Abstract
Insulin resistance underlies most glucose disorders in adults and is associated with an increased risk of cardiovascular disease. Alpha blockers decrease insulin resistance, whereas diuretics increase insulin resistance.
The authors studied the effects of these two classes of hypertension medications (doxazosin, an α blocker, and chlorthalidone, a diuretic) on cardiovascular disease outcomes in adults aged >55 years with hypertension and glucose disorders who were participants in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (8749 had known diabetes mellitus and 1690 had a newly diagnosed glucose disorder [fasting glucose ≥110 mg/dL]). There was no difference in either group between the chlorthalidone‐ and doxazosin‐based treatments with regard to fatal or nonfatal myocardial infarction or all‐cause mortality. There was, however, a difference for combined cardiovascular disease (myocardial infarction, revascularization procedures, angina, stroke, heart failure, and peripheral arterial disease) in favor of the diuretic. This difference was due primarily to an increased heart failure risk in those treated with doxazosin (relative risk, 1.85; 95% confidence interval, 1.56–2.19) in the known diabetes mellitus group and a relative risk of 1.63 (95% confidence interval, 1.05–2.55) in those with a newly diagnosed glucose disorder despite lower glucose levels on follow‐up in those treated with α blockers. The authors conclude that treatment of hypertension with doxazosin in adults with glucose disorders incurs the same risk of coronary heart disease as treatment with chlorthalidone; however, treatment with doxazosin increases the risk of combined cardiovascular disease and heart failure despite lower glucose levels.
Several attributes of α blockers make their use attractive for the treatment of hypertension in patients with diabetes mellitus. These include enhanced insulin sensitivity, 1 , 2 inhibition of vasoconstriction, 3 a favorable effect on lipid levels, 1 , 4 , 5 and no adverse effect on renal function. 4 In adults with diabetes and hypertension there is diminished insulin sensitivity, 6 increased vascular resistance, 7 abnormal lipid values, 8 and impaired renal function. 9 Little information is available on cardiovascular outcomes following the use of α blockers for the management of hypertension among persons with diabetes.
The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) was a National Heart, Lung, and Blood Institute‐sponsored study designed to determine whether the occurrence of fatal coronary heart disease (CHD) or nonfatal myocardial infarction is lower for high‐risk hypertensive patients treated with a calcium‐channel blocker (amlodipine), an angiotensin‐converting enzyme inhibitor (lisinopril), or an α blocker (doxazosin), each compared with diuretic treatment (chlorthalidone). An early report from ALLHAT has shown that doxazosin is not as effective as chlorthalidone for the treatment of hypertension in the entire cohort. 10 In this article we report on the doxazosin vs. chlorthalidone treatment outcomes in participants with diabetes mellitus, a subgroup prespecified for analysis. We also examine the effect of these treatments in participants with glucose disorders diagnosed at the time of enrollment, a post hoc subgroup. The report includes additional end points that were not available in the earlier report. 11 Given the growing prevalence of glucose disorders and insulin resistance and the need to find treatments to ameliorate their impact on cardiovascular health, we believe these analyses are important.
METHODS
Prior ALLHAT publications detail participant selection, criteria for entry into the trial, primary and add‐on medications used for hypertension treatment, primary and secondary cardiovascular trial outcomes, and statistical methods. 10 , 11 , 12 , 13
A participant's glucose status was categorized as known diabetes mellitus, that is, a diagnosis of diabetes mellitus on a checklist of eligibility criteria at trial entry (n=8749) (this was a prespecified group); newly diagnosed diabetes mellitus, that is, no diagnosis of diabetes mellitus at trial entry but baseline fasting glucose >125 mg/L (n=712); or impaired fasting glucose (IFG), that is, no diagnosis of diabetes mellitus at study entry but baseline glucose 110‐125 mg/dL (n=978). For the purposes of this analysis, these last two categories were combined under the heading new glucose disorder (n=1690), a post hoc subgroup. Persons with IFG are at risk for cardiovascular disease (CVD) as much as those with newly diagnosed diabetes mellitus. 14 Of subjects who entered the study without a diagnosis of diabetes mellitus, approximately 74% had a fasting glucose level available. It is therefore likely that some “normals” had undiagnosed diabetes mellitus or IFG.
RESULTS
An overview of the number of trial participants, events, and those lost to follow‐up is shown in Figure 1.
Figure 1.
