Abstract
Currently 247 million people are living with chronic HBV infection (CHB), and the development of novel curative treatments is urgently needed. Immunotherapy is an attractive approach to treat CHB, yet therapeutic approaches to augment the endogenous HBV-specific T cell response in CHB patients have demonstrated little success. Here, we show that strain 68–1 rhesus cytomegalovirus vaccine vectors expressing HBV antigens (RhCMV/HBV) engender HBV-specific CD8+ T cells unconventionally restricted by major histocompatibility complex (MHC) class II and the non-classical MHC-E molecule in rhesus macaques (RM). Surface staining of human donor (HD) and RM primary hepatocytes (PH) ex vivo revealed the majority of PH expressed MHC-E, but not MHC-II. HBV-specific, MHC-E-restricted CD8+ T cells from RhCMV/HBV-vaccinated RM recognized HBV-infected PH from both HD and RM. These results provide proof-of-concept that MHC-E-restricted CD8+ T cells could be harnessed for the treatment of CHB, either through therapeutic vaccination or adoptive immunotherapy.
INTRODUCTION
CHB is a major global health concern, affecting 247 million individuals worldwide and causing 887,000 deaths annually (1). While there is an effective prophylactic vaccine available, 10–15% of individuals do not respond adequately to vaccination and are not protected against HBV infection (2). CHB can lead to progressive liver dysfunction, cirrhosis, and in some cases hepatocellular carcinoma. There are multiple treatment options for CHB, including pegylated-IFNα and reverse-transcriptase inhibitors (3), but these treatments are rarely curative (4). Thus, there is an urgent global need to develop curative therapies for HBV.
Developing cellular immunotherapeutic strategies for CHB is supported by the fact that 90–95% of acutely HBV-infected adults mount broad, highly functional HBV-specific T cell responses and subsequently clear infection (5–7). In contrast, patients progressing to CHB exhibit narrowly focused, low-frequency, functionally exhausted HBV-specific T cell responses (8–10). Therefore, many immunotherapeutic strategies currently in development focus on augmenting HBV-specific T cell immunity.
T cell-based immunotherapies for CHB must overcome or circumvent T cell exhaustion and provide sustained viral suppression or clearance. Given the difficulty in reversing the immunological tolerance of established HBV-specific T cells in CHB patients, the most effective way to augment HBV-specific T cell immunity may be to engender or impart a completely unique set of HBV-specific T cell responses through therapeutic vaccination or adoptive T cell therapy. We recently discovered that strain 68–1 RhCMV vectors engineered to express antigenic targets elicit broad, effector-memory CD8+ T cell responses exclusively restricted by either MHC-II or by MHC-E, a highly conserved, monomorphic, “non-classical” MHC class Ib molecule (11). Importantly, RhCMV-based vaccines eliciting such unconventionally restricted CD8+ T cells have been shown to protect rhesus macaques (RM) against SIV, TB and malaria (12–14).
MHC-E (HLA-E in humans) is an MHC-Ib molecule that binds a conserved peptide (VMAPRTL(L/V/I)L, VL9) encoded in the signal sequence of polymorphic MHC-Ia molecules (15). The MHC-E/VL9 complex then serves as a ligand for the NK cell receptors NKG2A and NKG2C (16). Since MHC-E protects against NK cell activity by engaging the inhibitory receptor NKG2A it is often upregulated by chronic pathogens or in cancer (17–19). The unique ability of CMV-based vectors to elicit MHC-E-restricted CD8+ T cells enables the specific targeting of pathogens or cancer cells via MHC-E for the first time, thus potentially turning NK cell evasion into a T cell vulnerability.
Here, we explored the possibility that HBV-infected PH could be targeted by MHC-E-restricted CD8+ T cells. This innovative strategy is particularly attractive for HBV, since such T cells are not known to be naturally induced by HBV and as such would not be subjected to immunological tolerance. Moreover, the high conservation of MHC-E could allow for the use of universal T cell receptor-based therapies, overcoming the major limitation of disparate HLA genotypes in conventional T cell therapies. However, very little is known about MHC-E expression on HBV-infected PH. Moreover, it is unknown whether HBV antigen-derived peptides would be accessible to MHC-E-restricted CD8+ T cells. To address these questions, we elicited HBV-specific, MHC-E-restricted CD8+ T cells in RM using RhCMV-based vectors expressing HBV antigens. Importantly, we demonstrate that the majority of rhesus and human HBV-infected PH express MHC-E and can be recognized by HBV-specific MHC-E-restricted CD8+ T cells. These data thus suggest a new paradigm for the treatment of HBV infection that theoretically could be universally applied to all patients given the extreme conservation of MHC-E across humans and macaques (11).
MATERIALS AND METHODS
Construction of RhCMV/HBV vectors.
Genotype D, serotype ayw HBV core, polymerase and S antigen gene fragments were isolated by PCR from previously described plamids (kindly provided by Frank Chisari, Scripps Research Institute). The N-terminal 333 amino-acids (AA) of polymerase obtained from plasmid pCDNA3-POL/ENV (20) were C-terminally HA-epitope tagged and fused by PCR-mediated mutagenesis to the C-terminal 228 AA of the S-antigen obtained from plasmid pCMV-S2/S (21) to generate fusion S/PolN (left forward primer: CATCGAGCTAGCACCATGGAGAACATCACATCAGG, left reverse primer: GTGTTGATAGGATAGGGGAATGTATACCCAAAGAC; right forward primer: GTCTTTGGGTATACATTCCCCTATCCTATCAACAC, right reverse primer: GGAATCGTCGACTCAAGCGTAATCTGGAACATCGTATGGGTAAAGATTGACGATAAGGGAGAGGCAG). The final PCR product was blunt-end cloned into either pJet vector (Thermo Fisher Scientific) to be a template for BAC recombineering or into pORI to evaluate expression. The C-terminal 416 AA of polymerase obtained from plasmid pCDNA3-POL/ENV (20) was HA-epitope tagged by PCR-mediated mutagenesis and inserted into pORI (forward primer: GTGGTACCCTCGAGGATTGGGGACCCTGCGCTGAACATGGAG, reverse primer: TCAGTCGACCTAAGCGTAATCTGGAACATCGTATGGGTAC). The gene encoding Core was PCR amplified from plasmid pCDNA-CORE (22) and inserted into pORI (forward primer: CTGCTAGCATGGACATTGACCCTTATAAAGAATTTGG reverse primer: CTAGGTACCACATTGAGATTCCCGAGATTGAG). The C-terminal polymerase fragment was then inserted downstream of Core using KpnI and SalI to generate fusion protein Core/PolC. The KpnI site adds a two amino acids (GT) linker between the two proteins.
