Abstract
The Saudi Pediatric Pulmonology Association (SPPA) is a subsidiary of the Saudi Thoracic Society (STS), which consists of a group of Saudi experts with well-respected academic and clinical backgrounds in the fields of asthma and other respiratory diseases. The SPPA Expert Panel realized the need to draw up a clear, simple to understand, and easy to use guidance regarding the application of different aerosol therapies in respiratory diseases in children, due to the high prevalence and high economic burden of these diseases in Saudi Arabia. This statement was developed based on the available literature, new evidence, and experts' practice to come up with such consensuses about the usage of different aerosol therapies for the management of respiratory diseases in children (asthma and nonasthma) in different patient settings, including outpatient, emergency room, intensive care unit, and inpatient settings. For this purpose, SPPA has initiated and formed a national committee which consists of experts from concerned specialties (pediatric pulmonology, pediatric emergency, clinical pharmacology, pediatric respiratory therapy, as well as pediatric and neonatal intensive care). These committee members are from different healthcare sectors in Saudi Arabia (Ministry of Health, Ministry of Defence, Ministry of Education, and private healthcare sector). In addition to that, this committee is representing different regions in Saudi Arabia (Eastern, Central, and Western region). The subject was divided into several topics which were then assigned to at least two experts. The authors searched the literature according to their own strategies without central literature review. To achieve consensus, draft reports and recommendations were reviewed and voted on by the whole panel.
Keywords: Aerosol therapy, children, consensus, Saudi Arabia
The morbidity and mortality associated with respiratory diseases in children represent a major health problem all over the world.[1] In Saudi Arabia, respiratory diseases were reported by the Saudi Ministry of Health as the fifth leading cause of death in the Kingdom in 2014.[2] In Saudi Arabia, the contemporary lifestyle, including exposure to tobacco smoke and pets, can be the reason behind the significant increase in the prevalence of bronchial asthma (one of the most common chronic diseases among children) during the past years.[3]
Aerosolized drugs are frequently prescribed since ancient times to patients to treat bronchospasms, decrease airway inflammation, enhance mucus clearance, as well as prevent or treat an infection.[4]
The use of therapeutic aerosol was first proposed by the ancient Egyptians, dating back to ≈1554 BC by heating leaves of a specific plant and inhaling vapors produced during the heating process.[5] Abu'Ali al-Husayn ibn Sina described the use of opium for a variety of diseases, including severe cough by smoking or nasal inhalation.[6] Aerosol therapy was the first to be described for asthma back in India.[7] Hippocrates (460–377 BC) used a pot with a hole in the lid to deliver various vapors to treat several illnesses.[8] Galen of Pergamon, a Greek physician in the early second century, used inhaled powdered drugs to treat nasal and chest diseases.[9] Ma Huang remedies used by the Chinese 5000 years ago were shown to contain ephedrine, which was shown to be an effective treatment for asthma.[7,10] In 1885, Japanese chemist Nagayoshi Nagai continued to use ephedrine for the management of cough and other respiratory illnesses. In the first century AD, smoking Datura was considered as a therapeutic practice for treating asthma. Inhalation of steam to treat asthmatic episodes was a technique introduced by Roman physician Caelius Aurelianus during the 5th or 6th century AD.[11]
From the fall of Rome (476 AD) to the beginning of the industrial revolution (c. 1760 AD), there were no outstanding advances in inhalation and aerosol delivery devices, and people continued to rely on previously described techniques. Arab physician living in Baghdad from 850 to 932 AD, Rhazes described the use of arsenic to treat respiratory diseases using a sponge wetted with the substance and placed on the patient's mouth and nose. Inhalation of therapeutic aerosols was dramatically changed by Maimonides (1138–1204 AD), who was working for Saladin, the sultan of Egypt (1137 or 1138–1193 AD). He proposed inhaling fumes from burned leaves of herbs.[12] Christopher Bennet, an English physician, is credited with describing the oldest known inhalation device.[13]
Nebulizers and early dry powder inhalers (DPIs) were introduced with the emergence of the industrial revolution in 1760. English physicians Philip Stern and John Mudge described various inhalation techniques and devices that were targeting the general public.[14] Maw and Sons in London marketed a ceramic inhaler known as Nelson inhaler in the 1800s. Innovation in pharmaceutical aerosol delivery devices and techniques was reported in the last half of the 19th century. This period was marked by the introduction of nebulizers, DPIs, and asthma cigarettes.[15] Bleyer published a paper in the Annual Meeting of the American Medical Association describing drug delivery of various substances into bronchi in 1890.[16] Wyeth pencil inhaler was also introduced during this period. The device was intended to vaporize menthol for patients.[17]
Atomizers and nebulizers reshaped aerosol drug delivery. Dr. Auphon from France was the first to develop an atomizer device in 1849, followed by Jean Sales Girons, who made a portable device with a similar function.[18,19] Using Swiss physicist Daniel Bernoulli and Italian physicist Giovanni Battista Venturi principles, German physician Bergson developed the Hydrokonium, a rubber squeeze bulb device to deliver medications in the form of aerosol in 1862.[18] This paved the way for more advancement and allowed further development into portable devices in the early 20th century. Wright nebulizer was the first plastic nebulizer introduced in 1950.[20]
Currently, novel advances are available in inhaled drug delivery and its applications. This allowed several variant treatment options in the treatment and prevention of respiratory infections, using safer inhaled corticosteroids (ICSs), as well as systemic and gene replacement therapy. However, several challenges are associated with aerosolized drug delivery in children. Because there are no enough definitive data for children, aerosol practices in this population have been informed by data extrapolated from adult studies.
Consequently, this consensus report was developed by the Saudi Pediatric Pulmonology Association (SPPA) Expert Panel to provide healthcare providers in Saudi Arabia with consensus-based suggestions, regarding the application of aerosol therapies in respiratory diseases in children.
Methods
The Task Force was composed of 11 invited participants who were identified based on their expertise in pulmonary aerosol delivery. The subject was first divided into several topics, and each topic was assigned to at least two experts.
Topic writers searched the literature based on their own search strategies, and they determined their own databases. No attempt was made to grade evidence or recommendations. The literature search was completed in September 2019.
Draft reports written by the experts were then distributed to the entire expert panel, and comments were solicited in advance of meetings that were held at the 2019 European Respiratory Society Congress, a meeting on October 15, 2019, a meeting on May 17, 2019, as well as a meeting on May 4, 2019, in collaboration with the Chairman of the Pulmonology Section of the Moscow Society of Pediatric Physicians. During these meetings, the recommendations, along with the supporting evidence, were reviewed and discussed by the entire panel. Approval of the recommendations required consensus, which was defined as a majority approval. To accommodate any differences of opinion, the recommendations were revised until consensus was reached.
Despite differences between the guidelines and the available drugs and devices, the panel tried its best to develop a consensus statement to be valid worldwide.
Types of Nebulizing Inhalation Devices
Types of nebulizers include (a) pneumatic jet nebulizers, (b) ultrasonic nebulizers (USNs), and (c) vibrating mesh nebulizers (VMNs). If categorized based on their reservoir size, small-volume nebulizers (SVNs) typically hold 5–20 ml while large-volume nebulizers hold up to 200 ml. The latter is typically used for bland aerosol therapy of continuous nebulization of medication.[21,22,23]
Jet nebulizers
Gas flowing through a restricted orifice (jet) is used to operate standard SVNs under the control of a high-pressure gas source. This flow of gas through a narrow tube tends to draw a solution. As solution impacts against baffles, it is broken into smaller droplets ranging from 0.1 to 500 μm.[24] Figure 1 provides the structure of a jet nebulizer.
Figure 1.
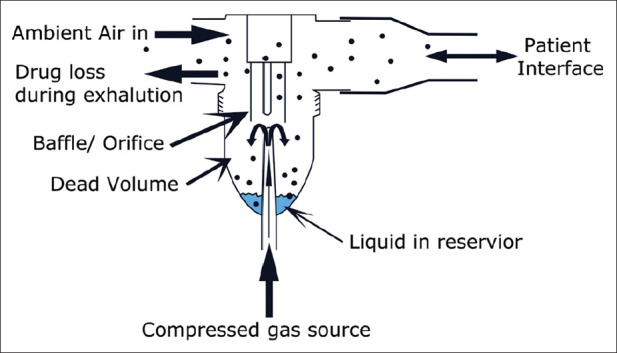
Structure of a jet nebulizer
Several factors affect nebulizer performance and drug delivery. These include (a) gas flow and pressure, (b) gas density, and (c) humidity and temperature. Higher flows produce smaller particle size droplets and reduce medication delivery time. This is also true when using higher driving pressure to operate the device. Consequently, devices that are designed to be operated using a higher pressure source may not be suitable for home use.[21,22,23,25] Jet nebulizers driven by a lower density gas source will have less aerosol impaction in airways with enhanced drug delivery to lungs. When using an oxygen–helium (Heliox) mixture gas, the flow should be corrected to avoid an associated drop in aerosol output. Flow should be twice to thrice of that used on a standard flowmeter.[26] Nebulizer output can be greatly reduced due to evaporation and reduced temperature during the operation of a nebulizer, leading to reduced particle size.[27] Jet nebulizers are classified into the following: (a) jet nebulizer with reservoir tube, (b) jet nebulizer with collection bag, (c) breath-actuated jet nebulizer, and (d) breath-enhanced jet nebulizer, with the former being the most commonly used device for drug delivery. Jet nebulizers offer an advantage with enhanced delivery of medication in the reservoir bag with consecutive inspiration. Aerosol delivery occurs only during inspiration when using breath-actuated nebulizers, contributing to less medication loss during expiration. Breath-enhanced nebulizers utilize a one-way valve to trap medications within the nebulizer, preventing loss of medication to the external environment.[28]
Ultrasonic nebulizers
USNs use high-frequency vibrations from an electrical source. Aerosol generation is believed to occur because of cavitation and/or acoustic streaming.[29] Several drugs can be delivered to the respiratory tract using USNs, which include bronchodilator, anti-inflammatory agents, and antibiotics. These devices are associated with a high cost and a low rate of reliability.[30] Figure 2 provides the structure of an ultrasonic nebulizer.
Figure 2.
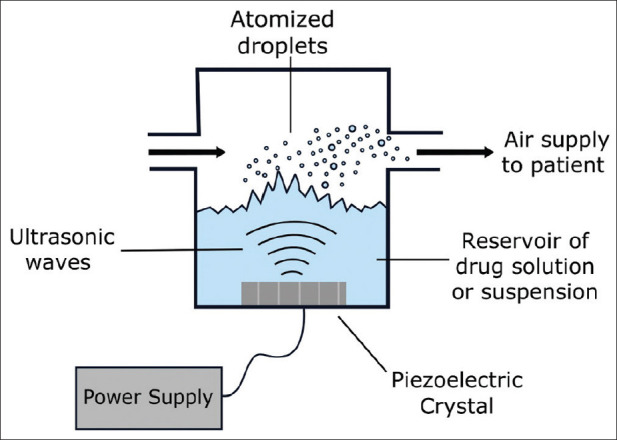
Structure of an ultrasonic nebulizer
Vibrating mesh nebulizers
VMNs utilize micropump technology to deliver medication. Vibrating piezoceramic elements vibrate aperture plate. Vibration rate of up to 130 kHz allows for the movement of plate up and down, resulting in an electronic pump.[21] These devices tend to produce consistent and enhanced aerosol generation efficiency. Particle size is usually exceptionally fine permitting enhanced delivery into the peripheral lung. Furthermore, low-drug volumes are needed. Optimal drug delivery can be increased by adjusting (a) pore size, (b) aerosol chamber reservoir, and (c) output rate.[30] Figure 3 provides the structure of a vibrating mesh nebulizer. Table 1 shows the characteristics of the commonly used nebulizers.[31,32]
Figure 3.
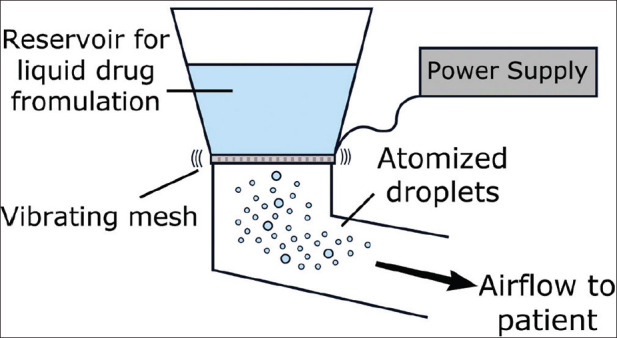
Structure of a vibrating mesh nebulizer
Table 1.
Characteristics of clinically commonly used nebulizers
| Type | Advantages | Disadvantages |
|---|---|---|
| Jet nebulizer | Simple structure, durable, widely used in clinical practice | Noisy |
| A nasal sinus jet nebulizer with superimposed oscillation waves can spread the drug through oscillation, effectively deposit in nasal sinus cavities and moisten nasal sinus mucosa, also suitable for children | Requiring compressed air supply or power (generally AC power) to drive | |
| When using nasal sinus jet nebulizers, it is required to close the soft palate and hold the breath, which is difficult to master; therefore, medical staff is needed to instruct before patients master the inhalation method | ||
| Ultrasonic nebulizer | Large amount of aerosol release, quiet, and noise free | Requiring power (generally AC power) |
| Easy drug degeneration | ||
| Easy inhalation of excessive moisture | ||
| Easily affect suspensions concentrations with different water solubilities | ||
| Vibrating mesh nebulizer | Quiet and noise free, compact, and lightweight, battery-driven | Requiring power (batteries) |
| Liquids can be placed above the breathing tube, without any backflow preventing contamination from the solution in the tube | Durability has not been confirmed, and there are limited types to choose from | |
| Amount of nebulizing inhalation of the drug can be adjusted at any time |
Lung Deposition
An aerosol is defined as “any system of solid particles or liquid droplets of sufficiently small diameter to maintain some stability as suspension in air.”[33] It can be classified into monodisperse where the particles have approximately the same size as well as heterodisperse or polydisperse when different sizes are involved. However, the perfect monodisperse system does not exist, and it is widely accepted that if a relative standard deviation percent is <20% p/p, an aerosol can be called monodisperse.[34]
For the treatment of respiratory disorders, different types of inhalers are used. Drug particles are deposited in the respiratory system, depending on the drug's physical and chemical properties and the host's physiology.
Mechanisms of deposition
Although there are many mechanisms for lung deposition, only three mechanisms are important: inertial impaction, gravitational sedimentation, and Brownian diffusion,[34,35] as shown in Figure 4.
Figure 4.
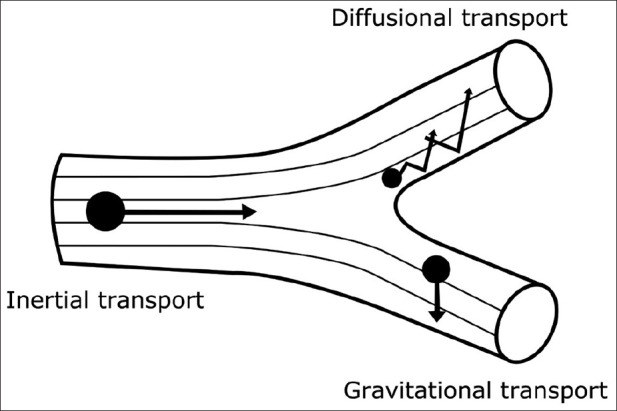
Illustration of particle transport onto airway surfaces
Inertial impaction
The deposition of majority of drug particles larger than a few micrometers occurs by inertial impaction. When the particles are heavy or traveling at high speed, this may lead to the particles being unable to follow a change in direction, and as a result, they will impact on the airway wall.[34,35]
Gravitational sedimentation
Particle sedimentation is driven by the gravitational force which is balanced by air resistance. Particle sizes range from 0.5 to 5 μm and may travel to peripheral parts of the lung where they can settle onto smaller airways. This can occur during quiet breathing or breath holding.[34,35]
Brownian diffusion
For particles smaller than 0.5 μm, Brownian diffusion is the most significant mechanism of deposition. Here, particles inside the airways may be displaced by the random bombardment of gas molecules which impact with the airway walls.[34,35]
Insignificant mechanisms
These include electrical charge force, diffusiophoresis, thermophoresis, and simple contact.[35]
Concept of aerodynamic particle diameter
The parameters that most effect particle transports into the respiratory tract are the particle size, density, velocity, and time. The particle size of an aerosol is a crucial physical property, affecting lung drug deposition. In addition, aerodynamic diameter controls particle deposition in the lungs, rather than geometric diameter. The aerodynamic diameter is a product of geometric diameter and the square root of density as indicated above (pd2), where p denotes particle density and d denotes geometric diameter.
Furthermore, particles with the same value of the product (pd2) exhibit identical deposition. On the other hand, a particle becomes less dense when it becomes more porous, resulting in decreased aerodynamic diameter. Accordingly, as density decreases, particles that are larger in geometric diameter can deposit deeper into the lung region, because they are smaller in aerodynamic diameter.[36]
Aerodynamic diameter of the particles can be defined as the diameter of a fictitious sphere of unit density, which settles with the same velocity as the particles in question under the action of gravity.[35]
Methods of studying deposition
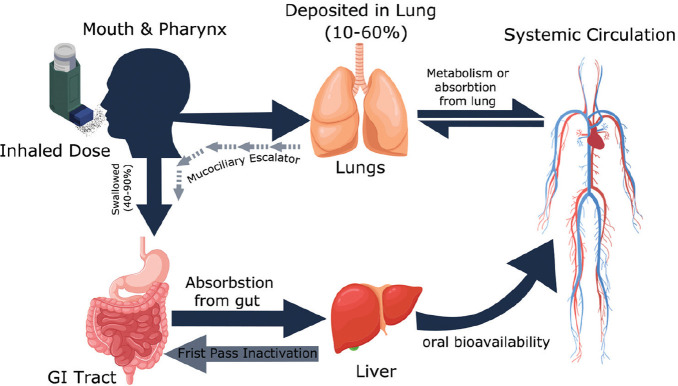
In many devices, after inhalation, no more than 20% of the inhaled dose reaches the lung, and 80% is deposited in the oropharyngeal region and therefore is swallowed. The fraction delivered to the lung is either cleared by the mucociliary escalator and swallowed or absorbed into the systemic circulation.[37] More details are shown in Figure 5.[38]
Figure 5.
