Abstract
MOTIVATION:
Studies have shown poor clinical effectiveness of the Epworth Sleepiness Scale (ESS) due to its ambiguity of items and cultural applicability. This study aimed to investigate the efficacy of a Visual Analog Scale (VAS) to assess sleepiness, compared to ESS.
METHODS:
Thirty-two obstructive sleep apnea (OSA) patients and 32 healthy participants completed two visits, 1 month apart, during which they completed both ESS and VAS. Patients diagnosed with OSA were treated with Continuous positive airway pressure (CPAP) between visits. The agreement between the ESS and VAS scores in both patients with OSA and healthy participants was investigated using Pearson correlation and Area Under the receiver operating characteristics.
RESULTS:
The (mean ± standard deviation) Oxygen Desaturation Index for patients with OSA was 18.5 ± 5.7 events/hour and 1.7 ± 1.0 events/hour in the healthy participants. A reduction in sleepiness, following CPAP treatment occurred in patients with OSA, using the ESS (11.2 ± 5.5–4.7 ± 5.0 points, P < 0.001) and the VAS (50.2 ± 3.0–21.9 ± 26.5 mm, P < 0.001). There was no significant change in sleepiness, in healthy participants using the ESS (3.91 ± 3.14–3.34 ± 3.27 points (P < 0.48) or the VAS (15.58 ± 21.21–12.05 ± 14.75 mm, (P < 0.44). A Likert scale showed that the VAS was easier to use compared to ESS in visit 1 (VAS: 8.7 ± 1.9 points, ESS: 7.7 ± 2.6 points, (P < 0.001), and visit 2 (VAS: 9.5 ± 1.4 points, ESS: 8.6 ± 1.5 points, P < 0.001).
CONCLUSION:
These preliminary results suggest that the VAS can detect a change in sleepiness after CPAP treatment in patients with OSA and that the VAS was also easier to use compared to ESS.
Keywords: Excessive daytime sleepiness, obstructive sleep apnea, visual analog scale
Introduction
Obstructive sleep apnea (OSA) is a common disease. A recent study estimated that the global prevalence of OSA was approximately 1 billion people worldwide,[1] though 60% of those patients have mild OSA (Apnoea-hypopnea index: AHI >5 to <15 events/hour). One of the most common daytime symptoms of OSA is excessive daytime sleepiness (EDS); approximately 42% of patients with OSA report symptoms of EDS.[2] It has long been accepted that continuous positive airway pressure (CPAP) is an effective treatment for patients with moderate-to-severe OSA and symptoms of EDS.[3] Recently, our group has reported that 3 months of CPAP treatment improved the quality of life in patients with mild OSA and daytime symptoms.[4] Furthermore, following the outcome of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, which did not show cardiovascular risk reduction, sleepiness is the principle indication for starting CPAP in the majority of OSA patients; therefore, its assessment is of clinical importance.[5]
The Epworth Sleepiness Scale (ESS) is the most frequently used questionnaire to measure subjective EDS.[6] However, clinical studies have reported difficulties associated with its use. These include, but are not limited to, challenges in the accurate completion of the scale due to illiteracy, the ambiguity of items, and cultural applicability.[7] A study by Ghiassi et al. reported that one-third of new patients and one-sixth of ESS-naive patients with OSA made errors or required assistance when completing the scale.[8] Examples of errors included leaving a question blank or writing “yes” or “no” instead of giving an item a score. Some patients also needed help with completing the questionnaire due to reading and writing difficulties or vision impairments. Bindi et al. investigated the challenges of translating the ESS into different languages and found that item seven in the ESS (”Sitting quietly after a lunch without alcohol”) was ambiguous either syntactically, semantically, or culturally in 66 languages.[9] Moreover, item eight in the ESS (”In a car, while stopped for a few minutes in the traffic”) does not make a clear distinction regarding whether the respondents should imagine themselves as drivers or passengers. This is important as the association between EDS and motor vehicle accidents is well-recognized.[10] Another study found that the contextual factors such as location, position, and level of interest during the completion of the questionnaire affected the report of the ESS score, and this led to high variability between patients.[11] Finally, the question that addresses the likelihood of dozing off in a theater may be ambiguous even to anglophones, since a 'theatre' in the UK is a venue for plays, whereas in Australia where the ESS originated it is a venue for films (i.e., a cinema).
