Abstract
To analyze the influence of sympathetic activity on blood pressure (BP) and its effects on urinary albumin excretion (UAE), the authors carried out a cross‐sectional study in their local health coverage area. The following variables were monitored in a representative sample of the general population made up of 495 individuals: anthropometric parameters; blood glucose, creatinine, and lipid levels; 24‐hour urinary albumin, norepinephrine, and epinephrine excretion; and BP of patients with known hypertension and newly discovered BP ≥140/90 mm Hg, evaluated by ambulatory monitoring. In the multivariate analysis, only gender, systolic BP, and UAE were associated with norepinephrine levels; only gender, systolic BP, and body mass index were associated with epinephrine. After excluding those patients with chronic kidney disease, the multivariate analysis showed a strong association between UAE ≥30 mg/d and elevated norepinephrine and epinephrine levels. The authors concluded that in the subject population there is an association between elevated adrenergic activity and higher UAE, independent of factors such as age and BP.
Many studies have documented the existence of an increase in sympathetic nervous system (SNS) activity in essential hypertension; this is present from an early stage and plays an important role in its development and progression. 1 , 2 , 3 , 4 , 5 , 6 It is also known that sympathetic activation is proportional to the rising slope of blood pressure (BP) and gives rise to a series of deleterious metabolic and cardiovascular effects, independent of the BP increase. 1 , 7 , 8 Higher SNS activity contributes, therefore, not only to the pathogenesis of hypertension but also to associated morbidity and mortality increases, as it promotes organ damage and causes an increase in cardiovascular risk.
The objective of this study was to analyze the influence of sympathetic tone on BP detected through occasional clinical measurements and ambulatory BP monitoring (ABPM), as well as its effects on early vascular damage measured by urinary albumin excretion (UAE). While the majority of studies in this field use samples of patients coming from hospitals or hypertension clinics, this study is based on a general population, after hypertension detection through office BP and ABPM. This has given us a platform for analyzing the relationship between the SNS and vascular damage produced by hypertension.
MATERIALS AND METHODS
Study Design
The study was carried out in the area of the Rio Hortega University Hospital in the province of Valladolid in northwestern Spain using a representative sample of the general population of 1500 individuals; the design and methodology have been previously published. 9 , 10 The following data were collected: (1) weight and height, (2) waist and hip circumferences, and (3) BP measurement with the patient seated for at least 5 minutes in a chair; an average of 2 readings was calculated using the automatic device Omron Hem‐706/711 (Omron Healthcare Inc, Bannockburn, IL) taken at a 2‐minute interval. 11 , 12 Blood samples were taken after a 12‐hour fasting period. Subjects were given written instructions on the 24‐hour urine collection procedure; urine volumes were measured and urine aliquots (10 mL) were taken from the 24‐hour collection and stored at −85°C. Urine specimens were then sent to a laboratory.
Patients reporting a prior hypertension diagnosis by a physician (known hypertension) and those who presented with a BP ≥140/90 mm Hg were requested to undergo ABPM. Monitoring was carried out in 71.1% and 77.9% of the hypertensive subjects, respectively, on weekdays with a validated noninvasive automatic device (Meditech ABPM‐04; Meditech Ltd, Budapest, Hungary). 13
All patients gave informed consent. A total of 495 individuals participated. To check for the existence of bias, the medical records of 1500 subjects were examined. A greater participation of patients with diabetes mellitus was verified, with a lesser prevalence among the nonparticipants (4.6% vs 7.4%; P=.016). There were no differences between the collaborating and noncollaborating subjects with respect to the other variables: age, gender, body mass index (BMI), education level, history of hypertension, dyslipidemia, active smoking habit, cerebrovascular disease or ischemic heart disease, basal glycemia and cholesterolemia, and number of hospital admissions within the past 5 years.