To generate 68–1 RhCMV/Core/PolC and 68–1 RhCMV/S/PolN we replaced the pp71-encoding Rh110 gene in the 68–1 RhCMV bacterial artificial chromosome (BAC) (23) using a modified galactokinase (galK) selection system, a two-step method that allows DNA modification without introducing unwanted heterologous sequences (24). We recently demonstrated that replacement of Rh110 can be used to elicit robust antigen responses while attenuating the 68–1 RhCMV vector (25). To delete Rh110, competent SW105 bacteria containing the 68–1 RhCMV BAC were electroporated with a PCR product containing a galK/kanamycin cassette with 50bp flanking homology to Rh110. The bacteria were plated on kanamycin/chloramphenicol LB agar at 30°C for positive selection. To replace the galK/kanamycin cassette with the HBV fusion genes a PCR product containing the HBV S-PolN fusion or HBV Core-PolC fusion with the same flanking homology to Rh110 was electroporated and the bacteria were plated on 2-deoxy-galactose (DOG) chloramphenicol minimal media plates with glycerol as the carbon source for negative selection. PCR primers for homologous recombination were as follows:
Rh110 S/PolN forward: GATCACGTCATTGACACCGGCCTCCCACCAGCTCTCACATTCTCCGCATCACCATGGAGAACATCACATCAGGAT
Rh110 S/PolN reverse:
CAAAATATTATTACATGGTACGCAATTTATTGTCTATTTTCGTTATTTGTTTATTCAAGCGTAATCTGGAACATCGTAT
Rh110 Core/PolC forward: GATCACGTCATTGACACCGGCCTCCCACCAGCTCTCACATTCTCCGCATCACCATGGACATTGACCCTTATAAAGAAT
Rh110 Core/PolC reverse: CAAAATATTATTACATGGTACGCAATTTATTGTCTATTTTCGTTATTTGTTTATCTAAGCGTAATCTGGAACATCGTAT
To generate 68–1 RhCMV/Core we amplified the HBV core gene from pCDNA-CORE and introduced an N-terminal FLAG-tag by PCR (forward primer: CTGCTAGCATGGATTACAAGGATGACAAGGACATCGACCCTTATAAAGAATTTGG; reverse primer: CTAGTCGACACATTGAGATTCCCGAGATTGAG). The amplified product was cloned into pORI downstream of the EF1α promoter. This expression cassette was inserted into Rh211 region of 68–1 RhCMV together with a Kan resistance cassette flanked by FRT sites by homologous recombination using primers containing 50bp homology to regions of Rh211 (forward primer: GGGAAATCACGTCATCAGGCTGGGTAGTCAACATGGGCATACGAAACTTGCCCGAATAGATGCTCTCACTTAACGGCTGACATG, reverse primer: CCAGAATGTGCTCTACTTTTTGGCCAGCGGGTTGGATGATTTCGCGCGTCATGGACTGCTTCACTGTAGCTTAGTACGTTAAAC). The PCR fragment was electroporated into EL250 bacteria containing the RhCMV 68–1 BAC for in vivo recombination and recombinants selected for Kan resistance. The Kan resistance cassette was removed by temperature-inducible FLIP recombination.
The resulting BACs were analyzed by restriction digest, PCR analysis of recombination sites and by next generation sequencing on an Illumina MiSeq sequencer. This sequence analysis revealed two point mutations in S/PolN that were introduced during PCR amplification resulting in amino acid exchanges A118T and T125M in the S-antigen.
BAC DNA was purified using alkaline lysis, phenol/chloroform extraction and isopropanol precipitation and virus was reconstituted by transfection of BAC DNA using Lipofectamine 2000 (following manufacturer’s protocol, ThermoFisher Scientific) of telomerized pp71 expressing rhesus fibroblasts (25) or primary rhesus fibroblasts.
Expression of HBV antigens was confirmed by infecting telomerized RM fibroblasts with 68–1 RhCMV/Surface/PolN or RhCMV/Core/PolC. Cells were harvested at full cytopathic effect and lysed in SDS sample buffer. 293T cells transfected (lipofectamine 2000) with the pORI expression plasmids containing the HA-tagged HBV proteins served as positive controls. After electrophoretic separation, immunoblots were performed with anti-HA antibody MMS-101P (Covance MMS).
Animals and RhCMV/HBV vaccinations.
A total of 8 Indian rhesus macaques (RM1-F-8yr, RM2-F-3yr, RM3-F-7yr, RM4-F-13yr, RM5-M-1yr, RM6-F-2yr, RM7-M-5yr, RM8-M-5yr) were inoculated subcutaneously with 1 × 107 plaque forming units of each RhCMV/HBV. RM are naturally resistant to HBV infection and therefore were not screened for pre-existing anti-HBV immune responses (26). At assignment, all study RM were free of cercopithicine herpesvirus 1, D-type simian retrovirus, simian T-lymphotrophic virus type 1, and Mycobacterium tuberculosis, but all were naturally RhCMV-infected. All study RM were housed at the Oregon National Primate Research Center (ONPRC) in Animal Biosafety level (ABSL)-2 rooms with autonomously controlled temperature, humidity, and lighting. RM were fed commercially prepared primate chow twice daily and received supplemental fresh fruit or vegetables daily. Fresh, potable water was provided via automatic water systems. Physical exams including body weight and complete blood counts were performed at all protocol time points. RM care and all experimental protocols and procedures were approved by the ONPRC Institutional Animal Care and Use Committee (IACUC). The ONPRC is a Category I facility. The Laboratory Animal Care and Use Program at the ONPRC is fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC) and has an approved Assurance (#A3304–01) for the care and use of animals on file with the NIH Office for Protection from Research Risks. The IACUC adheres to national guidelines established in the Animal Welfare Act (7 U.S.C. Sections 2131–2159) and the Guide for the Care and Use of Laboratory Animals (8th Edition) as mandated by the U.S. Public Health Service Policy.
Isolation of PH.
