [DOI] [PubMed] [Google Scholar]
- 39. Amar J, Vaur L, Perret M, et al. Hypertension in high‐risk patients: beware of the underuse of effective combination therapy (results of the PRATIK study). J Hypertens. 2002;20:779–784. [DOI] [PubMed] [Google Scholar]
- 40. Meredith PA. Angiotensin II receptor antagonists alone and combined with hydrochlorothiazide: potential benefits beyond the antihypertensive effect. Am J Cardiovasc Drugs. 2005;5:171–183. [DOI] [PubMed] [Google Scholar]
- 41. Neutel JM. Metabolic manifestations of low‐dose diuretics. Am J Med. 1996;101:71S–82S. [DOI] [PubMed] [Google Scholar]