Fate of inhaled drug
The drug proportion which reaches the systemic circulation has the potential to cause extrapulmonary adverse effects such as in case of corticosteroids which may cause osteoporosis and Cushing's syndrome. However, the corticosteroid absorbed from the gut undergoes first-pass metabolism and these adverse effects are minimized. Corticosteroids are generally highly affected by first-pass metabolism. As an example, budesonide is metabolized up to 89%, fluticasone >99%, and mometasone >99%.[39]
There are many methods to investigate drug deposition in the lung classified into two main types: in vivo such as the pharmacokinetic and scintigraphic methods and in vitro methods which have the potential value in predicting lung deposition. These methods have a major role in the quality control for inhaled formulations; examples include dose emission and particle size distribution.
The information that is available from these in vivo and in vitro studies includes total lung dose, extrapulmonary delivery, drug distribution within the respiratory system, relationship between lung dose and therapeutic effect, and influence of factors such as disease, inhalation technique, and intra- and inter-patient variability.[35]
In vivo methods
Imaging
There are three main methods for imaging, planar gamma scintigraphy, single photon emission computed tomography, and positron emission tomography. The main advantage of these imaging techniques over others is the ability to localize deposition within the body, including extrapulmonary and the distribution throughout the airways. However, there are safety issues. All imaging methods that use radionucleotides expose the subjects to health risks, and the risks are more pronounced in children than in adults.[35] In practice, imaging techniques are subject to a number of operational challenges including labeling of the drug formulation and interpretation of the images produced.
Pharmacodynamics methods
A good relationship between lung deposition and the effects for both bronchodilators and steroids is well documented.[40,41] In addition, some research has shown a relationship between the pattern of deposition and the pharmacodynamic effects, especially for inhaled steroids. There has, however, been limited work in this area because the therapeutic effect of inhaled steroids needs weeks to be seen, while for β2 agonists, doses are usually administered at close to/or supramaximal level, making the doses closer to the plateau of the dose–response curve.[35]
Pharmacokinetic methods
Pharmacokinetic methods are used to evaluate the lung deposition. Although they do not generally provide information on the distribution of drug into different regions of the lungs,[42] these methods estimate total systemic delivery via oral and inhaled routes by means of area under the curve data or urinary excretion of the drug.[37]
In vitro METHODS
In vitro methods carried out by pharmaceutical industry contribute in a major way to drug development. Alongside this, researchers use in vitro methods to predict the drug deposition in the lung. Pharmacological effects of an inhaled drug are greatly influenced by the amount of drug depositing in the lower airways.[43] Several methods can be utilized to characterize the particle size of a drug. In practice, it is possible to broadly categorize these into two areas: optical and inertial methods.
Optical methods
Optical methods include microscopy, time-of-flight (TOF) aerodynamic particle size analyzers, light interaction methods - optical particle counters, laser diffractometry, and phase Doppler particle analysis. These instruments provide rapid techniques, and in addition, TOF instruments measure the aerodynamic particle size. Disadvantages of these methods are that they are not drug specific and lack the capability of a direct assay for the active pharmaceutical ingredient (API), due to the inability to differentiate between drug particles and carrier particles.
Inertial impaction method
The inertial cascade impaction is the gold standard to determine the aerodynamic characteristic of emitted doses. Generally, it is the method most acceptable to the regulatory agencies as it is based on the inertial impaction concept. Since the inhaled formulations comprise a combination of the active pharmaceutical ingredient (API) and other excipients, it is important to measure the API. The method also uses the entire dose as sample and can measure the aerodynamic size. A disadvantage is that it is calibrated only at fixed-flow rates.
Pulmonary Delivery Devices
Inhaled drug products are exceedingly popular for drug delivery through the lung or nasal mucosa for local or systemic therapy. Inhaled bronchodilators and corticosteroids are the mainstay for the treatment of asthma.
The inhaled drug devices are classified into three main categories: nebulizer, pressurized metered dose inhaler (pMDI), or DPI. Most clinical evidence shows that all these devices will work for most situations of acute exacerbation and stable cases.[44]
Pressurized metered dose inhaler
pMDI was first introduced during the first half of the 20th century. At first, they were known as “MDI,” but the term “pMDI” has become more popular, to differentiate them from other nonpressurized metered dose devices such as DPIs and other multidose devices.[45] It has become the most popular dosage form for the delivery of drug to the respiratory tract. pMDI consists of propellants, drug formulation, a metering valve and actuator, as illustrated in Figure 6,[46] all of these play roles in the particle size, the spray formation, and as a result, in determining drug delivery to the lungs.
Figure 6.
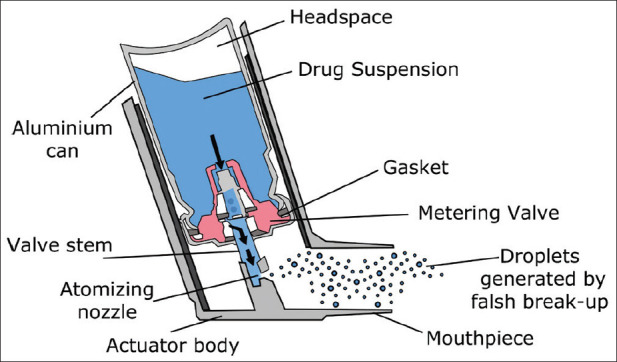
A schematic diagram of the pressurized metered dose inhaler
One of the most viable components of a pMDI is its propellant. The propellant creates the force needed to generate the aerosol cloud. Chlorofluorocarbons (CFCs) met these requirements and pMDIs have conventionally used CFC as the major propellant.[45]
Recently, pMDIs containing CFCs have been replaced because the use of CFCs was banned under international agreement because of their effect on the ozone layer
As result, hydrofluoroalkanes (HFAs) have replaced CFCs. Formulations containing HFAs either tetrafluoroethane (HFA-134a) or heptafluoropropane (HFA-227), are usually used. Overall pMDIs that use CFC-free propellants have continued to challenge formulation scientists to develop efficient pMDI devices.[45]
Actuator is usually made from plastic and its design is a factor in determining the aerosol particle size, particularly nozzle diameter, ranging between 0.14 and 0.6 mm, and length.[45,47]
Breath-actuated pressurized metered dose inhalers
Patient coordination of actuation with inhalation can be a problem with pMDIs, especially in certain groups of patients such as the young, elderly, or chronically ill. To overcome this problem, breath-actuated pMDIs can be used since they are sensitive to patient inhalation through the device and fire the inhaler simultaneously with patient's inhalation. Autohaler, Easibreathe, K-Haler, MD Turbo, Xcelovent, and Smartmist are the examples of such devices, and there are several more under development. Another mechanism to overcome this problem is the addition of a spacer device or integrated spacer mouthpiece. Examples include Aerohaler, Azmacort pMDI, and Spacehaler.[45]
Advantages and disadvantages of pMDIs are listed in Table 2.
Table 2.
Advantages and disadvantages of conventional pressurized metered dose inhalers
| Advantages | Disadvantages |
|---|---|
| Convenient, availability to use | Drug delivery is significantly affected by inhalation technique |
| Cost | Needs propellants |
| Number of doses may reach 100 | High velocity of particles leads to high oropharyngeal deposition |
| High pressure protects its contents against bacteria and moisture |
Adjunct devices
Spacers slow down the particles and make the coordination between actuation and inhalation less critical. In addition, spacers increase the proportion of the inhaled dose that is delivered to the airways to produce the needed effect of the drug and reduce the systemically absorbed proportion that usually causes unwanted effect. pMDIs with large-volume spacers deposit at minimum 30% more drug in the lung while deposit 60% less drug in the patient's body. This results in reducing oropharyngeal deposition as well as reducing the systemic side effects of steroids including growth restriction in children.[48,49,50,51]
Large spacers in most of the cases have a valve system to permit the drug to stay in suspension phase while it is being inhaled. The dose may be reduced due to the accumulation of electrostatic charge. Accordingly, the drug is absorbed on to the plastic surface. An optimal solution to this problem is to soak the spacer in a diluted household detergent solution and then allow drip-drying while rinsing the spacer with water or wiping it with towel. This will reduce deposition inside the spacer and increase the residence time.[52,53,54]
The British Thoracic Society (BTS) guidelines recommend the use of an pMDI along with a spacer in preference to a nebulizer for the treatment of a mild-to-moderate acute asthma attack.[55] This approach was also supported by a Cochrane systematic review which found that nebulizers were not significantly any better than pMDIs.[56] Advantages of using a large-volume spacers include (a) better pattern of deposition resulting in more effective treatment and less side effects, (b) largely overcoming problems of poor inhaler technique if spacers are used properly, (c) easily used by children and elderly (except those with weak or arthritic hands), (d) as effective as a nebulizer in terms of treating acute attacks while being light, cheap, maintenance-free, portable, and available on prescription, (e) useful for the management of first attacks of wheezing in patients who have never used inhalers, (f) useful for the administration of bronchodilator when testing reversibility to establish the diagnosis of asthma, and (g) reduced prescribing cost through basing the treatment on the cheap pMDIs. Cough after using a spacer and a pMDI is still a problem that may affect patient's compliance. In one study, cough affected 30% of children after using beta-agonists and 54.5% of children after using a steroid inhalation.[57] The latter study concluded that the type and volume of the spacer as well as a face mask or mouthpiece did not influence cough. They stated that cough after using spacer devices delivering corticosteroids is a common side effect in asthmatic pediatrics.
Dry powder inhalers
DPIs can be as small and portable as a pMDI, but they require less coordination as drug delivery is dependent on the patient's peak inspiratory-flow rate. DPIs are more expensive than pMDIs plus spacer, and BTS guidelines state that they are not more effective. Evidence suggests that newer DPIs are not more effective than older types.[55] DPIs do not include any propellant. Patients sometimes complain that they are not sure if they have taken the dose or not and that devices may be discarded before they are empty.
Nebulizers
Nebulization is a viable alternative for children who are not able to use a spacer device, and it is particularly relevant for “pre-schoolers.” Saudi Initiative for Asthma states that nebulized inhaled therapy is the only reliable alternative to pMDI with spacers.[58] Several factors should be considered in the administration of inhaled therapy in infants and young children, as they can affect the drug dose that is delivered to the lungs. These factors include nose breathing, small tidal volume (VT), no breath holding, no cooperation, and crying.[59]
Nebulizers are divided into three subgroups: (a) pneumatic jet nebulizers, (b) USNs, and (c) VMNs Check Section 1. If categorized based on their reservoir size, SVNs typically hold 5–20 ml, while large-volume nebulizers hold up to 200 ml. The latter is typically used for bland aerosol therapy and continuous nebulization of medication.[21,22,23] Table 3 lists the characteristics of aerosol inhalers.[60] Table 4 compares between pMDIs with holding chamber, DPIs, and nebulizers as aerosol delivery devices.[61]
Table 3.
Characteristics of aerosol inhalers
| pMDI | DPI | Nebuliser | |
|---|---|---|---|
| Technique of generation of aerosol | Propellant based | Patient driven | Bernoulli’s principle |
| Piezoelectric crystal | |||
| Particle size (m) | 1-10 | 1-10 | Variable |
| Drug deposition (%) | 5-10 | 9-30 | 2-10 |
| Oropharyngeal deposition | Significant | Variable | Insignificant |
| Patient coordination | Required | Not applicable | Not required |
| Breath hold | Required | Not required | Not required |
| Patient generation of flow | Not required | Required | Not required |
| Amount of drug | Small doses | Small doses | Large doses possible |
| Contamination | No | No | Possible |
| Use for chronic therapy | Yes | Yes | Rarely |
| Use for emergency management | No | No | Yes |
| Use for intubated patients | Preferred | No | Second choice |
pMDI=Pressurized metered dose inhaler, DPI=Dry powder inhaler
Table 4.
Comparison between pressurized metered dose inhalers with holding chamber, dry powder inhalers, and nebulizers as aerosol delivery devices
| pMDI/HC | DPIs | Nebulizer | |
|---|---|---|---|
| Performance | |||
| Majority of aerosol particles <5 mm in size | + | + | ± |
| High pulmonary deposition | + | ± | ± |
| Low mouth deposition | + | ± | − |
| Reliability of dose | + | ± | ± |
| Not compromised by humidity | + | − | + |
| Physical and chemical stability | + | + | + |
| Breath actuated | − | + | − |
| Low risk of contamination | + | + | − |
| Convenience | |||
| Lightweight, compact | + | + | − |
| Multiple doses | + | + | − |
| Dose counter | ± | + | − |
| Easy and quick operation | ± | ± | − |
| Suitable for all ages | + | − | + |
+: present; -: not present; ±: sometimes present, sometimes not. pMDI=Pressurized metered dose inhaler, DPIs=Dry powder inhalers, HC: Holding chamber
Choosing the Correct Device for the Management of Different Respiratory Disorders
Asthma
Short-acting β2-agonists and inhaled corticosteroids
For the management of asthma in the outpatient setting, both pMDIs (with or without spacer/holding chamber) and DPIs are appropriate for the delivery of short-acting β2-agonists (SABAs) and ICSs. The adequate selection of a certain type of aerosol delivery devices in this setting should be based on the patient's ability to use the device, the patient's preferences for the device, the availability of the drug device combination, the compatibility between the drug and the device, the time and skills needed to properly instruct and monitor the patient regarding the use of the device appropriately, the cost of the treatment, and the financial reimbursement.[62] A guide to the choice of delivery device in children of different ages is shown in Figure 7.[35] Table 5 includes the recommendations for ICS aerosol delivery devices.[63]
Figure 7.
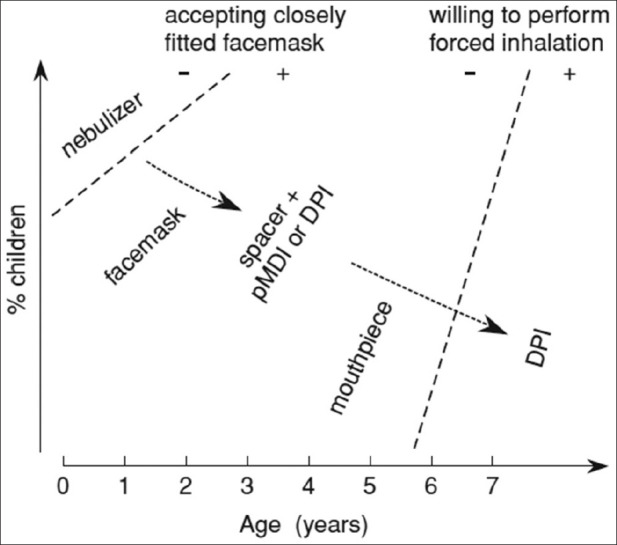
Choice of delivery device in children of different ages
Table 5.
Recommendations for delivery of inhaled corticosteroids aerosol delivery devices
| Nebulizer | pMDI + spacer | DPI | |
|---|---|---|---|
| Infants (<2 years) | Reliable | Unpredictable | Not appropriate |
| Easy to use | Requires training | ||
| Mask, tight seal not required | Mask, tight seal required | ||
| Preschool (2-5 years) | Effective | Effective | Not appropriate |
| Easy to use | Requires training | ||
| Mask, tight seal not required | Mask, tight seal required | ||
| Breath-enhanced mouthpiece | Mouthpiece | ||
| School age (>5 years) | Effective | Effective | Effective |
| Breath-enhanced mouthpiece | Mouthpiece | Easy to use | |
| Requires training and practice | Deposition varies by device |
pMDI=Pressurized metered dose inhaler, DPI=Dry powder inhaler
Use and care of spacers
When choosing a spacer, it must be compatible with the pMDI device. The drug needs to be administered through repeating single actuations of the drug into the spacer, and each actuation should be followed by inhalation. The delay between each actuation and the following inhalation should be minimized. It should be considered that tidal breathing has the same efficacy as single breaths. It is important to clean spacers (monthly not weekly) according to the manufacturer's instructions to preserve their performance.
Spacers should be washed using a detergent and let to air-dry. The mouthpiece should be wiped and cleaned of any detergent.
Drug delivery could vary significantly because of static charges. However, this is not the case with metal (or other antistatic spacers). It is important to replace plastic spacers at least annually. However, some types need to be changed every 6 months.[55]
Techniques to improve the adherence to aerosol medications[64]
Clear written explanation and demonstration should be given to the patient at the time the medication or device is prescribed
The patient should be asked to bring the medication during every appointment to make sure he/she is using the device appropriately
The patient should be asked about his/her adherence as well as any faced problems with the medication or the device
Thy physician should follow up with the patient on unfilled prescriptions as well as refills
The number of prescribed medications and devices should be minimized as much as possible
Parents should be aware that they are primarily responsible for the medications' administration by their child regardless of his/her age.
Bronchiolitis
Epinephrine
Some studies have revealed that epinephrine nebulization may result in reduced hospital admissions,[65] and one study showed that combined treatment of epinephrine and steroids reduced hospital admissions.[66] However, the evidence remains insufficient to support routine use of epinephrine in the emergency department. It may be reasonable to administer a dose of epinephrine and carefully monitor clinical response; however, unless there is clear evidence of improvement, continued use is not appropriate.
3% hypertonic saline nebulization
The efficiency of nebulized 3% hypertonic saline is widely debated, and definitive recommendations will likely require further accumulation of evidence. It is hypothesized that hypertonic saline enhances mucociliary clearance and rehydrates the airway surface. In addition, there is some evidence of reduced clinical severity among inpatient and outpatient populations with no reports of significant adverse events. A Cochrane review revealed that hypertonic saline nebulization may reduce the length of stay from more than 3 days to 1 day. The best treatment regimen is still unclear. However, the most commonly reported regimen in clinical trials was 3% saline, with or without a bronchodilator, delivered by jet nebulizer every 8 h.[67]
Cool mist, isotonic saline, and other therapies
Cool mist therapies and other aerosol therapies have been used for some time to manage bronchiolitis, with scant evidence supporting their efficacy. A Cochrane systematic review showed that there is no evidence to support or deny the efficacy of cool mist in the management of bronchiolitis.[68]
Other therapies can be used for the treatment of severe bronchiolitis in critically ill infants. These therapies include Heliox gas mixtures, nasal continuous positive airway pressure (CPAP), mechanical ventilatory support, and surfactant.
