Abstract
Prehypertension seems to be related to increased cardiovascular risk in healthy subjects, while hypoadiponectinemia and hyperresistinemia may contribute to insulin resistance and accelerated atherogenesis. This study investigated whether plasma levels of adiponectin (known to increase insulin sensitivity) and resistin (a protein possibly involved in inflammatory activities) are affected in healthy individuals with prehypertension, and to compare the findings to those of healthy normotensives matched for age, gender, and body mass index. Twenty‐six (14 men and 12 women) healthy individuals with prehypertension (mean age, 52±5 years; mean body mass index, 23±1.5 kg/m2) and 24 healthy normotensives (13 men and 11 women; mean age 53±6 years; body mass index 23.2±1.4 kg/m2) were studied. The adiponectin and resistin plasma levels were determined by the enzyme‐linked immunosorbent assay method. Plasma resistin levels were significantly higher, while adiponectin plasma levels were significantly lower, in prehypertensive subjects compared with normotensive subjects (10.62?3.17 ng/mL vs. 6.72±3.15 ng/mL and 6.26±2.18 μg/mL vs. 12.12±4.8 μg/mL; p<0.01, respectively). The findings suggest that healthy individuals with prehypertension have significantly higher resistin plasma levels and significantly lower adiponectin plasma levels compared with healthy normotensives. These findings may represent another possible mechanism that may increase the cardiovascular risk in this special group of patients, needing further investigation.
It is recognized that cardiovascular (CV) risk increases linearly at systolic blood pressure (SBP) of 130–135 mm Hg and diastolic blood pressure (DBP) of 80–85 mm Hg, levels lower than those that trigger the use of antihypertensive therapy. It is also well known that patients with high‐normal blood pressure (BP) have higher rates of CV events than those with normal BP. 1
Adiponectin and resistin have recently been described as secretory products of adipose tissue. Adiponectin is secreted by fat cells and circulates in the blood. Plasma adiponectin is reduced in obese animals and in patients with type 2 diabetes mellitus. Adiponectin stimulates fatty acid oxidation, decreases plasma triglycerides, and improves glucose metabolism by increasing insulin sensitivity. In addition, adiponectin inhibits the inflammatory process and, possibly, atherogenesis by suppressing the migration of monocytes and macrophages and their transformation into foam cells. 2
Resistin belongs to a newly discovered protein family with no homology to any previously known proteins. These proteins are members of a cysteine‐rich secretory protein family called “resistin‐like molecules” found in inflammatory zones. Resistin is expressed exclusively in white adipose tissue. Resistin is detectable in human adipose tissue at a very low level. There is no relationship between resistin expression and obesity. Resistin is also detected in peripheral blood monocytes, suggesting a possible role in inflammatory processes. 3
The aim of this study was to test the hypothesis that elevated resistin plasma levels and decreased adiponectin plasma levels exist in healthy subjects with prehypertension compared with healthy normotensives without any history of CV disease.
METHODS
Two groups of subjects were studied: 26 (14 men, 12 women) healthy people with prehypertension (mean age, 52±5 years; mean body mass index [BMI], 23±1.5 kg/m2) and 24 healthy normotensives (13 men, 11 women; mean age, 53?6 years; mean BMI, 23.2±1.4 kg/m 2 without any history of CV disease or diabetes mellitus). All participants were Greek Caucasians. The demographic characteristics and laboratory assessment of the participants are presented in Table I. At the baseline examination, all participants underwent a physical examination with a medical history, laboratory assessment of risk factors for CV disease, and routine electrocardiography. Because the mean age of the study population was older than 50 years, all patients underwent an exercise test.
Table I.
