Abstract
The PREMIER trial assessed the aggregate effect on blood pressure (BP) of nationally recommended lifestyle modifications in free‐living adults with high‐normal (stage 1) hypertension. Participants (N=810) were randomized to the advice‐only group; the established group (consisting of weight loss, increased physical activity, and reduced sodium and alcohol intake); or the established plus Dietary Approaches to Stop Hypertension (DASH) diet group (consisting of the established interventions in addition to the DASH dietary pattern). The primary outcome was change in systolic BP at 6 months. Net of advice only, mean systolic BP declined by 3.7 mm Hg for members of the established group (p<0.001) and 4.3 mm Hg for the established plus DASH group (p<0.001). The prevalence of hypertension decreased from a baseline of 38% to 17% in the established group (p=0.01) and to 12% in the established plus DASH group (p<0.001) compared with a decrease to 26% in the advice‐only group. The PREMIER trial demonstrated that persons with above‐optimal BP and stage 1 hypertension can make multiple lifestyle changes leading to better control of BP.
Hypertension is highly prevalent in the United States; approximately 25% of the population is affected. 1 This prevalence is expected to increase, and although hypertension affects a large portion of the population, recognition and adequate treatment are less than ideal. 2
Hypertension is an independent risk factor for cardiovascular disease with a continuous relationship between blood pressure (BP) level and the risk of cardiovascular disease events. 3 Recent studies have found an elevated risk for cardiovascular disease events at BP levels categorized as high‐normal by the sixth report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI). 4 , 5 In response to this evidence, the seventh report of the JNC (JNC 7) 2 created a new category of hypertension, “prehypertension,” which is defined as systolic BP of 120–139 mm Hg or diastolic BP of 80–89 mm Hg. 2 Patients in this category are at increased risk for progression to hypertension. 6
Current national guidelines recommend nonpharmacologic therapy for primary prevention and treatment of hypertension. 2 , 4 This includes lifestyle modifications such as weight loss in those who are overweight or obese, increased physical activity, dietary sodium reduction, limited alcohol consumption, and the Dietary Approaches to Stop Hypertension (DASH) diet pattern. 2 Although it was recommended by JNC VI to implement these lifestyle modifications as first‐line treatment for up to 6 months in patients with stage 1 hypertension and no evidence of target organ damage, no trial has tested the combined effect of simultaneously implementing all recommended interventions. 4 , 7 , 8 , 9 , 10 In addition, the efficacy of the DASH dietary pattern was originally studied in a controlled‐feeding trial 11 and its effectiveness in free‐living adults had not been evaluated.
The PREMIER trial was designed to assess the ability of free‐living adults with high‐normal or stage 1 hypertension to make multiple, simultaneous lifestyle modifications and to assess their aggregate effect on BP. In addition, the investigators sought to evaluate the combined effect on BP of the DASH diet with the other recommended lifestyle interventions. The major findings have been published 12 and are summarized in this article.
DESIGN OF STUDY
The study design has been discussed in detail elsewhere. 13 The PREMIER trial was a multicenter, randomized controlled trial designed to assess the combined effect of lifestyle interventions on BP in patients with high‐normal BP or stage 1 hypertension who met the criteria for a 6‐month trial of nonpharmacologic therapy as defined by JNC VI. 4 The National Heart, Lung, and Blood Institute sponsored and participated in the trial. Participating centers included Duke University Medical Center, Durham, NC; Kaiser Permanente Center for Health Research, Portland, OR; Johns Hopkins Medical Center, Baltimore, MD; and Pennington Biomedical Research Center, Baton Rouge, LA. The Center for Health Research also served as the coordinating center.
Participants were generally healthy, community‐dwelling adults with above‐optimal BP or stage 1 hypertension in whom a 6‐month trial of lifestyle modification was the initial step in management of elevated BP. Inclusion criteria included a systolic BP of 120–159 mm Hg and diastolic BP of 80‐95 mm Hg, without antihypertensive treatment, based on mean BP across three screening visits. Other inclusion criteria were age ≥25 years and body mass index between 18.5 and 45. Major exclusion criteria included evidence of target‐organ damage, clinical cardiovascular disease, or diabetes mellitus and regular use of antihypertensive medications.