Cystic fibrosis
Aerosol antibiotics
Aerosol antibiotics can be beneficial for patients requiring frequent antibiotic courses to treat a high burden of bacteria that are difficult to treat. Several aerosolized antibiotics are currently available such as tobramycin, aztreonam, and colistin.
A high concentration of antibiotics such as quinolones and aminoglycosides could be delivered to the proximal airway through aerosolization with minimum systemic effects.[69] Aerosol antibiotics tend not to reach deep into the lungs due to the large amount of pus as in cystic fibrosis (CF). Accordingly, the antibiotic concentration in the most involved areas become not sufficient to kill the resistant organisms, and this will eventually induce resistance.
In addition, antibiotic aerosols may be irritating and cause bronchospasm and cough.[70,71]
For an antibiotic to be used as an aerosol, it has to be soluble and effectively delivered when aerosolized, has concentration-dependent pharmacokinetics, can penetrate through and into sputum, not degraded after nebulization. Its activity should be maintained for hours after being nebulized. Additionally, it should not cause serious adverse events if administrated systemically; it should have minimal systemic absorption.[72]
Aminoglycosides were among the first antibiotics used in the form of aerosols due to their associated risks (such as renal dysfunction and hearing problems) when administered systematically. These risks are reduced but not eliminated when aminoglycosides are administered as aerosols.[73]
Tobramycin solution was the first antibiotic to be commercially available as an aerosol for the treatment of CF disease. When 300 mg dose of tobramycin solution is administered by jet nebulization twice daily, it can improve pulmonary function, decrease the risk of infectious exacerbations, and reduce the need for intravenous (IV) therapy.[74]
It was thought that administering the tobramycin aerosol for 28 days and stopping it for 28 days will reduce the risk of bacterial resistance. However, bacterial resistance continued to increase. Accordingly, the drug should be stopped for longer durations that are sufficient to reverse time-dependent bacterial resistance.[75] The risks of renal dysfunction and hearing loss associated with tobramycin are decreased but still not eliminated when administered as an aerosol.[76]
Inhaling cumulative doses of gentamicin can result in nephrotoxicity.
Mucoactive medications
Mucoactive medications are medications that influence the secretions or clearance of mucus.
A class of mucoactive drugs is mucolytics that are used to decrease the viscosity of mucous and enhance ciliary and cough clearance. N-acetyl cysteine is a classic mucolytic, but aerosol N-acetyl cysteine has not shown any effectiveness in the treatment of lung diseases. In addition, it may be damaging to the airway of CF patients as mucin protects the airway surface. The primary beneficial effect of acetyl cysteine is that it induces cough. The only approved peptide mucolytic drug for treating CF patients is dornase alfa. It improves pulmonary function and decreases the CF exacerbation frequency. Actin depolymerizing agents show synergistic action when used with dornase as they decrease the secondary polymer network.[77]
It is suggested that hypertonic solution can improve the pulmonary functions and decrease the frequency of pulmonary exacerbations compared to normal saline.[78] Results of small studies showed that the efficacy of hypertonic saline may not be the same as that of dornase alfa in terms of improving forced expiratory volume in 1 s (FEV1) in CF patients.[79]
Hyperosmolar saline should be administered in combination with a beta-agonist as it may cause bronchospasm. Dry powder mannitol inhalation can be effective in improving pulmonary functions, and it shows at least the same tolerability as hyperosmolar saline.[80]
Aerosol surfactants
Surfactants can mobilize secretions, stabilize airways, and have anti-inflammatory effects. Although they are difficult to be nebulized into the airway as they tend to foam and become highly viscous, surfactants could show efficacy in terms of secretion mobilization in intubated as well as ventilated patients where the airway is partly humidified.[64,81]
Anti-inflammatory drugs
ICSs used for treating asthma are the most used anti-inflammatory drugs. Other drugs such as recombinant secretory leukoprotease inhibitors, antineutrophil elastase, and alpha-1 antiprotease have been studied to be used as aerosols as they can reduce the activity of serine proteases in chronically inflamed airways. Antioxidants including recombinant superoxide dismutase and glutathione have been used as aerosols supported by the current studies to examine the efficacy of aerosolized glutathione as an adjuvant therapy in the treatment of CF disease.[64,82,83]
Cyclosporines also can be nebulized, and they may protect the airways against inflammation and allergic reactions. In addition, aerosolized cyclosporine may be effective in graft preservation in patients undergoing lung transplantation.[84,85]
Tracheostomized children
Aerosolized therapies are important treatments in the management of airway disorders that can deliver medication directly to where it is required.
Aerosolized treatment comes with several broad categories:
For bronchodilator treatment, most commonly, this would be an SABA such as albuterol, while longer-acting β2-agonists such as salmeterol may also be used. Anticholinergic therapy such as ipratropium bromide may form part of the acute treatment of asthma. These may be used in either nebulized or pMDI form
Inhaled steroids may also form part of antiasthma therapy, with inhaled beclomethasone, fluticasone, and budesonide being used. Combination inhalers (fluticasone + salmeterol) may form part of regular treatments
Inhaled antimicrobial therapy may be nebulized. This most commonly includes not only antibiotics such as the aminoglycosides – tobramycin, amikacin, and gentamicin, but also other antibiotics such as colomycin and meropenem. Antifungals such as amphotericin/ambisome can be nebulized as can anti-Pneumocystis jirovecii treatment such as pentamidine
Mucolytic therapy may be nebulized. This includes dornase alfa, hypertonic saline, N-acetyl cysteine, and carbocisteine
Pulmonary hypertension medication: Epoprostenol and prostacyclin
Palliative care: Fentanyl, diamorphine, and morphine.[86]
Neuromuscular diseases
Inhaled hypertonic saline is used in older children during acute respiratory illnesses in patients with neuromuscular diseases (NMDs). Hypertonic saline may have different mechanisms of action.[87] It is used as an expectorant to aid patients in producing sputum for examination.[88] This is probably due to its osmotic effect on increasing the depth of the airway surface fluid layer, which also improves mucociliary clearance. In addition, it may have mucolytic properties through disrupting the ionic bonds within the mucus gel and accordingly reducing entanglements and cross-linking. Similar to DNase, hypertonic saline can dissociate white cell DNA from mucoproteins, making them more amenable to being digested by proteolytic enzymes.[89] Inhaled hypertonic saline may have anti-inflammatory properties through increasing the level of glutathione and thiocyanate, which protect against oxidative injuries in the airway surface while possibly decreasing the level of interleukin-8 obtained from bronchoalveolar lavage fluid.
Regarding inhaled anticholinergic drugs such as ipratropium bromide, atropine, tiotropium, and glycopyrrolate, they block mucus hypersecretion that is triggered by the stimulation of M3 muscarinic receptors. Anticholinergic drugs do not increase the viscosity of mucous or reduce its normal production.[90,91] In hypersecretory conditions such as chronic obstructive pulmonary disease (COPD), their use has been shown to decrease sputum volume.[92] No such effect has been studied in patients with NMD. One of the adverse effects of these drugs is oral dryness, and they are frequently used enterally to treat sialorrhea.[93,94] They are less effective antisialagogues when used by spraying the inhalational form of the drug into the mouth.[95] Although excessive thickening of secretions is a common concern when using these medications enterally, it is not a frequently reported adverse effect.[94,96]
General instructions when choosing an aerosol device
Choose the most suitable device to your patient. The following points should be taken into consideration:
The choice of the device could be determined based on the drug to be used
If the patient is unable to use the device adequately, an alternative should be prescribed
The patient's ability to use a device needs to be ensured by a competent healthcare provider
The medications need to be titrated against the clinical response to ensure the optimum efficacy
In children aged <5 years, pMDIs and spacers are the preferred method to administer beta-agonists and ICSs
A face mask is needed to be used until the child can reproducibly breathe using the spacer mouthpiece
Nebulization is an effective alternative to pMDI and spacer.
Recommendations
Asthma
pMDI and DPI are appropriate for the delivery of SABA in the outpatient treatment of asthmatic patients
For ICS delivery, nebulizer is the most effective delivery device in all pediatric ages, while pMDI is not suitable for infants <2 years old, and DPI is only appropriate in children >5 years old.
Bronchiolitis
Insufficient evidence supports the routine use of epinephrine for bronchiolitis in the emergency department
Nebulized hypertonic saline reduced clinical severity scores with no significant adverse events reported, in addition to reduced length of stay. However, the optimal treatment regimen remains unclear
No evidence supports cool mist and other aerosols use in bronchiolitis.
Cystic fibrosis
Aerosol antibiotics are appropriate for CF patients, especially who require repeated courses of antibiotic therapy. To avoid antibiotic resistance, the chosen antibiotic should be soluble, should penetrate into pus, resist longer in the airway, should have minimal systemic effect
For mucolytic medications, dornase alfa is the only approved medication for CF treatment in improving pulmonary function and reducing the CF respiratory tract exacerbations frequencies
Acetyl cysteine could be damaging to the CF airway. However, its primary beneficial effect is by inducing a cough
Hypertonic saline study showed a significant improvement in FEV1 in subjects with CF and fewer pulmonary exacerbations. Because hyperosmolar inhaled saline can trigger bronchospasm, it is given along with a beta-adrenergic agonist
Inhaled dry powder mannitol is tolerated as hyperosmolar saline and the improvement of pulmonary function lasts for more than 18 months.
Tracheostomized children
In children with tracheostomies, nebulizers and pMDIs are encouraged to be used in almost all USA centers including ICSs, short-acting beta-agonists, combination therapy (long-acting bronchodilator + corticosteroid), mucolytics, and antibiotics.
Neuromuscular diseases
In older children with NMD having acute respiratory illnesses, inhaled hypertonic saline is used as a routine expectorant, as a mucolytic, and for its inflammatory effects
Inhaled anticholinergic has not been studied in patients with NMD.
Choosing the Best Aerosol Device by Age
Choosing an aerosol device for infants (birth to 4 years of age)
The nebulizer or pMDI with valved holding chambers (VHCs) should be selected in the administration of aerosol therapy to infants because a child younger than 4 years may not be able to practice specific breathing techniques.[97] While pMDI provides a faster and more convenient aerosol drug administration, some infants may tolerate nebulizers better. At low VTs, particularly, VHCs are the preferred method for pMDI delivery in infants and small children. The use of breath-actuated nebulizers, breath-actuated pMDIs, or DPIs may not be reliable in children under 4 years of age.
Children younger than 3 years of age may not be able to use a mouthpiece which necessities using a mask with nebulizers and pMDIs. However, the use of a face mask is not the only option for infants, especially when nebulizers are used for aerosol therapy. Previous literature reported that a hood may provide comparable efficacy compared with face mask. While research on drug administration via high-flow nasal cannula (HFNC) is limited, its efficiency during aerosol therapy has been documented.[86,98] Therefore, drug delivery through HFNC could be an alternative, especially for babies who cannot tolerate a mask during aerosol therapy. Even though clinicians use the blow-by technique in which the mask or open tube is held near the infant's nose and mouth to deliver aerosolized medications, using the blow-by technique should be discouraged because drug delivery is decreased as the distance from the child's face and device is increased.[99]
Inhaled drugs should only be given to infants when they are settled and breathing quietly. Studies demonstrate that the breathing pattern of quiet and sleeping infants results in greater inhaled drug dose than patterns of children who are awake. However, it is difficult to administer aerosol to a sleeping infant without waking them unless one is using a hood, a nasal cannula, or a stealthy placed mask. While a crying child apparently receives no aerosol drug into his lungs, most of the inhaled drug deposits in the upper airways, and much of it are then swallowed. Therefore, it is essential to develop innovative approaches to minimize distress before administering aerosol drugs.[100]
These approaches may include playing a game with the device to enhance its acceptance, comforting babies, as well as considering other effective forms of distraction. In addition, aerosol drugs can be given to infants when they sleep considering that the administration will not wake up or agitate the infant.
Choosing an aerosol device for preschool children (4–5 years of age)
Selection of an aerosol device is vital to ensure successful aerosol therapy in toddlers and preschool children. Nebulizers and pMDIs with VHC are recommended for use with preschool children, and drug delivery with both aerosol devices is similar.[63] However, longer treatment time and less portability make the nebulizer less desirable than the pMDI when drug administration is PRN (i.e. as needed). such as short-acting bronchodilators or dosing several times a day. Once children reach the age of 4 years and above, they may adequately understand the method of using pMDI or DPI successfully and may generate the sustained inspiratory-flow rate that is required for optimal use of the device.[97,101]
Choosing an aerosol device for young children (6–12 years of age)
Since young children between 6 and 12 years of age can often master complex inhalation techniques, it is possible to use a broader range of aerosol devices with this patient population.
For example, some children will be able to take slow inhalation and hold breath when using pMDI with or without VHC, while others can master the DPI. Breath-actuated pMDIs can eliminate hand–breath coordination problems and would be helpful for those who cannot achieve good hand–breath coordination with pMDIs. When patients stop inhaling, the Cold-Freon effect can reduce the inhaled dose. However, the cold air–apnea effect can be eliminated by means of VHC.[102]
It is important to realize that various types of aerosol devices deposit different fractions of the total prescribed dose (the “nominal” dose) of a given drug in the lungs. Moreover, different types of aerosol devices do not have the same nominal dose. For example, when using albuterol, the typical pMDI nominal dose is two actuations (about 200 μg) while the typical nominal dose from a nebulizer is 2.5 mg which is more than 12 times more drug.[25]
Recommendations
Aerosol device for infants (birth to 4 years of age)
The nebulizer or pMDI with VHC is the best aerosol therapy in infants <4 years old. Although nebulizers are more tolerable than pMDI, breath-actuated nebulizers, breath-actuated pMDIs, or DPIs are not reliable in this group age
With nebulizers and pMDIs, the mask is preferable than in children younger than 3 years of age
During aerosol therapy, when nebulizers are used, a hood may provide comparable efficacy compared with face mask. HFNC is an alternative way in children who cannot tolerate a mask
The greater inhaled drug dose is delivered when infants are settled and breathing quietly.
Aerosol device for preschool children (4–5 years of age)
As for infants, nebulizers and pMDIs with VHC are recommended for use with and drug delivery with both aerosol devices is similar. On the other hand, the nebulizer is less desirable than the pMDI in a regular treatment basis
For children aged 4 years or more, the method of using pMDI or DPI is applicable.
Aerosol device for young children (6–12 years of age)
A broader range of aerosol devices can be mastered in children between 6 and 12 years of age including pMDI with or without VHC, DPI, and breath-actuated pMDIs.
Aerosol Therapy in Pediatric Emergency Care
Pediatric respiratory diseases are among the most common challenges in pediatric emergency. The main backbone for the management for many pediatric pulmonary diseases in emergency is the inhaled therapy. Choosing the appropriate aerosol devices in emergency is critical for rapid and effective management of the patient.[103,104]
Types of inhalation devices used in pediatric emergency care
The main inhalation devices used for pediatric patients are pMDI, DPIs, liquid metered-dose inhaler (LMDI), and nebulizers.
However, the most used devices in the emergency department are either nebulizers or pMDI due to the ease of use and the fast effect in a crowdy area like the emergency room (ER).[105]
Management of the major diseases in the emergency room
Asthma
Initial management of asthma in the emergency department depends on severity of disease. The main aerosol devices used in emergency are nebulizer or pMDIs using pediatric respiratory assessment measure score or pulmonary index score to determine mild or moderate or severe exacerbation. The first-line inhaled therapy is SABA (salbutamol) while glucocorticoids are considered as the second-line treatment.[101,106,107]
Mild asthma
For the management of mild asthma, salbutamol inhaler can be administered through either nebulizer or pMDI where the latter is more recommended.
Nebulizer: Salbutamol 0.15 mg/kg (minimum 2.5 mg and maximum 5 mg per dose), repeat the doses according to the condition and need of the patient
pMDI ⅓ puff to ¼ puff/kg (minimum four puffs and maximum eight puffs per dose).[106,107]
Moderate asthma
For moderate exacerbation of asthma, the following is recommended:
Oxygen mask should be used if oxygen saturation (SaO2) level is below 92%
Albuterol nebulization (0.15 mg/kg, maximum 5 mg) should be used for three doses every 20–30 min
Ipratropium bromide nebulization (250 μg/dose if <20 kg; 500 μg/dose if >20 kg) is to be used every 20–30 min for three doses or continuously
Combination of inhaled anticholinergics and β2-agonist in children 1–18 years of age with mild, moderate, or severe exacerbations of asthma can improve lung function and decrease hospitalization[108,109]
If SaO2 is below 90%, using nebulizer is recommended.
Severe asthma
Oxygen mask should be used if SaO2 level is below 92%
Albuterol nebulization (0.15 mg/kg, maximum 5 mg) should be used for three doses every 20–30 min
Ipratropium bromide nebulization (250 μg/dose if <20 kg; 500 μg/dose if >20 kg) is to be used every 20–30 min for three doses or continuously
Patients who required more doses of salbutamol can have additional doses intermittently every 30–45 min or continuously inhaled salbutamol.
Continuous versus intermittent nebulization
Studies comparing continuous and intermittent nebulized delivery of beta-agonists have shown similar efficacy outcomes as well as safety profiles. We recommend using continuous therapy rather than intermittently nebulized or pMDI therapy for children with moderate or severe exacerbations. It is common to use continuous salbutamol nebulizer in the emergency department for severe asthma because it is effective and safe for children.[110,111]
Compared to intermittent salbutamol nebulizer, continuous salbutamol nebulizer provides the benefit of reduced cost and minimal time to prepare the inhaled medication in emergency situation.[112] A disadvantage of continuous nebulizer is the required special preparation and design in the emergency area to avoid the refilling medication during giving the nebulizer.
Inhaled corticosteroids for asthma management
According to the Global Initiative for Asthma 2018, some studies showed that inhaled glucocorticoids had almost the same effect compared to oral glucocorticoids.[113,114,115] Double-blind, placebo-controlled studies in children suggest that high-dose ICS, given within the 1st h after presentation to the emergency department, reduces the need for hospitalization, while for hospitalized patients, the addition of nebulized budesonide to existing treatment (including oral corticosteroids) has been shown to reduce the length of hospitalization.[116]
For the management of acute severe asthma in children, the Egyptian Scientific Society of Bronchology recommends using nebulized ICS (3 doses of budesonide add on therapy over 1 h via nebulization) within the 1st h of the event. The recommended dose of budesonide is 250 μg for children aged 0–5 years or 500 μg for children aged 6 years or older.[117]
Table 6 shows the Russian Expert Pediatric Consensus on using nebulized ICS in asthma exacerbations.[118]
Table 6.
