Abstract
The AVALON study was a randomized, multicenter trial to assess the efficacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia. Phase one was an 8‐week, double‐blind, double‐dummy, placebo‐controlled period whereby patients received amlodipine 5 mg, atorvastatin 10 mg, amlodipine 5 mg and atorvastatin 10 mg, or placebo. Thereafter, all patients received single‐blind amlodipine 5 mg and atorvastatin 10 mg for 8‐weeks, followed by 12 weeks of open‐label treatment where doses could be titrated to improve low‐density lipoprotein cholesterol and blood pressure control. A total of 847 patients entered the double‐blind phase. At Week 8, 45% of the patients receiving amlodipine 5 mg and atorvastatin 10 mg reached both their blood pressure and low‐density lipoprotein cholesterol goals, compared with 8.3% with amlodipine (p<0.001), 28.6% with atorvastatin (p<0.001), and 3.5% with placebo. At 28 weeks, 67.1% of patients coadministered amlodipine and atorvastatin (mean doses, 7.6 mg and 28.4 mg, respectively) achieved both targets. Framingham estimated 10‐year risk of coronary heart disease declined from baseline levels of 15.1% to 6.9% at Week 28. Following coadministered treatment, the adverse events reported were similar to either agent alone. Concomitant administration of amlodipine and atorvastatin is an effective and well tolerated treatment for coexisting hypertension and dyslipidemia.
Cardiovascular disease (CVD) is one of the leading contributors to global disease burden, accounting for 30% of total deaths worldwide 1 and 37% of deaths in the United States during 2003. 2 Hypertension (HTN), and particularly, elevated systolic blood pressure (BP), is a strong predictor of CVD. 3 Patients with HTN frequently have additional cardiovascular (CV) risk factors such as dyslipidemia (DYS). 4 , 5 Individual risk factors such as HTN and DYS interact to augment an individual's absolute CVD risk. 6 , 7 Accordingly, CVD risk management should focus on multifactorial therapeutic interventions to manage a patient's overall risk of CVD, rather than treating single CV risk factors. 8 , 9 Recent clinical trials have embraced the approach of overall CV risk management and have shown that a multifactorial approach reduces CV events. 10 , 11 , 12 , 13 Strategies that reduce BP and lipid levels simultaneously are likely to lead to a greater reduction in the incidence of CVD‐related events than treating either factor in isolation. 14
Treatment guidelines are increasingly recognizing the importance of modifying a patient's overall CV risk. 15 , 16 , 17 In practice, this can be achieved by treating patients to goals for both BP and lowdensity lipoprotein cholesterol (LDL‐C). However, despite the availability of many safe and efficacious treatments for lowering BP and lipids, the screening, diagnosis, and treatment rates of patients requiring antihypertensive and lipid‐lowering therapy are low. 18 , 19 , 20 , 21 , 22 , 23 , 24
One approach to help patients achieve goals for both BP and lipid disorders is the use of combination therapy to simultaneously target more than one CV risk factor. 8 Both amlodipine, a calcium channel blocker (CCB), and atorvastatin, a statin, have proven clinical benefits in the treatment of HTN and DYS, respectively, and have been shown to reduce CV events. 10 , 13 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The pharmacokinetics of these medications are favorable for combination into a single pill, with each possessing a long half‐life that enables once‐daily dosing, at any time of day, with or without food.34 An additional advantage of combining treatments into a single pill is the potential for an increase in patient adherence, since a lower pill burden and starting therapy simultaneously have been demonstrated to improve adherence to therapy. 35 , 36 , 37 , 38
The AVALON trial evaluated the efficacy and safety of coadministered amlodipine 5 mg and atorvastatin 10 mg as separate pills compared with either agent alone or placebo in the treatment of concomitant HTN and DYS in patients with varying degrees of CV risk.
METHOD
Design and Study Population
The AVALON trial was a multicenter, randomized, controlled trial conducted between February 2001 and May 2003 in 105 centers in the United States and Canada. Study participants were men and women, aged 18–75 years, of any ethnicity, with a diagnosis of concomitant HTN and DYS (defined in accordance with the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [JNC VI] 39 guidelines [the current standard when the study was designed] and the National Cholesterol Education Program Adult Treatment Panel III [NCEP ATP III] 15 guidelines, respectively). At screening, study participants were classified into one of three CV risk categories, based on the presence of other CV risk factors or coronary heart disease (CHD) in addition to HTN and DYS, and qualifying LDL‐C and BP measurements (Table–I). Patients taking antihypertensive and/or lipid‐lowering therapies completed a 2–6‐week washout period before randomization into the double‐blind phase of the study.
Table I.
Criteria for Allocation Into Cardiovascular (CV) Risk Categories and Recommended Blood Pressure (BP) and Low‐Density Lipoprotein Cholesterol (LDL‐C) Target Levels for Each Risk Group
| CV Risk Group | |||
|---|---|---|---|
| I | II | III | |
| CV risk factors* | HTN + DYS; no additional CV risk factors | HTN + DYS with ≥1 additional CV risk factor, excluding CHD and DM | HTN + DYS + CHD, DM, or other atherosclerotic disease |
| Entry criteria** | |||
| BP (mm Hg) | SBP 140–179 and/or DBP 90–109 | SBP 140–179 and/or DBP 90–109 | SBP 130–159 and/or DBP 85–99 |
| Fasting LDL‐C (mg/dL [mmol/L]) | 161–250 (4.1–6.5) | 131–250 (3.4–6.5) | 101–250 (2.6–6.5) |
| Treatment goals | |||
| BP (mm Hg)† | <140/90 | <140/90 | <130/85 |
| LDL‐C†† (mg/dL [mmol/L]) | <160(<4.1) | <130(<3.4) | <100(<2.6) |
| HTN=hypertension; DYS=dyslipidemia; DM=diabetes mellitus; SBP=systolic BP; DBP=diastolic BP; *includes: age ≥45 yr (male) or ≥55 yr (female), history of coronary heart disease (CHD) in parent or sibling before age 55 yr (male) or 65 yr (female), smoking (current), high‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL. Patients with HDL‐C ≥60 mg/dL required two additional CV risk factors; **average of measurements collected at two or three visits 7–14 days apart; †defined according to the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 39 except for group III patients, who were at lower goals for the purpose of this study; ††defined according to National Cholesterol Education Program Adult Treatment Panel III 15 | |||
Individuals were excluded if they had a history of intolerance to dihydropyridine CCBs and/ or statins. Additional exclusion criteria included pregnancy or lactation, impaired hepatic or renal function, a history of myocardial infarction within 6 months of screening, stroke, angina, coronary artery bypass or intracoronary interventions within 3 months of screening, a history of congestive heart failure or cardiomyopathy, hemodynamically significant valvular disease, secondary DYS or HTN of any etiology, diabetes mellitus, and any serious disorder that would interfere with the conduct of the study or interpretation of data.