A single lobe of RM liver was perfused with 200 mL pre-perfusion media (0.5 mM EGTA (Bio-World, cat#:40120128–1), 10 IU/mL heparin (Fresenius Kabi, cat#:C504730), HBSS with calcium and magnesium (Fisher Scientific, cat#:24–020-117)), followed by 200 mL HBSS without calcium and magnesium (Fisher Scientific, cat#:SH3003103) to remove remaining EGTA. Next 100 mL of collagenase media (DMEM/F12 (Gibco, cat#:11320–082), 1mM calcium chloride (Sigma-Aldrich, cat#:C5670–100G), 20mM HEPES (HyClone, cat#:SH30237.01) 1mg/mL collagenase IV (Sigma-Aldrich, cat#:C9722–50MG)) warmed to 42°C was perfused into the lobe and discarded. This was followed by re-circulation of 150 ml collagenase media through the liver lobe at 42 °C for 30 minutes to 1 h using a rate of 75–150 mL/min, depending on the size of the liver lobe. Following collagenase perfusion, the liver was filleted with scalpels, washed over with remaining collagenase media, and media was filtered through a tea strainer. PH were washed three times in wash media (DMEM/F12, 2% bovine growth serum (HyClone, cat#:SH3054103), 23 mM HEPES buffer, 0.6 mg/ml glucose, 2mM L-glutamine (HyClone, cat#:SH3003401), 1x antibiotic/antimycotic (HyClone, cat#:SV3007901), and 0.1 mg/mL Gentamicin (Life Technologies, cat#:15750–060)) at room temperature, with centrifugation between each wash at 50 x g for 3 min. Prior to the third wash spin, PH were passed through a 70 μM filter to ensure single-cell suspension. PH were then suspended in 20ml of 36% isotonic percoll (GE Healthcare, cat#:17–0891-01) in a 50ml conical using PH media as a diluent (DMEM/F12, 10% bovine growth serum, 23 mM HEPES buffer, 0.6 mg/ml glucose, 2mM L-glutamine, 1x antibiotic/antimycotic, and 0.1 mg/mL Gentamicin), and centrifuged at 200 x g for 7 min. The purified PH pellet was then resuspended in room temperature PH media and counted. Collagenized plates for the hepatocytes were prepared using 0.2 mg/mL collagen R in 0.01% acetic acid (Serva, cat#:47254), left on the plate for at least 20 min prior to washing with 1 mL HBSS immediately prior to plating at 2 × 105 PH per well in a 12-well plate. Plates were placed at 37°C, 5% CO2. The next day, wells were washed twice with HBSS and cultured in 1 ml PH media supplemented with 1.8% DMSO (PH-DMSO) for the remainder of the experiment. HD PH were isolated from murine humanized livers and purchased from Yecuris, Inc. Humanized murine donors were generated with cryopreserved primary hepatocytes collected from deceased patients with the following demographics: HD1 (13 year old, female, Hispanic, HBV naïve); HD2 (13 year old, female, Caucasian, HBV naïve); and HD3 (27 year old, male, Caucasian, HBV naïve).
HBV infection of HD and RM PH.
One day after plating RM PH (see ‘isolation of macaque PH’), replication-incompetent adenovirus serotype 5 expressing human NTCP (MOI 10) under the liver-specific TTR promoter was added to the culture for 2 days. On the second day, cells were re-fed with 1ml PH-DMSO media. On the fourth day following adenovirus transduction, PH were washed twice in 1 ml HBSS and overlaid with HBV-containing media at an MOI of 100 (PH-DMSO containing 4% PEG6000, Sigma-Aldrich, cat#:81253–250G) and incubated overnight. The next morning, wells were washed three times with 1 ml HBSS and then cultured in 1 ml PH-DMSO for the remainder of the experiment.
One day after plating, HD PH were overlaid with HBV-containing media at an MOI of 100 (PH-DMSO containing 4% PEG6000, Sigma-Aldrich, cat#:81253–250G) and incubated overnight. The next morning, wells were washed three times with 1 ml HBSS and then cultured in 1 ml PH-DMSO for the remainder of the experiment.
Analysis of MHC expression and HBV infection via FACS.
Prior to HBV infection, one well of PH was collected and stained as a baseline. Starting on day two post-infection, a well each of HBV-infected and HBV-naïve PH was collected with 0.5% trypsin-EDTA (Fisher Scientific, cat#: SH30236.01) and washed twice in ice cold FACS buffer (PBS, Fisher Scientific, cat#:SH30256FS, with 10% fetal bovine serum). Cells were incubated with anti-HLA-E antibody (clone: 4D12, Origene, cat#: LS-C179742) for 30 minutes at 4°C, washed twice in ice cold FACS buffer, and incubated with F(ab)2-Goat anti-mouse IgG (H L)-APC (Invitrogen, cat#: A10539) for 30 minutes at 4°C. Cells were then washed twice in ice cold PBS and incubated with pan-MHC-I-PerCP-Cy5.5 (clone: W6/32,Biolegend Inc., cat#: 311419), anti-HLA-DR Alexa 700 (clone: L243 (BD Biosciences, cat#: 560743), and Live/Dead fixable yellow (Invitrogen, cat#: L-34959) for 30 minutes at 4°C. Cells were washed in FACS buffer and fixed using Foxp3 / Transcription Factor Staining Buffer Set (eBioscience, cat#: 00–5523-00) for one hour at room temperature. Prior to fixation all wash spins were performed at 350 x g for 3 minutes. After fixation, cells were suspended in permeabilization buffer (eBioscience, cat#: 00–8333-56). All wash spins after fixation were performed at 830 x g for 3 minutes. PH were incubated for one hour at 4°C with Hepatitis B Virus Core Antigen Antibody (clone: 13A9, Fisher Scientific, cat#: MA1–7606) conjugated to R-phycoerythrin (PE) using the Lightning-Link R-PE kit (Innova Biosciences, cat#: 703–0005). Cells were washed three times in permeabilization buffer and then collected on a Becton-Dickenson LSR-II. Analysis was performed on FlowJo X (TreeStar Inc.). In all analyses, gating on the light scatter signature of large, complex PH was followed by assessment of specific MHC and HBV markers.
CD8+ T cell recognition assays.