Russian Expert Pediatric Consensus; place of nebulized inhaled corticosteroid in asthma exacerbations therapy
| Severity of exacerbations | Treatment options* |
|---|---|
| Mild | Budesonide suspension - 0.5 mg 2 times/day for 5-7 days, then lower the dose for 50% |
| Moderate | Budesonide suspension - 0.5 mg 2 times/day until symptom’s resolution |
| Severe** | Budesonide suspension - 1 mg 2 times/day until symptom’s resolution |
The term SABA is short for Short-acting β2-agonist. *Short-acting β2-agonist SABA or SABA + ipratropium bromide, **Systemic corticosteroids. SABA=Short-acting β2-agonist
Recommendations
The main inhaled therapy for acute asthma in the emergency department is inhaled beta-2 agonists as emergency treatment and can be intermittent nebulization, continuous nebulization, or by pMDI with a valve spacer
Both nebulizer and pMDI have similar effects in all pediatric ages with all grades of severity
pMDI had similar effects and may be more superior compared to nebulizer in treating bronchospasm and fewer side effects.
Advantage of nebulizer
Inhaled mediation can be given with oxygen at the same time
Inhaled medication can be given as continues nebulize medication
Salbutamol and ipratropium can be given together.
Advantage of pressurized metered dose inhaler
Equal or superior compared to nebulizer in infants with bronchopulmonary dysplasia. Further, in wheezy infants <1 year and young children with moderate-to-severe asthma[119]
Less side effect (vomiting, tremors, hypoxemia, and tachycardia) compared to nebulizers[120,121]
-
Reduce wheezing more than nebulizer and can reduce admission compared by nebulizer in young children with moderate or severe asthma (30% vs. 60%).[122,123]
- ICS is a treatment option for asthma exacerbations
- For mild asthma exacerbations, the recommended treatment is budesonide inhalation suspension 1.0 mg + SABA to be given every 4–6 h till the symptoms resolve
- For moderate-to-severe asthma exacerbations, ICS 1.0 mg + SABA are to be given every 30 min for a total of three doses.
Croup
Croup or (laryngotracheitis) is a disease of upper airway illness in patients aged 6 months to 3 years. Main presentations are inspiratory stridor, barking cough, and hoarseness. It is mainly caused by parainfluenza virus. Most croup presented to emergency are mild and does not need inpatient medical intervention and is self-limited. Emergency physician should make sure that the patient can be safely discharged. There is no clear treatment for croup, and only supportive therapy for moderate and severe croup is needed. The main treatment goals are to minimize upper airway edema and keep oxygen and hydration. Croup can be graded according to more than one scoring systems including Westley score [Table 7], which considers five component items making up a total score ranging from 0 to 17 points.[124]
Table 7.
Croup scoring system of Westley et al.
| Symptoms | Croup score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Stridor | None | With agitation only | At rest | |||
| Retractions | None | Mild | Moderate | Severe | ||
| Cyanosis | None | Cyanosis with agitation | Cyanosis at rest | |||
| Level of consciousness | Normal (including asleep) | Disorientated | ||||
| Air entry | Normal | Decreased | Markedly decreased | |||
Recommendations
Epinephrine nebulizer should not be used for mild croup (Westley score 0–2), and there is no need for any inhalation therapy
The main inhalation therapy for moderate (Westley score 3–5) and severe croup (Westley score 6–11) croup is epinephrine nebulization as soon as possible with the administration of oral, intramuscular (IM), or IV dexamethasone as appropriate in addition to keeping the child calm
-
Epinephrine nebulizer is recommended for all patients with moderate and severe croup according to the following[124,125,126,127]
- The dose for racemic epinephrine (2.25%) is 0.05 ml/kg per dose (maximum of 0.5 ml) and the dose for L-epinephrine 1:1000 or 1 mg/ml (IV) is 0.5 ml/kg per dose (maximum of 5 ml)
- Add 3 ml of normal saline for dilution
- Use the nebulizer for over 15 min
- Repeated doses can be done within 15 min
- The epinephrine nebulizer cannot be used for more than 2 h due to rebound phenomena where croup could be getting worse
- Consider observation in the ER for 3–4 h after nebulization.
Few studies done on inhaled beclomethasone (on small samples) showed the same improvement in the clinical croup score compared to IM dexamethasone[113]
-
If SaO2 is <92%, oxygen mask should be used
- Humidified air can be comforting for children, but it does not result in any significant improvement in croup score.[128]
Bronchiolitis
Bronchiolitis is one of the lower respiratory system diseases that affect children younger than 2 years, and it is a leading cause of hospital as well as intensive care unit (ICU) admission. It is mainly caused by respiratory syncytial virus (RSV).[129,130] The main treatment option for bronchiolitis is supportive therapy, and there are many conflicts in the management of bronchiolitis in the guidelines and in practice.[131]
Recommendations
- For nonsevere bronchiolitis:
- No routine inhaled therapy is recommended
- The management is mainly supportive in most of the cases
- No pharmacological intervention is needed
- Using bronchodilators or epinephrine nebulizers is not recommended.[114]
- For severe bronchiolitis:
- The supportive care is the mainstay for the treatment of severe bronchiolitis in the emergency department
- Inhaled bronchodilators are not recommended for the routine management of bronchiolitis, and they may cause adverse side effect in addition to the high cost with no clinical benefit[133]
- Inhaled epinephrine is not recommended
Aerosol Therapy in Pediatric Intensive Care Unit
Aerosol inhalation therapy is commonly used in pediatric critical care patients. Unlike in the outpatient setting, delivery of aerosols could be challenging and complex, particularly in ventilated patients.[140]
Successful delivery of aerosolized medications to patients in the pediatric ICU depends upon patient, inhalation device, and ventilation factors. Currently, there are limited data and information about the best evidence practice of aerosol therapy in pediatric critical care; therefore, healthcare provider must have the proper knowledge and skills for using inhalation therapy in critically ill patients, either breathing spontaneously or undergoing invasive or noninvasive ventilation (NIV).[141]
Pediatric patients receiving noninvasive ventilation
Noninvasive respiratory support is frequently used in pediatric critical setting in patients with impending respiratory failure to avoid intubation or to prevent extubation failure. Combining inhaled drug such as bronchodilators and anti-inflammatory drugs with NIV or HFNC to treat different pediatric lung diseases might be even attractive and of advantageous therapeutic benefits.[142,143,144]
In acute asthma, for example, there is an additional beneficial effect of combing the NIV with inhaled bronchodilators, and there is a dose relationship between pressure applied during NIV and bronchodilators response.[145,146,147]
There are several forms of pediatric NIV devices that can be connected with aerosol-generating apparatus to deliver aerosolized drugs via heated and humidified HFNC, nasal CPAP, and noninvasive positive pressure devices (NIV or bi-level positive airway pressure). Many of these devices use single-limb system with complicated gas-flow pathways and integrated leak valves; in addition, there is a concern about system triggering and proper drug delivery. These might require the infant and children to be disconnected from the noninvasive machine to deliver inhaled therapy.[4]
There are several factors that could significantly influence the efficiency of aerosol therapy in patients using NIV and HFNC. These include position of the aerosol in the ventilator and HFNC circuit, type of aerosol, position of leak port in the mask, level of pressure at inspiration and expiration, patient's breathing effort and pattern, cannula size, and flow rates of HFNC.[141,142,143,144,145,146,147,148]
Knowing the best technique to overcome this limitation is quite essential and would result in optimal drug delivery. For example, in NIV, placing the nebulizer after the exhalation leak port and as close as possible to the patient mask results in the greatest drug delivery (away from the ventilator). Further, more aerosol is delivered at higher level of inspiratory pressure support while less aerosol is delivered at higher level of expiratory pressure. The novel lightweight NIVO VMN (Philips Respironics, Murrysville, Pennsylvania, USA) could represent a suitable technology that allows a clinician to provide continuous NIV and medication delivery without disconnecting patient circuits and struggling with in-line nebulizer add-ons. It is easily adjusted and can remain attached when not in use or be easily removed between treatments. If it not feasible to use an NIVO VMN, then a VMN should be placed close to the patient as much as possible and after the exhalation leak valve.[141]
In contrast, HFNC delivery of aerosolized drugs may be effective if the drugs are given using VMN type of nebulizer and at low flow and with bigger cannula size.[149] Little is known however about the efficacy of drug delivery and safety using HFNC. Condensate from drug and humidity could accumulate in the cannula and aspirated into the nasal airway opening. In addition, potential skin and eye irritation from impacted aerosol leaking into the nose and face could also happen during this combined therapy. More clinical studies on aerosol delivery using HFNC are therefore needed before making robust recommendation.[115]
In vitro studies showed greater surge delivery during single-circuit variable-flow CPAP when placed proximal to the patient and even greater when placed before the humidifier in the dual-limb circuit with constant-flow bubble CPAP.[150] It is also advised to use chin straps to provide a better oral seal and prevent any leak in children using nebulizer with CPAP nasal or face mask.[151]
Recommendations
Clinical studies on aerosol delivery during NIV are encouraging and suggest that aerosol therapy can be delivered without discontinuation of NIV
During pediatric NIV, new VMNs can be integrated into full face masks. If these nebulizer devices or masks are unavailable, then a VMN should be placed as close as possible to the patient and after the exhalation leak valve
The best position for an aerosol generator is between the mask and the exhalation leak port. pMDI might be more effective than nebulizer if this port is in the mask
Care must be taken to avoid possible leak into eyes of the child and possible aspiration
Clinical studies on aerosol delivery during HFNC and nasal CPAP remain challenging and are not conclusive to make a recommendation for or against inhalation therapy
For HFNC, available evidence suggests better drug delivery using VMNs device type and at a low flow
For infant nasal CPAP, the administration and the placement of inhaled agents remain a challenge and of unknown efficacy.
Pediatric tracheostomy patients
Though commonly used, studies of using inhaler drugs through a tracheostomy tube in children are quite limited. Previous studies revealed that regardless of the used type of aerosol device, a measurable amount of the aerosol can be delivered through the tracheostomy tube. The proportion of aerosol delivery through the tracheostomy tube varied widely (from 1% to 45%) depending on the size of the tube, using an interface, presence of thick secretion, and oxygen-flow rate.[152,153]
Tracheostomy tubes with inner cannulas are commonly used for both spontaneously breathing and mechanically ventilated child. It is important to know that tracheostomy tube is shorter and more curved than an endotracheal tube (ETT), which could increase the resistance to air flow and increased drug deposition in the artificial airways and tracheobronchial region.[154,155]
A T-piece interface between the tracheostomy tube and the jet nebulizer has been found to be more effective compared to tracheostomy mask alone.[153,154,155] In addition, removal of the inner tracheostomy cannula and turning off the high-flow oxygen device before aerosol therapy in patients with tracheostomy are highly recommended for better delivery, especially for the smaller sized tubes.[152,153,154,155]
Alternatively, pMDI with a VHC can also be combined with a T-piece and manual resuscitation bag to deliver inhaler medication effectively.[153]
Recommendations
For spontaneously breathing patients with a tracheostomy tube, use a T-piece interface with the jet nebulizer without additional gas flow given by the oxygen system
Remove tracheostomy inner cannula before aerosol therapy is highly recommended.
Mechanically ventilated pediatric patients
Although data of using aerosol therapy in mechanically ventilated child are still limited, it is commonly used in critical care settings to deliver different drugs to treat different pediatric lung pathology. The use of this combination, though sound attractive, needs to be carefully guided and regulated.[4]
Inhaled bronchodilators, hypertonic saline, as well as corticosteroids and mucolytics still remain the most widely used inhaled drugs in mechanically ventilated pediatric patients. In small infants, they may improve lung compliance and VT, decrease pulmonary resistance, and enhance extubation, especially in infants with chronic lung disease.[156,157,158,159]
Studies have shown that the effectiveness of aerosol therapy is technique dependent and it is important to know that effective drug delivery to the lung and maximum concentration depend on several practical factors. These include patient, device type, drug, and mechanical factors. Critical care physicians need to be attentive to scientific basis of aerosol therapy, the proper technique of administration, patient's lung mechanics, ventilator model, mode of ventilation, aerosol generators, heating and humidification of the inspired gas, ETT size, position of the aerosol generator in the ventilator circuit, the proper VT, inspiratory flow, and bias-flow setting during the therapy so that they can provide the most effective, consistent, and precise delivery of aerosolized medications.[160,161,162]
Patient factors that could adversely affect drug delivery include supine position, presence of airway obstruction, abnormal airway and impaired mucociliary clearance, and presence of high turbulence. These will result in lower drug deposition in the distal airways and lung parenchyma. Drug delivery factors include size formulation, dose, and frequency applied.[141]
Ventilation and device factors
Successful delivery of aerosolized medications to pediatric critical care patients depends upon the type of the aerosol device, the technique used during therapy, installation position, circuit humidification, and the ventilator settings.[152]
Mechanical ventilation factors including unadjusted ventilatory circuit temperature, small ETT size, or ventilatory asynchrony, unsynchronized-flow pattern, and high respiratory rate could all adversely affect drug delivery. Future studies are needed to determine the optimal: TV, inspiratory time/rate setting combination, circuit size, and bias flow.[141]
Nebulizers and pMDIs, with and without spacers, by far are the two most common types of devices available for use in pediatric mechanically ventilated patients. DPIs are used mainly in stable and nonmechanically ventilated patient as its use in mechanically ventilated is quite variable in efficacy.[163,164]
It is quite challenging to suggest one nebulizer or circuit location with the array of different nebulizers, drugs, ventilators, and patient sizes. In general, pMDIs are easy to administer, less labor time, more reliable dosing, are also more cost-effective than nebulizers, and have minimal risk of bacterial contamination. In addition, the circuit does not need to be disconnected when pMDIs are used with a collapsible spacer. In vitro studies have shown improved aerosol delivery with large spacers compared with small spacers for pMDIs and VMNs.[140,165,166]
Nebulizers in contrast to other devices take longer time to deliver similar dose. In general, built-in nebulization in the newer ventilation offer the most effective way of drug delivery to the lung by synchronizing the nebulizer with the respiratory cycle; among three devices, there is a variation in efficiency between other nebulizer types with mesh nebulizers stand as the most efficient method of drug delivery.[167]
Jet nebulizers into a ventilator circuit may have many drawback and safety concerns related to inadvertent pressure and volume increase, which could affect triggering due to the additional flow. In addition, significant loss could occur during the exhalation phase as the drug is continuously delivered throughout the respiratory cycle. In addition, jet nebulizers require a gas source or a compressor which makes them less portable and more expensive to operate.[4]
This is in contrast with the VMNs which are unique in that it does not require a gas source, making it safe and effective. The high efficiency of this type of nebulizers is based on a very low residual drug volume (0.1 ml) that remains in the medication reservoir following a treatment.[4]
Based on the available evidence and clinical experience, VMNs though costly represent the most efficient and potentially safer drug delivery option than other nebulizer types during pediatric ventilation. VMN should be placed in the inspiratory limb proximal to the patient's Y-piece and not before the humidifier to optimize drug delivery during neonatal/pediatric ventilation compared to adults where a position of 15 cm from the Y-piece in the inspiratory limb of the circuit gives the best drug delivery.[167]
Drug delivery and device selection in high frequency ventilation
Evidence about using inhaled drugs with high-frequency ventilation (HFV) is quite controversial; this is mainly due to a concern that drug delivery to the lower respiratory tract could be negligible due to shorter inspiratory times, high bias flows, and low TV during this type of ventilation.[168] This however could be obviated in vitro model through combination of shorter inspiratory durations, higher frequencies, and active exhalation during this type of ventilation, hence making it less likely for the drug to be delivered to the expiratory limb, resulting in greater delivery to the lung than during conventional ventilation. Studies of in vitro lung models have shown that better drug delivery with a VMN is obtained during High-frequency ventilation (HFOV) compared to conventional neonatal and pediatric mechanical ventilation.[169]
Recommendations
VMN and pMDI/spacer represent two nebulizer devices that are commonly used in mechanically ventilated child due to patient acceptance and ability to integrate into a ventilator system
pMDIs with spacer are more effective than nebulizers in mechanically ventilated patients
For nebulizer, built-in nebulization in newer ventilation offers the most effective way of lung delivery followed by VMN
The best position for nebulizers is close to the ventilator
Clinical studies on aerosol delivery during HFV remain controversial and are not conclusive to make a recommendation for or against inhalation therapy.
Contemporary application and safety of inhalation therapy in pediatric critical care
Use of aerosol therapy is not completely safe and could be potentially harmful. Data about safety and efficacy of aerosol drug delivery using invasive and noninvasive respiratory support are still limited.[141]
Aerosol therapy in NIV and HFNC could result in significant skin and eye irritation from impacted bronchodilators that are running down the nose and face during aerosol treatment with HFNC. There is also risk of aspiration into the airway from the accumulating condensate in the cannula. Aerosol therapy in mechanically ventilated patients have potential general systemic side effects (e.g., nephrotoxicity by aminoglycosides) or local complications such as airway irritation, cough and often bronchospasm, worsening hypoxemia (and secondary arrhythmias), mechanical complications due to ventilator malfunction, and obstruction of expiratory filters. It may also increase the work of breathing and cause ventilator desynchrony requiring additional sedation. Aerosol therapy may also cause blockages in expiratory filters in ventilators and interfere with ventilator sensing. Careful monitoring therefore for the airway pressure and SaO2 is needed during aerosol delivery for all mechanically ventilated patients.[141,170,171,172,173]
Aerosols generated by patients may escape and carry a potential risk of transmission of airborne diseases such as H1N1 and tuberculosis. This necessitates using some form of high-efficiency particulate air filter in the expiratory limb of the ventilator circuit to reduce the escape of aerosols generated by the patient or an aerosol device to minimize the infection risk to both patient and healthcare provider.[171]
There are also concerns about hemodynamic instability and respiratory deterioration during ventilator interruption for inhaled drug administration. Frequent discontinuation in the circuit may increase the risk for colonization and hence ventilator-associated pneumonia. Furthermore, drug administration via manual bagging could result in advertent excessive VT and iatrogenic lung injury, especially in neonates and small infants. Short-term delivery has been shown to be efficient with in-line spacers. Caution should be exerted however if visible condensate occurs which could significantly reduce drug delivery by 50%.[174]
Standard jet nebulizers could result in poor triggering, inaccurate volume monitoring and have many concerns related to volume and pressure delivery in infant ventilation.