Additional antihypertensive or lipid‐regulating therapies, apart from study medications, or drugs known to affect lipid levels or to alter the absorption/metabolism of the study medications, were not permitted.
AVALON consisted of four phases, the first of which was an 8‐week, randomized, double‐blind, double‐dummy, placebo‐controlled phase in which patients were randomized to receive once‐daily treatment with amlodipine 5 mg and atorvastatin 10 mg, or amlodipine 5 mg and placebo, or atorvastatin 10 mg and placebo, or placebo and placebo (Figure 1). The second phase of the trial was a further 8‐week, single‐blind phase in which all patients received once‐daily amlodipine 5 mg plus atorvastatin 10 mg. The third phase was a 12‐week, open‐label phase during which patients' therapy could be titrated to the maximum doses of amlodipine 10 mg and atorvastatin 80 mg in an effort to attain recommended goals for BP and LDL‐C (Table I). The final phase was a 64‐week, open‐label extension phase that will be reported separately. Compliance was assessed by a tablet count at each visit and was defined as taking 80%‐120% of the protocol‐specified study medication.
Figure 1.
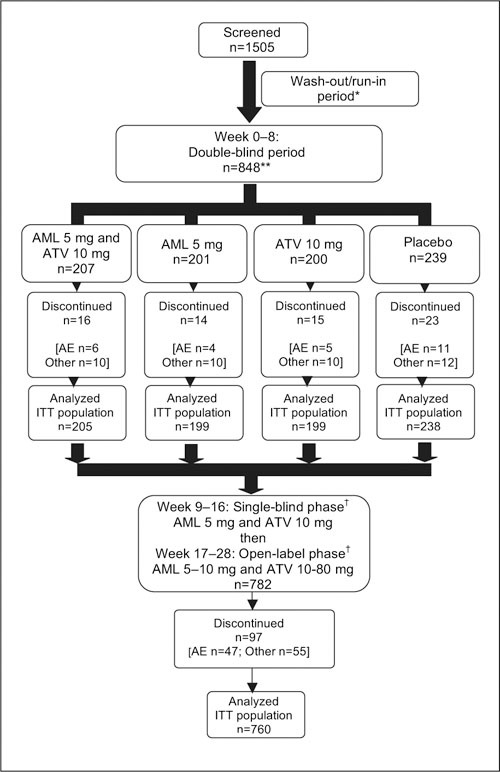
Study design and patient disposition. AE=adverse event; Other=reported lack of efficacy of drug, laboratory abnormalities, or patient defaulted (includes lack of treatment compliance); ITT=intent‐to‐treat; *duration 2–6 weeks depending on medication previously administered; **one patient randomized to treatment but did not receive drug; †amlodipine (AML) 5 mg and atorvastatin (ATV) 10 mg o.d. × 8‐week, single‐blind period, then up‐titrated to a maximum dose of AML 10 mg × ATV 80 mg, if required, during subsequent 12 weeks to achieve goals
Study End Points
The primary end points of this trial were to compare:
-
•
The percentage of patients in the amlodipine 5 mg plus atorvastatin 10 mg group to the percentage of patients in the atorvastatin 10 mg group who reached their recommended JNC VI BP goals at the end of Week 8
-
•
The percentage of patients in the amlodipine 5 mg plus atorvastatin 10 mg group to the percentage of patients in the amlodipine 5 mg group who reached their recommended NCEP ATP III LDL‐C goals at the end of Week 8 Secondary end points included:
-
•
The percentage of patients in each group who reached both NCEP ATP III and JNC VI goals at the end of each phase
-
•
The mean change from baseline to the end of each phase in BP and lipids (LDL‐C, total cholesterol, triglycerides, high‐density lipoprotein cholesterol [HDL‐C], and apolipoprotein B)
-
•
The change from baseline to the end of each phase in global risk factor score (converted to Framingham estimated 10‐year risk of CHD)
-
•
An evaluation of the safety profile of coadministered amlodipine and atorvastatin
The presence of CV risk factors was used to calculate the global risk factor score, based on a patient's gender, age, HDL‐C, BP, and smoking status. 40 Age and smoking were presumed to be constant during the study for the calculation. The global risk factor score was converted to the Framingham estimated 10‐year CHD risk percentage.
Safety
The severity, duration, date of onset, action taken (if any), and the suspected relationship to study drug of all adverse events (AEs) were recorded at each visit. The safety profile of coadministered amlodipine and atorvastatin treatment was compared with that of either treatment alone or placebo.
Statistical Analyses
The sample size, based on a two‐sided chi‐square test (p=0.05), was determined for the primary end point, such that a sample size of 1000 patients (250 patients per treatment group) would have approximately 90% power to detect an absolute 15% difference between coadministered amlodipine and atorvastatin compared with amlodipine therapy alone for reaching NCEP ATP III therapeutic goals, and 13% difference between coadministered amlodipine and atorvastatin compared with atorvastatin therapy alone for reaching JNC VI therapeutic goals.
Continuous data were summarized using descriptive statistics, and the treatment groups were compared using a 2 × 2 factorial analysis of covariance. Categoric data were analyzed using a Cochran‐Mantel‐Haenszel chi‐square test. Tests were two‐sided, with a significance level of α=0.05. All patients who were randomized and received at least one dose of assigned medication were included in the safety analysis. All patients who took at least one dose of the assigned treatment during each phase (double‐blind or single‐blind/open‐label) and had at least one efficacy assessment (BP or lipids) during this phase were evaluated for all efficacy analyses and constituted the intent‐to‐treat population. For patients withdrawn before the end of each phase, the last‐observation‐carried‐forward approach was used.
