Abstract
The relationship between dietary salt, blood pressure, and risk for cardiovascular disease has been debated for decades. Microalbuminuria is a biomarker for both cardiovascular and kidney disease. The presence of microalbuminuria correlates directly with the risk for myocardial infarction and stroke and indicates individuals at risk for the development of progressive kidney disease. Since patients with the metabolic syndrome, diabetes, or chronic kidney disease often are blood pressure salt sensitive, and it is well known that increasing dietary salt may offset both the antihypertensive and antiproteinuric effects of renin‐angiotensin system blocking drugs, physicians must consider increased salt intake as a potential modifiable risk factor for progression of chronic kidney disease and possibly even cardiovascular disease.
Chronic kidney disease (CKD) is not unusual in the general population. It is estimated that approximately 5% of the US population has a glo‐merular filtration rate below 60 mL/min. 1 In the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 2 involving more than 42,000 67‐year‐old patients with high blood pressure (BP), approximately one third of the patients had an estimated glomerular filtration rate below 60 mL/min. Microalbuminuria is also not unusual in the general population. In the Third National Health and Examination Survey (NHANES III) 3 dataset, which included 22,244 participants with and without diabetes, the overall prevalence of microalbuminuria was 7.8%. Among those individuals with diabetes, the prevalence of microalbuminuria was 28.8%. In this dataset, if one looked only at the patients with high BP, the incidence of microalbuminuria was 16%.
Patients with CKD and/or microalbuminuria are among those individuals at greatest risk for progression to both cardiovascular and renal events. 4 Consequently, there is a greater need to identify these patients early in their disease course and provide sufficient treatment to more intensively treat their global cardiovascular and renal disease risk. Nonpharmacologic therapy remains the foundation of our approach to the prevention and treatment of cardiovascular disease risk factors. Among the most important nonpharmacologic efforts that should be incorporated into the treatment paradigm is modification of dietary salt ingestion, since patients with CKD are more commonly BP salt sensitive. As it is well known that increasing dietary salt offsets both the antihypertensive and antiproteinuric effects of drugs such as angiotensin‐converting enzyme (ACE) inhibitors and angiotensin‐II receptor blockers, it is quite likely that increasing dietary salt may at least partially offset both the cardio‐ and renal‐protective properties of these drugs and thus increase the risk for progression of cardiorenal disease. Consequently, increased dietary salt intake should be considered a potentially modifiable risk factor for the progression of both cardiovascular and renal disease.
RENAL AUTOREGULATION
The presence of microalbuminuria in the urine indicates a disturbed blood urine interface that is representative of diffuse vascular disease throughout the circulation. This vascular disease may also affect the autoregulatory vascular beds that control glomerular capillary pressure—the afferent and efferent glomerular arterioles. The glomeruli effectively operate at a BP approximately one half to two thirds of systemic BP. 5 , 6
The afferent arteriole serves as the regulator of inflow to the glomerulus. By providing necessary preglomerular vasoconstriction, it reduces systemic pressures to ideal filtration pressures for the glomerular filter. The efferent arteriole selectively vasoconstricts during situations of diminished effective arterial blood volume, thus restoring proximal pressures within the glomerulus to facilitate ultrafiltration. This physiologic mechanism controls glomerular capillary pressure within about 5 mm Hg, despite a wide range of perfusion pressures. Inadequate autoregulation of these vascular beds results in increased glomerular capillary pressure with increasing likelihood for mechanical stretch and strain on vascular beds with subsequent injury. The presence of albumin in the urine indicates not only concerns about the vessels providing autoregulation but also the glomerular filtering vessels, which provide both pore‐ and charge‐selective restriction of plasma proteins like albumin.
DIETARY SALT AND BP SALT SENSITIVITY
For almost five millennia, it has been recognized that salt is essential for life, yet also may be associated with adverse effects on human health. This is a particularly important issue in the twenty‐first century, as there is an ongoing debate about salt and its contribution to disease, given the ubiquitous and often excessive intake of salt in contemporary Western diets. While it is beyond the scope of this review to address the relationship between salt and BP, it is important to note that the people primarily at risk would be those who demonstrate BP salt sensitivity. The mechanism underlying the differential response of BP with salt loading between salt‐sensitive and salt‐resistant patients is based on the concept that salt‐resistant hypertensives have elevated BP mediated by generalized vasoconstriction along with afferent (or preglomerular) vasoconstriction, which shifts the pressure‐natriuresis curve to the right but does not alter its slope. Thus there are individuals who can ingest large amounts of sodium without much change in BP (resistant) and others whose BPs will rise with even a minimal increase in salt intake (salt sensitive).