Demographic Characteristics and Laboratory Assessment
| Prehypertensive (n=26) | Normotensive (n=24) | |
|---|---|---|
| Age (yr) | 52±5 | 53±6 |
| Gender (n/n) (men/women) | 14/12 | 13/11 |
| Body mass index (kg/m2) | 23±1.5 | 23.2±1.4 |
| Serum glucose (mg/dL) | 0.95±0.8 | 0.93±0.75 |
| Serum creatinine (mg/dL) | 0.88±0.4 | 0.90±0.3 |
| Total cholesterol (mg/dL) | 230±15 | 229±12 |
| High‐density lipoprotein cholesterol (mg/dL) | 45±2 | 46±3 |
| Triglycerides (mg/dL) | 98±10 | 97±9 |
| All data are mean ± SD unless otherwise indicated. No significant differences were found between groups for these data. | ||
The classification of the participants was made by BP measurements in accordance with the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). 4
None of the subjects were taking medication or were smokers. All subjects were on a standardized diet before sampling and none had any thyroid abnormalities. Alcohol consumption was expressed in g/d, determined by a detailed questionnaire. Information concerning physical activity was obtained from a previously described questionnaire. 5 Written informed consent approved by the hospital review committee was obtained from each participant.
Measurements of BP and Laboratory Assessment
SBP and DBP were measured at the time of the first and fifth Korotkoff sounds, respectively. Measurements were made on the right arm to the nearest millimeter of mercury with the use of a mercury column sphygmomanometer. All measurements were made in the supine position after the patient had rested for 15 minutes. Results are the average of measurements obtained on at least three separate occasions, performed by the same trained nurse, who was not aware of the history of the subjects. If the SBP and DBP readings belonged to different JNC 7 categories, then the higher of the two categories was assigned. 6 Prehypertension was defined as SBP 120–139 mm Hg or DBP 80–89 mm Hg. Normotension was defined as SBP <120 mm Hg or DBP <80 mm Hg.
Blood sampling to determine adiponectin and resistin plasma levels was performed after 12 hours of fasting, between 8 a.m. and 9 a.m. The adiponectin plasma levels (RD195023100, Human Adiponectin, Elisa Bio Vendor Laboratory Medicine Inc., Brno, Czech Republic) and the resistin plasma levels (RD191016100, Human Resistin, Elisa Bio Vendor Laboratory Medicine Inc.) were measured by immunoassay. Investigators performing the assays were not aware of the sources of the samples studied. Results are reported as concentrations of adiponectin in μg/mL and concentrations of resistin in ng/mL.
Statistical Analyses
Values are expressed as mean ± SD. An unpaired Student t test was used to assess differences between the two groups. A p<0.05 was considered statistically significant. All analyses were performed with the SPSS statistical package (SPSS Inc., Chicago, IL).
RESULTS
As shown in Table I, there were no statistical differences according to age, gender, BMI, and other laboratory markers for CV disease between the two groups. As is shown in Table II and depicted in the Figure, subjects with prehypertension compared with normotensives had significantly lower mean adiponectin plasma levels (6.26±2.18 ng/mL vs. 12.12±4.8 ng/mL; p<0.01) and significantly higher mean resistin plasma levels (10.62±3.17 ng/mL vs. 6.72±3.15 ng/mL; p<0.01).
Table II.
Results and Comparison Between Groups
| Prehypertensive (n=26) | Normotensive (n=24) | p Value | |
|---|---|---|---|
| Systolic BP (mm Hg) | 133±2 | 116±3 | <0.001 |
| Diastolic BP (mm Hg) | 87±2 | 76±2 | <0.001 |
| Adiponectin (μg/mL) | 6.26±2.18 | 12.12±4.8 | <0.01 |
| Resistin (ng/mL) | 10.62±3.17 | 6.72±3.15 | <0.01 |
| All data are mean ± SD. BP=blood pressure | |||
Figure.