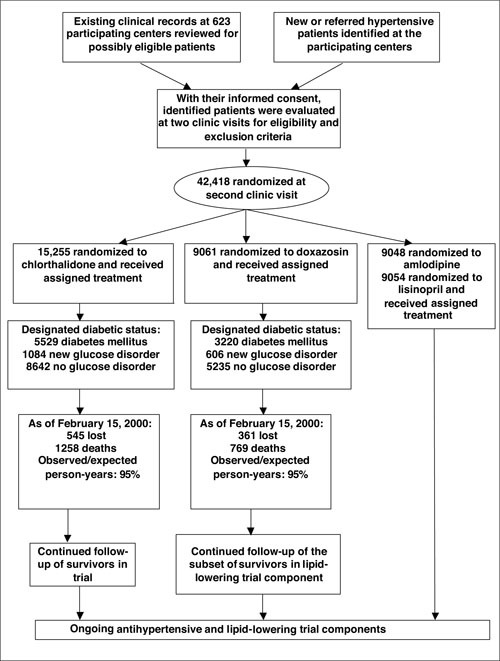
Flow chart of the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial
Baseline Characteristics
Table I shows baseline characteristics categorized by treatment assignment. There was an equal distribution of all characteristics between those randomized to chlorthalidone and to doxazosin in both glucose disorder groups. Compared with participants with no glucose disorder, those with known diabetes mellitus were more likely to be women, nonwhite, nonsmokers, and have a higher body mass index. Those with newly diagnosed glucose disorders were more likely to be men. Those with known diabetes mellitus were noted to unexpectedly have fewer identifiable coexisting cardiovascular comorbidities than those with no glucose disorder or those with a new glucose disorder.
Intermediate Outcomes on Follow‐Up
Duration of follow‐up was similar for both treatment groups (Table II). At 4 years, a higher proportion of those randomized to chlorthalidone was taking assigned medication compared with those randomized to doxazosin in all categories of diabetes status. Also, fewer subjects on chlorthalidone were taking step 2 and 3 study medications.
Table II.
Descriptive and Laboratory Values at Follow‐Up of Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial Participants With Newly Diagnosed Glucose Disorders and Known Diabetes Mellitus*
| New Glucose Disorder | Known Diabetes Mellitus | No Glucose Disorder | ||||
|---|---|---|---|---|---|---|
| Doxazosin n=606 | Chlorthalidone n=1084 | Doxazosin n=3220 | Chlorthalidone n=5529 | Doxazosin n=5235 | Chlorthalidone n=8642 | |
| Mean follow‐up (years±SD) | 3.25±1.18 | 3.10±1.20 | 3.09±1.24 | 3.13±1.22 | 3.13±1.14 | 3.13±1.14 |
| Participants on step 1 medication at year 4 (%) | 67 | 79 | 69 | 78 | 73 | 77 |
| Participants on step 2 and 3 medications at year 4 (%) | 50 | 44 | 43 | 41 | 47 | 40 |
| Mean SBP at year 4 (mm Hg±SD) | 139±17** | 134±16 | 137±17 | 136±16 | 137±18† | 135±16 |
| Mean DBP at year 4 (mm Hg±SD) | 77±11 | 75±9 | 75±11 | 75±10 | 77±11 | 77±10 |
| Mean fasting glucose at year 4 (mg/dL±SD) | 128±40 | 144±65 | 153±64 | 161±62 | 96±20 | 102±24 |
| Mean cholesterol at year 4 (mg/dL±SD) | 192±40 | 188±36 | 183±41 | 195±43 | 189±36 | 200±42 |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; *approximately 30% of patients were receiving atenolol as add‐on therapy; **doxazosin vs. chlorthalidone, p=0.002; †doxazosin vs. chlorthalidone, p<0.001 | ||||||
There was a decrease in systolic blood pressure (SBP) and diastolic blood pressure from baseline in both diabetic groups. There was, however, a greater decrease in SBP in those treated with chlorthalidone compared with those treated with doxazosin in all categories of diabetes status (p<0.01 for the new and no glucose disorder groups, nonsignificant for the known diabetes mellitus group). Patients on chlorthalidone had statistically nonsignificant higher fasting glucose levels than those on doxazosin in both glucose disorder groups and in nondiabetic individuals. There was no consistent relationship for serum cholesterol and treatment in the two diabetes groups.