Measurements
The anthropometric parameters were measured according to World Health Organization (WHO) guidelines 14 ; waist/hip ratio (WHR) (waist circumference/hip circumference) and BMI were calculated from the data received. Apart from UAE, the following hypertension complications were considered 15 : slight serum creatinine increase (1.2–2.0 mg/dL) or renal insufficiency (creatinine >2.0 mg/dL); and history of coronary heart disease or cerebrovascular disease, taken directly from case histories. Patients with BP ≥140/90 mm Hg on examination and mean daytime BP <135/85 mm Hg were classified as having white coat hypertension, and those with BP ≥140/90 mm Hg on examination and mean daytime BP ≥135/85 mm Hg were classified as having newly detected and previously unknown hypertension. Among the subjects with known hypertension, those with a mean ABPM BP <135/85 mm Hg were classified as controlled.
UAE was measured by immunoturbidimetry (Albumi Tina‐quant, E Hoffmann‐La Roche Ltd, Basel, Switzerland), and urine norepinephrine and epinephrine levels by high‐performance liquid chromatogra‐phy (Decade II, Antec Leyden BV, Zoeterwoude, The Netherlands). Norepinephrine values of 89.0–473.0 nmol/24 h and epinephrine values of 0.10–109.0 nmol/24 h were considered normal. 16
Statistical Methods
We analyzed the relation of urinary catecholamine values with age; gender; history of hypertension or diabetes mellitus; active smoking habit; presence of hypertensive complications; systolic BP (SBP); diastolic BP (DBP); pulse pressure (PP); BMI; WHR; glucose, total low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) cholesterol; triglycerides; and UAE. Following this, a multivariate analysis was carried out by means of a general lineal model to test the independent association of catecholamine levels with factors and covariables that had shown a relationship in one‐to‐one comparisons. The Bonferroni method was used for the post hoc comparisons.
RESULTS
Of the 495 subjects, 69 were patients with known hypertension; among these 69, 40.4% of those who underwent ABPM presented good BP control. Another 89 subjects had no previous diagnosis of hypertension but presented with BP values ≥140/90 mm Hg; after ABPM (70 individuals), 35 subjects in this group exhibited findings of white coat hypertension, while the other 35 presented persistently high BP. Table I shows global sample characteristics.
Table I.
Sample Characteristics
| Age, y | 47.2±17.1 |
| Range | 18–85 |
| Men | 46.8 |
| Education level | |
| Primary school | 46.9 |
| High school or university studies | 53.1 |
| Blood pressure, mm Hg | |
| Systolic | 126.6±19.9 |
| Diastolic | 79.0±10.3 |
| Pulse pressure | 47.6±15.8 |
| Obesity | |
| Body mass index, kg/m2 | 25.6±4.1 |
| Body mass index ≥30 kg/m2 | 12.7 |
| Central obesity* | 25.9 |
| Waist/hip ratio | 0.87±0.12 |
| Diabetes mellitus | 5.4 |
| Active smoking habit | 28.0 |
| Urinary albumin excretion ≥30 mg/d | 9.6 |
| Data are presented as mean ± SD or % except as noted. *Waist/hip ratio >1 in men and >0.85 in women. | |
A statistical correlation existed between UAE and values of SBP (rs [Spearman correlation coefficient]=0.183; P=.001) and PP (rs=0.258; P<.0001), but not with DBP. UAE correlated with nighttime SBP (rs=0.212; P=.035) and nighttime PP (rs=0.224; P=.028). No nighttime DBP or daytime value correlations were found.
Urinary norepinephrine levels were lower in normotensive subjects than in subjects with hypertension, whether known or unknown (235.7±139.8 nmol/24 h vs 317.3±178.2 nmol/24 h; P<.0001). There were no differences between the groups in epinephrine levels. Norepinephrine (336.1 nmol/24 h vs 239.1 nmol/24 h; P=.009) and epinephrine (53.91 nmol/24 h vs 39.43 nmol/24 h; P=.025) levels were higher in hypertensive patients than in subjects with white coat hypertension. There were no differences between known or previously undiagnosed hypertensive patients. Table II shows the correlations between BP and 24‐hour urine catecholamine measurements.
