Abstract
Endothelin‐1 stimulates collagen synthesis and is increased in hypertension, but its effect on collagen degradation remains unknown. The current study tested the hypothesis that elevated endothelin‐1 levels are associated with decreased collagenase activity, markers of collagen degradation, and arterial compliance in hypertensive patients. Normotensive (n=10) and hypertensive (n=13) patients who were not on any antihypertensive medication were recruited, and small and large artery elasticity index, systemic vascular resistance, pulse pressure, and blood pressure were determined using blood pressure waveform analysis. Large artery elasticity index and collagen degradation products were decreased whereas endothelin‐1, systemic vascular resistance, and pulse pressure were elevated in hypertensive patients. Plasma endothelin‐1 was negatively correlated with a cross‐linked C‐terminal telopeptide of collagen type I, a collagen degradation marker (r=−0.43; p=0.04), collagenase matrix metalloproteinase‐1 (r=−0.48; p=0.02), and large artery elasticity (r=−0.45; p=0.03) and positively correlated with pulse pressure (r=0.68; p=0.0005). These results suggest that endothelin‐1 contributes to decreased arterial compliance in hypertension via inhibition of collagen degradation.
Arterial hypertension is associated with increased risk of target organ damage, resulting in stroke, left ventricular hypertrophy, and coronary artery disease. 1 A number of observational and clinical studies, including the Framingham Heart Study, demonstrated that there is a strong association among blood pressure (BP), pulse pressure (PP), and adverse cardiac events. 2 , 3 , 4 , 5 Since BP and PP are related to the physical properties of elastic arteries, arterial stiffness and pulse wave velocity are now considered cardiovascular risk factors; assessment of arterial stiffness may improve efforts for optimal treatment of high BP and related complications. 5 , 6 In recent years, the development of noninvasive techniques such as brachial artery pulse wave analysis has enabled the rapid assessment of arterial stiffness. 7 , 8 , 9
Structural changes in the vessel wall such as increased extracellular matrix deposition (especially collagen type I), resulting in arterial fibrosis, contribute to increased stiffness. 10 , 11 , 12 Enhanced collagen deposition may be due to increased collagen synthesis and/or decreased degradation by matrix metalloproteinases (MMP), including collagenase MMP‐1. Laviades and colleagues 13 , 14 reported that plasma levels of MMP‐1 and a cross‐linked C‐terminal telopeptide of collagen type I (ICTP), a collagen type I degradation product, were decreased in patients with essential hypertension and left ventricular hypertrophy, whereas levels of endogenous inhibitors of matrix proteinases were increased. Using human arterial tissue, our group demonstrated that not only MMP‐1 and MMP‐9, but MMP inducer and activator proteins, are also down‐regulated in the hypertensive state. 15 However, the relationship of circulating levels of collagen synthesis and degradation products such as ICTP, as well as collagenase levels to arterial stiffness remained unknown.
While increased stiffness is mainly attributed to structural changes in the vessel wall, changes in smooth muscle tone also affect the stiffness of arteries. Vasoactive peptides such as endothelin‐1 (ET‐1) and angiotensin II are involved in this process. 8 McEniery et al. 16 reported that endogenous ET‐1 regulates arterial pulse wave velocity. In addition to vasoconstrictor effects, ET‐1 stimulates collagen synthesis. We and others have shown that ET receptor antagonism improves extracellular matrix deposition in experimental hypertension. 11 , 12 , 17 , 18 , 19 , 20 , 21 Thus, ET‐1 is an important vasoactive factor that can affect both functional and structural arterial stiffening. Accordingly, the goal of this study was to study the relationship among circulating ET‐1, markers of vascular remodeling, and indices of arterial elasticity in normotensive vs. hypertensive patients.
