Abstract
Isolated systolic hypertension is the most common form of hypertension, especially among patients 50 years or older. What is not appreciated is that there are secondary causes of isolated systolic hypertension. Hyperthyroidism increases systolic blood pressure by decreasing systemic vascular resistance, increasing heart rate, and raising cardiac output. Potential cardiovascular consequences of hyperthyroidism include atrial arrhythmias (especially atrial fibrillation), pulmonary hypertension, left ventricular hypertrophy, and heart failure. The prevalence of hypertension is greater among hyperthyroid patients than euthyroid patients. Whether there is a blunted nocturnal decline in ambulatory blood pressure among hyperthyroid patients is more controversial. Treatment is associated with a reduction in systolic blood pressure, heart rate, and cardiac output.
Isolated systolic hypertension (≥140/<90 mm Hg) is the most common form of hypertension. 1 The treatment of systolic blood pressure (SBP) has received increasing attention over the past 10 years because of its impact on coronary heart disease, stroke, heart failure, end‐stage renal disease, and total mortality. 2 Two trials, the Systolic Hypertension in the Elderly Program (SHEP) 3 and the Systolic Hypertension in Europe (Syst‐Eur) trial, 4 have documented cardiovascular benefits for lowering blood pressure (BP) in patients with isolated systolic hypertension. The increase in SBP occurs with aging and is associated with loss of the elastic properties of the aorta. 1 , 5 , 6 This amplifies SBP and reduces diastolic BP. 7
What is not appreciated is that there are secondary causes of isolated systolic hypertension. These include anemia, aortic insufficiency, Paget's disease, beriberi, and hyperthyroidism (Figure 1). They all share a common mechanism of an increase in cardiac output.
Figure 1.

Graves' disease. A 57‐year‐old man whose blood pressure had been controlled on verapamil sustained‐release 240 mg b.i.d. presented with a history of weight loss and tremulousness. His blood pressure was 159/80 mm Hg and his heart rate was 100 bpm. His laboratory values included thyroxine 21.5 μg/dL (normal, 5.0–9.0 μg/dL), triiodothyronine radioimmunoassay 241 ng/dL (110–200 ng/dL), and sensitive thyroidstimulating hormone <0.1 IU/mL (0.6–4.6 IU/mL).
EFFECT OF THYROID HORMONE ON THE CARDIOVASCULAR SYSTEM
Triiodothyronine (T3) is the active form of thyroid hormone. 8 It is produced in the liver by 5′‐monodeiodination of thyroxine (T4) that is synthesized by the thyroid gland. 9 T3 affects the basal metabolic rate by altering oxygen consumption in peripheral tissues, metabolic demands, and tissue thermogenesis.
In hyperthyroidism, T3 dilates resistance arterioles, reducing systemic vascular resistance by 300–1000 dynes•sec•cm−5. The decline in systemic vascular resistance stimulates renin release and sodium reabsorption, resulting in an expansion of blood volume by 5.5% and an increase in venous return to the heart. 10 Erythropoietin stimulation also contributes to blood volume. Heart rate and cardiac contractility are also changed with hyperthyroidism. Heart rate is raised by 4–58 bpm and is present at rest and during sleep. Cardiac output is increased by >1 L/min and may be up to 300% higher in patients with hyperthyroidism than in patients without the condition. The net effect of these hemodynamic changes is a rise in SBP and a widening of pulse pressure. Arterial stiffness is increased. 11 Other potential cardiovascular consequences of hyperthyroidism include atrial arrhythmias (especially atrial fibrillation 12 ), pulmonary hypertension, 13 left ventricular hypertrophy, 14 and heart failure. 8 , 15
EFFECT OF HYPERTHYROIDISM ON HORMONAL FACTORS
As previously noted, both renin and erythropoietin production is stimulated and enlarge blood volume. Because of the clinical manifestations of hyperthyroidism, it is surprising that catecholamines are normal or decreased. However, atrial natriuretic peptide, brain natriuretic peptide, endothelin‐1, and the vasodilating polypeptide adrenomedullin are higher in hyperthyroidism. 8 , 9
HYPERTENSION AND HYPERTHYROIDISM
An increase in diastolic BP is uncommon in hyperthyroidism because of the reduction in systemic vascular resistance. It is estimated that the prevalence of hypertension with thyrotoxicosis is 20–30%; however, there are limited data to be certain that this statistic is accurate since hypertension is a highly prevalent condition. In 1931, a study of 458 hyperthyroid patients observed a prevalence of 26% using an SBP of ≥150 mm Hg. 16 A report by Japanese investigators who compared 321 hyperthyroid patients with 324 euthyroid subjects reported a higher SBP with hyperthyroidism for each decade between 20 and 59 years of age. 17 A subsequent study by the same investigators evaluated 446 untreated hyperthyroid and 549 euthyroid patients. 18 The prevalence of hypertension was significantly higher in hyperthyroid patients who were 20–49 years of age (Figure 2).
Figure 2.