Table II.
Correlations Between Blood Pressure (BP) and 24‐Hour Urinary Catecholamine Levels
| Norepinephrine | Epinephrine | |||
|---|---|---|---|---|
| BP Parameter | RS | P Value | RS | P Value |
| Systolic | 0.321 | <.0001 | 0.062 | .248 |
| Diastolic | 0.244 | <.0001 | 0.100 | .063 |
| Pulse pressure | 0.248 | <.0001 | 0.046 | .393 |
| Daytime | ||||
| Systolic | 0.298 | .004 | 0.113 | .290 |
| Diastolic | 0.242 | .022 | 0.353 | .001 |
| Pulse pressure | 0.191 | .071 | −0.166 | .118 |
| Nighttime | ||||
| Systolic | 0.208 | .052 | 0.050 | .642 |
| Diastolic | 0.118 | .272 | 0.296 | .005 |
| Pulse pressure | 0.147 | .172 | −0.181 | .091 |
| Nonparametric correlation coefficients were calculated because norepinephrine and epinephrine variables did not follow a normal distribution, rs indicates Spearman correlation coefficient. | ||||
Norepinephrine and epinephrine levels were correlated (rs=0.579; P<.0001). The subjects with high norepinephrine levels (mean, 660 [range, 474–1713] nmol/24 h) were predominantly male in relation to the subjects with normal levels (mean, 233 [range, 7–467] nmol/24 h). They also showed a higher prevalence of UAE ≥30 mg/d (Table III). Norepinephrine levels were correlated with age (rs=0.263; P<.0001), BMI (rs=0.130; P=.016), WHR (rs=0.273; P<.0001), blood glucose (rs=0.186; P=.002), and UAE (rs=0.194; P<.0001) as well. Subjects with high epinephrine levels (mean, 163 [range, 114–284] nmol/24 h) were less often smokers when compared with those with normal levels (mean, 38 [range, 3–108] nmol/24 h) and showed a higher prevalence of UAE ≥30 mg/d. Likewise, epinephrine levels were correlated with WHR (rs=0.214; P<.0001) and blood glucose (rs=−0.124; P=.036). Catecholamine levels were not correlated with LDL or HDL cholesterol or triglyceride levels. Norepinephrine levels of the subjects in the first UAE quintile (Figure) were lower than those in the fifth quintile (215±98 nmol/24 h vs 305±258 nmol/24 h; P=.003); the same findings were noted with epinephrine levels (36±26 nmol/24 h vs 46±36 nmol/24 h; P=.036).
Table III.
Characteristics of Subjects With Normal or High Urinary Catecholamine Levels
| Norepinephrine | Epinephrine | |||
|---|---|---|---|---|
| High (n=19) | Normal (n=331) | High (n=14) | Normal (n=336) | |
| Age, y | 64.0±11.9 | 48.0±16.7* | 51.1 ± 15.6 | 48.8±17.0 |
| Men | 84.2 | 42.9* | 64.3 | 44.3 |
| Smoker | 21.1 | 26.8 | 0.0 | 27.6† |
| Diabetes mellitus | 10.5 | 6.3 | 6.7 | 6.8 |
| Hypertension | 68.4 | 22.7* | 35.7 | 24.6 |
| Systolic BP, mm Hg | 142±20 | 127±20‡ | 133±16 | 128±21 |
| Diastolic BP, mm Hg | 84±10 | 79±10 | 81±13 | 79±10 |
| Pulse pressure, mm Hg | 58±18 | 48±16‡ | 52±11 | 48±17 |
| Body mass index, kg/m | 26.3±5.1 | 25.9±4.1 | 24.0±3.7 | 26.0±4.2 |
| Waist/hip ratio | 0.94±0.12 | 0.87±0.11‡ | 0.87±0.10 | 0.87±0.11 |
| Blood glucose, mg/dL | 117±58 | 90±21§ | 96±18 | 91±26f |
| Hypertension complications∥ | 10.5 | 13.8 | 6.7 | 13.9 |
| Urinary albumin excretion ≥30 mg/d | 31.6 | 8.7§ | 28.6 | 8.6† |
| Data are presented as mean ± SD or %. BP indicates blood pressure. *P<.0001; †P<.05; ‡P<.01; §P<.001 in the comparisons between the groups with high or normal levels. ∥Coronary heart disease and/or cerebrovascular disease and/or serum creatinine ≥1.2 mg/dL. | ||||
Figure.