Eligible participants were randomized to one of three interventions: the advice‐only group; the established group, consisting of weight loss, increased physical activity, reduced dietary sodium intake, and reduced alcohol intake; or the established plus DASH group, consisting of the established‐group interventions as well as adoption of the DASH dietary pattern. An intensive intervention program occurred in the initial 6‐month period, followed by an additional, less intensive intervention period of 12 months. Participants randomized to the advice‐only intervention had a single, 30‐minute, individual session with a trained interventionist immediately following randomization. At this visit, participants received education on the recommended guidelines for nonpharmacologic BP control and were provided with printed educational materials. Upon completion of the 6‐month follow‐up measurements, advice‐only participants had another 30‐minute session with an interventionist.
In contrast to the advice‐only intervention, the active interventions consisted of 18 intervention contacts over 6 months (14 group meetings and four individual meetings). The established and established plus DASH intervention groups shared the same format and contact pattern. Goals for physical activity, sodium intake, alcohol intake, and weight loss were the same in these two groups (Table I). In addition, the established plus DASH intervention group participants received specific instructions about the DASH diet.
Table I.
Goal Lifestyle Changes in the Active Intervention Arms of the PREMIER Trial
| Intervention | Established | Established+ DASH |
|---|---|---|
| Fat (%) | ≤30 | ≤25 |
| Saturated fat (%) | ≤10 | ≤7 |
| Fruits/vegetables | Not specified | 9–12 Servings/d |
| Dairy | Not specified | 2–3 Servings/d |
| Physical activity | 180 minute/wk | |
| Sodium | ≤100 mmol/d | |
| Alcohol | ||
| Men | ≤1 oz/d | |
| Women | ≤0.5 oz/d | |
| Weight loss (BMI >24.9) | ≤15 lb at 6 months | |
| DASH=Dietary Approaches to Stop Hypertension diet; BMI=body mass index | ||
Trained, certified study personnel blinded to the participants' randomization assignments measured BP using a random zero sphygmomanometer. Baseline BP was the average of eight BP readings over four visits. BP reading at 6 months was the average of six BP readings over three visits.
The primary outcome of the study was change in systolic BP from baseline to 6 months. The advice‐only intervention group served as the control. The effects on systolic BP and other outcomes of the established group and established plus DASH group interventions were compared with that of the advice‐only intervention. In addition, effects of the established intervention were compared with the established plus DASH intervention. Secondary outcomes included diastolic BP at 6 months, systolic and diastolic BP at 18 months, and the incidence and prevalence of hypertension at 6 and 18 months. Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg.
RESULTS
Lifestyle Changes
A total of 810 participants were enrolled in the study. The baseline characteristics were similar among the randomized groups. Thirty‐eight percent of the participants had hypertension, with mean systolic and diastolic BPs of 143.9 mm Hg and 87.5 mm Hg, respectively. In the participants without hypertension, baseline mean systolic and diastolic BPs were 129.5 mm Hg and 83.2 mm Hg, respectively. For the entire study sample, mean age was 50.0 years, 62% were women, 34% were African American, and only 5% had a body mass index <25.
Several indicators of adherence revealed that many participants in all interventions were able to adopt multiple lifestyle changes. In the active intervention arms, intervention session attendance was high, with 70% of the established group and 78% of the established plus DASH group participants attending at least 15 sessions. Only 8% and 7% of the established and established plus DASH group participants, respectively, attended five sessions or fewer. Participants in all three interventions made lifestyle changes; however, more participants in the established and established plus DASH intervention groups achieved lifestyle modification goals (Table II). Participants in the established plus DASH intervention group had additional dietary goals for fruit/vegetable, dairy, and total fat/saturated fat intake (Table I). More than 33% of the participants in the established plus DASH intervention group consumed ≥9 servings/d of fruits and vegetables. Fifty‐nine percent reached dairy intake goals of ≥2 serving/d, whereas 58.7% and 46.5% met study goals for intake of total fat (≤25%) and saturated fat (≤7%), respectively.
