Abstract
Hypertensive heart disease encompasses anatomical changes and altered physiology of heart muscle, coronary arteries, and great vessels. Left ventricular hypertrophy is not only a target organ response to increased afterload, but is also the most potent cardiovascular risk factor. Regression of hypertrophy reduces morbidity and mortality. Heart failure may be present in the absence of a reduction of myocardial contractility. Ischemic heart disease occurs in the absence of epicardial coronary disease. Left atrial size and atrial fibrillation are associated. Potentially lethal ventricular arrhythmias and sudden cardiac death are more common in hypertensive patients. The relationship of aortic root size to blood pressure is weaker than expected; however, the relationship to aortic dissection is stronger. Careful attention and treatment of left ventricular hypertrophy, heart failure, ischemic heart disease, and atrial fibrillation will improve survival.
Hypertensive heart disease is the target organ response of systemic arterial hypertension. However, it is more than left ventricular hypertrophy (LVH) or heart failure (HF). It also includes ischemic heart disease, aortic root disease, left atrial enlargement, and arrhythmias.
LEFT VENTRICULAR HYPERTROPHY Pathophysiology
An increase in peripheral vascular resistance, the hallmark of established hypertension, alters wall stress in the left ventricle (Figure). Concentric LVH is the consequence of the neutralization of wall stress associated with increased impedance to ventricular emptying. The increase in wall thickness follows the law of Laplace (Figure). Wall stress stimulates sarcomeres to proliferate in parallel by increasing protein synthesis, which increases myocyte width. The net effect is an increase in the wall thickness/chamber dimension or relative wall thickness. However, when hypertrophy can no longer compensate for the increased afterload, then LV dilatation (eccentric hypertrophy) occurs and LV performance decreases (Figure).
Figure 1.
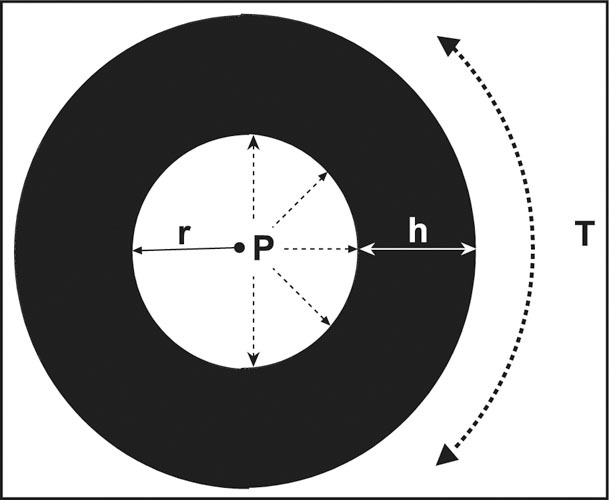
The Law of Laplace follows the formula, T=P x r/h: circumferential wall stress (T)=transmural distending pressure (P) x radius of chamber (r)÷wall thickness (h). To compensate for an increase in left ventricular pressure, wall thickness increases to reduce wall stress.
Figure 2.