HBV-specific CD8+ T cell responses were measured in mononuclear cell preparations from the peripheral blood (longitudinal and deconvolution) or spleens (MHC-restriction and HBV+ PH recognition) of RhCMV/HBV vaccinated RM by flow cytometric ICS, as previously described (27, 28). Briefly, splenocytes (E:T of 10:1) or isolated CD8β+ T cells (E:T of 5:1) were incubated with HBV-infected or HBV naïve PH targets and the co-stimulatory molecules CD28 and CD49d (BD Biosciences) for 1 hour, followed by addition of brefeldin A (Sigma-Aldrich) for an additional 8 hours. CD8β+ T cells were isolated by staining with anti-CD8β-PE (Beckman Coulter) and then secondarily staining with anti-PE beads before running over a positive selection magnetic column according to manufacturer’s instructions (Miltenyi Biotec). Co-stimulation without PH target co-culture served as a background control. The MHC-restriction of a response was determined by: a) pre-incubating PH targets for 1 hour at room temperature in the presence of pan anti-MHC-I antibody (25 μg/ml; clone: W6–32), VL9 peptide (20uM), CLIP peptide (MHC-II–associated in- variant chain, amino acids 89 to 100; 10 μg/ml) or anti-HLA-DR antibody (10μg/ml; clone: L243) before co-culturing with effector cells, or b) pre-incubating cell lines (K562- or .221-derived) expressing a single MHC molecule with HBV peptides for 1 hour at room temperature prior to co-culturing with effector cells (E:T of 10:1). K562 cell lines expressing HLA-E*01:03 or Mamu-E*02:20 were generated, cultured, and utilized in our assays as previously described (11). .221 cell lines expressing HLA-A*02:01 or HLA-C*06:02 were generated using lentiviral transduction as described in (29). HLA fragments were cloned into the modified pLVX-EF1α-IRES-Puro (Clontech) vector, in which EF1α was replaced with the SFFV promoter. The expression cassette encoded ZsGreen linked via self-cleaved P2A peptide to HLA with a FLAG-tag at its N-terminus. HLA-positive cells were selected using 0.25 μg/ml puromycin. Stimulated T cells were fixed, permeabilized, and stained as described (27, 28), and flow cytometric analysis was performed on an LSR-II instrument (BD Biosciences). Analysis was done using FlowJo X software (Tree Star, Inc.). In all analyses, gating on the light scatter signature of small lymphocytes was followed by progressive gating on the CD3+ population and then the CD4–/CD8+ T cell subset. Longitudinal antigen-specific response frequencies for CD8+ T cell populations were routinely determined by intracellular expression of IFN-γ following stimulation of PBMC with pools of overlapping 15-mer peptides corresponding to the HBV core ORF (Genscript; >70% purity). For all ICS results shown, we collected no less than 1 × 105 CD3+ T cell events to ensure the accuracy of our conclusions. CD8+ T cell responses were memory-corrected (naïve cells excluded) as previously described (28).
RESULTS
RhCMV/HBV inoculated RM mount MHC-E-restricted CD8+ T cell responses against HBV antigens.
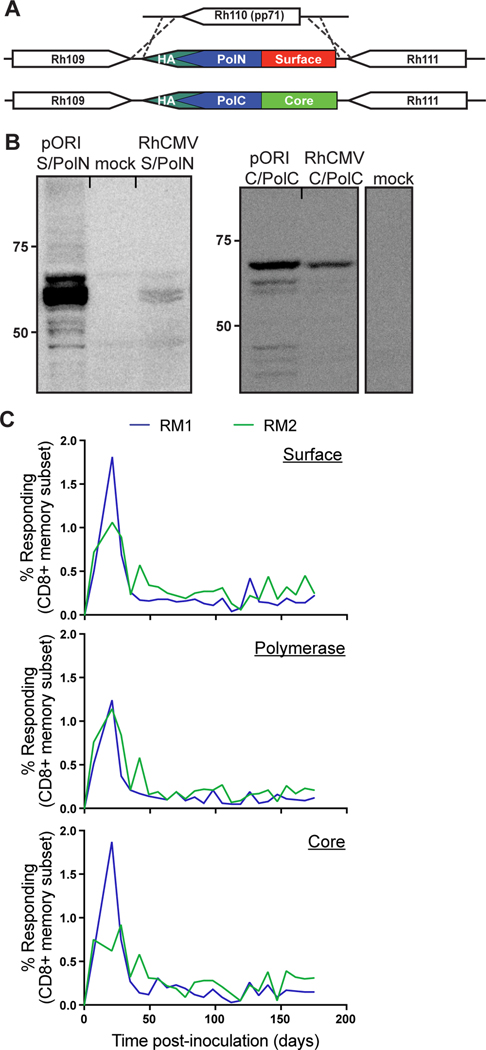
To elicit MHC-II and MHC-E-restricted HBV-specific CD8+ T cell responses in RM, we constructed two recombinant strain 68–1 RhCMV vectors expressing HBV genotype D serotype ayw core, surface, and polymerase antigens. Using BAC recombineering we replaced the gene Rh110 which encodes for the RhCMV homologue of HCMV pp71 with either an HA-tagged fusion protein of Core and the C-terminus of polymerase (Core/PolC) or an HA-tagged fusion protein of preS2/S-antigen and the N-terminus of polymerase (Surface/PolN) (Figure 1A). Upon reconstitution of recombinant RhCMV we confirmed fusion protein expression by immunoblot using HA-epitope specific antibodies (Figure 1B). We inoculated two RM (RM1, RM2) with both 68–1 RhCMV/Surface/PolN and 68–1 RhCMV/Core/PolC vectors and longitudinally monitored the CD8+ T cell response against each of the antigens by intracellular cytokine staining (ICS) using pools of overlapping 15mer peptides corresponding to each antigen. We found robust CD8+ T cell responses to all three HBV antigens in both animals (Figure 1C). In addition, we inoculated two additional RM (RM3, RM4) with a 68–1 RhCMV-based vector that expressed HBV Core under the EF1α promoter (Figure 2A) and observed longitudinal HBV Core antigen (HBcAg)-specific CD8+ T cell responses in the blood of both RM (Figure 2B). To verify that the CD8+ T cells elicited by the 68–1 RhCMV/Core/PolC and 68–1 RhCMV/Core vector were restricted by MHC-II and MHC-E, we characterized the breadth and MHC-restriction of HBcAg-specific CD8+ T cell responses in all four animals via ICS by using individual peptides and reagents that specifically block presentation by MHC-I, MHC-II, and MHC-E as previously described (Figure S1)(27, 28). We observed that, similar to strain 68–1 RhCMV vectors expressing SIV antigens under the endogenous Rh110 promoter (25) or EF1α promoter (27, 28), the HBcAg-specific CD8+ T cells targeted a broad array of HBcAg peptides that were presented either by MHC-II or MHC-E (Figure 2C). However, given that many of these responses are to consecutive 15-mers, it is likely that the true number of targeted HBV antigens is smaller. We next further confirmed the MHC-E-restriction of the RhCMV/HBV engendered CD8+ T cell responses by performing MHC-restriction assays using splenocytes from RM3 as effector cells co-cultured with K562 cells (MHC-null) expressing either a single human (HLA) or rhesus macaque (Mamu) MHC-E allele and pulsed with one of three individual HBcAg 15-mer peptides identified as MHC-E-restricted via blocking in Figure 2C. We found that these HBcAg-specific CD8+ T cells recognized their cognate antigen presented in the context of both HLA-E and Mamu-E (Figure 2D). Of note, these HBcAg-derived 15-mer peptides do not contain amino acid sequences matching the canonical leader sequence-like binding motif of MHC-E, in line with the alternate MHC-E binding repertoire that we, and others, have recently described (27, 30–32). These results demonstrate the presence of MHC-E-restricted, HBV-specific CD8+ T cells in 68–1 RhCMV/HBV inoculated RM, and further support the high functional conservation of primate MHC-E molecules as we have shown previously (11, 27). Since the surface and polymerase antigens are expressed by the same vector backbone in RM1 and RM2, it is highly likely that surface and polymerase-specific CD8+ T cells are similarly restricted by MHC-E and MHC-II.