Patients with salt‐sensitive increases in BP may have diminished nephron mass and an overall reduction in glomerular ultrafiltration capacity, in addition to enhanced sodium reabsorption, which changes the slope of the pressure‐natriuresis curve, as shown in Figure 1. It is likely that other hormonal systems such as the renin‐angiotensin system and the sympathetic nervous system also play a role in mediating salt sensitivity. Renin levels are inappropriately suppressed in patients with salt‐sensitive hypertension, and blockade of the renin‐angiotensin system assists in restoration of the BP natriuresis response. 7 Acute salt loading inhibits secretion of catecholamines in healthy individuals. On the other hand, in salt‐sensitive hypertensives, no inhibition occurs. 8 Thus there are clear differences in hormonal regulation of glomerular hemodynamics and the pressure‐natriuresis response, which is different in the salt‐sensitive individual. Despite these clear differences, there is limited objective scientific evidence to conclusively link alterations in these hormonal systems with the associated changes in systemic and glomerular hemodynamics. Of interest is the observation that salt‐sensitive individuals possess a cardiovascular risk‐clustering phenotype with left ventricular hypertrophy, microalbuminuria, dyslipid‐emia, glucose intolerance, and lack of nocturnal dip of BP in many cases. Consequently, although BP salt sensitivity per se may not be a pathophysiologic factor leading to cardiovascular disease, it may identify people at risk for the development of cardiovascular disease for a number of different reasons.
Figure 1.
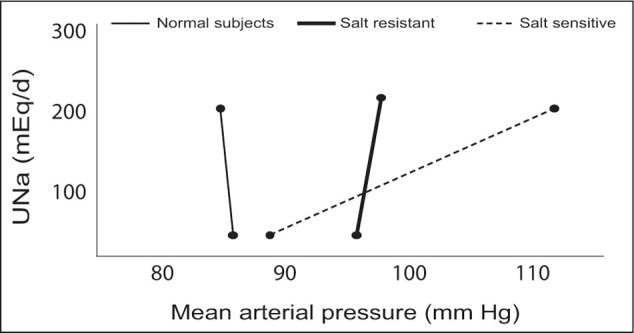
Pressure‐natriuresis curve among normal subjects and essential hypertensives who are either salt resistant or salt sensitive. UNa=urinary sodium Reproduced with permission from Kidney Int. 1982;21:371–378. 8
There is not an easy and simple test in clinical practice to identify those individuals who are sensitive to dietary salt and those who are not. One has to gauge the likelihood of salt sensitivity based on various demographic factors. Increasing age, obesity, African‐American ethnicity, diabetes, and CKD are more commonly associated with BP salt sensitivity. Thus, one can use the demographic background and comorbid diseases to predict the likelihood of dietary exposure to excess salt as a risk factor that could contribute to the progression of both cardiovascular and kidney disease.
Increasing dietary salt increases BP in salt‐sensitive individuals and results in an increase in the glomerular filtration fraction, 9 that is, the ratio of the glomerular filtration rate to the renal plasma flow. There is also a resultant increase in proteinuria. 9 Consequently, dietary salt‐induced changes in both systemic BP and glomerular capillary pressure may contribute to progressive renal injury. As depicted in Figure 2, middle‐aged, hypertensive patients were studied on a low‐salt and a high‐salt diet. Increasing dietary salt resulted not only in an increase of systolic BP but also an increased filtration fraction and proteinuria.
Figure 2.