The visual analog scale (VAS) is a simple method that has been previously used to measure subjective feelings.[12] It has been used and validated for the assessment of chronic symptoms such as anxiety,[13] mood,[12] depression,[14] pain,[15] and breathlessness.[16] Sleepiness is a subjective feeling, and therefore a VAS may be an effective measurement.[17] A VAS for the assessment of sleepiness was developed, with endpoints that closely match the ESS.
The primary aim of this study was to determine the relationship between the ESS and VAS in patients with OSA and healthy participants at baseline and after 1 month, during which patients with OSA were treated with continuous positive airway pressure (CPAP). Healthy participants were included in the follow-up, to provide insights into test, re-test variability. The secondary aims were to assess the ease of use and time taken to complete both the ESS and VAS.
Methods
Participants
Patients were recruited prospectively from the Centre for Sleep, at the Royal Brompton Hospital, London, UK, if they had a confirmed diagnosis of OSA (overnight respiratory polygraphy; Apnea link, ResMed, San Diego, California, US). Healthy participants were also recruited to the study from the Royal Brompton Hospital and Imperial College London staff.
The inclusion criteria for the patients with OSA were Oxygen Desaturation Index (ODI) >7.5 events/hour and for healthy participants ODI <5 events/hour. Exclusion criteria were coexisting sleep disorder, clinically diagnosed depression or anxiety disorder, prescription of psychotropic medications, or use of recreational drugs. All participants gave written informed consent, and the study was approved by National Research Ethics Services Committee South West-Cornwall and Plymouth 12/SW/0091.
Measurements
All participants were asked to complete the ESS and VAS questionnaires, at baseline and after 1 month. During this month, patients with OSA were treated with CPAP (ResMed, San Diego, California, US) and their adherence to the treatment was recorded using remote monitoring (Air view, ResMed, San Diego, California, US). Oxygen saturation was recorded overnight for all participants in their own homes at visit 1. Oxygen saturation was also recorded overnight at visit 2 for patients with OSA. ODI was measured through portable pulse oximetry overnight (Konica Minolta Sensing Inc, Tokyo, Japan).
Epworth Sleepiness Scale
A self-administered questionnaire that yields a score between zero (least sleepy) and 24 (most sleepy).[6] Participants were asked to mark on a scale of 0–3, how likely they are to doze off or fall asleep during eight different situations. The ESS scores can be interpreted as follow: 0–5 Lower Normal Daytime Sleepiness, 6–10 Higher Normal Daytime Sleepiness, 11–12 Mild Excessive Daytime Sleepiness, 13–15 Moderate Excessive Daytime Sleepiness, and 16–24 Severe Excessive Daytime Sleepiness.
Visual Analog Scale
A self-administered scale that consists of two opposing statements “I never fall asleep” and “I always fall asleep”, joined by a 100-mm line. These two statements were selected to resemble the scores from the ESS as closely as possible. The instructions that were given to the participants before completing the VAS were “place an X on the line below to show on average, over the past week, how likely you are to fall asleep during the day.”
The VAS was developed by a consensus of experts (four consultants at the Royal Brompton Hospital, two physiologists, and patients with OSA).
Both the ESS and VAS were recorded on paper. The VAS was measured by a ruler, and the score was to the nearest millimeter. Participants were also asked to rate the ease of use of both measures on the scale of zero (least easy) to 10 (most easy). The time taken to complete the ESS and VAS was also recorded on each occasion.
Sample size calculation and statistical analysis
An a priori sample size was estimated based on the study by McMillan et al., 2014.[18] These researchers investigated the impact of CPAP on daytime sleepiness in older patients with OSA, recording the ESS before and after CPAP treatment. They found a reduction in the ESS of two points following CPAP treatment (95% CI 2.8–1.2). Using these data, at 5% significance level and 80% power, a sample size of 64 participants were required for this study to show a similar reduction in the VAS after CPAP.
Normality of the data was investigated using Shapiro–Wilk's normality test.
The difference between the baseline and 1-month ESS and VAS scores were tested using a paired t-test in patients with OSA and in healthy participants. The relationship between the ESS and VAS scores in both patients with OSA and healthy participants was investigated using a Pearson correlation and Area Under the Receiver Operating characteristics (ROC) Curve. The cut-off values of <10 points in the ESS were used to determine the value in the VAS.