Figure 1. Design of 68–1 RhCMV/HBV vectors.
A) Schematic of 68–1 RhCMV/Surface/PolN and RhCMV/Core/PolC vectors. Rh110 was excised and replaced with HA-tagged HBV antigens. B) Western blots show HA-tagged HBV protein expression in 68–1 RhCMV/HBV-infected telomerized rhesus macaque fibroblasts or 293 cells transfected with a plasmid expressing the HBV fusion proteins. C) RMs inoculated with 68–1 RhCMV/Surface/PolN and RhCMV/Core/PolC mount sustained CD8+ T cell responses against HBV surface, polymerase and core. The memory-corrected frequencies of T cells responding to overlapping 15mer peptides corresponding to the indicated HBV proteins were determined at bi-weekly intervals by intracellular cytokine staining for IFNγ and TNFα.
Figure 2. 68–1 RhCMV/HBV inoculated RMs mount CD8+ T cell responses.
A) Schematic of 68–1 RhCMV/core vector. An expression cassette encompassing the cellular EF1α promoter and N-terminally FLAG-tagged HBV core was inserted into Rh211. B) 68–1 RhCMV/Core inoculated RMs mount sustained CD8+ T cell responses against HBcAg. C) Each CD8+ T cell response against an HBV core antigen 15-mer from 68–1 RhCMV/Core/PolC (RM1, RM2) or 68–1 RhCMV/core (RM3, RM4) inoculated RMs is indicated by a box, color coded as shown to indicate MHC restriction. D) The MHC-null cell line K562 was transfected with either HLA-E or Mamu-E and these cells were used to present HBcAg 15-mers to CD8+ T cells collected from RM3, which was 68–1 RhCMV/Core inoculated. Only cells expressing MHC-E can present HBcAg 15-mers to these CD8+ T cells. 15-mer peptide sequences are indicated on right.
HBV-infected PH express MHC-E in vitro.
We next set out to determine if PH express MHC-E. To this end, we isolated PH from three unrelated RM and three unrelated HD and examined surface expression of bulk MHC-I using antibody W6/32, MHC-II using antibody HLA-DR, and MHC-E using antibody 4D12 (33). We previously demonstrated that in addition to HLA-E, 4D12 specifically stains Mamu-E but not classical Mamu-Ia molecules (27). All three HD shared one HLA-A and one HLA-C allele (Table I). Thus, before proceeding we confirmed that the MHC-E-specific 4D12 clone stains only HLA-E and not the HLA-A or -C molecules shared between the three HD (Figure 3A). We then stained for expression of MHC-E and demonstrated that the majority of PH from both species expressed MHC-E (Figure 3B and 3C). To determine if HBV infection influences the expression of MHC-E on the surface of PH, we collected PH from the same three HD and infected these cells one day after plating with an MOI of 100 of HBV genotype D serotype ayw. HBV infection of HD PH was confirmed by measuring the level of HBV envelope antigen (HBeAg) in the supernatant prior to staining of the cells (Figure S2A). We co-stained for MHC markers (MHC-I, MHC-E, and MHC-II) along with intracellular HBcAg on day 4 post-HBV infection, since this was the first time point where intracellular HBcAg was detectable (Figure S2B). We observed staining with the 4D12 antibody on both HBV-infected and HBV-naïve PH, indicating moderate levels of MHC-E surface expression (Figures 3D, 3E, and S3). In contrast, HLA-DR expression was minimal or absent in all three HD PH samples, in line with previously published results (34). It is possible that our ex vivo manipulation of the PH prior to assessing surface MHC levels induced a fraction of these cells to lose MHC-E expression. Nevertheless, taken together, these results showed that HBV-infected PH express MHC-E and that MHC-E could represent a potential HBV-specific CD8+ T cell restriction element.
Table I. MHC genotypes of HD.
Grey highlighting indicates HLA alleles shared by all three HD.
| HLA-A | HLA-A | HLA-B | HLA-B | HLA-C | HLA-C | |
|---|---|---|---|---|---|---|
| HD1 | 02:17 | 02:01 | 40:02 | 50:01 | 03:05 | 06:02 |
| HD2 | 01:01 | 02:01 | 15:XX* | 37:XX* | 03:03 | 06:02 |
| HD3 | 02:01 | 02:01 | 57:01 | 27:05 | 02:02 | 06:02 |
XX - Allele indeterminate
Figure 3. PH express MHC-E in vitro.