Table II.
Intervention Outcomes in the PREMIER Trial: Mean Change From Baseline and Percent of Participants Meeting Intervention Goals at 6 Months
| Intervention Outcome | AdviceOnly | Established | Established+ DASH | Established vs . AdviceOnly | p Value* Established vs. + DASH AdviceOnly | Established vs .+ DASH Established |
|---|---|---|---|---|---|---|
| Weight loss | ||||||
| Mean change (kg) | −1.1 | −4.9 | −5.8 | <0.001 | <0.001 | 0.07 |
| Weight loss >15 lb (n/total [%]) | 15/242 (6.2) | 68/238 (28.6) | 80/233 (34.3) | <0.001 | <0.001 | 0.18 |
| Urinary sodium | ||||||
| Mean change (mEq/24 h) | −20.6 | −31.6 | −32.6 | 0.01 | 0.12 | 0.36 |
| Urinary Sodium ≤100 mmol/d (n/total [%]) | 42/215 (19.5) | 80/212 (37.7) | 59/211 (28) | <0.001 | 0.04 | 0.03 |
| Fruits and vegetables | ||||||
| Mean change (servings/d) | 0.5 | 0.5 | 3.0 | 0.79 | <0.001 | <0.001 |
| Fruits and vegetables ≥9 servings/d (n/total [%]) | 15/232 (6.5) | 14/227 (6.2) | 78/230 (33.9) | 0.9 | <0.001 | <0.001 |
| Dairy products | ||||||
| Mean change (servings/d) | 0.1 | −0.2 | 0.5 | 0.02 | <0.001 | <0.001 |
| Dairy products ≥2 servings/d (n/total [%]) | 78/232 (33.6) | 64/227 (28.2) | 136/230 (59.1) | 0.21 | <0.001 | <0.001 |
| Total fat | ||||||
| Mean change (% kcal) | −1.0 | −3.9 | −9.5 | <0.001 | <0.001 | <0.001 |
| Fat ≤25% kcal/d (n/total [%]) | 41/232 (17.1) | 66/227 (29.1) | 135/230 (58.7) | 0.004 | <0.001 | <0.001 |
| Saturated fat | ||||||
| Mean change (% kcal) | −0.4 | −1.5 | −3.3 | <0.001 | <0.001 | <0.001 |
| Saturated fat ≤7% kcal/d (n/total [%]) | 36/232 (15.5) | 60/227 (26.4) | 107/230 (46.5) | 0.004 | <0.001 | <0.001 |
| DASH=Dietary Approaches to Stop Hypertension diet; *corresponding pairwise differences in change for mean changes corresponding to pairwise differences at 6 months from logistic regression analysis for percent meeting goal lifestyle change Reprinted with permission from JAMA. 2003;289:2083–2093. 12 ©2003 American Medical Association. All rights reserved. | ||||||
Due to several comparable intervention goals, there were a number of similarities in intervention outcomes for the active intervention arms. There were no statistically significant differences between the established and established plus DASH intervention groups for physical activity, fitness, alcohol intake, or sodium intake. On average, all groups lost weight, with the established and established plus DASH participants losing a greater amount compared with the advice‐only participants. Compared with the advice‐only and established interventions, participants in the established plus DASH intervention group made statistically significant increases in intake of fruits, vegetables, and dairy. Changes in total fat and saturated fat intake were also greater for those in the established plus DASH intervention.
Blood Pressure Effects
During the 6‐month period, BP declined in each group (Figure 1). Mean BP decreased 6.6/3.8 mm Hg for the advice‐only group, 10.5/5.5 mm Hg for the established group, and 11.1/6.4 mm Hg for established plus DASH group. Patients with hypertension had greater reductions in both systolic and diastolic BP than those without hypertension. In participants with hypertension, mean systolic BP was reduced by 7.8, 12.5, and 14.2 mm Hg, respectively. Diastolic BP was reduced by 3.8, 5.8, and 7.4 mm Hg, respectively. Participants without hypertension reduced mean systolic and diastolic BPs by 5.8/3.8 mm Hg, 9.4/5.3 mm Hg, and 9.2/5.8 mm Hg, respectively.