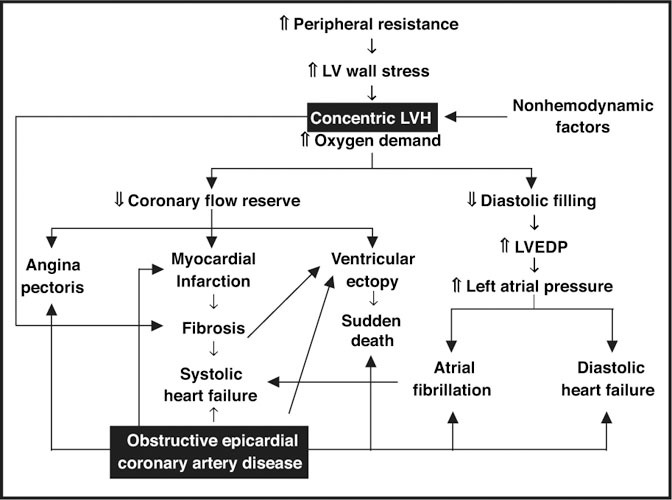
Pathophysiology of hypertensive heart disease. Common manifestations of hypertensive heart disease are displayed; note the similarity of manifestations of coronary artery disease and hypertensive heart disease. LV=left ventricular; LVH=left ventricular hypertrophy; LVED p=left ventricular end‐diastolic pressure
Ambulatory blood pressure (BP) correlates better with LVH than BP measured at a single visit or multiple office visits. However, a single ambulatory BP cannot assess the total impact of BP over months or years. However, despite the lack of long durations of assessment of BP, other nonhemodynamic variables (Figure), including body mass index, age, physical activity, sodium, parathyroid hormone, growth hormone, thyroid hormone, thyroid‐stimulating hormone, norepinephrine, renin, aldosterone, angiotensin II, intracellular calcium, atrial natriuretic peptide, blood viscosity, and arterial compliance correlate with LVH. In addition, other factors, such as obesity, can modify the heart's response to pressure overload.
Consequences
As the ventricle becomes thicker, the elasticity diminishes. Thus, filling during diastole becomes more difficult and depends on atrial systole. As a consequence, LV end‐diastolic pressure and left atrial pressure increases, resulting in enlargement of the left atrium, pulmonary venous congestion, and pulmonary congestion (Figure). Oxygen demand and basal coronary blood flow increases as myocardial hypertrophy increases; however, coronary blood flow per unit mass of muscle at rest is normal. Only with exercise or severe LVH will subendocardial blood flow be jeopardized, potentially causing myocardial infarction (MI) and fibrosis. Intramural small vessel disease has been shown to be present in patients with LVH.
Uncontrolled hypertension may be associated with HF, even with preserved LV function (diastolic HF). Increased LV end‐diastolic pressure and increased impedance to LV outflow result in the acute pulmonary congestion. However, with long‐standing untreated hypertension and associated MI, fibrosis and LV dilatation contractility diminishes and systolic HF ensues (Figure). The dilated left atrium and pulmonary veins that occur from increased LV end‐diastolic pressure causes atrial premature beats and atrial fibrillation (AF) (Figure). The presence of myocardial fibrosis predisposes patients to ventricular ectopy, as well as potentially lethal arrhythmias that may result in sudden cardiac death.
Clinical Findings
Most patients are asymptomatic with LVH. However, dyspnea, angina, HF, syncope, and sudden death can occur. The classic physical findings include an abnormal apical impulse and the S4 gallop. Normally, in the supine position: 1) the apical impulse is within the midclavicular line in the forth or fifth LV intercostal space, 2) the amplitude is small and brief in duration, and 3) the palpable area is less than 2.5 cm 2 . A sustained, enlarged (>3 cm diameter) apical impulse, which may be displaced outside the midclavicular line, is characteristic of isolated LVH. Using ultrafast computed tomographic cardiac measurements, the physical examination findings of a percussion distance greater than 10.5 cm in the sixth left intercostal space to detect an abnormal posterior wall thickness was 100% sensitive and 50.5% specific and an apical impulse greater than 3 cm to detect an increased left ventricular mass (LVM) was 100% sensitive and 40% specific.
An S4 gallop, best heard with the bell of the stethoscope in the left lateral decubitus position, is common in chronic hypertension. It reflects the decreased elasticity of a hypertrophied ventricle during the late filling of the ventricle following the atrial contraction. Occasionally, it may be palpable.
Diagnosis
The two most common tools used to define LVH are the electrocardiogram (ECG) and the echocardiogram. Each has prognostic significance, but provides different data. Echocardiography supplies precise information about LV wall thickness, left atrial size, LV function, and wall‐motion abnormalities. The ECG provides unique information on rhythm disturbances, hyperkalemia, PR interval, and QT interval that suggest a diagnosis or alteration of treatment.