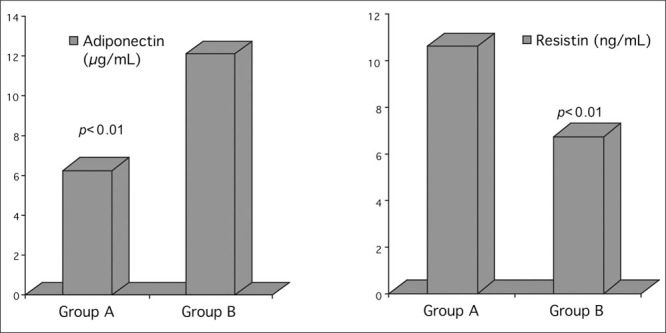
Adiponectin and resistin plasma levels in healthy subjects with prehypertension (Group A) and healthy normotensives (Group B)
DISCUSSION
The current study demonstrated that the mean adiponectin plasma levels were lower while the mean resistin plasma levels were higher in healthy individuals with prehypertension compared with healthy normotensives, even when age, gender, BMI, and the other laboratory markers for CV disease were matched for the two groups.
For a long time, white adipose tissue was regarded as a relatively passive site of energy storage. Recent studies indicate that white adipose tissue is an endocrine organ producing numerous proteins with broad biologic activity. The secretory products of white adipose tissue, collectively referred to as “adipocytokines,” include leptin, tumor necrosis factor‐α, interleukin‐6, transforming growth factor‐β, plasminogen activator inhibitor‐1, angiotensinogen, adiponectin, resistin, and metallothioneins. Proteins secreted by adipose tissue are involved in obesity‐associated complications such as insulin resistance, endothelial dysfunction, arterial hypertension, and atherosclerosis. 6
Adiponectin is the most abundant secretory protein of adipose tissue in humans, 7 with insulin sensitizing, anti‐inflammatory, and antiatherogenic effects. 8 Recent cross‐sectional studies in humans have reported a significant negative correlation between adiponectin plasma levels and obesity, waist‐hip ratio, insulin resistance, 9 diabetic dyslipidemia, 10 and CV disease. 11 , 12 A negative correlation between adiponectin and BP levels has been observed in normotensive populations. Results of recent studies on adiponectin in hypertension have been inconsistent. High, 13 low, 14 , 15 , 16 or normal adiponectin levels (only in patients without insulin resistance) are reported in patients with essential hypertension. Data concerning adiponectin plasma levels in patients with high‐normal BP are also extremely limited. 14 , 16 Our findings are in agreement with Iwashima et al. 14 and Kazumi et al., 16 and at variance with others who noticed a relationship between lower adiponectin levels and a predisposition to hypertension or high‐normal BP in men.
Resistin is a recently discovered adipocyte‐secreted polypeptide that has been implicated in the development of insulin resistance. It belongs to a family of cysteine‐rich secretory proteins termed the “resistin‐like molecules” family and is also described as “adipose tissue secretory factor” or “FIZZ” (found in inflammatory zones) protein. 16 , 17 , 18 , 19 Assays for human resistin are in their infancy. In the past year, several small studies have reported that circulating resistin levels are increased in human obesity and diabetes, although not all reports have been consistent. 20
In humans, resistin is expressed primarily in inflammatory cells. 21 Recombinant resistin up‐regulates cytokines and adhesion molecule expression on human endothelial cells, 22 suggesting a potential role in atherosclerosis. The relationship of resistin to inflammation, insulin resistance, and atherosclerosis in humans, however, remains largely unexplored.
Studies concerning resistin levels in patients with hypertension are limited. Furuhashi et al. 23 found that resistin levels are not related to insulin resistance, at least in patients with essential hypertension, while Zhang et al. 24 pointed out a relationship between fasting serum resistin and blood glucose in patients with essential hypertension and differing glucose tolerance, suggesting that resistin may be a mediator involved in the development of diabetes in humans. To the best of our knowledge, this is the first study of resistin plasma levels in healthy individuals with prehypertension.