RESULTS
Study Population
Of the 1505 patients screened, 848 met the inclusion criteria and were randomized to double‐blind treatment, and 847 took at least one dose of the study medication to which they were assigned (Figure 1). The treatment groups were balanced at baseline with respect to gender, race, age, CV risk, BP, and lipid levels (Table II). There were no statistically significant differences among the four treatment groups in LDL‐C levels, BP, or global risk scores at baseline. The majority of study participants (94%) had at least one CV risk factor, diabetes mellitus, or CHD in addition to HTN and DYS (i.e., CV risk groups II or III). Most patients were in CV risk group II (76%) and very few patients (6%) were classified into CV risk group I (i.e., no CV risk factors in addition to HTN and DYS). Because of the high proportion of patients assigned to CV risk group II, all analyses were performed on the overall study population. There was no significant difference between the numbers of patients from each of the CV risk groups assigned to the different treatment groups (p=0.224). A total of 782 patients progressed into the single‐blind and open‐label phases of the study.
Table II.
Baseline Characteristics of Study Population Prior to Randomization to Double‐Blind Treatment Groups (Week 0) and Prior to Entry Into Single‐Blind Phase (at the End of Week 8)
| Prior to Randomization to Double‐Blind Treatment Groups | |||||
|---|---|---|---|---|---|
| Characteristic | AML 5 mg+ ATV 10 mg(n=207) | AML 5 mg (n=201) | ATV io mg(n=200) | Placebo(n=239) | Prior to Single‐Blind, Open‐Label(n=782) |
| Gender (n [%]) | |||||
| Male | 135 (65.2) | 117(58.2) | 111 (55.5) | 151 (63.2) | 471 (60.2) |
| Female | 72 (34.8) | 84 (41.8) | 89 (44.5) | 88 (36.8) | 311 (39.8) |
| Age (yr) (M [SD]) | 55.6 (9.9) | 56.2 (10.3) | 55.1 (9.3) | 55.3 (9.2) | 55.5 (9.7) |
| Race (n [%]) | |||||
| White | 173 (83.6) | 169 (84.1) | 167 (83.5) | 193 (80.8) | 655 (83.8) |
| Black | 24 (11.6) | 18 (9.0) | 22 (11.0) | 27(11.3) | 82 (10.5) |
| Asian | 6 (2.9) | 4 (2.0) | 2 (1.0) | 3 (1.3) | 13 (1.7) |
| Other | 4 (1.9) | 10 (5.0) | 9 (4.5) | 16 (6.7) | 32 (4.1) |
| Weight (kg) (M [SD]) | |||||
| Male | 95.0 (17.2) | 93.3 (18.5) | 96.1 (18.1) | 94.0 (16.7) | – |
| Female | 81.4 (17.2) | 81.2 (17.8) | 83.4 (17.4) | 82.2 (17.2) | – |
| Duration of primary | |||||
| diagnoses (yr) | |||||
| (M [range]) | |||||
| Hypertension | 5.9 (0.0–41.1) | 5.9 (0.0–37.7) | 5.6 (0.0–49.2) | 7.6 (0.0–51.5) | – |
| Dyslipidemia | 4.5 (0.0–30.7) | 4.2 (0.0–31.9) | 4.2 (0.0–25.5) | 4.6 (0.0–25.7) | – |
| CV risk group (n [%])* | |||||
| I | 14 (6.8) | 18 (9.0) | 11 (5.5) | 9 (3.8) | 49 (6.3) |
| II | 155 (74.9) | 143 (71.1) | 160 (80.0) | 189 (79.1) | 598 (76.5) |
| III | 38 (18.4) | 40 (19.9) | 29 (14.5) | 41 (17.2) | 135 (17.3) |
| Eligibility parameters | |||||
| (M [SD])* | |||||
| LDL‐C (mg/dL) | 163.9 (25.0) | 164.3 (26.0) | 161.7 (24.6) | 163.3 (24.8) | 133.6 (39.2) |
| Systolic BP (mm Hg) | 146.6 (12.3) | 147.6 (10.0) | 147.1 (10.9) | 146.7 (10.8) | 137.1 (14.0) |
| Diastolic BP (mm Hg) | 92.1 (7.2) | 92.6 (6.9) | 91.4 (7.7) | 92.4 (6.2) | 85.9 (9.0) |
| Framingham 10‐yr risk | 15.9 (8) | 15.0 (7.3) | 14.5 (6.7) | 15.1 (7.1) | 11.0(6.7) |
| of CHD(%) | |||||
| (M [SD]) | |||||
| Drug therapy before | |||||
| enrollment (n [%]) | |||||
| Antihypertensive only | 54 (26.1) | 57 (28.4) | 46 (23.0) | 65 (27.2) | – |
| Lipid‐lowering only | 4 (1.9) | 6 (3.0) | 2 (1.0) | 6 (2.5) | – |
| Both | 17 (8.2) | 22 (10.9) | 18 (9.0) | 20 (8.4) | – |
| None | 132 (63.8) | 116(57.7) | 134 (67.0) | 148 (61.9) | – |
| AML=amlodipine; ATV=atorvastatin; M=mean; CV=cardiovascular; LDL‐C=low‐density lipoprotein cholesterol; BP=blood pressure; CHD=coronary heart disease; *no statistically significant differences among treatment groups for these parameters | |||||
Prior Medications
Prior to study initiation, 37% of patients were receiving treatment with antihypertensive or lipid‐lowering therapy (Table II). Of these, 26% were receiving antihypertensive treatment only; 2% were receiving lipid‐lowering therapy only; and 9% were receiving both antihypertensive and lipid‐lowering therapy.
Patient Compliance With Study Medication
Only seven patients (two on amlodipine plus atorvastatin; two on amlodipine, two on atorvastatin, one on placebo) were reported to be noncompliant during the double‐blind phase. During the single‐blind and open‐label phases, 27 patients were noncompliant (3.5%) and therefore discontinued from the study.
Administration of Study Drug During the Open‐Label Phase
At Week 17, the start of the open‐label phase, the majority of patients were treated with amlodipine 5 mg (60.0%) and atorvastatin 10 mg (96.2%). At the end of Week 28, the mean doses of study treatment were 7.6 mg and 28.4 mg, respectively. Similar numbers of patients were treated with 5 mg or 10 mg of amlodipine (50.2% and 49.8%, respectively). With respect to atorvastatin, the majority of patients were treated with atorvastatin 10 mg (32.1%) or 20 mg (32.0%). Only 12.7% of patients received the highest dose of atorvastatin (80 mg), while 23.2% had been titrated to receive atorvastatin 40 mg.