ELECTROCARDIOGRAM
The ECG is often used as an inexpensive, initial screening tool to assess target organ damage in a hypertensive patient. It can be used to assess the presence of left atrial enlargement, LVH, MI, myocardial ischemia, ventricular premature beats, and AF. Left atrial enlargement—one of the earliest ECG findings of hypertensive heart disease—is said to be present if the terminal portion of the P wave has a duration of 0.04 seconds and a depth of 1 mm or more or their product is ≥−0.04 mm × sec (Figure). ECG LVH with “strain pattern” is the most lethal classic Framingham cardiovascular risk factor (4, 5, 6, 7).
Figure 3.
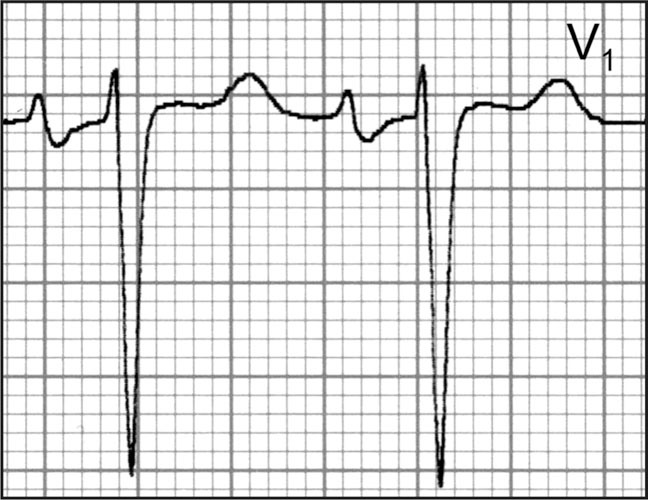
Left atrial enlargement is one of the earliest electrocardiographic findings of hypertensive heart disease. It is present if the terminal portion of the P wave has a duration of 0.04 seconds and a depth of 1 mm or more, or their product is ≥‐0.04 mm X s.
Figure 4.
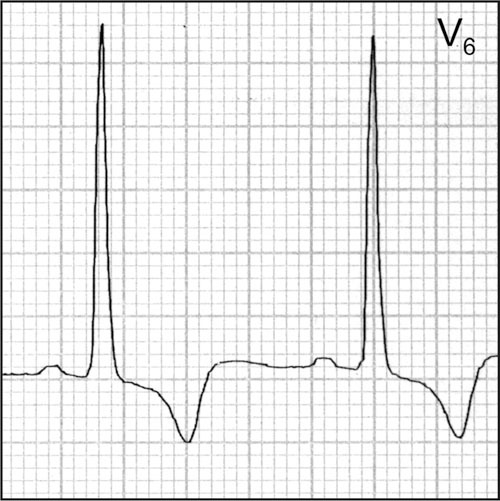
The strain pattern with left ventricular hypertrophy conveys increased risk beyond an increased left ventricular voltage.
Figure 5.
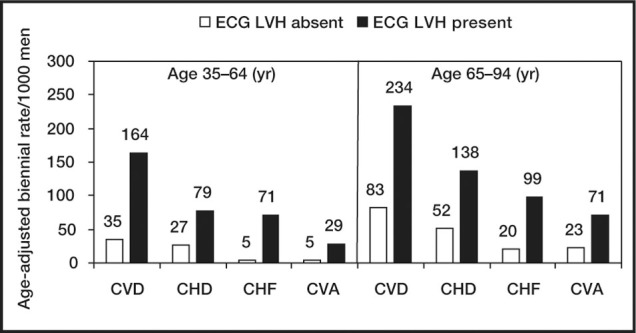
Risk of cardiovascular disease with or without electrocardiographs left ventricular hypertrophy after 34‐year follow‐up: the Framingham Study. The presence of ECG LVH increases risk of cardiovascular disease in younger and older persons. ECG LVH=electrocardiographic left ventricular hypertrophy; CVD=cardiovascular disease; CHD=coronary heart disease; CHF=congestive heart failure; CVA=cerebrovascular accident. Data derived from J Hypertens. 1991;9(suppl 2):S3‐S9. 2
Figure 6.