Primary and Secondary Outcomes on Follow‐Up
Event rates and relative risks (RRs) for primary and secondary outcomes by treatment assignment are shown in Table III. Among participants with known diabetes mellitus, there was no statistically significant difference in CHD or combined CHD outcomes, stroke, and all‐cause mortality. There was a significant difference in combined CVD outcomes, with doxazosin‐treated participants having a higher event rate and risk (RR, 1.22; 95% confidence interval [CI], 1.11–1.33). Further analysis showed that this difference was due to an increased rate of revascularization procedures and heart failure incidence. When analysis was confined to those with hospitalized or fatal heart failure, results were similar with a 70% greater risk in the doxazosin group (RR, 1.70; 95% CI, 1.41–2.06). In the much smaller new glucose disorder group, similar results with respect to CHD or combined CHD outcomes, stroke, and all‐cause mortality were found. There was no significant difference in combined CVD between treatment assignments. There remained, however, a persistent difference in heart failure risk. When analysis was confined to those with hospitalized or fatal heart failure, results showed a 54% risk difference between treatment assignments (RR, 1.54; 95% CI, 0.93–2.55); this was no longer significant because of small numbers.
Results similar to the known diabetes mellitus and the newly diagnosed glucose groups were observed in those without a glucose disorder with respect to CHD and all‐cause mortality. Combined CHD, stroke, and combined CVD were significantly higher for those treated with doxazosin compared with those treated with chlorthalidone. The latter finding was due primarily to an increased risk of heart failure in those treated with doxazosin. When analysis was confined to those with hospitalized or fatal heart failure, the increased risk of 74% remained significant (RR, 1.74; 95% CI, 1.43–2.13; p<0.01).
Further Analysis of Heart Failure
The 4‐year cumulative incidence of heart failure was 8.6% for those with known diabetes mellitus, 6.6% for those with a new glucose disorder, and 5.4% for those with no glucose disorder. Rates differed significantly between the treatment groups in each group (known diabetes mellitus, 11.7% doxazosin compared with 6.9% chlorthalidone, p<0.001; new glucose disorder, 8.1% doxazosin compared with 5.8% chlorthalidone, p=0.03; and no glucose disorder, 7.2% doxazosin compared with 4.3% chlorthalidone, p<0.001). The cumulative incidence of heart failure by treatment in each group is shown in Figure 2. There was an early separation in heart failure incidence in each group among the treatment groups. When analysis was confined to the period after the first year of follow‐up, a significant difference among treatment groups still existed (3‐year rate: diabetes mellitus, doxazosin 8.2% vs. 5.9% for chlorthalidone, RR, 1.33 [p=0.008]; new glucose disorder, doxazosin 5.0% vs. 5.4% for chlorthalidone, RR, 0.96 [p=0.87]; no glucose disorder, doxazosin 5.1% vs. 3.6% for chlorthalidone, RR, 1.52 [p<0.001]).
Figure 2.
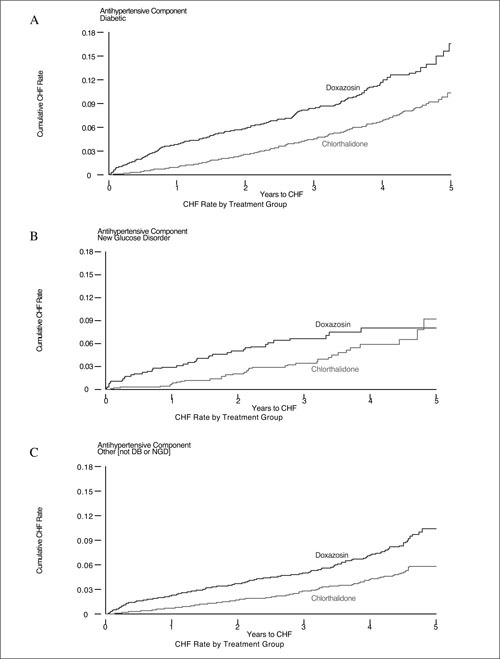
Cumulative incidence of heart failure in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial by type of antihypertensive treatment in those with A) diabetes mellitus (DB), B) new glucose disorder (NGD), and C) no glucose abnormality. CHF=congestive heart failure
To explore the heart failure findings further, RRs for incident heart failure among those treated with doxazosin vs. those treated with chlorthalidone by various characteristics are shown in Table IV. In all categories the use of doxazosin was associated with a higher risk of heart failure than patients treated with chlorthalidone.