Recommendations
Clinicians should use a device that delivers inhalation drugs safely and efficiently while minimizing potential harm that can develop due to infection, airway occlusion, and inadvertent airway pressure changes with the resultant hypoxia, equipment malfunction, and poor triggering of different ventilator devices
In-line spacers placed into the inspiratory limb of a ventilator can be used for pMDI administration to reduce the risk of circuit interruption
Standard jet nebulizers should be avoided in infant ventilation due to poor triggering.
Aerosol Therapy in In-Patient Settings
pMDIs with or without spacer are as effective as or superior to nebulizer method in aerosol delivery.[175]
In general, nebulizers are more expensive, cumbersome to use, and need outside electric or battery power. pMDI is a convenient device to use for quick relief of acute airway obstruction, but there can be problems of coordination between actuation and inhalation, particularly in small children who may not comprehend the instructions or whose hand-inspiration coordination may not yet be adequately developed.[176] Attachment of VHC with a one-way valve system to a pressurized aerosol device has been shown to increase the deposition of aerosol particles in the lungs and decrease upper airway aerosol deposition, as compared to the unaided use of an pMDI.[177]
Face masks should be used with nebulizers and VHC for infants and young children. Bronchodilator responses are the same whether masks or mouthpieces are used. The choice should therefore depend upon convenience – for example, masks are better for emergencies –and patient preference. Face masks should be tight-fitting. Patients should breathe with an open mouth. For ipratropium bromide, mouthpieces are preferred to masks if there is a possibility of glaucoma. For antibiotics, rhDNase, and corticosteroids, mouthpieces should be used.[178]
The selection of aerosol delivery system for hospitalized children can have a significant impact on the utilization of healthcare resources. The duration of treatment preparation and delivery was shown to be significantly lower with the pMDI-VHC than the nebulizer (2 min reduction in preparation time and 5 min reduction in delivery time).[179]
Caregivers mastered pMDI-VHC use after an average of two observed sessions, eliminating the need for respiratory therapy assistance during the hospital stay. Medication cost analysis showed savings in favor of pMDI-VHC. It has been shown in many studies that conversion to MDI-VHC mode of aerosolized therapy administration in hospitalized children can improve hospital resource utilization.[179]
Asthma
In children with stable asthma, equivalent percentages of total lung deposition of radiolabeled salbutamol aerosolized by either a nebulizer or pMDI-VHC. However, the delivery rate and the total dose of salbutamol deposited were significantly higher with the nebulizer.[180]
Many reports have suggested that there is little difference between the pMDI and nebulizer methods of treatment in asthmatic patients. Most of these reports measured only the forced vital capacity and FEV1 or the dyspnea score.[120,181,182,183] All the studies used oxygen as the driving gas in nebulizer treatment, and therefore, the SaO2 between the two methods was not comparable as no oxygen was supplied in the pMDI treatment. However, oxygen is not routinely given in nebulizer treatment of acute asthmatics in many pediatric emergency departments, even for those with severe asthma.[184] There have been some reports describing the occurrence of hypoxemia or oxygen desaturation after inhalation of selective β2-adrenergic agonists and perhaps more likely with the nebulized method as compared to pMDI.[185] Therefore, it is important that SaO2 is measured during their treatment and that oxygen should, if possible, be chosen as the driving gas in treating severe asthmatic patients given nebulizer treatment.[120]
In the setting of acute asthma management in children, both pMDI-VHC and nebulizer treatments were shown to be effective in treating clinical severity and airway obstruction in children with acute asthma, but the pMDI method was better than the nebulizer method, particularly for the improvement of the SaO2 and in some measurements of spirometry.[120]
Cystic fibrosis
In patients with CF, many therapies (including bronchodilators, corticosteroids, antibiotics, and mucolytics) can be administered using the inhalational technique. The advantages of inhaled medications include that their use can generate higher drug levels in the airways with limited systemic effect. They are relatively fast acting and their particle size enables them to be directed at the target site with optimal deposition.[186]
Although traditional jet nebulizers are the ones available in our region, the use of “intelligent nebulizers” such as the eFlow (PARI, Germany) and Ineb (Profile Pharma, Zambon SpA, Chichester, UK) both of which use the vibrating mesh technology (VMT) are more commonly used in the west. These are smaller, quicker devices that give improved deposition of many medications, reduce time of administration, and ultimately impact on reducing the burden of care in both inpatient and outpatient settings.[186]
Although nebulized antibiotics have been available for >30 years, the dry powder formulations can offer simple, fast, and convenient delivery of inhaled antibiotics, with similar efficacy to nebulized formulations. In addition, DPI requires minimal cleaning compared with a nebulizer system, which is also time-consuming and often not performed to recommended manufacturer's guidance.[186]
Mucolytic and mucoactive agents such as rhDNase and/or hypertonic saline are given using the nebulized method regularly in the outpatient setting, with increased frequency requirement in the inpatient setting during CF exacerbations. The advent of inhaled mannitol provided as a dry powder inhaler has demonstrated clinical efficacy and short duration of administration.[187]
Croup
In croup, the use of nebulized racemic epinephrine is typically reserved for patients in the hospital setting (in ER and inpatient) with moderate-to-severe respiratory distress. Nebulized epinephrine is generally used to avoid intubation, to stabilize children before transfer to intensive care, and to treat stridor following extubation. Its effectiveness is immediate with evidence of therapeutic benefit within the first 30 min and then a lasting effect for up to 2 h. Patients who receive nebulized racemic epinephrine in the ERs must be monitored for at least 3 h after last treatment because of concerns for a return of bronchospasm, worsening respiratory distress, or persistent tachycardia. Nebulized steroids may also reduce symptoms in croup in the first 2 h, but no data are available on long-term use effect on the eventual outcome. Mixture of Heliox is delivered via nasal cannula, face mask, or hood. Extremely limited studies discussed the effectiveness and safety of Heliox. The Cochrane database concluded that its safety and efficacy remain unclear. They stated that for children with mild croup, Heliox is not superior to 30% humidified oxygen. However, for children with moderate-to-severe croup who were treated with dexamethasone, it may be beneficial for short-term use. Further, its effect could be similar to 100% oxygen given with 1–2 doses of epinephrine.[188]
Bronchiolitis
In infants with bronchiolitis, bronchodilator therapy should not be used routinely, and there is a strong statement by the recent American Academy of Pediatrics Updates Guidelines on Bronchiolitis for not using salbutamol or adrenaline in bronchiolitis.[134] However, they are still widely used by clinicians for treating hospitalized patients. On a case-by-case basis, a trial of bronchodilators may be performed if there is a documented positive response from the treating team, or if a scoring system is used, a clear improvement is documented.[135]
There is growing evidence supporting the use of nebulized hypertonic saline in bronchiolitis. It has been shown to improve mucociliary function and reduce the length of hospitalization and illness severity. Due to its high safety profile and low cost, it looks reasonable to use hypertonic saline in the management of bronchiolitis.[134]
Inhaled steroids are not routinely recommended in previously healthy infants with acute bronchiolitis. They have no clinical benefit in reducing neither the rate of admission nor length of hospitalization. In addition, inhaled steroids that are prescribed in the acute phase are not beneficial in reducing the rate of postviral wheeze.[130,189,190,191]
Heliox could improve the severity score among infants with acute RSV bronchiolitis, especially if given during the 1st h after commencing inhalation therapy. It may be used as an adjunctive therapy in critically ill children who have RSV bronchiolitis.[192]
Nebulized ribavirin should not be used routinely but might be considered in infants with severe disease and in specific patients affected by severe immunodeficiency syndromes, or severe chronic lung disease, or hemodynamically significant congenital heart disease.[193] Its administration is by a small particle aerosol generator machine. However, ribavirin has not been shown to reduce the length of hospital stay or the need for oxygen or assisted ventilation.[194]
Recommendations
pMDI-VHC is superior to nebulizer method in aerosol delivery in terms of convenience, cost, and quicker relief of acute airway obstruction. Nevertheless, it is not suitable in younger children or in those without adequately developed hand inspiration coordination
For infants and young children, face masks should be used with nebulizers and spacers
Bronchodilator responses are the same whether masks or mouthpieces are used. For ipratropium bromide, mouthpiece is preferable than masks if there is a possibility of glaucoma. For antibiotics, rhDNase, and corticosteroids, mouthpieces should be used
pMDI-VHC has a significant lower duration of treatment preparation and delivery than the nebulizer
In hospitalized children, conversion to pMDI-VHC mode of aerosolized therapy administration can improve hospital resource utilization.
Asthma
In children with stable and acute asthma, the nebulizer or pMDI-VHC has equivalent percentages of total lung deposition of radiolabeled aerosolized salbutamol, and both treatments were found to be effective in treating clinical severity and airway obstruction. Yet, with the nebulizer, the delivery rate and the total dose of salbutamol deposited were substantially higher
SaO2 and oxygen should be measured during treatment, if possible, as there have been some reports of hypoxemia or oxygen desaturation occurrence after inhalation of selective β2-adrenergic agonists more likely with the nebulized method as compared to pMDI.
Cystic fibrosis
In patients with CF, aerosol therapies such as bronchodilators, corticosteroids, antibiotics, and mucolytics are administered. Mucolytic and mucoactive agents such as rhDNase and/or hypertonic saline are given more frequently in the inpatient setting during CF exacerbations
The intelligent nebulizers which use the VMT are smaller, quicker devices that give improved deposition of many medications, reduce time of administration, and ultimately impact on reducing the burden of care in both inpatient and outpatient settings than the traditional jet nebulizers
DPI offer simple, fast, and convenient delivery of aerosol antibiotics than nebulized formulations. In addition, it requires minimal cleaning.
Croup
In croup, the use of nebulized racemic epinephrine is typically reserved for patients in the hospital setting with moderate-to-severe respiratory distress
Nebulized steroids, in the first 2 h, may reduce symptoms in croup. For long-term outcome, no data are available
In very severe croup that failed to improve with racemic epinephrine, Heliox has been used and shown to improve symptoms but for a short term.
Bronchiolitis
Bronchodilator and adrenaline therapy should not be used routinely in bronchiolitis
On a case-by-case basis, a trial of bronchodilators may be performed if there is a documented positive response from the treating team if a scoring system is used and/or a clear improvement is documented
Nebulized hypertonic saline has been found to improve mucociliary function and reduce the length of hospitalization stay and illness severity
Inhaled steroids should not routinely be used in previously healthy infants with acute bronchiolitis
Heliox may be used as adjunctive therapy in critically ill children with RSV bronchiolitis
Nebulized ribavirin is not routinely recommended, but it could be considered in infants with severe disease and in specific patients affected by severe immunodeficiency syndromes, or severe chronic lung disease, or hemodynamically significant congenital heart disease.
Aerosol Therapy at Home: Education and Cleaning
Educating patients in correct use of aerosol devices
Several problems could occur when patients use aerosol devices. The knowledge of these problems could help healthcare providers to better instruct their patients about the appropriate methods used. In addition, recognition of these problems can direct healthcare providers when evaluating a patient with poor management of his/her airway disease. Both of poor adherences to the prescribed therapy or incorrect use of the aerosol devices may be behind the reduced effectiveness of the therapy.[25]
These two problems should be considered and well evaluated for each patient before thinking about changing his/her treatment.
Patient adherence
There are several ways to monitor the patient's adherence to prescribed aerosol therapy including patient self-report and dose counting. Failure of adherence to the prescribed therapy is classified as “unintentional” or “intentional.”[195]
Unintentional nonadherence may result from misunderstanding of the prescribed regimen that can result from poor communication between the patient and healthcare provider. Intentional nonadherence may be a result of patient's beliefs (e.g., I am not sick, I don't require regular medication, the drug is expensive, the drug has side effects,… etc.), forgetfulness, stress and busy life, complex regimen, or psychological factors (e.g., depression).[25]
Patient-centered care can help a healthcare provider to understand the patient's beliefs and concerns about their treatment that may be the cause of poor adherence. Motivational tools such as goal setting and action plan can be helpful in improving patient's adherence. They should be discussed with the patient in a bi-directional conversation.[196]
Common patient errors with pressurized metered dose inhalers
Errors encountered with the use of pMDIs include the lack of hand-breath coordination, improper inhalation, not holding breath after inhalation, and failure to shake the pMDI before use.[197,196]
Patients should be instructed to track the number of drug doses remaining in the inhaler as a device may still produce a spray of propellant with little or no drug when it is actuated for a number of times more than its rated capacity.[196] Inhalers without a counter are associated with significantly lower adherence rates (underuse and overuse) compared to those with a counter.[198]
Common patient errors with holding chambers/spacers
The most commonly encountered errors associated with using VHC and spacers include incorrect assembly of the add-on device, inability to remove electrostatic charges in nonelectrostatic holding chambers or spacers, delay between actuation and inhalation, too rapid inhalation, firing several puffs into the holding chamber or spacer before inhalation, and lack of patient's instructions on assembly or use. The ideal technique to use an inhaler with a VHC or a spacer is to place the mouthpiece between lips than to take a slow and deep inhalation that begins with pMDI actuation[25,54,199]
Common patient errors with dry powder inhalers
Common errors reported among patients using DPIs include the inability to hold the device correctly while loading the dose, inability to pierce or open the drug package, using the inhaler device in a wrong orientation, failure to prime, lack of adequate exhalation before inhalation, exhalation through the mouthpiece, inability to inhale with enough force, inadequate breath hold, exhalation into the mouthpiece after inhalation, using a multidose reservoir in high humidityas this may reduce the fine particle dose and finally lack of patient instructions in assembly or use.[25]
Common patient errors with small-volume nebulizers
The common errors encountered with SVNs are not related to the patient's use, but they are general disadvantages of these devices such as bulk and size of the equipment, the need for an external power source, and long-treatment durations.[200]
Nebulizers are the simplest devices for patients to use. Moreover, newer nebulizers' technology aims at reducing the overall devices' size, allowing shorter-treatment durations, eliminating the need for external power sources, as well as eliminating the drug loss during exhalation.[25]
Instructing and evaluating the patient in the use of inhaler devices
To ensure the correct patient use of inhalation devices, the following steps are recommended:
Review the device-specific instructions carefully, then practice using a placebo device before teaching your patients
Clearly demonstrate the correct methods of use and assembly of the device to your patients using a checklist
Make sure to provide your patient with written instructions about using the device including a plan to use the medication. Instructions should include illustrating pictures for patients who have low literacy
Observe your patient while practicing using the device for the first time and during each return visit
Revise the patient's understanding of using the inhaled medications during each return visit. This should include when to use the drug, purpose of using the drug, and the prescribed frequency
Make sure to assess the patient's adherence level and his/her ability to correctly use the device if airway disease is poorly managed.
Infection control
Aerosol generators may become contaminated with pathogens from several sources such as the patient himself/herself, the care provider, or the environment. Bacterial colonization of the respiratory tract can occur if infection control (IC) measures of the aerosol device are not adequately followed.[201,202] Accordingly, it is crucial to establish an IC management system to reduce the rate of nosocomial infections and reduce the length of stay in hospitals and associated costs.[202,203]
Cleaning and maintenance of aerosol generators
Cleaning
The cleaning instructions specific for each type of aerosol generators are shown below.
The plastic container of pMDIs must be cleaned once per week at least as shown in Table 8[25,204]
If VHC will be used with a pMDI, it has to be cleaned before the first use and periodically cleaned as per the manufacturer's instructions. Table 9 provides the instructions for cleaning the accessory devices of the pMDI[25]
DPIs should never be submerged in water. In addition, they should be kept dry because moisture can hinder drug delivery. There are no clear instructions regarding DPI cleaning. However, each manufacturer has specific recommendations to be followed for the regular cleaning of the DPI device
At home, it is important to clean nebulizers after every treatment.
Table 8.
Cleaning instructions for the pressurized metered dose inhale
| Clean one time per week and when required. Look at the hole where the drug sprays out from the inhaler |
| If you see any powder in the hole from which the drug is sprayed out or around it, the device should be cleaned |
| Remove the canister of the pMDI from the plastic container to keep it dry |
| The plastic container should be rinsed with warm water then shaken out to remove any excess water |
| Let the device dry overnight |
| Put the canister again inside the mouthpiece then recap the mouthpiece |
pMDI=Pressurized metered dose inhaler
Table 9.
Cleaning instructions for the pressurized metered dose inhaler chamber
| The chamber device should be cleaned every 2 weeks and when needed |
| The device should be disassembled for cleaning |
| The spacer parts are soaked in warm water containing a liquid detergent. Both pieces should then be gently shaken back and forth |
| Excess water is then removed by shaking the parts out |
| Spacer parts should be air dried overnight in a vertical position |
| It is not allowed to dry the spacer using a towel because this will produce static charges and hinder dose delivery |
| When the spacer becomes dry, reassemble the parts |
As per the CF Foundation guidelines,[205] parts of the aerosol generators should be washed using soap and hot water after each use. Cleaning instructions of jet nebulizers are shown in Table 10,[25] while VMNs and USNs should be cleaned and disinfected according to the manufacturer's instructions. Touching the mesh of mesh nebulizers during its cleaning can damage the unit.
Table 10.