Ease of use ratings and time taken to complete the ESS and VAS were analyzed through independent T-tests. All statistical analysis was conducted using IBM SPSS Statistics for Windows Version 24.0 . Armonk, NY: IBM Corp.
Results
Sixty-seven participants were assessed for eligibility to participate in the study; of which 64 participants were included; 32 (ten females) patients diagnosed with OSA (age [mean ± standard deviation (SD)] 55.8 ± 13.4 years) and 32 (19 female) healthy participants (age: 36.7 ± 11.7 years). Details of the recruitment are presented in the Consort diagram [Figure 1].
Figure 1.
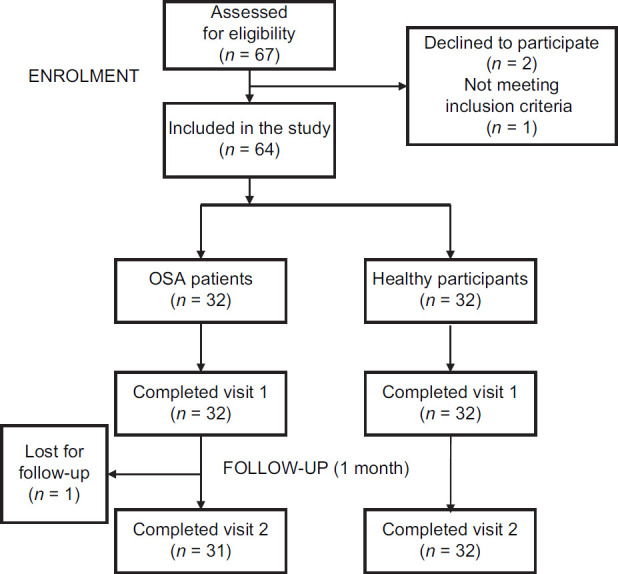
Consort diagram displaying method of recruitment and study of obstructive sleep apnea patients and healthy participants
Baseline characteristics of the participants of the patients with OSA and healthy participants are presented in Table 1.
Table 1.
Baseline characteristics of the participants
| Total sample (n=64) | Healthy participants (n=32) | OSA patients (n=32) | |
|---|---|---|---|
| Age (years) | 46±15 | 36±11 | 56±13 |
| ODI >4% at baseline (events/hour) | 10.10±9.36 | 1.73±0.95 | 18.47±5.70 |
| ODI >4% after CPAP treatment (events/hour) | N/A | N/A | 5.89±5.49 |
| BMI (kg/m2) | 28.72±5.83 | 26.26±5.12 | 31.17±5.52 |
| Period between visits 1 & 2 (days) | 34±19 | 26±10 | 42±22 |
| Medical history (n) | |||
| Hypertension | 14 | 0 | 14 |
| Heart failure | 4 | 0 | 4 |
| Other cardiac diagnosis | 8 | 1 | 7 |
| Epilepsy or other neurological diagnosis | 2 | 0 | 2 |
| Asthma | 15 | 6 | 9 |
| COPD or other respiratory disorders | 10 | 0 | 10 |
| Diabetes | 7 | 1 | 6 |
Values are given as mean±standard deviation (SD), or sample number (n).
Comparison between Epworth Sleepiness Scale and Visual Analog Scale at baseline and after 1 month
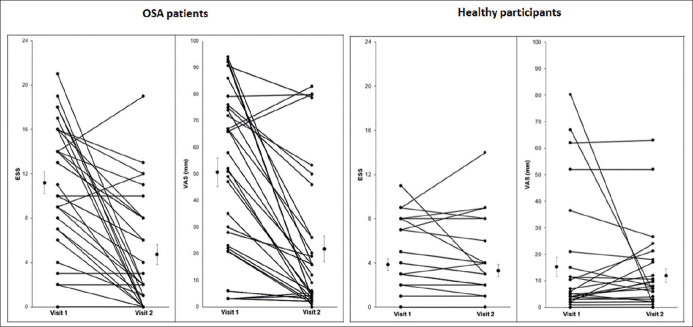
In patients with OSA, the (mean ± SD) score of ESS at visit 1 was 11.16 ± 5.53 points, which indicated that our patients were mildly sleepy. At visit 2, after 1 month of CPAP, the ESS score had decreased to 4.74 ± 5.01 points. Concurrently, the score of the VAS at visit 1 for patients with OSA was 50.22 ± 30.38 mm. At visit 2 after 1 month of CPAP, the VAS had decreased to 21.90 ± 26.92 mm [Figure 2].