Goal Attainment
At the end of 8 weeks of double‐blind treatment, 45.5% of patients coadministered amlodipine 5 mg and atorvastatin 10 mg had attained both their BP and LDL‐C therapeutic targets (Figure 2, left panel), which was significantly greater than those treated solely with either atorvastatin or amlodipine (p<0.001). At the end of 8 weeks, as expected, a significantly greater percentage of patients coadministered amlodipine 5 mg and atorvastatin 10 mg attained their JNC VI goal BP, compared with patients who had received atorvastatin 10 mg alone (p<0.001) (Figure 3, left panel). Similarly, as expected, significantly more patients who had received the two medications reached their NCEP ATP III goal LDL‐C, compared with patients who had received amlodipine 5 mg alone (p<0.001) (Figure 4, left panel).
Figure 2.
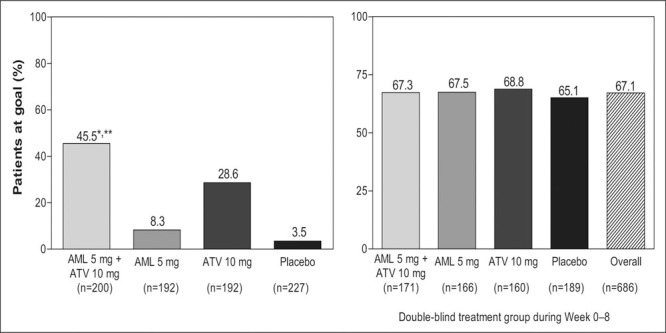
Percentage of patients within the four different treatment groups that reached target levels for both blood pressure and low‐density lipoprotein cholesterol at Week 8 (left panel) and Week 28 (right panel, stratified by the original randomized double‐blind treatment groups); data for risk groups I, II, and III combined. At Week 28, all patients were receiving coadministered amlodipine (AML) and atorvastatin (ATV). *p<0.001 vs. amlodipine alone; **p<0.001 vs. atorvastatin alone
Figure 3.
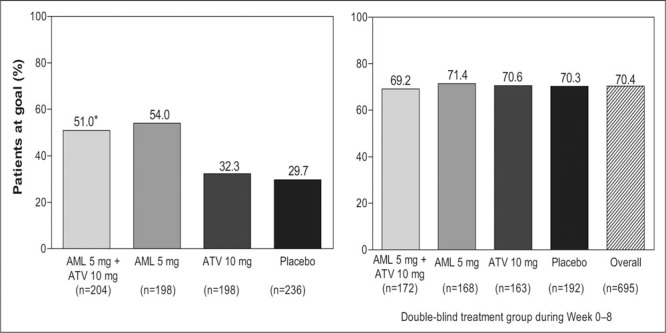
Percentage of patients within the four different treatment groups that reached target levels for both blood pressure and low‐density lipoprotein cholesterol at Week 8 (left panel) and Week 28 (right panel, stratified by the original randomized double‐blind treatment groups); data for risk groups I, II, and III combined. At Week 28, all patients were receiving coadministered amlodipine (AML) and atorvastatin (ATV). *p<0.001 vs. amlodipine alone; **p<0.001 vs. atorvastatin alone
Figure 4.
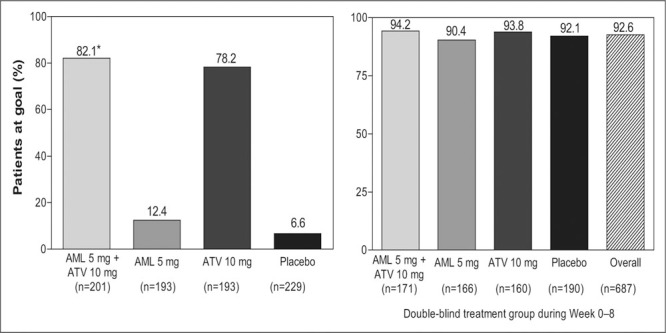
Percentage of patients within the four different treatment groups that reached target levels for low‐density lipoprotein cholesterol at Week 8 (left panel) and Week 28 (right panel, stratified by the original randomized double‐blind treatment groups); data for risk groups I, II, and III combined. At Week 28, all patients were receiving coadmin‐istered amlodipine (AML) and atorvastatin (ATV). *p<0.001 vs. amlodipine alone
At the end of the single‐blind phase (Week 16), when all patients had been treated with coadministered amlodipine 5 mg and atorvastatin 10 mg for 8 weeks, 48.5% of all patients achieved both BP and LDL‐C therapeutic goals, while 55.3% of patients achieved BP goals and 81.0% achieved LDL‐C goals. At the end of the open‐label phase (Week 28), coadministered amlodipine and atorvastatin (dosage titrated to achieve BP and LDL‐C goals) resulted in 70.4% of patients achieving BP, LDL‐C (92.6%), or both (67.1%) therapeutic goals. There were no significant differences in goal attainment at Week 16 or 28 when patients were stratified by their original double‐blind randomization treatment groups (2, 3, 4).
Effect on BP
At Week 8, patients coadministered amlodipine 5 mg and atorvastatin 10 mg, as expected, experienced a significantly greater reduction in BP (−12.7/−8.2 mm Hg) when compared with patients treated with atorvastatin alone (−5.9/−4.2 mm Hg) or placebo (−5.4/−3.3 mm Hg) (p<0.001). The mean treatment effect (after accounting for the placebo treatment effect) was −7.3/−4.9 mm Hg for coadministered amlodipine 5 mg and atorvastatin 10 mg, compared with −8.9/−5.6 mm Hg for amlodipine alone. Thus, mean reductions in BP were similar among patients treated with coadministered amlodipine and atorvastatin therapy and those treated with amlodipine alone (amlodipine alone, −14.3/−8.9 mm Hg).
At the end of the single‐blind phase (Week 16), treatment with amlodipine 5 mg and atorvastatin 10 mg was associated with a mean change in BP of −13.1/−8.1 mm Hg from baseline in all patients (n=760). From baseline to the end of the open‐label phase (Week 28), the mean change in BP was −16.3/−10.6 mm Hg. From Week 17 to Week 28, titrating the dosages of coadministered amlodipine and atorvastatin led to an additional reduction in mean BP of −3.0/−2.2 mm Hg. The mean decrease in BP at Week 28 was similar in all groups when patients were stratified by their original double‐blind treatment groups (Figure 5).