MATERIALS AND METHODS
Patient Enrollment and Pulse Contour Analyses
The study protocol was approved by the Human Assurance Committee at the Medical College of Georgia. Ten normotensive and 13 hypertensive patients who were not receiving any antihypertensive medication were recruited from the hypertension clinic. BP >160 mm Hg systolic and/or 95 mm Hg diastolic was considered hypertensive. Exclusion criteria included renal failure (serum creatine >2 mg/dL), pulmonary edema, asthma (exacerbation within the past month), recent myocardial infarction (<6 months) or angina (<1 month), cardiogenic shock, and heart failure. Demographics of patients are summarized in the Table. After patient consent was obtained, pulse contour analysis was measured using a CVProfilor DO‐2020 (Hypertension Diagnostics, Inc., Eagan, MN) cardiovascular profiling system. Patients remained supine for 5 minutes before measurement of arterial elasticity. The BP cuff was placed on the left upper arm. A wrist stabilizer was positioned on the patient's right wrist to stabilize the position of the radial artery. The arterial sensor was placed over the right radial artery at the point of maximal pulsation to capture an analog BP waveform data signal and adjusted to the highest relative signal strength. Three measurements approximately 1 minute apart were performed.
Blood Chemistry
Upon completion of compliance measurements, blood was collected in ethylenediaminetetraacetic acid tubes. Plasma ET‐1, MMP‐1, and collagen degradation and synthesis products ICTP and procollagen type I N‐terminal peptide, respectively (DiaSorin, Stillwater, MN), were determined as we have described previously. 15
Data Analyses
Comparisons between hypertensive and nonhypertensive groups were made using Student t tests. Pearson correlations were used to assess the linear association between plasma ET‐1 levels and other variables. Statistical significance was set at p<0.05. SAS version 8.2 (SAS Institute, Inc., Cary, NC) was used for all analyses.
RESULTS
There were significant differences only in systolic and diastolic BP, PP, and systemic vascular resistance between normotensive and hypertensive patients, which showed a significant increase in hypertensive patients as expected (Figure 1). Large artery elasticity index was significantly decreased (p=0.043), and there was a trend for decreased small artery elasticity index in hypertensive patients (p=0.11) (Figure 2).
Figure 1.
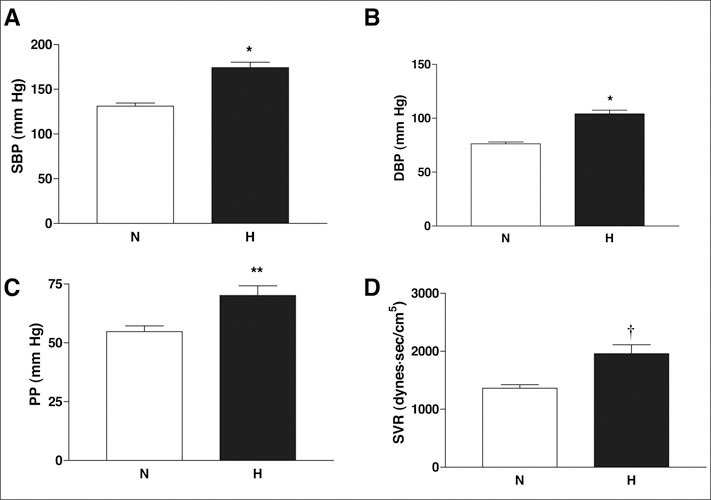
Comparison of hemodynamic parameters in normotensive (N) and hypertensive (H) patients. Systolic blood pressure (SBP) (A), diastolic blood pressure (DBP) (B), pulse pressure (PP) (C), and systemic vascular resistance (SVR) (D) were significantly elevated in the hypertensive group. Results are given as mean ± SEM. *p<0.001; **p=0.008;†p=0.003 vs. normotensive
Figure 2.