Figure 2.
Individual Epworth Sleepiness Scale and Visual Analog Scale results for obstructive sleep apnea patients (n = 31) and healthy participants (n = 32) collected at visits 1 and 2
In healthy participants, the (mean ± SD) score of ESS at visit 1 was 3.91 ± 3.14. At visit 2, after 1 month of no intervention, the ESS score was 3.34 ± 3.27 (P = 0.48). Concurrently, the score of the VAS at visit 1 for healthy participants was 15.58 ± 21.21 mm. At visit 2, after 1 month of no intervention, the VAS score was 12.05 ± 14.75 (P = 0.44) [Figure 2]. These data show that there were minimal change in the measurement of sleepiness using ESS and VAS after 1 month in the healthy participants.
In patients with OSA, the mean percentage change from visit 1 to visit 2 for the ESS was 57.5% ±35% compared to 56.4% ± 29.1% (P = 0.253) for the VAS scores. These results show that there was no significant difference in the percentage reduction in sleepiness measured using the ESS and VAS. Therefore, the use of both ESS and VAS detects a similar magnitude of change in sleepiness in patients with OSA after they have been treated with CPAP.
Correlation between Epworth Sleepiness Scale and Visual Analog Scale results
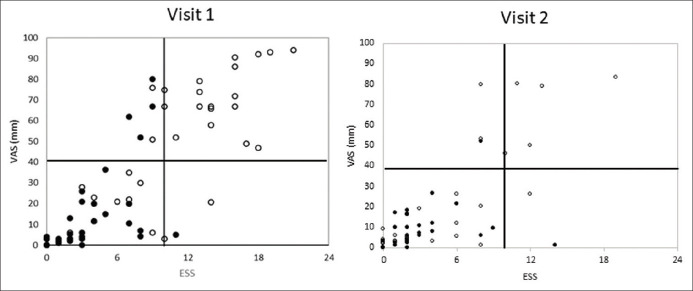
The VAS correlated significantly with ESS at both visits among patients with OSA (visit 1: r = 0.77, P < 0.001, visit 2: r = 0.82, P < 0.001) and healthy participants (visit 1: r = 0.60, P < 0.001, visit 2: r = 0.71, P < 0.001). When the data for patients with OSA and healthy participants were combined, to examine the performance of the VAS scale at different levels of sleepiness, the correlation between the ESS and VAS was r = 0.82 (P < 0.001) for visit 1 and r = 0.72 (P < 0.001) for visit 2. Figure 3 shows the scatter plots for visit 1 and visit 2.
Figure 3.
Correlation between the Epworth Sleepiness Scale and Visual Analog Scale results for patients with obstructive sleep apnea (open circle) and healthy participants (closed circle) for Visit 1 and visit 2. Solid lines denote ESS score of 11, considered the minimum for excessive daytime sleepiness, and corresponding Visual Analog Scale result of 40 mm
The Area Under ROC curve of the VAS for detecting sleepiness (ESS > 10) was 0.84, which means that the probability that VAS predicted sleepiness correctly was 84% [Figure 4].
Figure 4.
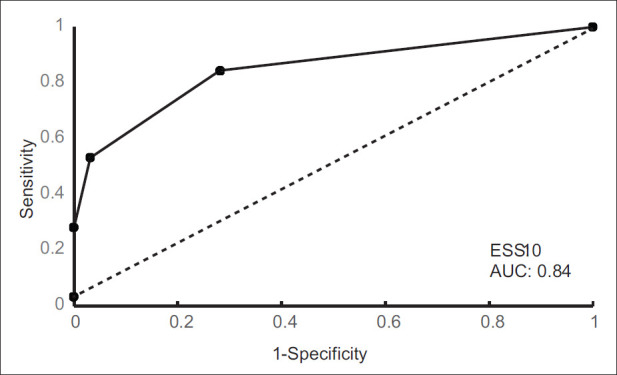
The Receiver Operating Characteristic curve. Sensitivity (y-axis) plotted against 1 - specificity (x-axis), demonstrating the predictive value of the cut-off score of the Epworth sleepiness scale. An Epworth Sleepiness Scale score of 10 had an area under the curve of 0.84, which means that the probability that Visual Analog Scale predicted sleepiness correctly was 84% when a cut-off Epworth Sleepiness Scale score of 10 was used
The ease of use and time taken to complete the Epworth Sleepiness Scale and Visual Analog Scale
The VAS was easier to use compared with the ESS for both patients with OSA (Visit 1: VAS: 8.29 ± 2.34; ESS: 7.74 ± 2.84, P < 0.001, Visit2: VAS: 9.71 ± 0.59, ESS: 8.97 ± 1.22, P < 0.001) and for healthy participants (Visit 1: VAS: 9.22 ± 1.43; ESS: 7.72 ± 2.34, P < 0.001, Visit2: VAS: 9.29 ± 1.94, ESS: 8.36 ± 1.75, P < 0.001). Table 2 summarizes the results for the ease of use of the VAS and ESS between visits for both patients with OSA and healthy participants.