A) 4D12 staining of MHC-transfected cell lines. MHC-null cells were transfected with a single MHC allele and 4D12 staining was performed. 4D12 staining was compared to matched IgG-isotype control. B) Anti-MHC-E antibody clone 4D12 binds to HD and RM PH. Primary hepatocytes from human and rhesus macaques were stained with the anti-MHC-E antibody clone 4D12 one day after liver perfusion and plating. Mouse IgG1 isotype was used to control for non-specific antibody binding by primary hepatocytes. C) 4D12 staining of PH from multiple HD and RM reveals high expression of MHC-E in vitro. D) Co-staining for surface MHC and intracellular HBcAg at day 4 post-HBV infection reveals high levels of MHC-E on HBV infected PH. E) Three different HD samples show high MHC-E expression on HBV-infected PH.
MHC-E-restricted CD8+ T cells from RhCMV/HBV inoculated RM recognize HBV-infected allogeneic and xenogeneic PH.
Our MHC study revealed MHC-E expression on HD and RM PH, regardless of HBV infection. However, given the dominance of VL9 peptide binding to MHC-E it was unclear whether hepatocyte MHC-E would present HBV-derived peptides and whether these non-canonical MHC-E/peptide complexes could be recognized by RhCMV/HBV vector-induced, MHC-E-restricted, HBV-specific CD8+ T cells. We thus determined whether HBcAg-specific CD8+ T cells from 68–1 RhCMV/Core inoculated RM would recognize allogeneic, HBV-infected RM PH taking advantage of the high functional conservation of MHC-E in primates (11). Strikingly, we found that CD8+ T cells (bulk splenocytes and purified CD8β+ T cells) from RM3 and RM4 recognized HBV-infected PH from two unrelated RM donors, but did not respond to uninfected PH (Figure 4A).
Figure 4. MHC-E-restricted CD8+ T cells from 68–1 RhCMV/Core inoculated RM recognize HBV-infected allogeneic and xenogeneic PH.
A) Splenocytes from 68–1 RhCMV/Core inoculated RM3 and RM4 were incubated with HBV naive or HBV-infected PH from RM7 and RM8 and then stained for CD3, CD8, and intracellularly for IFN-γ. In addition, reagents to block either all MHC class I (W6/32 – also blocks MHC-E), MHC-E only (peptide VL9), or MHC class II (CLIP, HLA-DR) were included to determine the restriction of these CD8+ T cell responses. B) Same as A, but with CD8β-sorted effectors. C) Splenocytes from 68–1 RhCMV/Core inoculated RM3 and RM4 were incubated with HBV naive or HBV-infected PH from HD1 and HD2 and then stained for CD3, CD8, and intracellularly for IFN-γ. In addition, reagents to block either all MHC class I (W6/32 – also blocks MHC-E), MHC-E only (peptide VL9), or MHC class II (CLIP, HLA-DR) were included to determine the restriction of these CD8+ T cell responses. D) Same as C, but with CD8β-sorted effectors.
In order to more comprehensively determine the MHC-restriction of CD8+ T cells recognizing HBV-infected PH targets, we performed a series of recognition experiments using the same MHC-specific blocking reagents used in Figure 2C and as we have previously described (27, 28). HBV-infected or HBV-naïve targets were collected at day 6 post-HBV infection (MOI = 100), incubated with the blocking agents W6/32 antibody (pan MHC-I), VL9 peptide (MHC-E), CLIP (MHC-II), or HLA-DR antibody (MHC-II), and then co-cultured with splenocytes or isolated CD8β+ T cells overnight. Following co-culture, CD8+ T cells were stained intracellularly for IFN-γ and TNF-α to assess recognition of the targets. We discovered that CD8+ T cell recognition of HBV-infected RM PH was blocked with W6/32 antibody and VL9 peptide, but not with CLIP or HLA-DR, indicating that the entirety of the response to HBV-infected targets was MHC-E-restricted (Figure 4A).
Because MHC-E is functionally conserved across primates (11), and since we demonstrated that the HBV-specific RM CD8+ T cells recognized HLA-E expressing K562 cells (Figure 2D), we hypothesized that CD8+ T cells from 68–1 RhCMV/Core inoculated RM would also recognize HBV-infected HD PH. To test this hypothesis, we performed similar recognition experiments incubating splenocytes and purified CD8β+ T cells from the same 68–1 RhCMV/Core inoculated macaques (RM3, RM4) with HBV-infected HD PH. As hypothesized, these CD8+ T cells recognized HBV-infected, xenogeneic HD PH (Figure 4B). As described above for RM PH target co-culture experiments, the CD8+ T cell recognition of HBV-infected HD PH was completely blocked by the MHC-E-binding VL9 peptide. Taken together, these results definitively show that HBV-infected PH present HBV antigen in the context MHC-E, indicating that this pathway can be exploited to target CD8+ T cells to HBV-infected cells.
DISCUSSION
Immunotherapies currently under investigation are designed to harness the immune system to better target HBV-infected hepatocytes and include immune stimulation with pattern recognition receptor agonists, check point inhibitor blockades, therapeutic vaccines, and adoptive T cell therapy (35). A common hurdle facing HBV immunotherapies is T cell immunotolerance (36–38). The initial triggers of immunotolerance, which distinguish patients that successfully clear acute HBV viremia from those that do not, are not completely understood. However, it is likely in part a consequence of the immunotolerant environment of the liver. Thus, in order to successfully clear CHB via immunotherapy, T cell immunotolerance must be overcome. Unfortunately, no immunotherapies to date have consistently achieved this goal, and this reality has been exacerbated by the lack of physiologically relevant animal models of CHB.
We report here on a completely new set of CD8+ T cell responses against HBV, which paves the way for development of innovative HBV therapies. While MHC-E-restricted CD8+ T cell responses have been occasionally identified in natural viral infections with CMV, EBV, TB, and HCV (39), no reports of MHC-E-restricted CD8+ T cells responses against HBV have been published. Moreover, it was unclear whether HBV-infected hepatocytes would be able to present HBV antigen in the context of MHC-E. We show here that MHC-E does present HBV antigens on the surface of HBV-infected hepatocytes and that CD8+ T cells from a completely distinct primate species can recognize these MHC-E:peptide complexes. Since we previously showed that MHC-E-restricted CD8+ T cells can recognize CD4+ T cells infected with SIV (27), these results suggest that endogenous peptide presentation by MHC-E seems to be a common occurrence in virally infected cells. Thus, although 68–1 RhCMV is currently the only vector that can be “programmed” to elicit MHC-E-restricted CD8+ T cells to any given target antigen, these T cells seem to be able to recognize, and possibly eliminate, pathogens via presentation of endogenous peptides by MHC-E.