DISCUSSION
In this, the largest study to date to examine the treatment of hypertension in people with glucose disorders, the use of a doxazosin‐based treatment regimen incurred the same risk of nonfatal myocardial infarction and CHD death (the primary end point of the study) as was incurred with the use of a chlorthalidone‐based treatment program. This was true overall and within each of the glucose subgroups. Further, there were only minor differences overall and within glucose subgroups with respect to combined CHD (the primary outcome plus revascularization procedures and hospitalized angina) and no differences for all‐cause mortality. Of importance, there were significant differences overall and within some glucose subgroups with regard to combined CVD—which included all the above plus stroke, lower extremity revascularization procedures, nonhospitalized angina, heart failure, and peripheral arterial disease. Patients treated with doxazosin were at higher risk compared with those receiving chlorthalidone. The combined CVD finding was due primarily to an increased risk of heart failure in those receiving doxazosin. During the course of ALLHAT, heart failure became the most frequent Medicare diagnostic‐related group due to the aging of the population and improved survival for those with CHD.
In the new glucose disorder group there was a 13% increased risk of stroke, and in the known diabetes mellitus group there was a 21% increased risk for those treated with doxazosin vs. those treated with chlorthalidone. In both groups the increased risk did not reach statistical significance. Nevertheless, there were no significant interactions between treatment type and glucose subgroups for any of the CVD outcomes, including stroke. Therefore, the best estimate of effect is that of the group with no glucose disorder in which doxazosin use was associated with a statistically significant 35% increased risk of stroke vs. chlorthalidone use.
The question arises of how to interpret the increased risk of heart failure in those receiving doxazosin. One possibility is that the effect was due to the less well controlled blood pressure compared with those receiving chlorthalidone. This explanation is unlikely. While the degree of SBP lowering in those with new glucose disorders was 5 mm Hg less with doxazosin than with chlorthalidone, there was only a 1 mm Hg difference in SBP in the known diabetes mellitus group. In this latter group, risk of heart failure was 85% higher for those treated with doxazosin. In the Systolic Hypertension in Elderly Study (SHEP), 15 which studied an elderly hypertensive cohort, a 2–4 mm Hg higher SBP explained only a 10%–20% increase in heart failure risk. Further analyses from ALLHAT show that controlling for this difference in follow‐up blood pressure measurements had little effect on the results. 16 Lastly, a meta‐analysis based on 61 prospective hypertension trials has recently shown that a 20‐mm Hg change in SBP is necessary for a doubling in heart failure mortality. 17
Another explanation is that doxazosin increased heart failure risk. Possible mechanisms for this are that α blockers stimulate sympathetic nervous system activity, increase catecholamine levels, and lead to fluid retention. 18 A third explanation is that chlorthalidone may prevent heart failure whereas doxazosin does not. Further analysis of ALLHAT data has shown that after the heart failure diagnosis, the use of heart failure treatments and the death rate on either medication was similar. 19 Thus, chlorthalidone, relative to doxazosin, delays the onset of heart failure. Similar to the total ALLHAT cohort, 11 the use of doxazosin was associated with a higher risk of heart failure even after the first year of follow‐up, suggesting that the difference in outcomes between the two treatments was not due to unmasking of undiagnosed heart failure in the doxazosin participants after withdrawal of baseline diuretic therapy.
Two other points regarding our results should be mentioned. First, there was more add‐on medication use among those randomized to doxazosin than among those randomized to chlorthalidone. This would have the effect of underestimating the true differences in combined CVD risk between the two treatments. Second, participants treated with chlorthalidone had higher fasting glucose levels at follow‐up than those treated with doxazosin. Despite this effect, there were no differences in CHD or all‐cause mortality outcomes between the two treatment groups. Moreover, rates of heart failure were lower among chlorthalidone‐treated participants. Elevated glucose levels are associated with heart failure risk. 20 Thus, the higher glucose levels associated with chlorthalidone, in the short run, did not translate into more negative CVD outcomes.
This study was office‐based and reflected medical practice in the community. There was more than 95% follow‐up, and events were verified. The known diabetes mellitus subgroup was designated a priori for analysis so that biases associated with post hoc analysis are not present. The large sample size, the double blind randomization, and the prespecified definition of heart failure and its validation are further strengths. The disadvantages of ALLHAT are that factors of interest, such as glycosylated hemoglobin, insulin levels, and proteinuria, were not measured. The new glucose disorder group was a post hoc subgroup. Also, the known diabetes mellitus cohort entered ALLHAT because of a diagnosis of diabetes mellitus. Efforts at ascertaining concomitant CVD were most likely not as vigorous as those made for participants without diabetes mellitus who entered the trial for other reasons, like CVD. This may account for the lower than expected prevalence of CVD at baseline in the known diabetes mellitus cohort compared with the other two cohorts.