A limitation of our study is that we have not measured insulin resistance, which is a factor known to influence plasma adiponectin concentration. 9 It should be emphasized, however, that none of the subjects studied were diabetic. The limitations of BMI in estimating body fat are known, and it is possible that more accurate measures of adipose tissue mass might be necessary to categorically exclude obesity as a common antecedent. Therefore, we cannot exclude that prehypertensive subjects had greater body fat mass than normotensive subjects, and that increased body fat mass is the cause of decreased plasma adiponectin concentration in prehypertensive subjects. Because this study is limited due to the small number of subjects enrolled, it is unreliable to compare hormone levels between males and females.
CONCLUSIONS
The present study suggests that in the absence of major CV risk factors, persons with prehypertension have significantly higher resistin plasma levels and lower adiponectin plasma levels compared with normotensives. Although we don't know the exact mechanism underlying these differences and recognize that they should be further investigated, we believe that subjects with prehypertension should be encouraged to modify their lifestyle to avoid further development of metabolic and subsequent CV diseases.
References
- 1. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001; 345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 2. Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004; 89:447–452. [DOI] [PubMed] [Google Scholar]
- 3. Beltowski J. Adiponectin and resistin—new hormones of white adipose tissue. Med Sci Monit. 2003; 9:RA55–RA61. [PubMed] [Google Scholar]
- 4. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 5. Seidell JC, Cigolini M, Deslypere JP, et al. Body fat distribution in relation to physical activity and smoking habits in 38‐year‐old European men. Am J Epidemiol. 1991; 133:257–265. [DOI] [PubMed] [Google Scholar]
- 6. 1999 World Health Organization International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J. Hypertens. 1999; 17:151–183. [PubMed] [Google Scholar]
- 7. Coppack SW. Pro‐inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001; 60:349–356. [DOI] [PubMed] [Google Scholar]
- 8. Maeda K, Okubo K, Shimomura I, et al. cDNA cloning and expression of a novel adipose‐specific collagen‐like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996; 221:286–289. [DOI] [PubMed] [Google Scholar]
- 9. Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte‐derived plasma protein adiponectin. Circulation. 1999; 100:2473–2476. [DOI] [PubMed] [Google Scholar]
- 10. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001; 86:1930–1935. [DOI] [PubMed] [Google Scholar]
- 11. Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002; 87:2764–2769. [DOI] [PubMed] [Google Scholar]
- 12. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000; 20:1595–1599. [DOI] [PubMed] [Google Scholar]
- 13. Mallamaci F, Zoccali C, Cuzzola F, et al. Adiponectin in essential hypertension. J Nephrol. 2002; 15:507–511. [PubMed] [Google Scholar]
- 14. Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004; 43:1318–1323. [DOI] [PubMed] [Google Scholar]
- 15. Adamczak M, Wiecek A, Funahashi T, et al. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003; 16:72–75. [DOI] [PubMed] [Google Scholar]
- 16. Kazumi T, Kawaguchi A, Sakai K, et al. Young men with high‐normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002; 25:971–976. [DOI] [PubMed] [Google Scholar]
- 17. Steppan CM, Brown EJ, Wright CM, et al. A family of tissue‐specific resistin‐like molecules. Proc Natl Acad Sci U S A. 2001; 98:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001; 409:307–312. [DOI] [PubMed] [Google Scholar]
- 19. Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine‐rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000; 19:4046–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005; 111:932–939. [DOI] [PubMed] [Google Scholar]
- 21. Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine‐endothelial interaction [published correction appears in Circulation. 2004;109(18):2254]. Circulation. 2003;108:736–740. [DOI] [PubMed] [Google Scholar]
- 22. Kawanami D, Maemura K, Takeda N, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine‐endothelial cell interactions. Biochem Biophys Res Commun. 2004; 314:415–419. [DOI] [PubMed] [Google Scholar]
- 23. Furuhashi M, Ura N, Higashiura K, et al. Circulating resistin levels in essential hypertension. Clin Endocrinol (Oxf). 2003; 59:507–510. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J‐L, Qin Y‐W, Zheng X, et al. Serum resistin level in essential hypertension patients with different glucose tolerance. Diabet Med. 2003; 20:828–831. [DOI] [PubMed] [Google Scholar]