Figure 5.
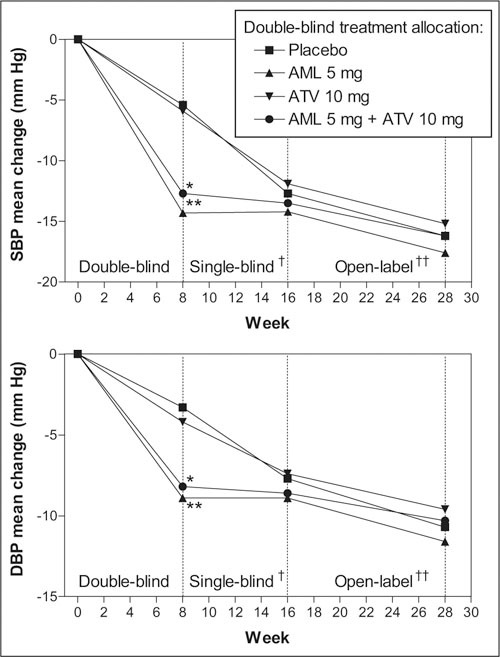
Least square mean changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) among the four treatment groups; data for groups I, II, and III combined. *p<0.001 vs. placebo; **p<0.001 vs. atorvastatin alone; †all patients received coadministered amlodipine (AML) 5 mg and atorvastatin (ATV) 10 mg; †all patients received coadministered AML 5–10 mg and ATV 10‐80 mg titrated to achieve goals
Effects on Blood Lipid Levels
At Week 8, as expected, coadministered amlodipine 5 mg and atorvastatin 10 mg treatment had significantly greater effects on all lipid parameters compared with treatment with amlodipine alone or placebo (p<0.001) (Table III). In comparison with atorvastatin alone, coadministered amlodipine 5 mg and atorvastatin 10 mg led to significantly greater reductions in LDL‐C (p=0.007), total cholesterol (p<0.001), and apolipoprotein B (p=0.013). Patients treated with atorvastatin therapy alone showed significantly greater changes in all lipid parameters compared with placebo‐treated patients (pp<0.001). For LDL‐C reductions, the mean treatment differences, after accounting for the placebo treatment effect, were −61.1 mg/dL for coadministered amlodipine and atorvastatin, −56.5 mg/dL for atorvastatin alone, and −3.4 mg/dL for amlodipine alone.
Table III.
Least Square Mean Percentage Changes (+ Standard Error) From Baseline in Lipid Parameters of Entire Study Population (Risk Groups I, II, and III Combined)
| Double‐Blind Treatment Group* | |||||
|---|---|---|---|---|---|
| Lipid Parameter | AML 5 mg+ ATV 10 mg | AML 5 mg | ATV 10 mg | Placebo | Single‐Blind, Open‐Label** |
| LDL‐C | −37.2±0.9†,†† | −1.8±0.9 | −33.9±0.9‡ | 0.2±0.8 | −41.0±0.4 |
| Patients (n) | 201 | 193 | 193 | 229 | 687 |
| Total cholesterol | −27.7±0.6‡‡†† | −2.1±0.6 | −24.4±0.6‡ | −0.9±0.6 | −31.0±0.4 |
| Patients (n) | 201 | 193 | 193 | 229 | 687 |
| Triglycerides | −23.0*±2.2†† | −2.3±2.3 | −17.2±23‡ | −0.1±2.1 | −29.0±1.1 |
| Patients (n) | 201 | 193 | 193 | 229 | 687 |
| HDL‐C | 5.0±1.0†† | 0.0±1.0 | 4.1±1.0§ | 0.2±0.9 | 5.2±0.6 |
| Patients (n) | 201 | 193 | 193 | 229 | 687 |
| Apolipoprotein B | −30.7±0.8§§†† | −1.7±0.8 | −27.9±0.8‡ | −1.0±0.7 | −34.5±0.4 |
| Patients (n) | 189 | 185 | 183 | 210 | 687 |
| LDL‐C=low‐density lipoprotein cholesterol; HDL‐C=high‐density lipoprotein cholesterol; *from baseline to the end of the doubleblind phase (Week 8); **from baseline to the end of the open‐label phase (Week 28); † p=0.007 vs. atorvastatin alone; †† p<0.001 vs. amlodipine alone; ‡ p<0.001 vs. placebo; ‡‡ p<.001 vs. atorvastatin alone; § p=0.005 vs. placebo; §§ p=0.013 vs. atorvastatin alone | |||||
At Week 28, coadministered amlodipine and atorvastatin led to incremental improvements in all lipid parameters from baseline (Table III). Changes in lipid parameters were generally similar in all groups when stratified by their original double‐blind, randomized treatment group.
Reduction in Estimated Framingham 10‐Year CHD Risk
At Week 8, coadministered amlodipine 5 mg and atorvastatin 10 mg treatment resulted in a significant (p<0.0001) reduction in the mean Framingham estimated 10‐year CHD risk, from 15.9% at baseline to 8.6% (an absolute reduction of 7.3%) in those patients categorized into CV risk groups I and II (n=178). Furthermore, the reduction in Framingham score was significantly greater (p<0.0001) than treatment with either drug alone. At the end of Week 28, the mean Framingham risk score was reduced to 6.9% in patients from CV risk groups I and II (n=629) from baseline levels of 15.1%.
Safety and Tolerability
During the double‐blind phase, the rate of discontinuations for any reason was similar following treatment with the coadministration of the two medications (7.7%), amlodipine 5 mg alone (7.0%), and atorvastatin 10 mg alone (7.5%). Treatment with placebo led to a slightly higher rate of discontinuations (9.6%). Only 26 patients discontinued treatment due to an AE during double‐blind treatment (six coadministered amlodipine 5 mg and atorvastatin 10 mg, four amlodipine alone, five atorvastatin alone, and 11 placebo). During the single‐blind and open‐label phases, 97 (12.4%) patients discontinued treatment for any reason and 47 (6%) patients discontinued from the study due to at least one AE. The majority of these discontinuations due to AEs were because of peripheral edema (2%) or myalgia (0.5%).