Figure 1.
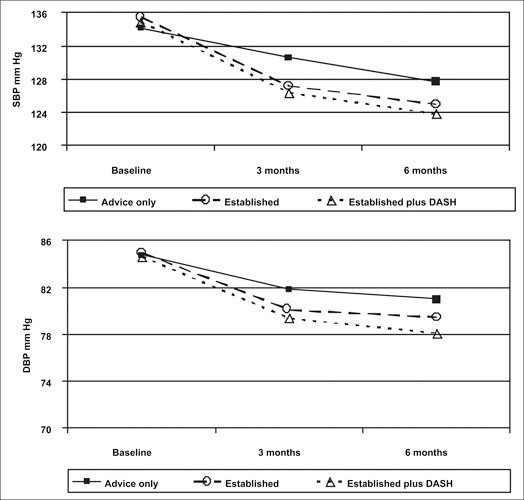
Mean systolic blood pressure (SBP) and diastolic blood pressure (DBF) over time by randomization group. DASH=Dietary Approaches to Stop Hypertension. Reprinted with permission from JAMA. 2003;289:2083–2093. 12 ©2003 American Medical Association. All rights reserved.
Comparing BP reduction achieved in the advice‐only intervention, the decrease in the mean systolic and diastolic BPs in both intervention arms was statistically significant (Table III). The significant decrease in systolic and diastolic BPs in the active intervention arms was consistent across all participants (including persons with and without hypertension). Although the reduction in mean systolic and diastolic BP was consistently greater in the established plus DASH intervention compared with the established intervention, this did not reach statistical significance.
Table III.
Mean Between‐Group Differences in Blood Pressure (BP) Change in All Participants, Participants Without Hypertension, and Participants With Hypertension in the PREMIER Trial
| Change in Established Minus | Change in Established+ DASH | Change in Established+ DASH | ||||
|---|---|---|---|---|---|---|
| Change in Advice Only | Minus Change in Advice Only | Minus Change in Established | ||||
| Mean | p Value | Mean | p Value | Mean | p Value | |
| Systolic BP | ||||||
| All | −3.7 | <0.001 | −4.3 | <0.001 | −0.6 | 0.43 |
| Nonhypertensives | −3.1 | 0.003 | −3.1 | 0.002 | 0 | 0.97 |
| Hypertensives | −4.6 | <0.001 | −6.3 | <0.001 | −1.7 | 0.2 |
| Diastolic BP | ||||||
| All | −1.7 | 0.002 | −2.6 | <0.001 | −0.9 | 0.11 |
| Nonhypertensives | −1.6 | 0.027 | −2 | 0.005 | −0.4 | 0.53 |
| Hypertensives | −2 | 0.025 | −3.6 | <0.001 | −1.6 | 0.08 |
| DASH=Dietary Approaches to Stop Hypertension trial Reprinted with permission from JAMA. 2003;289:2083–2093. 12 ©2003 American Medical Association. All rights reserved. | ||||||
At the end of 6 months, the prevalence of hypertension declined from a baseline of 38% in all three arms. Those who received advice only had a prevalence of hypertension of 26%. Compared with the advice‐only intervention group, the prevalence of hypertension at 6 months was significantly lower in both active intervention arms: 17% in the established group (p=0.01) and 12% in the established plus DASH group (p<0.001) (Figure 2). Among patients with hypertension at randomization, 52% still had hypertension at 6 months in the advice‐only arm, whereas only 34% of participants in the established group (p=0.01 vs. advice only) and 23% of participants in the established plus DASH arms remained hypertensive (p<0.001 vs. advice only; p=0.10 vs. established). Incident hypertension developed in 11% of patients without hypertension in the advice‐only arm at 6 months whereas only 8 % in the established group and 6% in the established plus DASH group developed hypertension. The differences in incident hypertension between treatment arms were not statistically significant.