Outside of representing a completely unique type of CD8+ T cells response against HBV, the breadth of epitopes targeted within HBcAg indicates that therapeutic vaccination with CMV/HBV vectors would elicit broadly-targeted CD8+ T cell responses. While this broad targeting has been shown previously against SIV (27), Mycobacterium tuberculosis (40), and malaria (12), it may be particularly efficacious against HBV, since the vast majority of the HBV genome is comprised almost exclusively of sequence-constrained overlapping reading frames. Here, we present evidence for the first time that MHC-E-restricted CD8+ T cells can be harnessed for the treatment of CHB, either through therapeutic vaccination or adoptive immunotherapy. These results warrant the continued study of these unconventional CD8+ T cell responses in terms of in vivo HBV targeting and suppression.
Supplementary Material
KEY POINTS.
RhCMV/HBV vectors engender MHC-E-restricted CD8+ T cell responses in RM.
HD and RM PH express MHC-E.
PH present HBV antigen to MHC-E-restricted CD8+ T cells from RhCMV/HBV inoculated RM.
ACKNOWLEDGEMENTS
We thank Mary N. Carrington for providing single MHC expressing cell lines and Daniel E. Geraghty for the MHC-E mAb 4D12 hybridoma. We thank Frank Chisari for providing plasmids POL/ENV, pCMV-S2/S and pCDNA-CORE. We thank Ulla Protzer for providing cell-culture derived HBV genotype D serotype ayw.
OHSU and Drs. Picker, Hansen, Sacha, Burwitz, and Früh have a significant financial interest in Vir Biotechnology, Inc., a company that may have a commercial interest in the results of this research and technology. The potential individual and institutional conflicts of interest have been reviewed and managed by OHSU.
This work was supported by Vir SRA-17-111 (awarded to BJB), Oregon Nanoscience & Microtechnologies Institute SRA-13-053-B (awarded to KF), the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01 AI144008 (awarded to BJB), R01 AI117802, R01 AI129703 and R01 AI140888 (awarded to JBS), and P51OD011092 (awarded to the Oregon National Primate Research Center).
Abbreviations:
- CHB
chronic HBV infection
- RhCMV/HBV
rhesus macaque cytomegalovirus expressing HBV antigens
- MHC
major histocompatibility complex
- RM
rhesus macaque
- HD
human donor
- PH
primary hepatocytes
- SIV
simian immunodeficiency virus
- PH-DMSO
primary hepatocyte media containing DMSO
- HBcAg
HBV core antigen
- HLA
human leukocyte antigen
- Mamu
Macaca mulatta
- HBeAg
HBV envelope antigen
REFERENCES
- 1.World Health Organization. Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en/
- 2.Joint Committee on Vaccination and Immunisation. Hepatitis B. In Immunisation Against Infectious Disease, 3rd ed. p. 468. [Google Scholar]
- 3.Bhattacharya D, and Thio CL 2010. Review of hepatitis B therapeutics. Clin. Infect. Dis. 51: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Chen B, Wang L, Chi J, Song S, Liu M, and Zhao Z. 2016. HBsAg seroclearance or seroconversion induced by peg-interferon alpha and lamivudine or adefovir combination therapy in chronic hepatitis B treatment: a meta-analysis and systematic review. Rev Esp Enferm Dig 108: 263–270. [DOI] [PubMed] [Google Scholar]
- 5.Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C, Williams R, Vergani D, and Bertoletti A. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 117: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 6.Phillips S, Chokshi S, Riva A, Evans A, Williams R, and Naoumov NV 2010. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J. Immunol. 184: 287–295. [DOI] [PubMed] [Google Scholar]
- 7.Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, and Ferrari C. 2009. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 58: 974–982. [DOI] [PubMed] [Google Scholar]
- 8.Bertoletti A, and Ferrari C. 2016. Adaptive immunity in HBV infection. JOURNAL OF HEPATOLOGY 64: S71–S83. [DOI] [PubMed] [Google Scholar]
- 9.Rehermann B, and Bertoletti A. 2015. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 61: 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurktschiev PD, Raziorrouh B, Schraut W, Backmund M, Wächtler M, Wendtner C-M, Bengsch B, Thimme R, Denk G, Zachoval R, Dick A, Spannagl M, Haas J, Diepolder HM, Jung M-C, and Gruener NH 2014. Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction. J. Exp. Med. 211: 2047–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu HL, Wiseman RW, Hughes CM, Webb GM, Abdulhaqq SA, Bimber BN, Hammond KB, Reed JS, Gao L, Burwitz BJ, Greene JM, Ferrer F, Legasse AW, Axthelm MK, Park BS, Brackenridge S, Maness NJ, McMichael AJ, Picker LJ, O’Connor DH, Hansen SG, and Sacha JB 2018. The Role of MHC-E in T Cell Immunity Is Conserved among Humans, Rhesus Macaques, and Cynomolgus Macaques. J. Immunol. 200: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen SG, Womack J, Scholz I, Renner A, Edgel KA, Xu G, Ford JC, Grey M, St Laurent B, Turner JM, Planer S, Legasse AW, Richie TL, Aguiar JC, Axthelm MK, Villasante ED, Weiss W, Edlefsen PT, Picker LJ, and Früh K. 2019. Cytomegalovirus vectors expressing Plasmodium knowlesi antigens induce immune responses that delay parasitemia upon sporozoite challenge. PLoS ONE 14: e0210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Früh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, and Picker LJ 2013. Immune clearance of highly pathogenic SIV infection. Nature 502: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Lifson JD, Nelson JA, Jarvis MA, and Picker LJ 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. 15: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braud V, Jones EY, and MCMICHAEL A. 1997. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 27: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 16.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, López-Botet M, and Geraghty DE. 1998. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proceedings of the National Academy of Sciences 95: 5199–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen A, Shen Y, Xia M, Xu L, Pan N, Yin Y, Miao F, Shen C, Xie W, and Zhang J. 2011. Expression of the nonclassical HLA class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma 58: 371–376. [DOI] [PubMed] [Google Scholar]