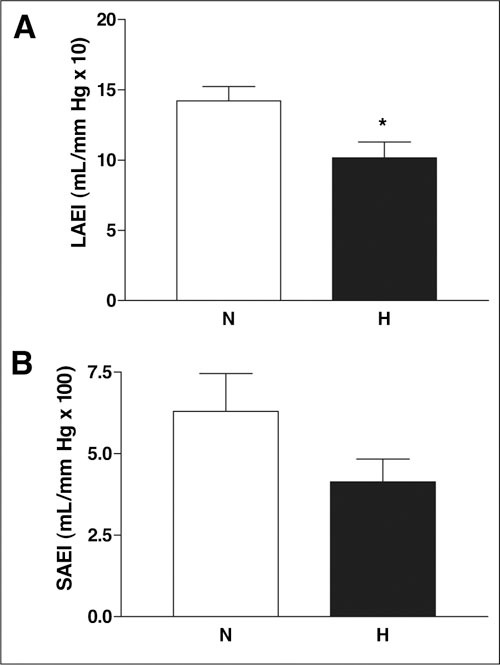
Arterial elasticity measurements in normotensive (N) and hypertensive (H) patients. Large artery elasticity index (LAEI) (A) is decreased and there is a trend for decreased small artery elasticity index (SAEI) (B) in the hypertensive group. Results are given as mean ± SEM. *p=0.043 vs. normotensive
Circulating markers of vascular remodeling were also analyzed. Collagen degradation marker ICTP was significantly decreased in hypertensive patients (p=0.005), but there was no change in a collagen synthesis marker between groups (Figure 3A and B). Consistent with decreased collagen markers, fibrillar collagenase MMP‐1 (Figure 3C) was also decreased in hypertensive patients (p<0.001). Collagen degradation levels showed an inverse relationship with increased systolic BP (r=−0.57; p=0.006), diastolic BP (r=−0.55; p=0.008), and PP (r=−0.48; p=0.024). Decreased matrix levels were also associated with increased systolic BP (r=−0.67; p=0.0006), diastolic BP (r=−0.72; p=0.0002), and PP(r=−0.49; p=0.021).
Figure 3.
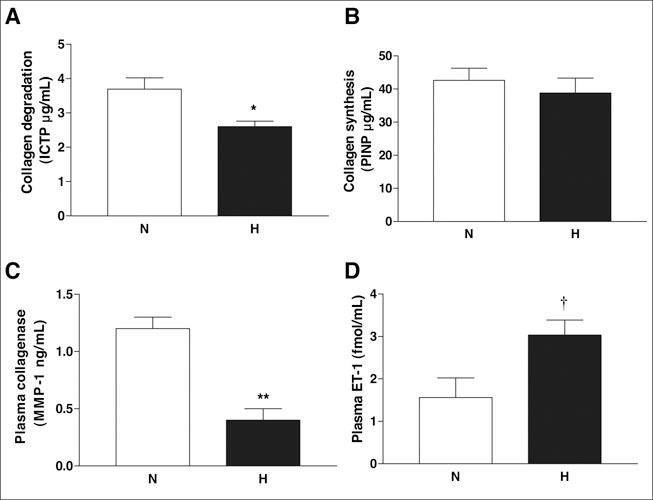
Plasma levels of vascular remodeling markers and endothelin‐1 (ET‐1) in normotensive (N) and hypertensive (H) patients. Collagen degradation product cross‐linked C‐terminal telopeptide of collagen type I (ICTP) and collagenase matrix metalloproteinase (MMP‐1) are decreased, whereas ET‐1 levels are elevated in hypertensive patients. Results are given as mean ± SEM. *p=0.005; **p<0.001;†p=0.018 vs. normotensive
As shown in Figure 3D, plasma ET‐1 levels were also elevated in the hypertensive group (p=0.018). Pearson correlations demonstrated that plasma ET‐1 was negatively correlated with collagen degradation product ICTP (r=−0.43; p=0.04), collagenase MMP‐1 (r=−0.48; p=0.02), and large vessel elasticity (r=−0.45; p=0.03), and positively correlated with PP (r=0.68; p=0.0005).