Figure 2.
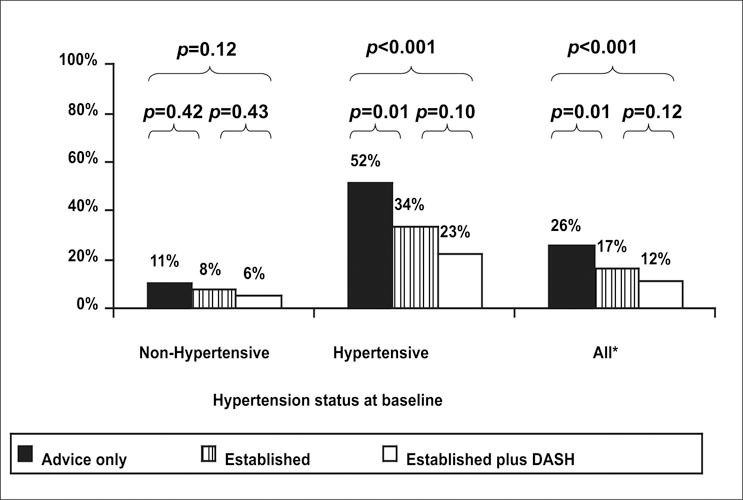
Percentage of participants with hypertension at 6 months by randomization group. DASH=Dietary Approaches to Stop Hypertension; *baseline prevalence of hypertension was similar (37%–38%) in each group. Reprinted with permission from JAMA. 2003;289:2083–2093. 12 ©2003 American Medical Association. All rights reserved.
Optimal BP control (BP <120/80 mm Hg) was achieved in significantly more participants in the two active intervention arms (30% in the established group, 35% in the established plus DASH group) than in the advice‐only arm (19%) (Figure 3). This effect was seen regardless of hypertension status at baseline. Twelve and 13% of participants with hypertension achieved optimal BP in the established and established plus DASH interventions, respectively. Only 3% of advice‐only participants with hypertension at baseline had optimal BP at 6 months. In participants with prehypertension, 48% of those in the established plus DASH group, 40% of those in the established group, and 29% of those in the advice‐only group achieved optimal BP at 6 months.
Figure 3.
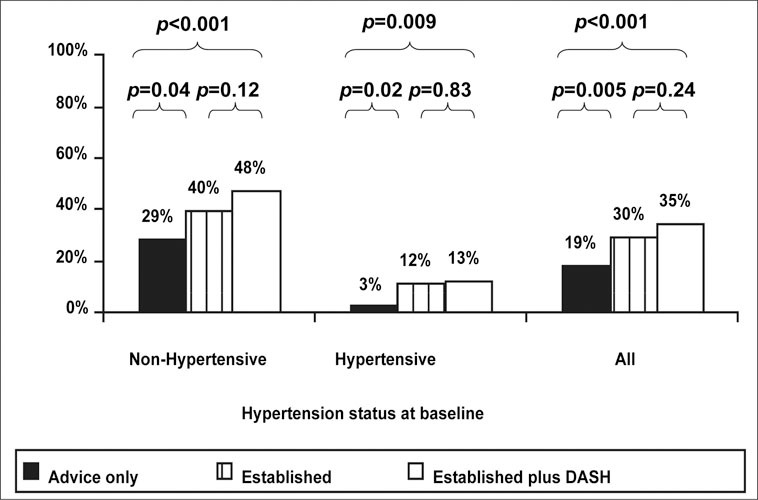
Percentage of participants with optimal blood pressure at 6 months by randomization group. DASH=Dietary Approaches to Stop Hypertension. Reprinted with permission from JAMA. 2003;289:2083–2093. 14 ©2003 American Medical Association. All rights reserved.