The use of the α blocker doxazosin as an initial treatment for hypertension in older adults with glucose disorders results in similar CHD, stroke, and all‐cause mortality outcomes as the use of chlorthalidone. On the other hand, treatment with doxazosin results in a higher risk of heart failure compared with treatment with chlorthalidone even though there is a more favorable metabolic profile with its use. Doxazosin should not be a first‐line single agent choice for the treatment of hypertension in adults with glucose disorders.
Acknowledgment and disclosure: Vincent DeQuattro, MD, participated in conducting the study; he was deceased at the time of writing of the article. This study was supported by contract NO1‐HC‐35130 from the National Heart, Lung, and Blood Institute. ALLHAT investigators received contributions of study medications from Pfizer (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol‐Myers Squibb (pravastatin), and financial support from Pfizer.
References
- 1. Giordano M, Matsuda M, Sanders L, et al. Effects of angiotensin‐converting enzyme inhibitors, Ca2+ channel antagonists, and α‐adrenergic blockers on glucose and lipid metabolism in NIDDM patients with hypertension. Diabetes. 1995;44:665–671. [DOI] [PubMed] [Google Scholar]
- 2. Glanz M, Garber AJ, Mancia G, et al. Meta‐analysis of studies using selective alpha 1‐blockers in patients with hypertension and type 2 diabetes. Int J Clin Pract. 2001;55:694–701. [PubMed] [Google Scholar]
- 3. Julius S. Are different hemodynamic patterns of antihypertensive drugs clinically important? Eur J Clin Pharmacol. 1990;38:S125–S128. [DOI] [PubMed] [Google Scholar]
- 4. Grimm RA Jr, Flack JM, Schoenberger JA, et al. Alpha‐blockade and thiazide treatment of hypertension. A double‐blind randomized trial comparing doxazosin and hydrochlorothiazide. Am J Hypertens. 1996;9:445–454. [DOI] [PubMed] [Google Scholar]
- 5. Maheux P, Facchini F, Jeppesen J, et al. Changes in glucose, insulin, lipid, lipoprotein, and apoprotein concentrations and insulin action in doxazosin‐treated patients with hypertension. Comparison between nondiabetic individuals and patients with non‐insulin‐dependent diabetes mellitus. Am J Hypertens. 1994;7:416–424. [DOI] [PubMed] [Google Scholar]
- 6. Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(suppl 6a):9S–14S. [DOI] [PubMed] [Google Scholar]
- 7. McVeigh GE. Arterial compliance in hypertension and diabetes mellitus. Am J Nephrol. 1996;16:217–222. [DOI] [PubMed] [Google Scholar]
- 8. Verges BL. Dyslipidaemia in diabetes mellitus. Review of the main lipoprotein abnormalities and their consequences on the development of atherogenesis. Diabetes Metab. 1999;25(suppl 3):32–40. [PubMed] [Google Scholar]
- 9. Ritz E, Rychlik I, Locatelli F, et al. End‐stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. [DOI] [PubMed] [Google Scholar]
- 10. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 11. Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group . Diuretic versus alpha‐blocker as first‐step antihypertensive therapy: final results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2003;42:239–246. [DOI] [PubMed] [Google Scholar]
- 12. Davis BR, Cutler JA, Gordon DJ, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Am J Hypertens. 1996;9:342–360. [DOI] [PubMed] [Google Scholar]
- 13. Barzilay JI, Jones CL, Davis BR, et al. Baseline characteristics of the diabetic participants in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care. 2001;24:654–658. [DOI] [PubMed] [Google Scholar]
- 14. Barzilay JI, Spiekerman CF, Kuller LH, et al. Prevalence of clinical and isolated subclinical cardiovascular disease in older adults with glucose disorders: The Cardiovascular Health Study. Diabetes Care. 2001;24:1233–1239. [DOI] [PubMed] [Google Scholar]
- 15. Kostis J, Davis BR, Cutler JA, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 16. Davis BR, Cutler JA, Furberg CD, et al. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Ann Intern Med. 2002;137:313–320. [DOI] [PubMed] [Google Scholar]
- 17. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002:360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 18. Riegger GA, Haeske W, Kraus C, et al. Contribution of the renin‐angiotensin‐aldosterone system to development of tolerance and fluid retention in chronic congestive heart failure during prazosin treatment. Am J Cardiol. 1987;59:906–910. [DOI] [PubMed] [Google Scholar]
- 19. Piller LB, Davis BR, Cutler JA, et al. Validation of heart failure events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants assigned to doxazosin and chlorthalidone. Curr Cont Trials Cardiovasc Med. 2002;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults, ≥65 years: the Cardiovascular Health Study. J Am Coll Cardiol. In press. [DOI] [PubMed] [Google Scholar]