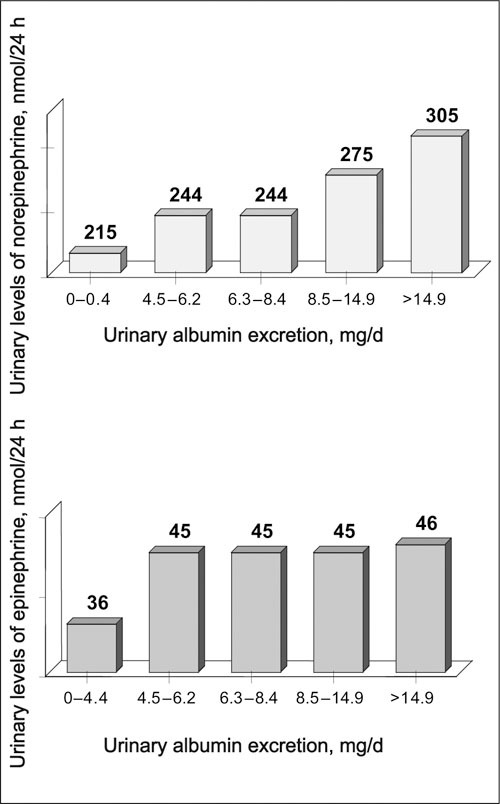
Urinary levels of norepinephrine (top) and epinephrine (bottom) by quintiles of urinary albumin excretion (n=70 in each quintile). Values in nmol/24 h are shown at the top of each histogram. Differences between first vs fifth urinary albumin excretion quintile: norepinephrine, P=.003; epinephrine, P=.036.
Among the categoric variables associated with catecholamine levels in one‐to‐one (gender, smoking, and UAE ≥30 mg/d) and continuous comparisons (age, SBP, PP, BMI, WHR, and blood glucose) only gender, SBP, and UAE were associated with norepinephrine levels in the multivariate analysis. Gender, SBP, and BMI were associated with epinephrine levels (Table IV).
Table IV.
General Lineal Model* Variables Associated With Urinary Catecholamine Levels
| Urinary Albumin Excretion | Differences in Means | ||||
|---|---|---|---|---|---|
| Catecholamines, nmol/24 h | ≥30 mg/d (X) | <30 mg/d (X) | 95% CI | P Value | |
| Entire sample | |||||
| Norepinephrine | 344±34 | 245±12 | 100 | 31–169 | .005 |
| Epinephrine | 57±8 | 46±3 | 11 | −5 to 26 | .173 |
| Subjects with creatinine clearance ≥60 mL/min | |||||
| Norepinephrine | 438±41 | 239±12 | 199 | 115–284 | <.0001 |
| Epinephrine | 73±9 | 45±3 | 28 | 9–46 | .003 |
| Data are presented as mean ± SD. CI indicates confidence interval. *After adjusting for the following variables included in the model: age, gender, smoking, systolic blood pressure, pulse pressure, body mass index, waist/hip ratio, and blood glucose. | |||||
Of all participants in the study, 8.1% showed a creatinine clearance <60 mL/min. After excluding these subjects in the univariate analysis, we obtained similar results to those mentioned above with variables associated with high catecholamine levels. A general lineal model showed a strong association between UAE ≥30 mg/d and elevated norepinephrine and epinephrine levels (Table IV).