DISCUSSION
The important findings of this study are that collagen degradation, and not synthesis, is altered in association with elevated ET‐1 levels, and that elevated ET‐1 levels are associated with decreased large vessel elasticity and increased PP in hypertensive patients. These results suggest that ET‐1 contributes to decreased arterial elasticity in hypertension via inhibition of collagen degradation.
It has long been recognized that PP is an indicator of arterial stiffness and that arterial stiffness is an important predictor of cardiovascular risk. 2 , 3 , 4 , 5 Increased arterial stiffness results in decreased vascular compliance, which reflects the inability of vessels to adapt to changes in BP. Therefore, in recent years, evaluation of arterial compliance in addition to BP measurements has been recommended by some investigators not only to monitor the effectiveness of antihypertensive therapy but also to determine early vasculopathy. 7 , 8 , 22 , 23 , 24 , 25 We and others have shown that both large and small artery elasticity can be easily assessed by noninvasive pulse wave analysis. 7 , 9 , 22 The current study provided evidence that large artery elasticity is significantly decreased in patients whose BP is not currently under control. Decreased arterial elasticity may arise from pathologic vascular remodeling of arteries in hypertension, which involves growth, extracellular expansion, and fibrosis. 26 , 27 , 28 Alterations in the abundance and properties of structural elements such as collagen and elastin are important determinants of vascular stiffness. MMPs regulate this process in a complex manner by degrading matrix proteins such as collagen and elastin. 29 In our previous study, we demonstrated that both inducer and activator proteins of the arterial MMP system are reduced in hypertension, leading to enhanced matrix deposition. 15 The current study provides evidence that circulating MMP‐1 levels are also decreased in hypertensive patients and that this is correlated with decreased collagen degradation markers as well as decreased arterial elasticity.
Several other studies have reported changes in the soluble markers of vascular remodeling in hypertension. 13 , 14 , 30 , 31 , 32 A recent report provided evidence that 6‐month treatment with felodipine restored decreased MMP levels in hypertensive patients, whereas diltiazem had no effect. 32 Yasmin et al. 33 investigated plasma proteinase levels in isolated systolic hypertension as well as in healthy individuals and found that aortic stiffness is related to these not only in patients with systolic hypertension but also in healthy individuals—indicating the importance of this system in structural properties of the vessel wall. The current study complements these findings and provides evidence that decreased circulating MMP‐1 levels in hypertensive patients are associated with increased arterial stiffness.
In addition to the structural properties of the vasculature, it is reasonable to speculate that smooth muscle tone can also affect arterial stiffness. Early studies suggested that ET‐1, the most potent vasoconstrictor with mitogenic and profibrotic properties, contributes to basal tone of large arteries, and that circulating ET‐1 levels are correlated with decreased arterial elasticity. 34 , 35 ET‐1 is also known to stimulate the synthesis of collagen. In an experimental model of borderline hypertension, we reported that ET‐1 regulates the activation of MMPs in the aorta. 17 These past studies suggest that ET‐1 regulates MMP expression/activity, leading to augmented collagen deposition in a complex and time‐dependent manner. 36 The current study extends the findings obtained with experimental models to clinical studies and demonstrates that elevated ET‐1 levels are associated with decreased arterial elasticity, plasma MMP‐1, and collagen degradation markers in hypertensive patients. As we previously reported, plasma ET‐1 levels are elevated to a greater degree in African‐American patients; in this study there are more African‐American individuals in the hypertensive group than in the normotensive group. 37 Whether this imbalance contributes to the findings of increased artery stiffness in the hypertensive group remains to be determined.