Cleaning instructions for the jet nebulizer
| Cleaning instructions after each use | Cleaning instructions once or twice per week |
|---|---|
| Wash your hands before handling any equipment | Wash your hands before handling any equipment |
| Disassemble the parts after every use | Disassemble the parts after every use |
| The tubing should be removed from the compressor and set aside | The tubing should be removed from the compressor and set aside |
| The tubing should never be washed or rinsed | The tubing should never be washed or rinsed |
| The nebulizer cup and the mouthpiece should be rinsed using sterile or distilled water | Nebulizer parts should be washed in warm water containing liquid dish soap |
| Excess water should be shaken off | The nebulizer should be disinfected according to the manufacturer’s instructions. Parts of the nebulizers could be soaked in one of the following solutions |
| One-part household bleach and 50-part water (3 min) | |
| 70% isopropyl alcohol (5 min) | |
| 3% hydrogen peroxide (30 min) | |
| One-part distilled white vinegar in three-part hot water (1 h). This is not recommended for CF patients | |
| Washed parts should be left to dry in air on an absorbent towel | Nebulizer’s parts should then be rinsed with sterile water or distilled water |
| The nebulizer cup should be stored in a zippered plastic bag | Excess water should be shaken off. The washed parts are placed on a clean paper towel |
| The parts should be left to dry in air on an absorbent towel | |
| The nebulizer should then be reassembled and stored in a clean and dry bag container |
CF: Cystic fibrosis
Disinfection
It is highly recommended to periodically disinfect and replace the nebulizers to minimize any possible contamination. It is important to follow the disinfection methods suggested by the manufacturers. It is important to discard all used solutions after the disinfection process. Disinfection includes heat methods and cold methods. The nebulizer should be disinfected once or twice per week.[25]
Preventing infection and malfunction of aerosol generators at hospitals or clinics
Aerosol generators
Bacterial contamination of the nebulizers can cause nosocomial infections.[196] The Centers for Disease Control and Prevention recommends that nebulizers should be cleaned, rinsed using sterile water, and left to dry in air between treatments.[206] An infection surveillance program should be present in every hospital or clinic to determine the required IC practices, according to the local infection data. It is important to change nebulizers every 24 h.[207,208] Aerosol generators designed “For Single Patient Use” should only be used for a single patient and discarded.[25]
Pressurized metered dose inhaler-common canister use
There is weak evidence that the common canister protocol practiced in some hospitals can lead to cross-contamination.[209] In contrast, a recent study revealed that common canister protocol resulted in significant cost savings with similar rates of ventilator-associated pneumonia, mortality, as well as hospital length of stay, compared to single-patient pMDI.[210] Accordingly, each hospital should evaluate the risk–benefit ratio before following a common canister protocol.[25]
Inhaled drugs
Nebulizers could get contaminated when multidose drug container is used which can be a source of nosocomial infection.[211] Therefore, it is recommended to use unit-dose medications when possible.[206]
Infection transmission
Infections could be transmitted from healthcare providers to their patients, and this could be minimized with proper hand washing using water and soap or using hand sanitizers before and after each treatment.[212] Using gloves and changing them between patients should be considered as a supportive measure to hand hygiene. Hands must be cleaned after removing gloves because they create a warm and moist environment which supports microbial growth and contamination.[213,214,215]
Infection control (IC) management system
It is important for each healthcare setting to establish an IC management system and ensure that the healthcare team is fully knowledgeable about its effective implementation. This could be achieved by the continuous training and monitoring of the IC management system implementation.[25]
Occupational health and safety of respiratory therapists
In addition to the risks of exposure to inhaled medications during aerosol therapy, respiratory therapists may inhale pathogens.
Health assessment and immunization
Respiratory therapists should be screened for infection and must be adequately immunized during the whole period of employment.
Hand hygiene
Hand hygiene is a highly effective measure in minimizing the transmission of airway viruses as inadequate hand hygiene is considered to be the leading cause of nosocomial infections.[216]
Protective equipment
Using goggles, face shields, and face masks alone or in combination can protect against airborne pathogen inhalation.[25] Ventilation systems are effective in removing 99.9% of the airborne contaminants for 69 min.[217] When a filter is placed on the nebulizer's exhalation part, it could protect therapists from infection.[25]
Recommendations
Infection control
IC management system must be established for aerosol generators to reduce the rate of acquired infections and accordingly the length of stay in hospitals and associated costs.
Cleaning and disinfection of aerosol
The cleaning and disinfection of aerosol is crucial in its routine use. Periodically, cleaning, disinfecting, and replacing the nebulizers are highly recommended to minimize any possible contamination by following the manufacturers' disinfection methods
A feasible general cleaning and disinfection method is applicable for all common aerosol's generators. Cleaning of nebulizers with soap and hot water is recommended after every use. pMDI container is cleaned once weekly. There is no clear method for DPI cleaning, although it should be kept dry. For home use of nebulizers, a weekly disinfection is advised.
Preventing infection and malfunction of aerosol generators at hospitals or clinics
For hospital use, nebulizers should be changed daily
For pMDI, risk–benefit ratio has to be evaluated considering cost saving and cross-contamination while using common canister protocol
In inhaled dugs, unit-dose medications are recommended if possible
Washing hands with hot and soap or sanitizers before and after treatment, and after removing gloves, is fundamental in minimizing infection transmission from healthcare professional and their patients
Continuous training of the caregivers and monitoring of the hospital IC management system implementation is considered.
Occupational health and safety of respiratory therapists
Respiratory therapists, as their patients, are at risk of infection transmission. Accordingly, they have to be screened and immunized for the risk of inhaled pathogens. Frequent hand hygiene is essential. Wearing protective personnel equipment is advised. Maintaining good ventilation can protect from infection.
Educating patients in correct use of aerosol devices
Poor adherence or inappropriate use of the aerosol devices is one of the reasons for reduced therapy effectiveness. Raising the awareness of the aerosol devices use problems may help the healthcare provider to better instruct their patients about the appropriate methods of use
Self-report and dose counting can help in monitoring patient's adherence to prescribed therapy
Motivational tools can be helpful in improving patient's adherence
Patient-centered care can help healthcare providers understand the patient's beliefs and concerns about their treatment that may be the cause of poor adherence
Patients should be instructed on the common patient errors with aerosol's generators to avoid them
Patients should be instructed clearly about correct use of inhalation devices and practice it for the first time and during each return visit in front of healthcare providers. In addition, patients should be provided with written instructions about using the device with illustrating pictures for low-literacy patients. Additionally, patients' adherence level should be assessed during each visit.
Financial support and sponsorship
This manuscript received financial and logistic support from the Saudi Thoracic Society.
Conflicts of interest
The committee is fully sponsored by the Saudi Thoracic Society.
Acknowledgment
Authors would like to thank the Saudi Thoracic Society for the support for this manuscript.
References
- 1.Mulholland K. Global burden of acute respiratory infections in children: Implications for interventions. Pediatr Pulmonol. 2003;36:469–74. doi: 10.1002/ppul.10344. [DOI] [PubMed] [Google Scholar]
- 2.Alsubaiei ME, Cafarella PA, Frith PA, McEvoy RD, Effing TW. Factors influencing management of chronic respiratory diseases in general and chronic obstructive pulmonary disease in particular in Saudi Arabia: An overview. Ann Thorac Med. 2018;13:144–149. doi: 10.4103/atm.ATM_293_17. doi: 10.4103/atm.ATM_293_17. PMID: 30123332; PMCID: PMC6073786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Frayh AR, Shakoor Z, Gad El Rab MO, Hasnain SM. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001;86:292–6. doi: 10.1016/s1081-1206(10)63301-7. [DOI] [PubMed] [Google Scholar]
- 4.DiBlasi RM. Clinical controversies in aerosol therapy for infants and children. Respir Care. 2015;60:894–914. doi: 10.4187/respcare.04137. [DOI] [PubMed] [Google Scholar]
- 5.The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes. JAMA: The Journal of the American Medical Association. 1931;96:1534. [Google Scholar]
- 6.Heydari M, Hashempur MH, Zargaran A. Medicinal aspects of opium as described in Avicenna's Canon of Medicine. Acta Med Hist Adriat. 2013;11:101–12. [PubMed] [Google Scholar]
- 7.Sanders M. Inhalation therapy: An historical review. Prim Care Respir J. 2007;16:71–81. doi: 10.3132/pcrj.2007.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson PJ. History of aerosol therapy: Liquid nebulization to MDIs to DPIs. Respir Care. 2005;50:1139–50. [PubMed] [Google Scholar]
- 9.Shehata M. History of inhalation therapy. Internet J Health. 2008;9:1–9. [Google Scholar]
- 10.Lee MR. The history of Ephedra (Ma-Huang) J R Coll Physicians Edinb. 2011;41:78–84. doi: 10.4997/JRCPE.2011.116. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan R. Historical aspects of bronchial asthma. Indian J Chest Dis. 1964;6:165–9. [Google Scholar]
- 12.Brenner BE. Where have we been? The history of acute asthma. In: Brenner BE, editor. Emergency Asthma. New York: Marcel Dekker, Inc; 1999. pp. 1–32. [Google Scholar]
- 13.Gandevia B. Historical review of the use of parasympatholytic agents in the treatment of respiratory disorders. Postgrad Med J. 1975;51:13–20. [PubMed] [Google Scholar]
- 14.Mudge J. A Radical and Expeditious Cure for a Recent Catarrhous Cough. 2nd ed. London: E Allen; 1778. pp. 131–47. [Google Scholar]
- 15.Warren I. Boston: Higgins, Bradley, and Dayton; 1989. The Household Physician: For the Use of Families, Planters, Seamen, and Travellers. [Google Scholar]
- 16.Bleyer JM. A new method of laryngeal and bronchial medication by means of a spray and tube during the act of deep inspiration: Read in the Section of Laryngology and Otology at the Forty-First Annual Meeting of the American Medical Association, Nashville, Tenn, May, 1890. J Am Med Assoc. 1890;15:634–6. [Google Scholar]
- 17.Wyeth J. Philadelphia, PA: John Wyeth & Brother, Inc; 1901. An Epitome of Therapeutics with Special Reference to the Laboratory Products of John Wyeth and Brother; pp. 251–2. [Google Scholar]
- 18.Nikander K, Sanders M. The early evolution of nebulizers. Medicamundi. 2010;54:47–53. [Google Scholar]
- 19.Abramson HA. Principles and, practice of aerosol therapy of the lungs and bronchi. Ann Allergy. 1946;4:440–56. [PubMed] [Google Scholar]
- 20.Rau JL. The inhalation of drugs: Advantages and problems. Respir Care. 2005;50:367–82. [PubMed] [Google Scholar]
- 21.Kacmarek R, Stoller J, Heuer A. 12th ed. Elsevier Health Sciences; 2020. Egan's Fundamentals of Respiratory Care [Internet] Available from: https://www.elsevier.com/books/egansfundamentals-of-respiratory-care/kacmarek/978-0-323-51112-4 . Last cited on 2021 Mar 24. [Google Scholar]
- 22.Gardenhire D. Rau's Respiratory Care Pharmacology E-book [Internet] 9th ed. Elsevier Health Sciences; 2015. Available from: https://www.elsevier.com/books/raus-respiratory-carepharmacology/unknown/978-0-323-29968-8 . Last cited on 2021 Mar 24. [Google Scholar]
- 23.Mosby's Respiratory Care Equipment E-book [Internet] 10th ed. Elsevier Health Sciences; 2017. Available from: https://www.elsevier.com/books/mosbys-respiratory-care-equipment/cairo/978-0-323-41636-8 . Last cited on 2021 Mar 24. [Google Scholar]
- 24.Nerbrink O, Dahlbäck M, Hansson HC. Why do medical nebulizers differ in their output and particle size characteristics. J Aerosol Med. 1994;7:259–76. doi: 10.1089/jam.1994.7.259. [DOI] [PubMed] [Google Scholar]
- 25.Gardenhire DS, Burnett D, Strickland S, Myers TR. A Guide to Aerosol Delivery Devices for Respiratory Therapists. 4th ed. Irving, Texas: American Association for Respiratory Care; 2017. [Google Scholar]
- 26.Goode ML, Fink JB, Dhand R, Tobin MJ. Improvement in aerosol delivery with helium-oxygen mixtures during mechanical ventilation. Am J Respir Crit Care Med. 2001;163:109–14. doi: 10.1164/ajrccm.163.1.2003025. [DOI] [PubMed] [Google Scholar]
- 27.Phipps PR, Gonda I. Droplets produced by medical nebulizers Some factors affecting their size and solute concentration. Chest. 1990;97:1327–32. doi: 10.1378/chest.97.6.1327. [DOI] [PubMed] [Google Scholar]
- 28.De Benedictis FM, Selvaggio D. Use of inhaler devices in pediatric asthma. Paediatr Drugs. 2003;5:629–38. doi: 10.2165/00148581-200305090-00005. [DOI] [PubMed] [Google Scholar]
- 29.Yeo LY, Friend JR, McIntosh MP, Meeusen EN, Morton DA. Ultrasonic nebulization platforms for pulmonary drug delivery. Expert Opin Drug Deliv. 2010;7:663–79. doi: 10.1517/17425247.2010.485608. [DOI] [PubMed] [Google Scholar]
- 30.Thomas SH, O’Doherty MJ, Page CJ, Treacher DF, Nunan TO. Delivery of ultrasonic nebulized aerosols to a lung model during mechanical ventilation. Am Rev Respir Dis. 1993;148:872–7. doi: 10.1164/ajrccm/148.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 31.Michotte JB, Jossen E, Roeseler J, Liistro G, Reychler G. In vitro comparison of five nebulizers during noninvasive ventilation: Analysis of inhaled and lost doses. J Aerosol Med Pulm Drug Deliv. 2014;27:430–40. doi: 10.1089/jamp.2013.1070. [DOI] [PubMed] [Google Scholar]
- 32.Dolovich MB, Dhand R. Aerosol drug delivery: Developments in device design and clinical use. Lancet. 2011;377:1032–45. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 33.Stuart BO. Deposition of inhaled aerosols. Arch Intern Med. 1973;131:60–73. [PubMed] [Google Scholar]
- 34.Newman SP, Agnew JE, Pavia D, Clarke SW. Inhaled aerosols: Lung deposition and clinical applications. Clin Phys Physiol Meas. 1982;3:1–20. doi: 10.1088/0143-0815/3/1/001. [DOI] [PubMed] [Google Scholar]
- 35.Bisgaard H, O’Callaghan C, Smaldone GC. New York: Marcel Dekker; 2002. Drug Delivery to the Lung; p. 162. [Google Scholar]
- 36.Mandal TK. Inhaled insulin for diabetes mellitus. Am J Health Syst Pharm. 2005;62:1359–64. doi: 10.2146/ajhp040249. [DOI] [PubMed] [Google Scholar]
- 37.Chrystyn H. Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol. 2001;51:289–99. doi: 10.1046/j.1365-2125.2001.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strong P, Ito K, Murray J, Rapeport G. Current approaches to the discovery of novel inhaled medicines. Drug Discov Today. 2018;23:1705–17. doi: 10.1016/j.drudis.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell EJ. Review of the unique properties of budesonide. Clin Ther. 2003;25(Suppl C):C42–60. doi: 10.1016/s0149-2918(03)80305-3. [DOI] [PubMed] [Google Scholar]
- 40.Borgström L, Derom E, Ståhl E, Wåhlin-Boll E, Pauwels R. The inhalation device influences lung deposition and bronchodilating effect of terbutaline. Am J Respir Crit Care Med. 1996;153:1636–40. doi: 10.1164/ajrccm.153.5.8630614. [DOI] [PubMed] [Google Scholar]
- 41.Thorsson L, Edsbäcker S, Conradson TB. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 42.Derendorf H, Hochhaus G, Möllmann H. Evaluation of pulmonary absorption using pharmacokinetic methods. J Aerosol Med. 2001;14:9–17. doi: 10.1089/08942680150506295. [DOI] [PubMed] [Google Scholar]
- 43.Van Oort M. In vitro testing of dry powder inhalers. Aerosol Sci Technol. 1995;22:364–73. [Google Scholar]
- 44.Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care. 2005;50:1313–21. [PubMed] [Google Scholar]
- 45.Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50:1177–90. [PubMed] [Google Scholar]
- 46.Mundy L, Sellvaraj U, Victoria N, Jotsingani N. Coping with Asthma. [Last accessed on 2020 Oct 04]. Available from: http://asthmanagement.weebly.com/pmdi.html .