DISCUSSION
We have performed an observational study using a representative sample of the general population in our locale. Office and ambulatory BP readings, as well as UAE and catecholamine values in 24‐hour urine samples, were evaluated.
Numerous methods have been developed to study adrenergic activity. 17 Determination of catecholamine levels in 24‐hour urine samples allows us to estimate the mean sympathetic tone during a 24‐hour period, although the inaccuracies and inconveniences of collection are well known. 17 , 18 Limitations of the 24‐hour collection include: (1) it provides a static vision of sympathetic function, (2) it does not allow for detection of differences in the sympathetic tone in different organs, an inherent limitation in all techniques evaluating sympathetic function globally, and (3) its results are not reliable in subjects with renal insufficiency, as urinary catecholamine excretion depends closely on glomerular filtration rate. Advantages of catecholamine determination in 24‐hour urine include its capacity to evaluate the mean sympathetic tone during the entire day. This is also a noninvasive technique with a low economic cost.
The independent relationship that exists between 24‐hour urine norepinephrine values and those of office BP 19 is well known. In our study, catecholamine urine levels also correlated with daytime ABPM BP values. Norepinephrine and epinephrine levels were higher in hypertensive patients than in subjects with white coat phenomenon. Pierdomenico et al 20 also detected lower urinary norepinephrine and 3‐methoxy‐4‐hydroxymandelic (vanillylmandelic) acid levels in a group of 12 patients with white coat hypertension than in another group of 12 patients with sustained hypertension.
We found a significant statistical correlation between UAE and BP and between office SBP, DBP, and PP values and norepinephrine levels, while the two catecholamines analyzed were correlated with ABPM DBP values (Table II). The main finding of the study is that the association between increased UAE and higher urinary catecholamine levels is independent of BP values. This finding is even more evident when patients with chronic kidney disease (defined as a glomerular filtration rate <60 mL/min) are excluded from the analysis. One possible explanation for this is shown in studies that demonstrate glomerular protein leakage by sympathetic stimulus. 21 On another hand, microalbuminuria forms a part of the insulin resistance syndrome 22 ; it is known that a high sympathetic tone is responsible for many of the metabolic syndrome abnormalities. 8 It has been shown that vasoconstriction produced by SNS activation reduces glucose uptake by skeletal muscle, increasing insulin resistance and hyperinsulinemia. 5 Sechi et al 23 showed a positive correlation between norepinephrine in 24‐hour urine and blood glucose, both at fasting and after oral glucose load. Obesity is also characterized by an increase in adrenergic activity, as has been shown in skeletal muscle by means of microneurography 24 ; the correlation between urinary catecholamine levels and BMI is known. 23 , 25 In our sample, we have verified a strong relationship between catecholamine levels and different variables closely connected with the insulin resistance syndrome, such as BP, blood glucose, BMI, and WHR.
In conclusion, a relationship between adrenergic activity increases and higher UAE exists in the population in our area, independent of possible confounders such as BP values and the higher sympathetic tone associated with aging. 26 , 27 Given the cross‐sectional character of the study and the limitations associated with the methodology used to assess sympathetic activity, this finding should be interpreted with care and should be evaluated in other studies in the future.
Disclosure: This study was supported by grants from the Castellano‐Leonesa Cdntabra Society of Internal Medicine.