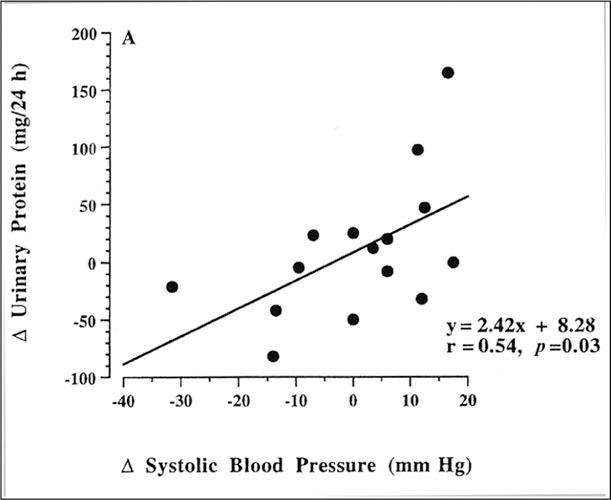
Scatterplots showing correlation between dietary salt‐mediated increase in systolic blood pressure with change in proteinuria (systolic, r=0.S4, p=0.03). Reprinted with permission from Hypertension. 1995;25:1339–1344. 9
CLINICAL IMPLICATIONS FOR THE MICROALBUMINURIC PATIENT
Microalbuminuria identifies patients with the risk clustering phenotype incorporating salt sensitivity, as well as the metabolic syndrome (obesity, systolic BP >130 mm Hg, glucose intolerance, high‐density lipoprotein cholesterol below 45 mg/dL, and triglycerides above 150 mg/dL). Its presence helps the clinician to identify those individuals with greater cardiovascular risk factors and a greater need for improved BP, cholesterol, and glucose control. Since salt sensitivity is common in these patients, advice with regard to modifying dietary salt intake is of great importance. The Dietary Approaches to Stop Hypertension (DASH) diet (fruits, vegetables, low‐fat dairy products, and a low sodium intake) has clearly demonstrated the important utility of dietary salt restriction in reducing BP in hypertensive subjects. 10 Moreover, these studies have also demonstrated the added benefits of ingesting legumes and diminished saturated fat in conjunction with dietary salt restriction in lowering BP. The advantage of legumes may be a result of increased dietary potassium, which may offset the hypertensive effects of dietary salt.
The other important clinical implication of microalbuminuria relates to the choice and dosing of antihy‐pertensive medications and the understanding of the impact of dietary salt. Increased dietary salt offsets at least some of the antihypertensive and antiproteinuric effects of ACE inhibitors, angiotensin receptor block‐ers, and nondihydropyridine calcium blockers. 11 , 12 In a large study 13 of salt‐sensitive hypertensive patients from different ethnic backgrounds, the patients demonstrated large, additional reductions in BP (systolic BP >10 mm Hg) with dietary salt restriction while on ACE inhibitor therapy compared to being on high salt. In perspective, dietary sodium modification is always advantageous regardless of chosen pharma‐cotherapy. Calcium channel blockers do maintain a greater ability to lower BP in the face of a high‐salt diet than other drugs.
Many clinicians are not aware that increasing dietary salt in the common range seen in the community can offset the antiproteinuric effects of the renin‐angiotensin system blockers. 11 , 12 In a carefully controlled clinical trial, 11 patients with nephropathy and approximately 6 g of urine protein/d were studied on a 50 mEq sodium diet and a 200 mEq sodium diet while on 20 mg of the ACE inhibitor, lisinopril. During the low‐salt diet, proteinuria was reduced by 50% to 3 g (Figure 3). Conversely, with the 200 mEq sodium diet, which is quite typical for a Western diet, there was no antiproteinuric effect with lisinopril and there was no difference when patients were compared with being treated with a‐methyldopa. Similar studies have been performed in patients taking angiotensin‐II receptor blockers and nondihydropyridine calcium channel blockers. It was demonstrated that dietary salt can offset the antiproteinuric effects of these drugs as well. These observations, in conjunction with recent post hoc analyses demonstrating that reducing proteinuria is associated with a reduction in both renal and cardiovascular events, 14 ' 15 strongly implicate dietary salt consumption as a potentially modifiable risk that may protect patients from cardiovascular and renal disease progression. Despite the lack of scientific evidence correlating BP salt sensitivity with adverse clinical outcomes, one has to question whether salt consumption represents an unrecognized and important risk factor for accelerated loss of kidney function in patients with CKD, particularly those with early CKD accompanied by microalbuminuria.
Figure 3.