- 47.Newman S, Pitcairn G, Steed K, Harrison A, Nagel J. Deposition of fenoterol from pressurized metered dose inhalers containing hydrofluoroalkanes. J Allergy Clin Immunol. 1999;104:S253-7. doi: 10.1016/s0091-6749(99)70042-4. [DOI] [PubMed] [Google Scholar]
- 48.Newman SP, Woodman G, Clarke SW, Sackner MA. Effect of InspirEase on the deposition of metered-dose aerosols in the human respiratory tract. Chest. 1986;89:551–6. doi: 10.1378/chest.89.4.551. [DOI] [PubMed] [Google Scholar]
- 49.Newman SP, Newhouse MT. Effect of add-on devices for aerosol drug delivery: Deposition studies and clinical aspects. J Aerosol Med. 1996;9:55–70. doi: 10.1089/jam.1996.9.55. [DOI] [PubMed] [Google Scholar]
- 50.Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. Am Rev Respir Dis. 1984;129:723–9. doi: 10.1164/arrd.1984.129.5.723. [DOI] [PubMed] [Google Scholar]
- 51.Kim CS, Eldridge MA, Sackner MA. Oropharyngeal deposition and delivery aspects of metered-dose inhaler aerosols. Am Rev Respir Dis. 1987;135:157–64. doi: 10.1164/arrd.1987.135.1.157. [DOI] [PubMed] [Google Scholar]
- 52.Wildhaber JH, Devadason SG, Hayden MJ, James R, Dufty AP, Fox RA, et al. Electrostatic charge on a plastic spacer device influences the delivery of salbutamol. Eur Respir J. 1996;9:1943–6. doi: 10.1183/09031936.96.09091943. [DOI] [PubMed] [Google Scholar]
- 53.Piérart F, Wildhaber JH, Vrancken I, Devadason SG, Le Souëf PN. Washing plastic spacers in household detergent reduces electrostatic charge and greatly improves delivery. Eur Respir J. 1999;13:673–8. doi: 10.1183/09031936.99.13367399. [DOI] [PubMed] [Google Scholar]
- 54.Wildhaber JH, Devadason SG, Eber E, Hayden MJ, Everard ML, Summers QA, et al. Effect of electrostatic charge, flow, delay and multiple actuations on the in vitro delivery of salbutamol from different small volume spacers for infants. Thorax. 1996;51:985–8. doi: 10.1136/thx.51.10.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.British Thoracic Society Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax. 2008;63(Suppl 4) doi: 10.1136/thx.2008.097741. iv1-121. doi: 10.1136/thx.2008.097741. PMID: 18463203. [DOI] [PubMed] [Google Scholar]
- 56.Cates CJ, Crilly JA, Rowe BH. Cochrane Database Syst Rev. 2006. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma; p. CD000052. doi: 10.1002/14651858.CD000052.pub2. Update in: Cochrane Database Syst Rev. 2013;9:CD000052. PMID: 16625527. [DOI] [PubMed] [Google Scholar]
- 57.Dubus JC, Mély L, Huiart L, Marguet C, Le Roux P Réseau de Recherche Clinique en Pneumologie Pédiatrique. Cough after inhalation of corticosteroids delivered from spacer devices in children with asthma. Fundam Clin Pharmacol. 2003;17:627–31. doi: 10.1046/j.1472-8206.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 58.Al-Moamary MS, Alhaider SA, Alangari AA, Al Ghobain MO, Zeitouni MO, Idrees MM, et al. The Saudi Initiative for Asthma – 2019 Update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2019;14:3–48. doi: 10.4103/atm.ATM_327_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amirav I, Newhouse MT, Minocchieri S, Castro-Rodriguez JA, Schüepp KG. Factors that affect the efficacy of inhaled corticosteroids for infants and young children. J Allergy Clin Immunol. 2010;125:1206–11. doi: 10.1016/j.jaci.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 60.Khilnani GC, Banga A. Aerosol therapy. Indian J Chest Dis Allied Sci. 2008;50:209. [Google Scholar]
- 61.Rubin BK, Fink JB. Aerosol therapy for children. Respir Care Clin N Am. 2001;7:175. doi: 10.1016/s1078-5337(05)70030-7. [DOI] [PubMed] [Google Scholar]
- 62.Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–71. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 63.Chipps BE, Bacharier LB, Farrar JR, Jackson DJ, Murphy KR, Phipatanakul W, et al. The pediatric asthma yardstick: Practical recommendations for a sustained step-up in asthma therapy for children with inadequately controlled asthma. Ann Allergy Asthma Immunol. 2018;120:559–79.e11. doi: 10.1016/j.anai.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Rubin BK. Pediatric aerosol therapy: New devices and new drugs. Respir Care. 2011;56:1411–21. doi: 10.4187/respcare.01246. [DOI] [PubMed] [Google Scholar]
- 65.Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011 Jun;15(6):CD003123. doi: 10.1002/14651858.CD003123.pub3. doi: 10.1002/14651858.CD003123.pub3. PMID: 21678340. [DOI] [PubMed] [Google Scholar]
- 66.Plint AC, Johnson DW, Patel H, Wiebe N, Correll R, Brant R, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–89. doi: 10.1056/NEJMoa0900544. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2008 Oct;8(4):CD006458. doi: 10.1002/14651858.CD006458.pub2. doi: 10.1002/14651858.CD006458.pub2. Update in: Cochrane Database Syst Rev. 2013;7:CD006458. PMID: 18843717. [DOI] [PubMed] [Google Scholar]
- 68.Umoren R, Odey F, Meremikwu MM. Steam inhalation or humidified oxygen for acute bronchiolitis in children up to three years of age. Cochrane Database Syst Rev. 2011 Jan;19(1):CD006435. doi: 10.1002/14651858.CD006435.pub2. doi: 10.1002/14651858.CD006435.pub2. PMID: 21249676. [DOI] [PubMed] [Google Scholar]
- 69.Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B, Döring G, et al. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol. 1998;25:88–92. doi: 10.1002/(sici)1099-0496(199802)25:2<88::aid-ppul3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 70.Jensen T, Pederson SS, Garne S, Heilmann C, Høiby N, Koch C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother. 1987;19:831–8. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 71.Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 2002;20:658–64. doi: 10.1183/09031936.02.00248102. [DOI] [PubMed] [Google Scholar]
- 72.Rubin BK. Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. J Aerosol Med Pulm Drug Deliv. 2008;21:71–6. doi: 10.1089/jamp.2007.0652. [DOI] [PubMed] [Google Scholar]
- 73.Ring E, Eber E, Erwa W, Zach M S. Urinary N-acetyl-beta-D-glucosaminidase activity in patients with cystic fibrosis on long-term gentamicin inhalation. Arch Dis Child. 1998;78:540–3. doi: 10.1136/adc.78.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 75.Smith AL, Ramsey BW, Hedges DL, Hack B, Williams-Warren J, Weber A, et al. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr Pulmonol. 1989;7:265–71. doi: 10.1002/ppul.1950070413. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann IM, Rubin BK, Iskandar SS, Schechter MS, Nagaraj SK, Bitzan MM. Acute renal failure in cystic fibrosis: Association with inhaled tobramycin therapy. Pediatr Pulmonol. 2002;34:375–7. doi: 10.1002/ppul.10185. [DOI] [PubMed] [Google Scholar]
- 77.Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care. 2007;52:859–65. [PubMed] [Google Scholar]
- 78.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 79.Suri R, Metcalfe C, Lees B, Grieve R, Flather M, Normand C, et al. Comparison of hypertonic saline and alternate-day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: A randomised trial. Lancet. 2001;358:1316–21. doi: 10.1016/S0140-6736(01)06412-1. [DOI] [PubMed] [Google Scholar]
- 80.Wills PJ. Inhaled mannitol in cystic fibrosis. Expert Opin Investig Drugs. 2007;16:1121–6. doi: 10.1517/13543784.16.7.1121. [DOI] [PubMed] [Google Scholar]
- 81.Murgia X, Gastiasoro E, Mielgo V, Alvarez-Diaz F, Lafuente H, Valls-i-Soler A, et al. Surfactant and perfluorocarbon aerosolization by means of inhalation catheters for the treatment of respiratory distress syndrome: An in vitro study. J Aerosol Med Pulm Drug Deliv. 2011;24:81–7. doi: 10.1089/jamp.2010.0831. [DOI] [PubMed] [Google Scholar]
- 82.Brand P, Schulte M, Wencker M, Herpich CH, Klein G, Hanna K, et al. Lung deposition of inhaled alpha1-proteinase inhibitor in cystic fibrosis and alpha1-antitrypsin deficiency. Eur Respir J. 2009;34:354–60. doi: 10.1183/09031936.00118408. [DOI] [PubMed] [Google Scholar]
- 83.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med. 2002;165:922–6. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 84.Eckstein JW, Fung J. A new class of cyclosporin analogues for the treatment of asthma. Expert Opin Investig Drugs. 2003;12:647–53. doi: 10.1517/13543784.12.4.647. [DOI] [PubMed] [Google Scholar]
- 85.Groves S, Galazka M, Johnson B, Corcoran T, Verceles A, Britt E, et al. Inhaled cyclosporine and pulmonary function in lung transplant recipients. J Aerosol Med Pulm Drug Deliv. 2010;23:31–9. doi: 10.1089/jamp.2009.0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amirav I, Newhouse MT. Aerosol therapy in tracheotomized children: Time for guidelines! Respir Care. 2012;57:1350. doi: 10.4187/respcare.02005. [DOI] [PubMed] [Google Scholar]
- 87.Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care. 2007;52:1176–93. [PubMed] [Google Scholar]
- 88.Brightling CE. Clinical applications of induced sputum. Chest. 2006;129:1344–8. doi: 10.1378/chest.129.5.1344. [DOI] [PubMed] [Google Scholar]
- 89.Elkins MR, Bye PT. Mechanisms and applications of hypertonic saline. J R Soc Med. 2011;104:S2-5. doi: 10.1258/jrsm.2011.s11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol. 2015;118:1483–90. doi: 10.1152/japplphysiol.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bateman ED, Rennard S, Barnes PJ, Dicpinigaitis PV, Gosens R, Gross NJ, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009;22:533–42. doi: 10.1016/j.pupt.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Tagaya E, Yagi O, Sato A, Arimura K, Takeyama K, Kondo M, et al. Effect of tiotropium on mucus hypersecretion and airway clearance in patients with COPD. Pulm Pharmacol Ther. 2016;39:81–4. doi: 10.1016/j.pupt.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Blasco PA, Stansbury JC. Glycopyrrolate treatment of chronic drooling. Arch Pediatr Adolesc Med. 1996;150:932–5. doi: 10.1001/archpedi.1996.02170340046009. [DOI] [PubMed] [Google Scholar]
- 94.Garnock-Jones KP. Glycopyrrolate oral solution: For chronic, severe drooling in pediatric patients with neurologic conditions. Paediatr Drugs. 2012;14:263–9. doi: 10.2165/11208120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 95.Thomsen TR, Galpern WR, Asante A, Arenovich T, Fox SH. Ipratropium bromide spray as treatment for sialorrhea in Parkinson's disease. Mov Disord. 2007;22:2268–73. doi: 10.1002/mds.21730. [DOI] [PubMed] [Google Scholar]
- 96.Mier RJ, Bachrach SJ, Lakin RC, Barker T, Childs J, Moran M. Treatment of sialorrhea with glycopyrrolate: A double-blind, dose ranging study. Arch Pediatr Adolesc Med. 2000;154:1214–8. doi: 10.1001/archpedi.154.12.1214. [DOI] [PubMed] [Google Scholar]
- 97.Ahrens RC. The role of the MDI and DPI in pediatric patients: Children are not just miniature adults. Respir Care. 2005;50:1323–8. [PubMed] [Google Scholar]
- 98.Bhashyam AR, Wolf MT, Marcinkowski AL, Saville A, Thomas K, Carcillo JA, et al. Aerosol delivery through nasal cannulas: An in vitro study. J Aerosol Med Pulm Drug Deliv. 2008;21:181–8. doi: 10.1089/jamp.2007.0662. [DOI] [PubMed] [Google Scholar]
- 99.Lin HL, Restrepo RD, Gardenhire DS, Rau JL. Effect of face mask design on inhaled mass of nebulized albuterol, using a pediatric breathing model. Respir Care. 2007;52:1021–6. [PubMed] [Google Scholar]
- 100.Everard ML. Trying to deliver aerosols to upset children is a thankless task. Arch Dis Child. 2000;82:428. doi: 10.1136/adc.82.5.428a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2007. Aug, [Last accessed on 2021 Mar 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7232/ [Google Scholar]
- 102.Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: which to choose, why and how to achieve effective aerosol therapy. Expert Rev Respir Med. 2011;5:561–72. doi: 10.1586/ers.11.49. [DOI] [PubMed] [Google Scholar]
- 103.Choi J, Lee GL. Common pediatric respiratory emergencies. Emerg Med Clin North Am. 2012;30:529–63. doi: 10.1016/j.emc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–8. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Chinese College of Emergency Physicians (CCEP), Emergency Committee of PLA, Beijing Society for Emergency Medicine, Chinese Emergency Medicine. Expert consensus on nebulization therapy in pre-hospital and in-hospital emergency care. Ann Transl Med. 2019;7:487. doi: 10.21037/atm.2019.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sawicki G, Haver K. Acute asthma exacerbations in children younger than 12 years: Home/office management and severity assessment. U: UpToDate. In: TePas E, editor. UpToDate. Waltham, MA: UpToDate; 2020. [Last accessed on 2020 Jun 15]. Available from: https://www.uptodate.com/contents/acute-asthma-exacerbationsin-childrenyounger-than-12-years-emergency-departmentmanagement . [Google Scholar]
- 107.Network British Guideline. British Guideline on the Management of Asthma. 2019. [Last accessed on 2020 Oct 20]. Available from: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/
- 108.Plotnick LH, Ducharme FM. Cochrane Database Syst Rev. 4. 2000. Combined inhaled anticholinergics and beta2-agonists for initial treatment of acute asthma in children; p. CD000060. doi: 10.1002/14651858.CD000060. Update in: Cochrane Database Syst Rev. 2013;8:CD000060. PMID: 11034671. [DOI] [PubMed] [Google Scholar]
- 109.Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med. 1998;339:1030–5. doi: 10.1056/NEJM199810083391503. [DOI] [PubMed] [Google Scholar]
- 110.Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med. 1993;21:1479–86. doi: 10.1097/00003246-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 111.Katz RW, Kelly HW, Crowley MR, Grad R, McWilliams BC, Murphy SJ. Safety of continuous nebulized albuterol for bronchospasm in infants and children. Pediatrics. 1993;92:666–9. [PubMed] [Google Scholar]
- 112.McPeck M, Tandon R, Hughes K, Smaldone GC. Aerosol delivery during continuous nebulization. Chest. 1997;111:1200–5. doi: 10.1378/chest.111.5.1200. [DOI] [PubMed] [Google Scholar]
- 113.Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacyof nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respir Med. 2000;94:1206–14. doi: 10.1053/rmed.2000.0952. [DOI] [PubMed] [Google Scholar]
- 114.Matthews EE, Curtis PD, McLain BI, Morris LS, Turbitt ML. Nebulized budesonide versus oral steroid in severe exacerbations of childhood asthma. Acta Paediatr. 1999;88:841–3. doi: 10.1080/08035259950168757. [DOI] [PubMed] [Google Scholar]
- 115.Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: A controlled comparative study with oral prednisolone. J Allergy Clin Immunol. 1998;102:605–9. doi: 10.1016/s0091-6749(98)70276-3. [DOI] [PubMed] [Google Scholar]
- 116.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma. 2018. [last accessed on 2021 Mar 24]. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf .
- 117.Management of Acute Asthma Exacerbations in the Emergency Department and Hospital-Based Care. A consensus statement of the Egyptian Scientific Society of Bronchology (ESSB) [Internet]. ESSB. 2015. Available from: https://www.essb-eg.org/wpcontent/uploads/2019/12/AZ-%EF%80%A0Respiratory-ESSB%EF%80%A0-Booklet-Guide_A5-3ml-Bleed-5.pdf . last cited on 2021 Mar 24.