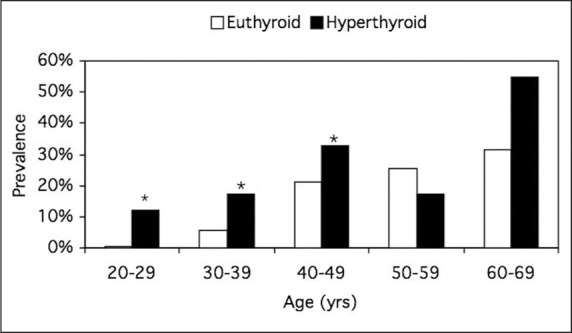
Prevalence of hypertension in 594 euthyroid and 446 hyperthyroid patients. The prevalence of hypertension was significantly higher in patients younger than 50 years of age. *p<0.05. Reproduced with permission from Endocrinol Metab Clin North Am. 1994;23:379–386. 18
Polish researchers examined 51 untreated hyperthyroid study participants and 30 healthy controls younger than 50. 19 SBP was significantly higher (p<0.001) and diastolic BP significantly lower (p<0.01) in the hyperthyroid cohort (132/72 mm Hg) than the normal individuals (115/79 mm Hg). Furthermore, among the hyperthyroid subjects, cardiac output was 3.38 L/min higher (p<0.001) and total peripheral resistance was 632 dynes•sec•cm−5 lower (p<0.001). After treatment of hyperthyroidism, SBP and cardiac output declined and total peripheral resistance diastolic blood increased. Other researchers have reported a similar decline in SBP and heart rate (Figure 3). 17
Figure 3.

Change in systolic blood pressure and heart rate in 321 hyperthyroid patients treated with antithyroid drugs, radioactive iodine, or subtotal thyroidectomy. Systolic blood pressure and heart rate decreased with treatment of hyperthyroidism, but there was a greater reduction in both parameters in younger patients. Data derived from J Am Geriatr Soc. 1985;33:19–22. 17
A blunted nocturnal decline in ambulatory BP is associated with increased target organ damage and mortality. 20 Whether there is a blunted nocturnal decline in BP with hyperthyroid patients is controversial. A German study measured 24‐hour ambulatory BP in 176 normotensive, 460 mild‐to‐moderate hypertensive, and 14 hyperthyroid patients. 21 The day/night change in BP was similar between normotensive (−14/−13 mm Hg) and hypertensive (−15/−14 mm Hg) study participants, but was significantly less among hyperthyroid patients (−6/−8 mm Hg; p<0.05). Another study compared 20 normotensive hyperthyroid and 15 healthy euthyroid patients using ambulatory BP monitoring. 22 Hyperthyroidism was due to Graves' disease in all but one patient. The 24‐hour SBP and heart rate were significantly higher in hyperthyroid subjects (125.3 mm Hg and 87.8 bpm, respectively) than euthyroid subjects (115.2 mm Hg and 72.4 bpm, respectively). Both daytime and nocturnal SBP and heart rate were higher among the hyperthyroid cohort. However, there was no significant difference between the groups for the mean nocturnal fall in BP or heart rate. After control of thyroid function, weight increased 5.7 kg, 24‐hour SBP declined 5.1 mm Hg, and 24‐hour heart rate dropped 15.2 bpm. Spanish investigators used thyrotropin‐suppressive doses of thyroxine in patients with differentiated thyroid cancer. 23 They studied 12 thyroid cancer patients and 16 control subjects with ambulatory BP. There were more nondippers among the treated cancer patients than the control patients (58.3% vs. 6.3%; p<0.05).
DIAGNOSIS
The clinical symptoms of hyperthyroidism may include palpitations, nervousness, sweating, weight loss with normal or increased appetite, frequent bowel movements, heat intolerance, and insomnia. Typical physical findings consist of tachycardia; atrial arrhythmias, including atrial fibrillation; widened pulse pressure; hand tremors; eyelid retraction; conjunctival injection; exophthalmos (Figure 1); and infiltrative dermopathy. In the elderly, many of the usual manifestations of thyrotoxicosis are absent, and tachyarrhythmias and weight loss may be the only abnormality. This is termed as apathetic or masked hyperthyroidism.
A low or undetectable thyroid‐stimulating hormone and elevated free T4 levels are usually present. Total serum T4 levels are dependent on thyroxine‐binding globulin; therefore, free T4, which represents the metabolically active fraction, is a better measurement. If the free T4 level is normal, then the free T3 level is measured to exclude T3 thyrotoxicosis, which accounts for a small percentage of hyperthyroid cases. Thyroidal radioactive iodine uptake and scan are performed in vivo to directly assess the level of the gland activity and integrity of hormone biosynthesis. Radioactive iodine uptake is increased in Graves' disease, apathetic hyperthyroidism, toxic multinodular goiter, and toxic adenoma. Low radioactive iodine uptake, as seen in subacute thyroiditis, indicates thyrotoxicosis in the absence of hyperthyroidism. In subacute thyroiditis, the gland is usually painful but not overactive; rather, it is injured and leaks thyroid hormones into the blood stream. Radioactive iodine uptake is also decreased in factitious thyrotoxicosis. Patients with low thyroid‐stimulating hormone, but normal free T4 and T3 levels, are classified as having subclinical hyperthyroidism, an early stage of hyperthyroidism. These patients are still at risk for cardiac complications including atrial fibrillation and left ventricular hypertrophy.