DISCUSSION
The PREMIER trial showed that free‐living adults with above‐optimal BP and stage 1 hypertension were able to implement multiple lifestyle modifications concurrently. Participants in the established and established plus DASH interventions lost weight and reduced dietary intake of sodium, fat, and saturated fat. In the established plus DASH intervention, participants significantly increased their fruit, vegetable, and dairy intake. These interventions significantly reduced BP, resulting in a decreased prevalence of hypertension and increased numbers of participants with optimal BP levels.
PREMIER participants represented a diverse population both demographically and geographically. Four centers were involved with participants from the south, mid‐Atlantic, and northwest regions of the United States. A range of educational and income levels were represented. More than half of the participants were women and more than one third were African American. Adherence to recommended lifestyle modifications was significant in both active interventions and across all sex‐race subgroups. The effectiveness of the PREMIER interventions in this diverse group supports the applicability of current recommendations for lifestyle modifications to the general public.
The reduction in BP achieved with the combined lifestyle interventions was essentially equivalent to that obtained with antihypertensive medication monotherapy. When implemented as first‐line therapy in patients with stage 1 hypertension, the established and established plus DASH interventions reduced the number of participants who met criteria to initiate antihypertensive therapy at 6 months by 66% and 77%, respectively. The active interventions were also effective in preventing hypertension. Implementing these interventions in the general population could potentially decrease the need for medications and associated monitoring for medication side effects, and ultimately, could result in prevention of cardiovascular events. 14 Further study of the effect of lifestyle modification on the prevention of cardiovascular events needs to be conducted.
A subadditive effect of multiple lifestyle modifications was seen in the PREMIER trial. This finding is consistent with other trials that implemented the combined effects of more than one lifestyle modification. 7 , 8 , 9 , 10 The established plus DASH intervention group participants did not have a statistically significant reduction in BP end points compared with the established intervention. Whereas the DASH trial was an efficacy study with controlled‐feeding design, the PREMIER trial was an effectiveness study that assessed the ability of free‐living adults to implement this dietary pattern. Despite marked improvements in fruit and vegetables intake, most participants did not reach the goal of nine servings a day achieved in the DASH trial. Decreased adherence may have resulted in a smaller dose, of sorts, of the DASH dietary pattern, thereby diminishing the full BP effect seen in the original DASH trials. Subadditivity may also have been caused by the inability of participants to fully adhere to multiple lifestyle interventions simultaneously. However, there was a trend toward greater reductions in BP and hypertension prevalence, with resulting increases in those with optimal BP in the established plus DASH group compared with the established group. This suggests there is likely an additional BP benefit with the addition of the DASH dietary pattern to other nonpharmacologic recommendations. Although addition of the DASH diet did not lead to statistically significant reductions in mean BP levels compared with the established‐group's lifestyle modifications alone, the DASH diet has health benefits beyond BP reduction. It favorably affects lipids, 15 homocysteine levels, 16 and possibly reduces osteoporosis risk. 17 In addition, eating a prudent dietary pattern similar to the DASH diet may lead to reduced cardiovascular disease events and mortality. 18 , 19 Thus, the DASH diet should be recommended as a lifestyle modification because of its ability to both improve overall health and BP.
Translating the PREMIER interventions into a traditional clinic setting may be difficult given time and resource constraints. A key component of the interventions in the trial was weekly group sessions led by a trained interventionist. Until intervention programs are widely available, clinicians and other members of the medical care team can use one‐on‐one counseling to play a significant role in educating patients about healthful lifestyles. An encouraging fact is that participants in the advice‐only arm of the PREMIER trial made lifestyle modifications using this type of intervention. A few extra moments spent counseling patients on nonpharmacologic interventions for BP control could translate into clinically significant reductions in BP. Resources to help educate patients on lifestyle modifications and the DASH dietary pattern are available from government sources at no cost (www.nhlbi.nih.gov/health/public/heart/hbp/dash/).