Table 2.
The ease of use for the ESS and VAS score in visit 1 & 2 for patients with OSA
| Ease of use for patients with OSA | ||||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Change | P | |
| ESS (points) | 7.74 ± 2.84 | 8.97 ± 1.22 | - 1.23 ± 2.64 | 0.033 |
| VAS (points) | 8.29 ± 2.34 | 9.71 ± 0.59 | - 1.42 ± 2.33 | 0.002 |
| Ease of use for healthy participants | ||||
| Visit 1 | Visit 2 | Change | P | |
| ESS (points) | 7.72 ± 2.34 | 8.47 ± 1.67 | - 0.75 ± 2.08 | 0.145 |
| VAS (points) | 9.22 ± 1.43 | 9.25 ± 1.87 | - 0.03 ± 1.69 | 0.940 |
ESS: Epworth sleepiness scale, VAS: visual analogue scale. The ease of use was measured using Likert scale between zero (least easy) to 10 (most easy).
The time taken to complete the VAS was also reduced significantly compared with the time taken to complete the ESS in patients with OSA (Visit 1: VAS: 14.92 ± 13.76 seconds, ESS: 38.75 ± 20.01 seconds, P < 0.001, Visit 2: VAS: 5.67 ± 3.39 seconds, ESS: 31.33 ± 23.68, P < 0.001) and in healthy participants (Visit 1: VAS: 17.32 ± 31.47 s, ESS: 62.32 ± 62.44 s, P < 0.001, Visit 2: VAS: 10.16 ± 9.43 s, ESS: 38.00 ± 21.90, P < 0.001). Table 3 summarises the results for the time taken to complete the ESS and VAS between visits for both patients with OSA and healthy participants.
Table 3.
The time taken to complete the ESS and VAS score in visit 1 & 2 for patients with OSA
| Time taken to complete the ESS and VAS for patients with OSA | ||||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Change | P | |
| ESS (seconds) | 39±20 | 31±24 | - 7±4 | 0.416 |
| VAS (seconds) | 15±13.76 | 6±3 | - 9±10 | 0.043 |
| Time taken to complete the ESS and VAS for healthy participants | ||||
| Visit 1 | Visit 2 | Change | P | |
| ESS (seconds) | 62±62 | 38±21 | - 24±40 | 0.118 |
| VAS (seconds) | 17±31 | 10±9 | - 7±22 | 0.349 |
ESS: Epworth sleepiness scale, VAS: visual analogue scale. The ease of use was measured using Likert scale between zero (least easy) to 10 (most easy).
Discussion
The main finding of this study was that in patients with OSA, symptoms of daytime sleepiness could be measured with both the ESS and VAS; the VAS measure was able to differentiate patients with OSA from healthy participants. The VAS was also easier to use, compared with the ESS over a 1-month period when patients with OSA were treated with CPAP and no intervention was applied to the healthy participants.
In this study, the VAS was used to assess sleepiness since it had been proved to be a simple and reliable method to evaluate feelings such as anxiety,[13] depression,[14] pain,[15] and breathlessness.[16] Different versions of the VAS have been used in the literature: Horizontal or vertical lines, numerical ratings, and a facial scale. However, the most widely used is the horizontal line version with no categories on the line. Where categories are used (for example, no pain, mild pain, moderate pain, severe pain, and very severe pain), and the true value falls in-between these categories, participants have shown a systematic bias either to the lower or higher score. It was also found that this bias decreases as the number of categories increases.[19] Furthermore, previous studies that used VAS for the assessment of sleepiness in patients with OSA utilized either different VAS version or used VAS that was originally constructed to measure different attributes. For example, Sauter et al. 2000., investigated a VAS that was originally constructed to assess mood, specifically tiredness (VAS-T) and performance (VAS-P). They showed that the VAS-P, VAS-T, and ESS were moderately correlated among patients with OSA, which indicate that they measure similar, but not identical attributes. Johnson et al., 1990 used a VAS version to assess EDS in combination with the Stanford Sleepiness Scale (SSS). The anchor points they used were “very alert on the left end and very sleepy on the right end”. These two anchors resemble the statements from the SSS, which previous studies showed that SSS statements refer to fatigue or boredom rather than sleepiness (Minkwitz et al., 2020).