Treatment of hyperthyroidism is aimed at decreasing the synthesis and release of thyroid hormones by antithyroid medications, radioactive iodine ablation, or surgical thyroidectomy. Beta blockers are effective in blocking peripheral manifestations of the thyroid hormone excess. A nonselective P blocker is given to block tachycardia and tremor. 24
SUMMARY
A common cause of isolated systolic hypertension is hyperthyroidism. In hyperthyroidism, T3 dilates resistance arterioles, reducing systemic vascular resistance, and increases cardiac output and pulse pressure. Hypertension appears to be more common among hyperthyroid patients. Treatment narrows the pulse pressure, decreases heart rate, and reduces cardiac output.
References
- 1. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 2. Gifford RW, Prisant LM. The importance of hypertension in the geriatric population. In: Prisant LM, ed. Hypertension in the Elderly. 1st ed. Totowa, NJ: Humana Press; 2005:3–9. [Google Scholar]
- 3. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group . JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 4. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 5. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. [DOI] [PubMed] [Google Scholar]
- 6. Franklin SS, Wong ND, Larson MG, et al. How important is pulse pressure as a predictor of cardiovascular risk? Hypertension. 2002;39:E12–E13. [PubMed] [Google Scholar]
- 7. Izzo JL. Aging, arterial stiffness, and systolic hypertension. In: Prisant LM, ed. Hypertension in the Elderly. 1st ed. Totowa, NJ: Humana Press; 2005:23–34. [Google Scholar]
- 8. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 9. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513–520. [DOI] [PubMed] [Google Scholar]
- 10. Resnick LM, Laragh JH. Plasma renin activity in syndromes of thyroid hormone excess and deficiency. Life Sci. 1982;30:585–586. [DOI] [PubMed] [Google Scholar]
- 11. Palmieri EA, Fazio S, Palmieri V, et al. Myocardial contractility and total arterial stiffness in patients with overt hyperthyroidism: acute effects of beta1‐adrenergic blockade. Eur J Endocrinol. 2004;150:757–762. [DOI] [PubMed] [Google Scholar]
- 12. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. [DOI] [PubMed] [Google Scholar]
- 13. Marvisi M, Brianti M, Marani G, et al. Hyperthyroidism and pulmonary hypertension. Respir Med. 2002;96:215–220. [DOI] [PubMed] [Google Scholar]
- 14. Tamer I, Sargin M, Sargin H, et al. The evaluation of left ventricular hypertrophy in hypertensive patients with sub‐clinical hyperthyroidism. Endocr J. 2005;52:421–425. [DOI] [PubMed] [Google Scholar]
- 15. Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94–98. [DOI] [PubMed] [Google Scholar]
- 16. Hurxthal LM. Blood pressure before and after operation in hyperthyroidism. Arch Intern Med. 1931;47:167–181. [Google Scholar]
- 17. Saito I, Ito K, Saruta T. The effect of age on blood pressure in hyperthyroidism. J Am Geriatr Soc. 1985;33:19–22. [DOI] [PubMed] [Google Scholar]
- 18. Saito I, Saruta T. Hypertension in thyroid disorders. Endocrinol Metab Clin North Am. 1994;23:379–386. [PubMed] [Google Scholar]
- 19. Marcisz C, Jonderko G, Kucharz E. Changes of arterial pressure in patients with hyperthyroidism during therapy. Med Sci Monit. 2002;8:CR502–CR507. [PubMed] [Google Scholar]
- 20. Prisant LM. Blunted nocturnal decline in blood pressure. J Clin Hypertens (Greenwich). 2004;6:594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Middeke M, Schrader J. Nocturnal blood pressure in normotensive subjects and those with white coat, primary, and secondary hypertension. BMJ. 1994;308:630–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iglesias P, Acosta M, Sanchez R, et al. Ambulatory blood pressure monitoring in patients with hyperthyroidism before and after control of thyroid function. Clin Endocrinol (Oxf). 2005;63:66–72. [DOI] [PubMed] [Google Scholar]
- 23. Botella‐Carretero JI, Gomez‐Bueno M, Barrios V, et al. Chronic thyrotropin‐suppressive therapy with levothyroxine and short‐term overt hypothyroidism after thyroxine withdrawal are associated with undesirable cardiovascular effects in patients with differentiated thyroid carcinoma. Endocr Relat Cancer. 2004;11:345–356. [DOI] [PubMed] [Google Scholar]
- 24. Prisant LM. Pharmacology of antihypertensive drugs. In: Weir MR, ed. Hypertension. Philadelphia, PA: American College of Physicians; 2005. [Google Scholar]