References
- 1. Mancia G, Di Rienzo M, Parati G, et al. Sympathetic activity, blood pressure variability and end organ damage in hypertension. J Hum Hypertens. 1997;11(suppl 1):S3–S8. [PubMed] [Google Scholar]
- 2. Mancia G. Bjorn Folkow Award Lecture . The sympathetic nervous system in hypertension. J Hypertens. 1997;15:1553–1565. [DOI] [PubMed] [Google Scholar]
- 3. Jennings GL. Noradrenaline spillover and microneurography measurements in patients with primary hypertension. J Hypertens Suppl. 1998;16:S35–S38. [PubMed] [Google Scholar]
- 4. Rahn KH, Barenbrock M, Hausberg M. The sympathetic nervous system in the pathogenesis of hypertension. J Hypertens Suppl. 1999;17:S11–S14. [PubMed] [Google Scholar]
- 5. Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13(6 pt 2):99S–105S. [DOI] [PubMed] [Google Scholar]
- 6. Wyss JM, Carlson SH. The role of the nervous system in hypertension. Curr Hypertens Rep. 2001;3:255–262. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Grassi G, Giannattasio C, et al. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. [DOI] [PubMed] [Google Scholar]
- 8. Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13(6 pt 2):112S–122S. [DOI] [PubMed] [Google Scholar]
- 9. Mena‐Martin FJ, Martin‐Escudero JC, Simal‐Blanco F, et al. Health‐related quality of life of subjects with known and unknown hypertension: results from the population‐based Hortega Study. J Hypertens. 2003;21:1283–1289. [DOI] [PubMed] [Google Scholar]
- 10. Martin Escudero JC, Simal Blanco F, Carretero Ares JL, et al. Prevalencia, grado de deteccion, tratamiento y control de la hipertension arterial en la poblacion general. Estudio Hortega. Hipertension 2003;20:148–154. [Google Scholar]
- 11. European Society of Hypertension‐European Society of Cardiology Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 12. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien E, Waeber B, Parati G, et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. Technical Report Series No. 854. [PubMed] [Google Scholar]
- 15. 1999 World Health Organization‐International Society of Hypertension: Guidelines Subcommittee . Guidelines for the management of hypertension. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 16. Tietz NW Clinical Guide to Laboratory Tests. 3rd ed. Philadelphia, PA: WB Saunders Co; 1995. [Google Scholar]
- 17. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. [DOI] [PubMed] [Google Scholar]
- 18. Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–365. [PubMed] [Google Scholar]
- 19. Ward KD, Sparrow D, Landsberg L, et al. Influence of insulin, sympathetic nervous system activity, and obesity on blood pressure: the Normative Aging Study. J Hypertens. 1996;14:301–308. [DOI] [PubMed] [Google Scholar]
- 20. Pierdomenico SD, Bucci A, Constantini F, et al. Twenty‐four‐hour autonomic nervous function in sustained and “white coat” hypertension. Am Heart J. 2000;140:672–677. [DOI] [PubMed] [Google Scholar]
- 21. Hoogenberg K, Sluiter WJ, Navis G, et al. Exogenous norepinephrine induces an enhanced microproteinuric response in microalbuminuric insulin‐dependent diabetes mellitus. J Am Soc Nephrol. 1998;9:643–654. [DOI] [PubMed] [Google Scholar]
- 22. Alberti KGMM, Zimmet PZ, for the WHO Consultation . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional Report of a WHO Consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 23. Sechi LA, Catena C, Zingaro L, et al. Hypertension and abnormalities of carbohydrate metabolism possible role of the sympathetic nervous system. Am J Hypertens. 1997;10:678–682. [DOI] [PubMed] [Google Scholar]
- 24. Grassi G, Colombo M, Seravalle G, et al. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity and congestive heart failure. Hypertension. 1998;31:64–67. [DOI] [PubMed] [Google Scholar]
- 25. Young JB, Troisi RJ, Weiss ST, et al. Relationship of cat‐echolamine excretion to body size, obesity, and nutrient intake in middle‐aged and elderly men. Am J Clin Nutr. 1992;56:827–834. [DOI] [PubMed] [Google Scholar]
- 26. Giannattasio C, Ferrari AU, Mancia G. Alterations in neural cardiovascular control mechanisms with ageing. J Hypertens Suppl. 1994;12:S13–S17. [PubMed] [Google Scholar]
- 27. Mazzeo RS, Rajkumar C, Jennings G, et al. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J Appl Physiol. 1997;82:1869–1874. [DOI] [PubMed] [Google Scholar]