In the present study, it was found that the VAS was sensitive to changes in sleepiness levels before and after CPAP treatment, with the same magnitude as shown for the ESS. The sensitivity of the ESS to changes in levels of sleepiness before and after CPAP treatment has been reported in previous research,[20,21,22] and the majority of these studies found a clinically significant change of 2–3 points in the ESS.[23] In this study, we observed a change of six points in the ESS score after CPAP treatment. One explanation for the larger figure, compared with that reported in the literature is that our patients were recruited from a specialist sleep clinic, and had been prescribed CPAP treatment with close monitoring of the compliance. In addition, the short follow-up time (one month) may have captured a “rebound” in sleepiness levels. Interestingly, McMillan et al. reported that the change in ESS after 3 months of CPAP treatment (−3.8 points) was greater than the change after 12 months (−3.4 points).[18]
One limitation of our study was that although the sample size was objectively calculated, there are still other important assumptions were not answered by this study such as patients with severe OSA who are also severely sleepy. Furthermore, we did not test how the VAS will change over longer period between visit 1 and visit 2. These questions are still not answered by the current study. Another limitation was that the mean age of our patients with OSA was higher than the mean age of the healthy participants. However, the purpose of adding healthy participants in this study was to show how the VAS can detect sleepiness among the spectrum of participants with different sleep iness levels. Therefore, the healthy participants were not “control group.” Instead, there were added to provide more meaningful data about the use of the VAS in participants with different level of sleepiness.
The use of the internet and modern technology in recent years has not only enabled health promotion but also has facilitated the collection of big data. A potential development of the present study would be to digitize the VAS and make it publicly available, hence an online version of the VAS was developed [Figure 5]. Such development may aid awareness among the public regarding symptoms of sleepiness and aid the diagnosis of sleep disorders in a timely manner. For example, In a previous study in a large number of participants (39,448 participants), Boyes et al., found that an online version of the ESS enabled an assessment of sleepiness symptoms of >1000 participants per month.[24] Such data can pave the way toward speeding up clinic visits. It might also be used to enable the motor vehicle agencies to screen for fitness to drive. Future work will investigate the feasibility of using the online version of the VAS as a measure for sleepiness.
Figure 5.
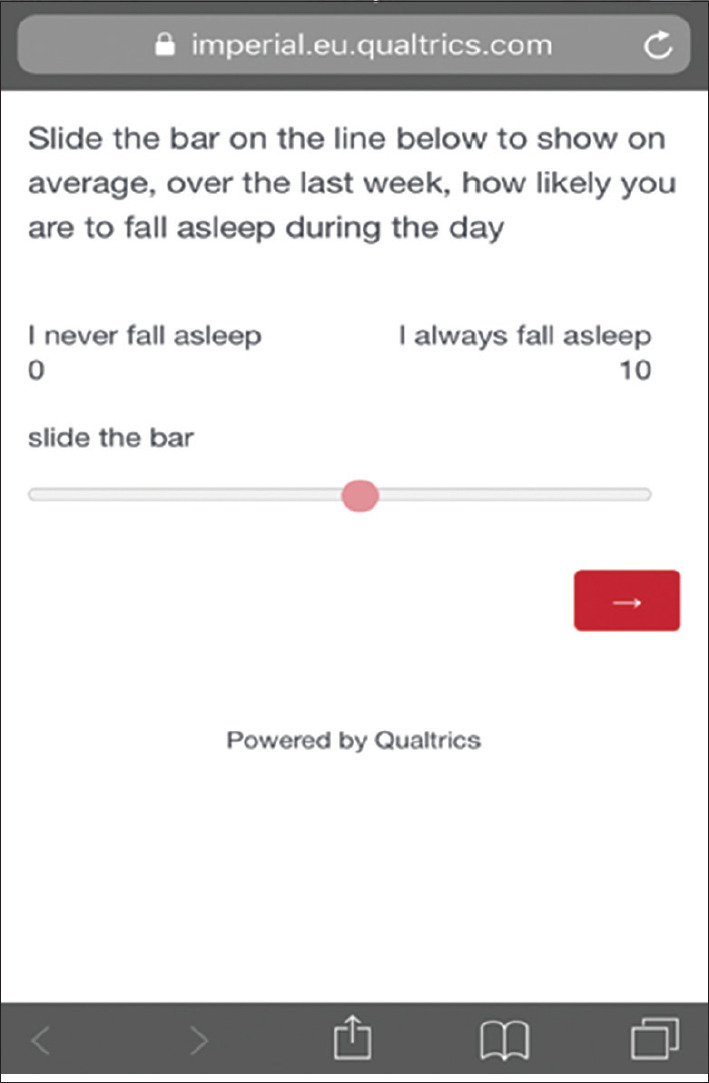
A screenshot of the online version of the Visual Analog Scale
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MS, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med. 2019;7:687–98. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur Respir J. 2014;44:1600–7. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: A systematic review and economic analysis. Health Technol Assess. 2009;13 doi: 10.3310/hta13040. iii-iv, xi-xiv, 1-119, 143-274. [DOI] [PubMed] [Google Scholar]