During the double‐blind phase, a total of 435 (51.4%) patients reported at least one incidence of an AE, which was similar across all treatment groups (amlodipine and atorvastatin treatment, 50.2%; amlodipine treatment alone, 50.7%; atorvastatin treatment alone, 52.0%; placebo, 52.3%). The proportion of patients who had at least one treatment‐emergent AE was similar across the four treatment groups (Table IV). The most common AEs reported among patients coadministered amlodipine 5 mg and atorvastatin 10 mg vs. placebo‐treated patients during the double‐blind phase were peripheral edema (5.3% vs. 2.1%), myalgia (4.8% vs. 2.1%), and sinusitis (2.9% vs. 0.8%) (Table IV). During the single‐blind and open‐label phases, the most frequently reported AEs were peripheral edema (17.0%), respiratory tract infection (13.7%), arthralgia (7.4%), and headache (6.8%) (Table IV).
Table IV.
Incidence of Treatment‐Emergent Adverse Events (All Causes, Occurring in ≥2% of Combination‐Treated Patients During Weeks 0–8)
| Double‐Blind Treatment Group* | |||||
|---|---|---|---|---|---|
| Adverse Event (n [%]) | AML 5 mg+ ATV | AML 5 mg | ATV 10 mg | Placebo | Single‐Blind, Open‐Label** (n=775) |
| Respiratory infection | 15 (7.2) | 17 (8.5) | 12 (6.0) | 17 (7.1) | 107 (13.7) |
| Headache | 14 (6.8) | 14 (7.0) | 20 (10.0) | 24 (10.0) | 53 (6.8) |
| Peripheral edema | 11 (5.3) | 11 (5.5) | 1 (0.5) | 5 (2.1) | 133 (17.0) |
| Myalgia | 10 (4.8) | 5 (2.5) | 5 (2.5) | 5 (2.1) | 33 (4.2) |
| Accidental injury | 8 (3.9) | 3(1.5) | 5 (2.5) | 6 (2.5) | 52 (6.6) |
| Asthenia | 7 (3.4) | 6 (3.0) | 8 (4.0) | 11 (4.6) | 45 (5.8) |
| Sinusitis | 6 (2.9) | 2 (1.0) | 2 (1.0) | 2 (0.8) | 26 (3.3) |
| Pain | 5 (2.4) | 5 (2.5) | 4 (2.0) | 5 (2.1) | 36 (4.6) |
| Flatulence | 5 (2.4) | 3(1.5) | 6 (3.0) | 6 (2.5) | 9 (1.2) |
| Arthralgia | 4 (1.9) | 9 (4.5) | 3(1.5) | 8 (3.3) | 58 (7.4) |
| *Weeks 0–8; **Weeks 9–28 | |||||
Serious AEs were experienced by 12 patients during the double‐blind phase and 18 patients during the single‐blind or open‐label phases. None of these serious AEs were considered by the investigator to be related to the study medications.
DISCUSSION
The AVALON trial demonstrates that the coadministration of amlodipine with atorvastatin over a short term of 7 months is a safe and effective treatment for patients with various levels of CV risk. The majority of study participants had, in addition to HTN and DYS, at least one other CV risk factor and were, therefore, considered at higher risk of CVD, and classified into CV risk groups II and III. The high frequency of concomitant CV risk factors observed in this study population is not surprising, given the tendency of CV risk factors to cluster in patients with HTN. 41
Patients who received coadministered amlodipine and atorvastatin therapy were significantly more likely to attain recommended therapeutic goals for both BP and LDL‐C compared with patients taking either therapy alone or placebo. At the end of Week 8, nearly half of all patients who received combined amlodipine 5 mg and atorvastatin 10 mg achieved their BP and LDL‐C goals, and at the end of Week 28 (when the mean dosages were amlodipine 7.6 mg and atorvastatin 28.4 mg), over two thirds of all patients achieved both BP and LDL‐C therapeutic goals. The present study confirms the results of a previously reported trial in which amlodipine and atorvastatin were coadministered as a single pill, 34 a treatment approach that may improve the goal achievement rates currently observed for HTN and DYS. 20 , 21 , 42 , 43 , 44 , 45
In the current study, coadministration with atorvastatin did not affect the BP‐lowering efficacy of amlodipine. However, coadministration of amlodipine 5 mg and atorvastatin 10 mg led to a significantly greater effect on LDL‐C, total cholesterol, and apolipoprotein B levels at Week 8, compared with atorvastatin treatment alone. This finding was unexpected, and further studies would be needed to explore this observation.
Significant reductions in Framingham risk score were observed. Framingham 10‐year risk estimates have been included in recent treatment guidelines, and have been used to assess the benefits of multi‐factorial interventions on a patient's CV risk factor profile. 46 , 47 The coadministration of a CCB and a statin in this study resulted in a reduction in the 10‐year CHD risk among patients without existing CHD or risk equivalent (i.e., CV risk groups I and II). These results were comparable to risk score reductions observed in the Respond trial, 48 which found a mean decrease in 10‐year CHD risk of almost 9 percentage points following 8 weeks of combined amlodipine 5 mg and atorvastatin 10 mg treatment, and a mean decrease of 11% when the doses of amlodipine and atorvastatin were increased to 10 and 80 mg, respectively. 48 It should be noted that reductions in estimated CHD risk predicted from the present study are theoretic and may not necessarily translate into real reductions in CV events, since this apparent risk reversal is based on short‐term therapy. These CHD risk‐reduction predictions could be validated only in prospective randomized trials, rather than assessing risk estimates from epidemiologic data. It is possible that the drug combination utilized in this study may influence other factors that are not included in the Framingham risk equations.
Combination treatment with amlodipine and atorvastatin for up to 28 weeks was safe and well tolerated. The reported incidences of peripheral edema and myalgia in the patients coadministered these two medications are of similar or lower frequency to those reported for amlodipine 49 and atorvastatin 50 individually.