Perspectives
There are several limitations of our study that should be recognized. First, the results of this study do not provide evidence of a cause‐and‐effect relationship between increased arterial stiffness and ET‐1 or MMPs. The current findings suggest that elevated ET‐1 levels down‐regulate collagenase expression/activity, which results in decreased collagen turnover and, ultimately, increased arterial stiffness. Second, we evaluated only collagenase‐and gelatinase‐type MMPs; other factors in this group remain to be studied. Third, we measured the indices of vascular compliance and plasma markers at only one time point. Hypertensive patients who participated in the study were not receiving any antihypertensive treatment when they were enrolled in the study. However, there is limited information regarding the duration of hypertension or the previous use of antihypertensive medications in this cohort. Finally, the number of subjects is small. Nevertheless, the importance of the current results is three‐fold. First, recent studies have identified plasma MMP‐9 as a novel predictor of cardiovascular mortality. 38 , 39 , 40 Thus, evaluation of MMPs involved in vascular remodeling may serve as a marker of vascular compliance and cardiovascular risk. Second, assessment of arterial stiffness may be important for the management of hypertension and may provide a noninvasive means to monitor BP control. The third and the most speculative interpretation of the current data is that ET receptor antagonism may provide BP control and vascular protection by improving arterial elasticity in hypertensive patients.
Disclosure: Dr. Ergul is the recipient of research awards from the American Diabetes Association, University of Georgia Research Foundation, Philip Morris USA Inc., Philip Morris International, and the National Institutes of Health (R15HL76236 and RO1 DK074385).
References
- 1. American Heart Association. Heart and stroke statistical update. Available at: www.americanheart.org. Accessed June 6, 2006.
- 2. Black HR, Kuller LH, O'Rourke MF, et al. The first report of the Systolic and Pulse Pressure (SYPP) Working Group. J Hypertens Suppl. 1999;17:S3–S14. [PubMed] [Google Scholar]
- 3. Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–360. [DOI] [PubMed] [Google Scholar]
- 4. Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–564. [DOI] [PubMed] [Google Scholar]
- 5. Nichols WW Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. [DOI] [PubMed] [Google Scholar]
- 6. Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19:341–352. [DOI] [PubMed] [Google Scholar]
- 7. Winer N, Folker A, Murphy JA, et al. Effect of fixed‐dose ACE‐inhibitor/calcium channel blocker combination therapy vs. ACE‐inhibitor monotherapy on arterial compliance in hypertensive patients with type 2 diabetes. Prev Cardiol. 2005;8:87–92. [DOI] [PubMed] [Google Scholar]
- 8. McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin‐converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91:30H–37H. [DOI] [PubMed] [Google Scholar]
- 9. Prisant LM, Pasi M, Jupin D, et al. Assessment of repeatability and correlates of arterial compliance. Blood Press Monit. 2002;7:231–235. [DOI] [PubMed] [Google Scholar]
- 10. Endemann DH, Pu Q, De Ciuceis C, et al. Persistent remodeling of resistance arteries in type 2 diabetic patients on anti‐hypertensive treatment. Hypertension. 2004;43:399–404. [DOI] [PubMed] [Google Scholar]
- 11. Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone‐infused rats: role of endothelin‐1. Am J Hypertens. 2002;15:164–169. [DOI] [PubMed] [Google Scholar]
- 12. Ammarguellat FZ, Gannon PO, Amiri F, et al. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA‐salt hypertensive rats: role of ET (A) receptors. Hypertension. 2002;39:679–684. [DOI] [PubMed] [Google Scholar]
- 13. Laviades C, Varo N, Fernandez J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–540. [DOI] [PubMed] [Google Scholar]
- 14. Diez J, Laviades C, Mayor G, et al. Increased serum concentrations of procollagen peptides in essential hypertension. Relation to cardiac alterations. Circulation. 1995;91:1450–1456. [DOI] [PubMed] [Google Scholar]