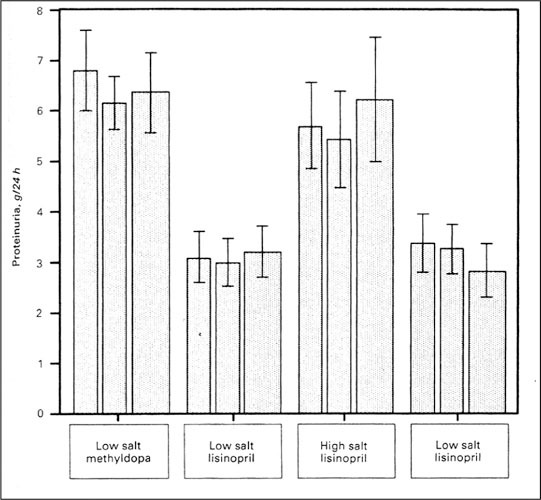
Urine protein excretion in nine patients with nephropathy depending on salt intake and antihypertensive therapy. The bars represent mean±SEM of 24‐hour urine protein in the last month of the study periods. Wide bars represent the mean of three samples before visits, whereas the narrow bars indicate values from preceding visits. Reprinted with permission from Kidney Int. 1989;36:272–279. 11
CONCLUSIONS
Patients with the metabolic syndrome and microalbuminuria are more likely to be BP salt sensitive than salt resistant. The etiology of this salt sensitivity is unknown. It may relate to early kidney disease with reduced neph‐ron mass. There are also theories supported by elegant scientific data to suggest that increasing dietary salt may increase the production of reactive oxygen species in the kidney, which may result in a diminished capability to excrete salt. 16 With the consequent increase in both systemic and glomerular capillary pressure associated with increased salt intake, there is a greater risk for the microalbuminuric patient for both cardiovascular and CKD progression. Thus future clinical trials need to focus on examining salt‐consumption patterns, perhaps by measuring urinary electrolyte excretion in patients with microalbuminuria and early evidence of CKD to evaluate their cardiovascular event risks longitudinally, which may provide some understanding about the possible advantage of modest dietary salt restriction. In the meantime, it would be prudent to consider dietary interventions in these patients to assist in achieving better BP goals and reduction in albuminuria. Future clinical trials will also need to address methods to overcome the effects of salt on BP and microalbuminuria if dietary efforts are not sufficient, perhaps by employing higher doses of ACE inhibitiors or angiotensin‐II receptor blockers, either alone or in conjunction with one another. More studies should be conducted with thiazide diuretics to see if they will amplify the antipro‐teinuric effects of these drugs, even in the face of greater salt consumption. Small studies do indicate that they may help. 17 Unfortunately, we do not have an answer on how much salt is too much in a given patient. Until we know, modest avoidance of foods rich in salt content is advisable in the patient with metabolic syndrome or microalbuminuria or both.
References
- 1. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 2. Rahman M, Brown CD, Coresh J, et al. The prevalence of reduced glomerular filtration rate in older hypertensive patients and its association with cardiovascular disease: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Arch Intern Med. 2004;164:969–976. [DOI] [PubMed] [Google Scholar]
- 3. Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. [DOI] [PubMed] [Google Scholar]
- 4. Weir MR. Microalbuminuria in type 2 diabetics: an important, overlooked cardiovascular risk factor. J Clin Hypertens (Greenwich). 2004;6:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Just A, Ehmke H, Toktomambetova L, et al. Dynamic char acteristics and underlying mechanisms of renal blood flow autoregulation in the conscious dog. Am J Physiol Renal Physiol. 2001;280:F1062–F1071. [DOI] [PubMed] [Google Scholar]
- 6. Sorensen CM, Leyssac PP, Skott O, et al. Role of the renin‐angiotensin system in regulation and autoregulation of renal blood flow. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1017–R1024. [DOI] [PubMed] [Google Scholar]
- 7. Van Paassen P, De Zeeuw D, Navis G, et al. Does the renin‐angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension. 1996;27:202–208. [DOI] [PubMed] [Google Scholar]
- 8. Campese VM, Romoff MS, Levitan D, et al. Abnormal relationship between sodium intake and sympathetic ner vous system activity in salt‐sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. [DOI] [PubMed] [Google Scholar]
- 9. Weir MR, Dengel DR, Behrens MT, et al. Salt‐induced increases in systolic blood pressure affect renal hemodynam‐ics and proteinuria. Hypertension. 1995;25:1339–1344. [DOI] [PubMed] [Google Scholar]
- 10. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 11. Heeg JE, De Jong PE, Van Der Hem GK, et al. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int. 1989;36:272–279. [DOI] [PubMed] [Google Scholar]
- 12. Bakris GL, Smith A. Effects of sodium intake on albumin excretion in patients with diabetic nephropathy treated with long‐acting calcium antagonists. Ann Intern Med. 1996;125:201–204. [DOI] [PubMed] [Google Scholar]
- 13. Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin‐converting enzyme inhibitor or a calcium chan nel antagonist in salt‐sensitive hypertensives. Hypertension. 1998;31:1088–1096. [DOI] [PubMed] [Google Scholar]
- 14. De Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 dia betic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. [DOI] [PubMed] [Google Scholar]
- 15. De Zeeuw D, Remuzzi G, Parving G, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;l 10:921–927 [DOI] [PubMed] [Google Scholar]
- 16. Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep. 2002;4:160–166. [DOI] [PubMed] [Google Scholar]
- 17. Buter H, Hemmelder MH, Navis G, et al. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998;13:1682–1685. [DOI] [PubMed] [Google Scholar]