Over the past 5 years, several trials have demonstrated that amlodipine and atorvastatin are effective in significantly reducing CV events, in addition to improving BP and lipids to target levels, respectively. 13 , 25 , 26 , 28 , 29 , 51 Our results appear to confirm that concurrent treatment of multiple risk factors can improve a patient's CV risk factor profile 10 , 11 , 12 , 52 and reduce the Framingham risk score. This approach, if adopted by physicians, is likely to improve the management of patients at risk for CVD. Initiating statin treatment with antihypertensive therapy has been suggested as a positive step toward treating a patient's overall CV risk. 9
The results from the AVALON study, with those from the Respond trial, 48 , 53 reinforce the efficacy and safety of coadministering amlodipine and atorvastatin for simultaneous treatment of HTN and DYS, and highlight the suitability of combining these two agents into a single pill for the effective management of CVD risk. Whether the results of the AVALON study apply to other combinations of dihydropyridine CCBs and statins can be determined only with additional trials
Acknowledgments and disclosure: The authors wish to acknowledge the support of the individual principal investigators at each site (see Appendix) and Pia Bruscbi, MS (Pfizer Inc., New York, NY), Colleen Gorman, BS (Pfizer Inc., New York, NY), Caridad Casteneda, BA (Pfizer Inc., New York, NY), David Smith, MD (Integrium, Tustin, CA), Marisa Cesari, RN (Pfizer Canada), and Suzanne Ranger, PhD (Pfizer Canada). The authors also wish to thank Sarah Shearing, PhD for her editorial support during the development of the manuscript. This study was supported by Pfizer Inc.
APPENDIX
Principal investigators in the AVALON study: D.C. Abella, MD; J.D. Altholz, MD; M.‐C. Audet, MD; G.L. Bakris, MD; L. Breger, MD; M.E. Brewer, MD, PhD; CD. Brown, MD; MX. Buchhuber, MD; M. Budoff, MD; E. Burgess, MD; R.J. Butcher, MD; D.K. Buth, MD; A.A. Carr, MD; J.E. Carter Jr., MD; N.F. Carulli, MD; R.S. Castaldo, MD; D. Cheung, MD; J.N. Cohn, MD; F.N. Cole, MD; G.E. Cooke, MD; R.W. Craft Sr., MD; F.E. Cummins, MD; V.B. Dave, MD; M.H. Davidson, MD; M.I. DeBruin, DO; J.M. DeCara, MD; A. DeFranco, MD; J.J. DelGiomo, MD; D. Dion, MD; E.M. Dodin, MD; H.J. Downey, MD; W. Drummond, MD; S.E. ElHafi, MD; F. Elijovich, MD; S.K. Elliot, MD; D.L. England, MD; D. Strutin, MD; K.W. Faistl, MD; C. Ferrario, MD; R.D. Ferrera, MD; J.A. Fialkow, MD; J.I. Fidelholtz, MD; J.M. Flack, MD, MPH; H. Fleming, MD; J.G. Fodor, MD; C. Fortin, MD; N.J. Fraser, MD; C. Gagne, MD; D. Gaudet, MD, PhD; G. Girard, MD; S. Glasser, MD; D. Gossard, MD; D.W. Gottlieb, MD; G.M. Gottschlich, MD; R. Grimm, MD, PhD; J.‐M. Guise, MD, MPH; J.S. Hagedorn, MD; I.U. Haque, MD; B.J. Harrison, MD; G.P. Heyrich, MD; J.F. Holcomb, MD; J.J. Holland, MD; J.C. Holler, MD; J. Holtzman, MD, PhD; M.C. Houston, MD; MX. Issacson, MD; B.K. Jackson, MD; R.A. Kaplan, MD; HX. Katzeff, MD; E.M. Kerwin, MD; M.A. Khan, MD; R. Khant, MD; N. Kopyt, MD; M.J. Koren, MD; D.J. Korn, MD; M.E. Kutner, MD; Y. Lacourciere, MD; A. Lade, MD; B. Lasko, MD; T.R. Lee, MD; L. Leiter, MD; T.W. Littlejohn III, MD; I.K. Loh, MD; H. Luque, DO; R.D. Madder, DO; F.P. Maggiacomo, DO; M.J. McCartney, MD; D.C McCluskey, MD; J.F. McNeer, MD; F.H. Messerli, MD; S.V. Michael, MD; A.B. Miller, MD; JX. Miller, MD; R.E. Mills, MD; K.M. Mimnagh, MD; A.M Mitchell, MD; M.I. Mulford, MD; P. Narayan, MD; J. Neutel, MD; JG. Nuckolls, MD; E.O. Ofili, MD, MPH; S. Oparil, MD; J.‐P. Ouellet, MD; J.A. Peacock, MD; G.E. Peterson, DO; R.J. Petrella, MD; R.W. Powell, MD; H.A. Punzi, MD, FCP; A. Puopolo, MD; S. Qadir, MD; D.M. Quillen, MD, PhD; R. Ramirez, MD; B.G. Rankin, DO; R.A. Reines, MD; TX. Richardson, MD; K.W. Rictor, MD; L.D. Riffel, MD; S. Rockson, MD; L.S. Sadler, MD; E. Saunders, MD; M.R. Seidner, MD; I. Pendrak, DO; A.M.M. Shepherd, MD, PhD; L.D. Sher, MD; K.D. Sherif, MD; D. Shu, MD; J. Siemienczuk, MD; W.D. Sinclair, MD; W.B. Smith, MD; B.D. Snyder, MD; D. Spence, MD; RX. Stoltz, MD; K.A. Stone, MD; J.A. Strom, MD; D.H. Sugimoto, MD; A.A. Taylor, MD, PhD; G. Tellier, MD; J.C. Thomas, MD; S. Tobe, MD; D.K.H. Tweel, MD; J. Vassall, MD; J.B. Vazquez‐Tanus, MD; R.M. Vicari, MD; N.K. Vijay, MD; L. Warrick, MD; M.H. Weinberger, MD; R.J. Weiss, MD; P.F. Whitsitt, MD; W Wickenmeyer, MD; M.R. Wofford, MD, MPH; N. Wright, MD; F. Yemofio, MD; H. Zaharowitz, MD; J.H. Zavoral, MD; J.J. Zerbe, MD
References
- 1. World Health Organization . The World Health Report 2003: Shaping the Future. Geneva, Switzerland: World Health Organization;2003. [Google Scholar]
- 2. Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics‐2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–el51. [DOI] [PubMed] [Google Scholar]
- 3. Flack JM, Neaton J, Grimm R Jr, et al. Blood pressure and mortality among men with prior myocardial infarction. Multiple Risk Factor Intervention Trial Research Group. Circulation. 1995;92:2437–2445. [DOI] [PubMed] [Google Scholar]
- 4. Greenlund KJ, Zheng ZJ, Keenan NL, et al. Trends in selfreported multiple cardiovascular disease risk factors among adults in the United States, 1991–1999. Arch Intern Med. 2004;164:181–188. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens. 2000;14:83–90. [DOI] [PubMed] [Google Scholar]
- 6. Thomas F, Bean K, Guize L, et al. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002;23:528–535. [DOI] [PubMed] [Google Scholar]
- 7. Neaton JD, Wentworth D. Serum cholesterol, blood pres sure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group . Arch Intern Med. 1992;152:56–64. [PubMed] [Google Scholar]
- 8. Jackson R, Lawes CM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet. 2005;365:434–441. [DOI] [PubMed] [Google Scholar]
- 9. Williams B. Recent hypertension trials: implications and controversies. J Am Coll Cardiol. 2005;45:813–827. [DOI] [PubMed] [Google Scholar]