- 15. Ergul A, Portik‐Dobos V, Hutchinson J, etal. Downregulation of vascular matrix metalloproteinase inducer and activator proteins in hypertensive patients. Am J Hypertens. 2004;17:775–782. [DOI] [PubMed] [Google Scholar]
- 16. McEniery CM, Qasem A, Schmitt M, et al. Endothelin‐1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. [DOI] [PubMed] [Google Scholar]
- 17. Ergul A, Portik‐Dobos V, Giulumian AD, et al. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol. 2003;285:H2225–H2232. [DOI] [PubMed] [Google Scholar]
- 18. Schiffrin EL. Role of endothelin‐1 in hypertension. Hypertension. 1999;34(part 2):876–881. [DOI] [PubMed] [Google Scholar]
- 19. Intengan HD, Schiffrin EL. Collagen degradation is diminished in mesenteric arteries of spontaneously hypertensive rats after hypertension is established [abstract]. Hypertension. 1999;34:329. [Google Scholar]
- 20. Schiffrin EL. Role of endothelin‐1 in hypertension and vascular disease. Am J Hypertens. 2001;14:83S–89S. [DOI] [PubMed] [Google Scholar]
- 21. Touyz RM, Chen X, Tabet F, et al. Expression of a functionally active gp91phox‐containing neutrophil‐type NAD (P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. [DOI] [PubMed] [Google Scholar]
- 22. Prisant LM, Resnick LM, Hollenberg SM. Arterial elasticity among normotensive subjects and treated and untreated hypertensive subjects. Blood Press Monit. 2001;6:233–237. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell GF, Lacourciere Y, Arnold JM, et al. Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension. 2005;46:1111–1117. [DOI] [PubMed] [Google Scholar]
- 24. Izzo JL Jr. Pulse contour analysis and augmentation index: it's time to move beyond cuff blood pressure measurement. Am J Hypertens. 2005;18:1S–2S. [DOI] [PubMed] [Google Scholar]
- 25. Izzo JL Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med. 2001;2:29–34, 37–40. [PubMed] [Google Scholar]
- 26. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. [DOI] [PubMed] [Google Scholar]
- 27. Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension. Hypertension. 2000;36:312–318. [DOI] [PubMed] [Google Scholar]
- 28. Ammarguellat F, Larouche II, Schiffrin EL. Myocardial fibrosis in DOCA‐salt hypertensive rats: effect of endothelin ET (A) receptor antagonism. Circulation. 2001;103:319–324. [DOI] [PubMed] [Google Scholar]
- 29. Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. [DOI] [PubMed] [Google Scholar]
- 30. Diez J, Panizo A, Gil MJ, et al. Serum markers of collagen type I metabolism in spontaneously hypertensive rats: relation to myocardial fibrosis. Circulation. 1996;93:1026–1032. [DOI] [PubMed] [Google Scholar]
- 31. Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17:119–124. [DOI] [PubMed] [Google Scholar]
- 32. Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase‐2 and ‐9 in essential hypertension. Am J Hypertens. 2004;17:273–276. [DOI] [PubMed] [Google Scholar]
- 33. Yasmin, Wallace S, McEniery CM, et al. Matrix metallo‐proteinase‐9 (MMP‐9), MMP‐2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vase Biol. 2005;25:372–378. [DOI] [PubMed] [Google Scholar]
- 34. Kyriakides ZS, Kremastinos DT, Bofilis E, et al. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart. 2000;84:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heintz B, Dorr R, Gillessen T, et al. Do arterial endothelin 1 levels affect local arterial stiffness? Am Heart J. 1993;126:987–989. [DOI] [PubMed] [Google Scholar]
- 36. Harris AK, Hutchinson JR, Sachidanandam K, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin‐1. Diabetes. 2005;54:2638–2644. [DOI] [PubMed] [Google Scholar]
- 37. Ergul S, Parish CD, Puett D, et al. Racial differences in plasma endothelin‐1 concentrations in individuals with essential hypertension. Hypertension. 1996;28:652–655. [DOI] [PubMed] [Google Scholar]
- 38. Sundstrom J, Evans JC, Benjamin EJ, et al. Relations of plasma matrix metalloproteinase‐9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. [DOI] [PubMed] [Google Scholar]
- 39. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 40. Medley TL, Cole TJ, Dart AM, et al. Matrix metalloproteinase‐9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vase Biol. 2004;24:1479–1484. [DOI] [PubMed] [Google Scholar]