- 4.Wimms AJ, Kelly JL, Turnbull CD, McMillan A, Craig SE, O’Reilly JF, et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): A multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:349–58. doi: 10.1016/S2213-2600(19)30402-3. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 6.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth sleepiness scale: A systematic review. Sleep Med Rev. 2014;18:321–31. doi: 10.1016/j.smrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Ghiassi R, Murphy K, Cummin AR, Partridge MR. Developing a pictorial Epworth Sleepiness Scale. Thorax. 2011;66:97–100. doi: 10.1136/thx.2010.136879. [DOI] [PubMed] [Google Scholar]
- 9.Bindi P, Bayles A, Acquadro C, Johns M. Challenges in translating the epworth sleepiness scale into 66 languages. Value Health. 2016;19:A93. [Google Scholar]
- 10.Rizzo D, Libman E, Creti L, Baltzan M, Bailes S, Fichten C, et al. Determinants of policy decisions for non-commercial drivers with OSA: An integrative review. Sleep Med Rev. 2018;37:130–7. doi: 10.1016/j.smrv.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Sharafkhaneh A, Hirshkowitz M. Contextual factors and perceived self-reported sleepiness: A preliminary report. Sleep Med. 2003;4:327–31. doi: 10.1016/s1389-9457(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 13.Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients’ anesthetic concerns. Anesth Analg. 2000;90:706–12. doi: 10.1097/00000539-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 14.Lees N, Lloyd-Williams M. Assessing depression in palliative care patients using the visual analogue scale: A pilot study. Eur J Cancer Care (Engl) 1999;8:220–3. doi: 10.1046/j.1365-2354.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 16.Mador MJ, Kufel TJ. Reproducibility of visual analog scale measurements of dyspnea in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:82–7. doi: 10.1164/ajrccm/146.1.82. [DOI] [PubMed] [Google Scholar]
- 17.Thorarinsdottir EH, Bjornsdottir E, Benediktsdottir B, Janson C, Gislason T, Thor A, et al. Definition of excessive daytime sleepiness in the general population: Feeling sleepy relates better to sleep-related symptoms and quality of life than the Epworth Sleepiness Scale score. Results from an epidemiological study. J Sleep Res. 2019;28:e12852. doi: 10.1111/jsr.12852. [DOI] [PubMed] [Google Scholar]
- 18.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): A 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–12. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz N. Self-reports: How the questions shape the answers. Am Psychol. 1999;54:93. [Google Scholar]
- 20.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: Failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 21.Ballester E, Badia JR, Hernández L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 22.Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40:386–93. doi: 10.1183/09031936.00177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel S, Kon SS, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The Epworth Sleepiness Scale: Minimum Clinically Important Difference in Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2018;197:961–3. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyes J, Drakatos P, Jarrold I, Smith J, Steier J. The use of an online Epworth Sleepiness Scale to assess excessive daytime sleepiness. Sleep Breath. 2017;21:333–40. doi: 10.1007/s11325-016-1417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]