- 10. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid Lowering Arm (ASCOT‐LLA) a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 11. Gaede P, Vedel P, Larsen N, et al. Multifactorial interven tion and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 12. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in moder ately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT‐LLT). JAMA. 2002;288:2998–3007. [DOI] [PubMed] [Google Scholar]
- 13. Dahlöf B, Sever PS, Poulter N, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 14. Wong ND, Pio JR, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol. 2003;91:1421–1426. [DOI] [PubMed] [Google Scholar]
- 15. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 16. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Clin Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 17. Giles TD, Berk BC, Black HR, et al. Expanding the defini tion and classification of hypertension. J Clin Hypertens (Greenwich). 2005;7:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turnbull F. Managing cardiovascular risk factors: the gap between evidence and practice. PLoS Med. 2005;2:el31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Wyk JT, Picelli G, Dieleman J, et al. Management of hypertension and hypercholesterolaemia in primary care in the Netherlands. Curr Med Res Opin. 2005;21:839–848. [DOI] [PubMed] [Google Scholar]
- 20. Johnson ML, Pietz K, Battleman DS, et al. Therapeutic goal attainment in patients with hypertension and dyslipidemia. Med Care. 2006;44:39–46. [DOI] [PubMed] [Google Scholar]
- 21. Wolf‐Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. [DOI] [PubMed] [Google Scholar]
- 22. O'Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dys lipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–1318. [DOI] [PubMed] [Google Scholar]
- 23. Ford ES, Mokdad AH, Giles WH, et al. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–2189. [DOI] [PubMed] [Google Scholar]
- 24. De Backer G, for the EUROASPIRE II Study Group . Evidence‐based goals versus achievement in clinical practice in secondary prevention of coronary heart disease: findings in EUROASPIRE II. Atheroscler Suppl. 2002;2:13–17. [DOI] [PubMed] [Google Scholar]
- 25. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 26. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292:2217–2225. [DOI] [PubMed] [Google Scholar]
- 27. Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. [DOI] [PubMed] [Google Scholar]
- 28. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 29. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐con trolled trial. Lancet. 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 31. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. [DOI] [PubMed] [Google Scholar]
- 32. Koren MJ, Hunninghake DB. Clinical outcomes in managed‐care patients with coronary heart disease treated aggressively in lipid‐lowering disease management clinics: the ALLIANCE study. J Am Coll Cardiol. 2004;44:1772–1779. [DOI] [PubMed] [Google Scholar]
- 33. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 34. Blank R, LaSalle J, Reeves R, et al. Single‐pill therapy in the treatment of concomitant hypertension and dyslipid‐emia (the Amlodipine/Atorvastatin Gemini Study). J Clin Hypertens (Greenwich). 2005;7:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care. 2000;9:2–6. [PubMed] [Google Scholar]
- 36. Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid‐lowering therapy. Arch Intern Med. 2005;165:1147–1152. [DOI] [PubMed] [Google Scholar]
- 37. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 38. Nichol M, Patel B, Thiebaud P, et al. A single pill com bining antihypertensive and statin therapies improves patient adherence compared with multi‐drug combinations: results from the Caduet Adherence Research Program and Education (CARPE) ‐PBM Adherence Study [abstract]. J Clin Hypertens. 2006;8:456. Abstract P‐526A. [Google Scholar]
- 39. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 40. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 41. Wilson PW, Kannel WB, Silbershatz H, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. [DOI] [PubMed] [Google Scholar]
- 42. Wong ND, Lopez V, Franklin S, et al. Prevalence, treatment status, and control of concomitant hypertension and dyslip‐idemia in US adults in 2001–2002 [abstract]. Circulation. 2005;112(suppl 2):831. [Google Scholar]
- 43. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 44. Muls E, De Backer G, Brohet C, et al. The efficacy of atorvastatin in treating patients with hypercholesterolaemia to target LDL‐cholesterol goals: the LIPI‐GOAL trial. Acta Cardiol. 2001;56:109–114. [DOI] [PubMed] [Google Scholar]
- 45. Lipitor (atorvastatin calcium) tablets. US Prescribing Information. New York, NY: Pfizer Inc;2005. [Google Scholar]
- 46. Lindholm LH, Ekbom T, Dash C, et al. Changes in car diovascular risk factors by combined pharmacological and nonpharmacological strategies: the main results of the CELL Study. J Intern Med. 1996;240:13–22. [DOI] [PubMed] [Google Scholar]
- 47. Hellenius ML, de Faire U, Berglund B, et al. Diet and exer cise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis. 1993;103:81–91. [DOI] [PubMed] [Google Scholar]
- 48. Preston RA, Harvey P, Herfert O, et al. Reduction in Framingham cardiovascular risk with concomitant treatment of hypertension/dyslipidemia with amlodipine/atorvastatin [abstract]. Am J Hypertens. 2005;18:A226. [Google Scholar]
- 49. Norvasc (amlodipine besylate) tablets. US Prescribing Information. New York, NY: Pfizer Inc; 2005. [Google Scholar]
- 50. Newman C, Tsai J, Szarek M, et al. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97:61–67. [DOI] [PubMed] [Google Scholar]
- 51. Jones PH, McKenney JM, Karalis DG, et al. Comparison of the efficacy and safety of atorvastatin initiated at different starting doses in patients with dyslipidemia. Am Heart J. 2005;149:el–e8. [DOI] [PubMed] [Google Scholar]
- 52. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22. 12114036 [Google Scholar]
- 53. Preston RA, Harvey P, Herfert O, et al. The efficacy and safety of fixed‐dose combinations of amlodipine and atorvastatin in the treatment of patients with concomitant hypertension and dyslipidemia [abstract]. Am J Hypertens. 2004;17:185A. [Google Scholar]