- 18.Pietra G, Romagnani C, Mazzarino P, Falco M, Millo E, Moretta A, Moretta L, and Mingari MC 2003. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proceedings of the National Academy of Sciences 100: 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, and Hayashi N. 2004. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. The Journal of Immunology 173: 6072–6081. [DOI] [PubMed] [Google Scholar]
- 20.Kakimi K, Isogawa M, Chung J, Sette A, and Chisari FV. 2002. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. Journal of Virology 76: 8609–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel ML, Davis HL, Schleef M, Mancini M, Tiollais P, and Whalen RG 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proceedings of the National Academy of Sciences 92: 5307–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isogawa M, Furuichi Y, and Chisari FV 2005. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity 23: 53–63. [DOI] [PubMed] [Google Scholar]
- 23.Chang WLW, and Barry PA 2003. Cloning of the full-length rhesus cytomegalovirus genome as an infectious and self-excisable bacterial artificial chromosome for analysis of viral pathogenesis. Journal of Virology 77: 5073–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warming S, Costantino N, Court DL, Jenkins NA, and Copeland NG 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Research 33: e36–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall EE, Malouli D, Hansen SG, Gilbride RM, Hughes CM, Ventura AB, Ainslie E, Selseth AN, Ford JC, Burke D, Kreklywich CN, Womack J, Legasse AW, Axthelm MK, Kahl C, Streblow D, Edlefsen PT, Picker LJ, and Früh K. 2019. Enhancing safety of cytomegalovirus-based vaccine vectors by engaging host intrinsic immunity. Science Translational Medicine 11: eaaw2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burwitz BJ, Wettengel JM, Muck-Hausl MA, Ringelhan M, Ko C, Festag MM, Hammond KB, Northrup M, Bimber BN, Jacob T, Reed JS, Norris R, Park B, Moller-Tank S, Esser K, Greene JM, Wu HL, Abdulhaqq S, Webb G, Sutton WF, Klug A, Swanson T, Legasse AW, Vu TQ, Asokan A, Haigwood NL, Protzer U, and Sacha JB 2017. Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques. Nature Communications 8: 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, Ford JC, Selseth AN, Pathak R, Malouli D, Legasse AW, Axthelm MK, Nelson JA, Gillespie GM, Walters LC, Brackenridge S, Sharpe HR, López CA, Früh K, Korber BT, McMichael AJ, Gnanakaran S, Sacha JB, and Picker LJ 2016. Broadly targeted CD8⁺ T cell responses restricted by major histocompatibility complex E. Science 351: 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Früh K, and Picker LJ 2013. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340: 1237874–1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Beltran WF, Hölzemer A, Martrus G, Chung AW, Pacheco Y, Simoneau CR, Rucevic M, Lamothe-Molina PA, Pertel T, Kim T-E, Dugan H, Alter G, Dechanet-Merville J, Jost S, Carrington M, and Altfeld M. 2016. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat Immunol 17: 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurtrey C, Harriff MJ, Swarbrick GM, Duncan A, Cansler M, Null M, Bardet W, Jackson KW, Lewinsohn DA, Hildebrand W, and Lewinsohn DM 2017. T cell recognition of Mycobacterium tuberculosis peptides presented by HLA-E derived from infected human cells. PLoS ONE 12: e0188288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampen MH, Hassan C, Sluijter M, Geluk A, Dijkman K, Tjon JM, de Ru AH, van der Burg SH, van Veelen PA, and van Hall T. 2013. Alternative peptide repertoire of HLA-E reveals a binding motif that is strikingly similar to HLA-A2. Molecular Immunology 53: 126–131. [DOI] [PubMed] [Google Scholar]
- 32.Walters LC, Harlos K, Brackenridge S, Rozbesky D, Barrett JR, Jain V, Walter TS, O’Callaghan CA, Borrow P, Toebes M, Hansen SG, Sacha JB, Abdulhaqq S, Greene JM, Früh K, Marshall E, Picker LJ, Jones EY, McMichael AJ, and Gillespie GM 2018. Pathogen-derived HLA-E bound epitopes reveal broad primary anchor pocket tolerability and conformationally malleable peptide binding. Nature Communications 9: 3137–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes BF, Reisner EG, Hemler ME, Strominger JL, and Eisenbarth GS 1982. Description of monoclonal antibody defining an HLA allotypic determinant that includes specificities within the B5 cross-reacting group. Human Immunology 4: 273–285. [DOI] [PubMed] [Google Scholar]
- 34.Senaldi G, Lobo-Yeo A, Mowat AP, Mieli-Vergani G, and Vergani D. 1991. Class I and class II major histocompatibility complex antigens on hepatocytes: importance of the method of detection and expression in histologically normal and diseased livers. J. Clin. Pathol. 44: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill US, and Kennedy PTF. 2017. Current therapeutic approaches for HBV infected patients. JOURNAL OF HEPATOLOGY 67: 412–414. [DOI] [PubMed] [Google Scholar]
- 36.Zong L, Peng H, Sun C, Li F, Zheng M, Chen Y, Wei H, Sun R, and Tian Z. 2019. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nature Communications 10: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong X, Sun R, Chen Y, Wei H, and Tian Z. 2014. γδT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J. Immunol. 193: 1645–1653. [DOI] [PubMed] [Google Scholar]
- 38.Milich DR 2016. The Concept of Immune Tolerance in Chronic Hepatitis B Virus Infection Is Alive and Well. Gastroenterology 151: 801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joosten SA, Sullivan LC, and Ottenhoff THM 2016. Characteristics of HLA-E Restricted T-Cell Responses and Their Role in Infectious Diseases. Journal of Immunology Research 2016: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, Gilbride RM, Hughes CM, Ventura AB, Ainslie E, Randall KT, Selseth AN, Rundstrom P, Herlache L, Lewis MS, Park H, Planer SL, Turner JM, Fischer M, Armstrong C, Zweig RC, Valvo J, Braun JM, Shankar S, Lu L, Sylwester AW, Legasse AW, Messerle M, Jarvis MA, Amon LM, Aderem A, Alter G, Laddy DJ, Stone M, Bonavia A, Evans TG, Axthelm MK, Früh K, Edlefsen PT, and Picker LJ 2018. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nature Publishing Group 24: 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.