- 118.Geppe NA, et al. Ros Vestn Perinatoli Pediatr. 2018;63(3):125–132. in Russ. doi: 10.21508/1027-4065-2018-63-3-125-132. [Google Scholar]
- 119.Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21:301–9. doi: 10.1002/(SICI)1099-0496(199605)21:5<301::AID-PPUL5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 120.Lin YZ, Hsieh KH. Metered dose inhaler and nebuliser in acute asthma. Arch Dis Child. 1995;72:214–8. doi: 10.1136/adc.72.3.214. doi: 10.1136/adc.72.3.214. PMID: 7741566. PMCID: PMC1511067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deerojanawong J, Manuyakorn W, Prapphal N, Harnruthakorn C, Sritippayawan S, Samransamruajkit R. Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler-spacer vs. jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39:466–72. doi: 10.1002/ppul.20204. [DOI] [PubMed] [Google Scholar]
- 122.Leversha AM, Campanella SG, Aickin RP, Asher MI. Costs and effectiveness of spacer versus nebulizer in young children with moderate and severe acute asthma. J Pediatr. 2000;136:497–502. doi: 10.1016/s0022-3476(00)90013-1. [DOI] [PubMed] [Google Scholar]
- 123.Wildhaber JH, Devadason SG, Hayden MJ, Eber E, Summers QA, LeSouëf PN. Aerosol delivery to wheezy infants: A comparison between a nebulizer and two small volume spacers. Pediatr Pulmonol. 1997;23:212–6. doi: 10.1002/(sici)1099-0496(199703)23:3<212::aid-ppul7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 124.Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup: A double-blind study. Am J Dis Child. 1978;132:484–7. doi: 10.1001/archpedi.1978.02120300044008. [DOI] [PubMed] [Google Scholar]
- 125.Bjornson C, Russell KF, Vandermeer B, Durec T, Klassen TP, Johnson DW. Nebulized epinephrine for croup in children. Cochrane Database Syst Rev. 2011 Feb;16(2):CD006619. doi: 10.1002/14651858.CD006619.pub2. doi: 10.1002/14651858.CD006619.pub2. Update in: Cochrane Database Syst Rev. 2013;10:CD006619. PMID: 21328284. [DOI] [PubMed] [Google Scholar]
- 126.Lee JH, Jung JY, Lee HJ, Kim DK, Kwak YH, Chang I, et al. Efficacy of low-dose nebulized epinephrine as treatment for croup: A randomized, placebo-controlled, double-blind trial. Am J Emerg Med. 2019;37:2171–6. doi: 10.1016/j.ajem.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 127.Waisman Y, Klein BL, Boenning DA, Young GM, Chamberlain JM, O’Donnell R, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup) Pediatrics. 1992;89:302–6. [PubMed] [Google Scholar]
- 128.Moore M, Little P Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006 Jul;19(3):CD002870. doi: 10.1002/14651858.CD002870.pub2. doi: 10.1002/14651858.CD002870.pub2. Update in: Cochrane Database Syst Rev. 2011;(6):CD002870. PMID: 16855994. [DOI] [PubMed] [Google Scholar]
- 129.Henrickson KJ. Advances in the laboratory diagnosis of viral respiratory disease. Pediatr Infect Dis J. 2004;23:S6-10. doi: 10.1097/01.inf.0000108187.63151.ea. [DOI] [PubMed] [Google Scholar]
- 130.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115:878–84. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 132.Eboriadou M, Chryssanthopoulou D, Stamoulis P, Damianidou L, Haidopoulou K. The effectiveness of local corticosteroids therapy in the management of mild to moderate viral croup. Minerva Pediatr. 2010;62:23–8. [PubMed] [Google Scholar]
- 133.Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014 Jun;17 doi: 10.1002/14651858.CD001266.pub4. 2014(6):CD001266. doi: 10.1002/14651858.CD001266.pub4. PMID: 24937099. PMCID: PMC7055016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. American Academy of Pediatrics Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 135.Alharbi AS, Alqwaiee M, Al-Hindi MY, Mosalli R, Al-Shamrani A, Alharbi S, et al. Bronchiolitis in children: The Saudi Initiative of Bronchiolitis Diagnosis, Management, and Prevention (SIBRO) Ann Thorac Med. 2018;13:127–43. doi: 10.4103/atm.ATM_60_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panitch HB. Respiratory syncytial virus bronchiolitis: Supportive care and therapies designed to overcome airway obstruction. Pediatr Infect Dis J. 2003;22:S83-7. doi: 10.1097/01.inf.0000053890.66801.97. [DOI] [PubMed] [Google Scholar]
- 137.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 138.Cade A, Brownlee KG, Conway SP, Haigh D, Short A, Brown J, et al. Randomised placebo controlled trial of nebulized corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000;82:126–30. doi: 10.1136/adc.82.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blom D, Ermers M, Bont L, van Aalderen WM, van Woensel JB. Inhaled corticosteroids during acute bronchiolitis in the prevention of post-bronchiolitic wheezing. Cochrane Database Syst Rev. 2007 Jan;24(1):CD004881. doi: 10.1002/14651858.CD004881.pub2. doi: 10.1002/14651858.CD004881.pub2. Update in: Cochrane Database Syst Rev. 2011;(1):CD004881. PMID: 17253528. [DOI] [PubMed] [Google Scholar]
- 140.Dhand R. Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care. 2007;13:27–38. doi: 10.1097/MCC.0b013e328012e022. [DOI] [PubMed] [Google Scholar]
- 141.Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA. Fundamentals of aerosol therapy in critical care. Crit Care. 2016;20:269. doi: 10.1186/s13054-016-1448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 143.Hess DR. Aerosol therapy during noninvasive ventilation or high-flow nasal cannula. Respir Care. 2015;60:880–91. doi: 10.4187/respcare.04042. [DOI] [PubMed] [Google Scholar]
- 144.Cabrini L, Landoni G, Oriani A, Plumari VP, Nobile L, Greco M, et al. Noninvasive ventilation and survival in acute care settings: A comprehensive systematic review and metaanalysis of randomized controlled trials. Crit Care Med. 2015;43:880–8. doi: 10.1097/CCM.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 145.Nava S, Karakurt S, Rampulla C, Braschi A, Fanfulla F. Salbutamol delivery during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease: A randomized, controlled study. Intensive Care Med. 2001;27:1627–35. doi: 10.1007/s001340101062. [DOI] [PubMed] [Google Scholar]
- 146.Galindo-Filho VC, Brandão DC, Ferreira Rde C, Menezes MJ, Almeida-Filho P, Parreira VF, et al. Noninvasive ventilation coupled with nebulization during asthma crises: A randomized controlled trial. Respir Care. 2013;58:241–9. doi: 10.4187/respcare.01371. [DOI] [PubMed] [Google Scholar]
- 147.Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55:536–43. [PubMed] [Google Scholar]
- 148.Reminiac F, Vecellio L, Heuze-Vourc'h N, Petitcollin A, Respaud R, Cabrera M, et al. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J Aerosol Med Pulm Drug Deliv. 2016;29:134–41. doi: 10.1089/jamp.2015.1219. [DOI] [PubMed] [Google Scholar]
- 149.Perry SA, Kesser KC, Geller DE, Selhorst DM, Rendle JK, Hertzog JH. Influences of cannula size and flow rate on aerosol drug delivery through the Vapotherm humidified high-flow nasal cannula system. Pediatr Crit Care Med. 2013;14:e250-6. doi: 10.1097/PCC.0b013e31828a7f79. [DOI] [PubMed] [Google Scholar]
- 150.Sunbul FS, Fink JB, Harwood R, Sheard MM, Zimmerman RD, Ari A. Comparison of HFNC, bubble CPAP and SiPAP on aerosol delivery in neonates: An in-vitro study. Pediatr Pulmonol. 2015;50:1099–106. doi: 10.1002/ppul.23123. [DOI] [PubMed] [Google Scholar]
- 151.Bachour A, Hurmerinta K, Maasilta P. Mouth closing device (chinstrap) reduces mouth leak during nasal CPAP. Sleep Med. 2004;5:261–7. doi: 10.1016/j.sleep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 152.Ari A. Aerosol therapy in pulmonary critical care. Respir Care. 2015;60:858–74. doi: 10.4187/respcare.03790. [DOI] [PubMed] [Google Scholar]
- 153.Piccuito CM, Hess DR. Albuterol delivery via tracheostomy tube. Respir Care. 2005;50:1071–6. [PubMed] [Google Scholar]
- 154.Pitance L, Vecellio L, Delval G, Reychler G, Reychler H, Liistro G. Aerosol delivery through tracheostomy tubes: An in vitro study. J Aerosol Med Pulm Drug Deliv. 2013;26:76–83. doi: 10.1089/jamp.2011.0962. [DOI] [PubMed] [Google Scholar]
- 155.Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: Historical challenges and new directions. Eur J Pediatr. 2011;170:433–44. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ari A, Harwood RJ, Sheard MM, Fink JB. An in vitro evaluation of aerosol delivery through tracheostomy and endotracheal tubes using different interfaces. Respir Care. 2012;57:1066–70. doi: 10.4187/respcare.01167. [DOI] [PubMed] [Google Scholar]
- 157.Hintz SR. Pharmacology review: Bronchodilator therapy in the preterm infant. Neoreviews. 2003;4:e245-9. [Google Scholar]
- 158.Sosulski R, Abbasi S, Bhutani VK, Fox WW. Therapeutic value of terbutaline in bronchopulmonary dysplasia. Pediatr Pulmonol. 1986;2:269–273. doi: 10.1002/ppul.1950020504. [DOI] [PubMed] [Google Scholar]
- 159.Gappa M, Gärtner M, Poets CF, von der Hardt H. Effects of salbutamol delivery from a metered dose inhaler versus jet nebulizer on dynamic lung mechanics in very preterm infants with chronic lung disease. Pediatr Pulmonol. 1997;23:442–8. doi: 10.1002/(sici)1099-0496(199706)23:6<442::aid-ppul8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 160.Diblasi RM. Clearing the mist from our eyes: Bronchodilators, mechanical ventilation, new devices, locations, and what you should know about bias flow. Respir Care. 2010;55:942–6. [PubMed] [Google Scholar]
- 161.Dhand R. Bronchodilator therapy. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. 2nd ed. New York: McGraw-Hill; 2006. pp. 1277–310. [Google Scholar]
- 162.Dhand R. Aerosol delivery during mechanical ventilation: From basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21:45–60. doi: 10.1089/jamp.2007.0663. [DOI] [PubMed] [Google Scholar]
- 163.Selroos O, Borgström L, Ingelf J. Use of dry powder inhalers in acute exacerbations of asthma and COPD. Ther Adv Respir Dis. 2009;3:81–91. doi: 10.1177/1753465809103737. [DOI] [PubMed] [Google Scholar]
- 164.Thomas M, Williams AE. Are outcomes the same with all dry powder inhalers. Int J Clin Pract Suppl. 2005;149:33–5. doi: 10.1111/j.1368-504X.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 165.Fink JB, Rubin BK. Problems with inhaler use: A call for improved clinician and patient education. Respir Care. 2005;50:1360–74. [PubMed] [Google Scholar]
- 166.Boukhettala N, Porée T, Diot P, Vecellio L. In vitro performance of spacers for aerosol delivery during adult mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2015;28:130–6. doi: 10.1089/jamp.2013.1091. [DOI] [PubMed] [Google Scholar]
- 167.Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55:845–51. [PubMed] [Google Scholar]
- 168.Garner SS, Wiest DB, Bradley JW. Albuterol delivery by metered-dose inhaled in a pediatric high-frequency oscillatory ventilation mode. Crit Care Med. 2000;28:2086–9. doi: 10.1097/00003246-200006000-00070. [DOI] [PubMed] [Google Scholar]
- 169.DiBlasi RM, Crotwell DN, Shen S, Zheng J, Fink JB, Yung D. Iloprost drug delivery during infant conventional and high-frequency oscillatory ventilation. Pulm Circ. 2016;6:63–9. doi: 10.1086/685080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ari A, Harwood RJ, Sheard MM, Fink JB. Pressurized metered-dose inhalers versus nebulizers in the treatment of mechanically ventilated subjects with artificial airways: An in vitro study. Respir Care. 2015;60:1570–4. doi: 10.4187/respcare.04125. [DOI] [PubMed] [Google Scholar]
- 171.Katzman M, Meade W, Iglar K, Rachlis A, Berger P, Chan CK. High incidence of bronchospasm with regular administration of aerosolized pentamidine. Chest. 1992;101:79–81. doi: 10.1378/chest.101.1.79. [DOI] [PubMed] [Google Scholar]
- 172.Zitter JN, Maldjian P, Brimacombe M, Fennelly KP. Inhaled Dornase alfa (Pulmozyme) as a noninvasive treatment of atelectasis in mechanically ventilated patients. J Crit Care. 2013;28:218.e1–7. doi: 10.1016/j.jcrc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 173.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Me. 2011;184:561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin HL, Fink JB, Zhou Y, Cheng YS. Influence of moisture accumulation in inline spacer on delivery of aerosol using metered-dose inhaler during mechanical ventilation. Respir Care. 2009;54:1336–41. [PubMed] [Google Scholar]
- 175.Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76–80. doi: 10.1001/archpedi.157.1.76. [DOI] [PubMed] [Google Scholar]
- 176.Macfarlane JT, Lane DJ. Irregularities in the use of regular aerosol inhalers. Thorax. 1980;35:477–8. doi: 10.1136/thx.35.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Newman SP, Millar AB, Lennard-Jones TR, Moren F, Clarke SW. Improvement of pressurized aerosol deposition with Nebuhaler spacer device. Thorax. 1984;39:935–41. doi: 10.1136/thx.39.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nebulizer therapy. Guidelines. British Thoracic Society Nebulizer Project Group. Thorax. 1997;52(Suppl 2):S4–24. doi: 10.1136/thx.52.2008.s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alhaider SA, Alshehri HA, Al-Eid K. Replacing nebulizers by MDI-spacers for bronchodilator and inhaled corticosteroid administration: Impact on the utilization of hospital resources. Int J Pediatr Adolesc Med. 2014;1:26–30. doi: 10.1016/j.ijpam.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wildhaber JH, Dore ND, Wilson JM, Devadason SG, LeSouëf PN. Inhalation therapy in asthma: Nebulizer or pressurized metered-dose inhaler with holding chamber? In vivo comparison of lung deposition in children. J Pediatr. 1999;135:28–33. doi: 10.1016/s0022-3476(99)70323-9. [DOI] [PubMed] [Google Scholar]
- 181.Salzman GA, Steele MT, Pribble JP, Elenbaas RM, Pyszczynski DR. Aerosolized metaproterenol in the treatment of asthmatics with severe airflow obstruction. Comparison of two delivery methods. Chest. 1989;95:1017–20. doi: 10.1378/chest.95.5.1017. [DOI] [PubMed] [Google Scholar]
- 182.Colacone A, Afilalo M, Wolkove N, Kreisman H. A comparison of albuterol administered by metered dose inhaler (and holding chamber) or wet nebulizer in acute asthma. Chest. 1993;104:835–41. doi: 10.1378/chest.104.3.835. [DOI] [PubMed] [Google Scholar]
- 183.Kerem E, Levison H, Schuh S, O’Brodovich H, Reisman J, Bentur L, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr. 1993;123:313–7. doi: 10.1016/s0022-3476(05)81710-x. [DOI] [PubMed] [Google Scholar]
- 184.Ros SP. Emergency management of childhood bronchial asthma: A multicenter survey. Ann Allergy. 1991;66:231–4. [PubMed] [Google Scholar]
- 185.Tal A, Pasterkamp H, Leahy F. Arterial oxygen desaturation following salbutamol inhalation in acute asthma. Chest. 1984;86:868–9. doi: 10.1378/chest.86.6.868. [DOI] [PubMed] [Google Scholar]
- 186.Agent P, Parrott H. Inhaled therapy in cystic fibrosis: Agents, devices and regimens. Breathe (Sheff) 2015;11:110–8. doi: 10.1183/20734735.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nevitt SJ, Thornton J, Murray CS, Dwyer T. Cochrane Database Syst Rev. 2018. Feb, Inhaled mannitol for cystic fibrosis. 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. PMID: 29424930. PMCID: PMC6491147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moraa I, Sturman N, McGuire TM, van Driel ML. Heliox for croup in children. Cochrane Database Syst Rev. 2018 Oct; doi: 10.1002/14651858.CD006822.pub5. 29;10(10):CD006822. doi: 10.1002/14651858.CD006822.pub5. PMID: 30371952. PMCID: PMC6516979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cruz AT, Cazacu AC, Greer JM, Demmler GJ. Performance of a rapid assay (Binax NOW) for detection of respiratory syncytial virus at a children's hospital over a 3-year period. J Clin Microbiol. 2007;45:1993–5. doi: 10.1128/JCM.00279-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–5. doi: 10.1542/peds.2007-1135. [DOI] [PubMed] [Google Scholar]
- 191.Lehtinen P, Jartti T, Virkki R, Vuorinen T, Leinonen M, Peltola V, et al. Bacterial coinfections in children with viral wheezing. Eur J Clin Microbiol Infect Dis. 2006;25:463–9. doi: 10.1007/s10096-006-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Al-Ansari K, Sakran M, Davidson BL, El Sayyed R, Mahjoub H, Ibrahim K, et al. Nebulized 5% or 3% hypertonic or 0.9% saline for treating acute bronchiolitis in infants. J Pediatr. 2010;157:630–4. doi: 10.1016/j.jpeds.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 193.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 194.Guerguerian AM, Gauthier M, Lebel MH, Farrell CA, Lacroix J. Ribavirin in ventilated respiratory syncytial virus bronchiolitis. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160:829–34. doi: 10.1164/ajrccm.160.3.9810013. [DOI] [PubMed] [Google Scholar]
- 195.Rau JL. Determinants of patient adherence to an aerosol regimen. Respir Care. 2005;50:1346–59. [PubMed] [Google Scholar]
- 196.Grieble HG, Colton FR, Bird TJ, Toigo A, Griffith LG. Fine-particle humidifiers. Source of Pseudomonas aeruginosa infections in a respiratory-disease unit. N Engl J Med. 1970;282:531–5. doi: 10.1056/NEJM197003052821003. [DOI] [PubMed] [Google Scholar]
- 197.Sanchis J, Gich I, Pedersen S Aerosol Drug Management Improvement Team (ADMIT) Systematic review of errors in inhaler use: Has patient technique improved over time. Chest. 2016;150:394–406. doi: 10.1016/j.chest.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 198.Koehorst-Ter Huurne K, Movig K, van der Valk P, van der Palen J, Brusse-Keizer M. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Eur Respir J. 2013;42:4146. doi: 10.1517/17425247.2016.1130695. [DOI] [PubMed] [Google Scholar]
- 199.Dennis JH. Standardization issues: In vitro assessment of nebulizer performance. Respir Care. 2002;47:1445–58. [PubMed] [Google Scholar]
- 200.Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51:158–72. [PubMed] [Google Scholar]
- 201.Vassal S, Taamma R, Marty N, Sardet A, d'Athis P, Brémont F, et al. Microbiologic contamination study of nebulizers after aerosol therapy in patients with cystic fibrosis. Am J Infect Control. 2000;28:347–51. doi: 10.1067/mic.2000.110214. [DOI] [PubMed] [Google Scholar]
- 202.Hutchinson GR, Parker S, Pryor JA, Duncan-Skingle F, Hoffman PN, Hodson ME, et al. Home-use nebulizers: A potential primary source of Burkholderia cepacia and other colistin-resistant, gram-negative bacteria in patients with cystic fibrosis. J Clin Microbiol. 1996;34:584–7. doi: 10.1128/jcm.34.3.584-587.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saiman L, Siegel J Cystic Fibrosis Foundation. Infection control recommendations for patients with cystic fibrosis: Microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24:S6-52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 204.Chew NY, Reddel HK, Bosnic-Anticevich SZ, Chan HK. Effect of mouthpiece washing on aerosol performance of CFC-free Ventolin. J Asthma. 2004;41:721–7. doi: 10.1081/jas-200027958. [DOI] [PubMed] [Google Scholar]
- 205.Stopping the spread of germs [Internet] Cystic Fibrosis Foundation. 2009. [Last accessed 2021 Mar 24]. Available from: http://www.cfcareli.com/images/downloadCenter/StoppingTheSpreadOfGerms.pdf .
- 206.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health care – Associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1 36. [PubMed] [Google Scholar]
- 207.O’Malley CA, VandenBranden SL, Zheng XT, Polito AM, McColley SA. A day in the life of a nebulizer: Surveillance for bacterial growth in nebulizer equipment of children with cystic fibrosis in the hospital setting. Respir Care. 2007;52:258 62. [PubMed] [Google Scholar]
- 208.American Association for Respiratory Care. AARC Clinical Practice Guideline. Selection of aerosol delivery device. American Association for Respiratory Care. Respir Care. 1992;37:891–7. [PubMed] [Google Scholar]
- 209.Larson T, Gudavalli R, Prater D, Sutton S. Critical analysis of common canister programs: A review of cross functional considerations and health system economics. Curr Med Res Opin. 2015;31:853 60. doi: 10.1185/03007995.2015.1016604. [DOI] [PubMed] [Google Scholar]
- 210.Gowan M, Bushwitz J, Watts P, Silver PC, Jackson M, Hampton N, et al. Use of a shared canister protocol for the delivery of metered dose inhalers in mechanically ventilated subjects. Respir Care. 2016;61:1285 92. doi: 10.4187/respcare.04550. [DOI] [PubMed] [Google Scholar]
- 211.Rau JL, Restrepo RD. Nebulized bronchodilator formulations: Unit dose or multi dose. Respir Care. 2003;48:926 39. [PubMed] [Google Scholar]
- 212.Boyce JM, Pittet D Healthcare Infection Control Practices Advisory Committee, HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/ APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1–CE4. [PubMed] [Google Scholar]
- 213.Rhinehart E, Friedman MM. Infection Control in Home Care (An Official APIC Publication) Gaithersburg MD: Aspen Publishers, Inc; 2006. Personal protective equipment and staff supplies; pp. 61–9. [Google Scholar]
- 214.Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251 69. doi: 10.1016/0196-6553(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 215.Siegel JD, Rhinehart E, Jackson M, Chiarello L Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65 164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.WHO Guidelines on Hand Hygiene in Health Care: a Summary [Internet] World Health Organization; 2009. Available from: https://www.who.int/gpsc/5may/tools/who_guidelineshandhygiene_summary.pdf . Last cited on 2021 Mar 24. [Google Scholar]
- 217.Segal-Maurer S, Kalkut GE. Environmental control of tuberculosis: Continuing controversy. Clin Infect Dis. 1994;19:299–308. doi: 10.1093/clinids/19.2.299. [DOI] [PubMed] [Google Scholar]