In addition, physicians can help patients make lifestyle modifications by utilizing several simple behavior‐counseling techniques. 20 , 21 , 22 First, physicians should assess a patient's readiness to change. Second, physicians should help patients set a realistic goal and formulate an action plan to achieve that goal. Third, physicians should help patients self‐monitor progress toward the goal. Physician‐initiated lifestyle modification can be followed‐up on with the help of ancillary personnel such as nurses, dietitians, and pharmacists.
Although PREMIER was a well designed effectiveness trial, further studies of translation and dissemination into realistic clinic settings are needed to provide additional insight into the implications for nonpharmacologic management of hypertension. Studies of cost‐effectiveness will also be beneficial to encourage insurers to provide this program as a benefit.
CONCLUSION
The PREMIER trial showed that comprehensive lifestyle modifications are achievable and effective. These results are applicable to the general population. The effect of adding the DASH dietary pattern to previously established nonpharmacologic recommendations for BP control was subadditive. However, the DASH diet has numerous potential health benefits and comprehensive nonpharmacologic measures should be promoted for prevention of hypertension and as adjunctive treatment in hypertension management.
References
- 1. Muntner P, He J, Roccella EJ, et al. The impact of JNC‐VI guidelines on treatment recommendations in the US population. Hypertension. 2002;39:897–902. [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 4. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 6. Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in nonhypertensive participants in the Framingham Heart Study. Lancet. 2001;358:1682–1686. [DOI] [PubMed] [Google Scholar]
- 7. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure . The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group . Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 8. Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group . JAMA. 1998;279:839–846. [DOI] [PubMed] [Google Scholar]
- 9. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 10. Gordon NF, Scott CB, Levine BD. Comparison of single versus multiple lifestyle interventions: are the antihypertensive effects of exercise training and diet‐induced weight loss additive? Am J Cardiol. 1997;79:763–767. [DOI] [PubMed] [Google Scholar]
- 11. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;36:1117–1124. [DOI] [PubMed] [Google Scholar]
- 12. Writing group of the PREMIER Collaborative Research Group . Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 13. Svetkey LP, Harsh DW, Vollmer WM, et al. PREMIER: a clinical trial of comprehensive lifestyle modification for blood pressure control: rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–471. [DOI] [PubMed] [Google Scholar]
- 14. Erlinger TP, Vollmer WM, Svetkey LP, et al. The potential impact of nonpharmacologic population‐wide blood pressure reduction on coronary heart disease events: pronounced benefits in African‐Americans and hypertensives. Prev Med. 2003;37:327–333. [DOI] [PubMed] [Google Scholar]
- 15. Obarzanek E, Sacks FM, Vollmer WM, et al. Effects on blood lipids of a blood pressure‐lowering diet: the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Clin Nutr. 2001;74:80–89. [DOI] [PubMed] [Google Scholar]
- 16. Appel LJ, Miller ER, Jee SJ, et al. Effect of dietary patterns on serum homocysteine: results of a randomized, controlled feeding study. Circulation. 2000;102:852–857. [DOI] [PubMed] [Google Scholar]
- 17. Lin PH, Appel LJ, Aickin M, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133:3130–3136. [DOI] [PubMed] [Google Scholar]
- 18. Kant AK, Schatzkin A, Graubard BI, et al. A prospective study of diet quality and mortality in women. JAMA. 2000;283:2109–2115. [DOI] [PubMed] [Google Scholar]
- 19. Hu FB, Rimm EB, Stampfer MJ, et al. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. [DOI] [PubMed] [Google Scholar]
- 20. Watson DL, Tharp RG. Self‐Directed Behavior: Self‐Modification for Personal Adjustment. 5th ed. Pacific Grove , CA : Brooks/Cole; 1989. [Google Scholar]
- 21. Prochaska JO, DiClemente CC. Stages and process of self‐change in smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. [DOI] [PubMed] [Google Scholar]
- 22. Marcus BH, Banspach SW, Pefebvre RC, et al. Using the stages of change model to increase the adoption of physical activity among community participants. Am J Health Promot. 1992;6:424–429. [DOI] [PubMed] [Google Scholar]