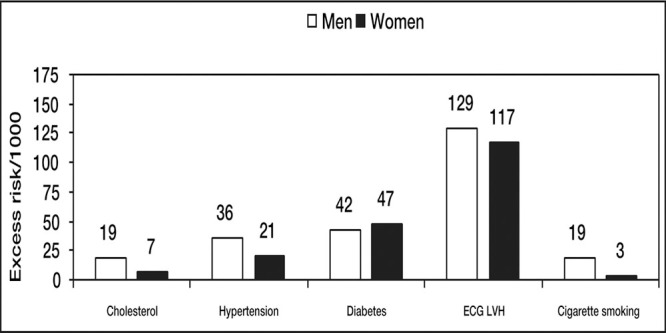
36‐Year follow‐up Framingham Study in subjects 35‐64 years of age. Electrocardiographic left ventricular hypertrophy (ECG LVH) is the most potent of traditional cardiovascular risk factors. Data derived from J Hypertens. 1991;9(suppl 2):S3‐S9. 2
Figure 7.
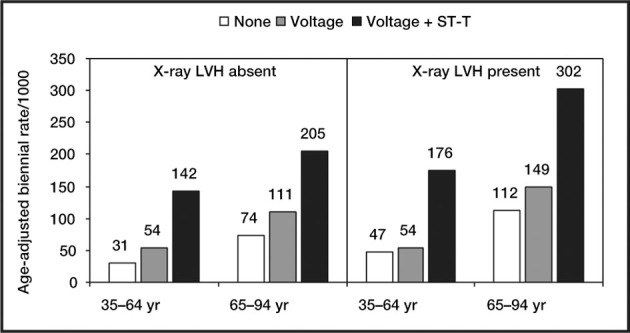
Risk of cardiovascular disease by x‐ray and electrocardiographs left ventricular hypertrophy in a 34‐year Framingham Study follow‐up in men. The presence of ST‐T changes (i.e., “strain” pattern) on an electrocardiogram conveys additional risk beyond excess voltage. LVH=left ventricular hypertrophy. Data derived from J Hypertens. 1991;9(suppl 2):S3‐S9. 2
The QRS voltage increases with both thickening of the wall (pressure overload) and dilatation of the chamber (volume overload) of the left ventricle. There is significant day‐to‐day variability in QRS voltage due to lead placement, respiration, and body position. In addition, young black males may have increased voltage in the absence of hypertension. There are various voltage criteria that have been used to diagnose LVH, but most have a poor sensitivity for the detection of LVH. The gender‐specific Cornell voltage‐QRS duration product has emerged as the criteria with the optimum operating characteristics. It is calculated as: men (RaVL + SV3) × (QRS duration); women: (RaVL + SV3+ 6) × (QRS duration).
LVH is present if the product exceeds 2440 mm × msec. The “strain pattern” is characterized by ST depression ≥1 mm in lateral leads I, aVL, and V4 to V6 (most commonly just V5 or V6 is used). The direction of the T wave is in the opposite of the direction of the upright QRS complex (Figure). Furthermore, the T wave is asymmetric with a gradual down slope (upward convex) followed by a rapid up slope and a terminal positive overshoot. The presence of a “strain pattern” implies a poor prognosis (Figures 5 and 7). Increased LVM and coronary artery disease (CAD) are associated with the strain pattern. Subendocardial ischemia may be the cause of the strain pattern.
ECHOCARDIOGRAM
An echocardiogram provides information that is useful in assessing symptoms of hypertensive patients. It is not considered appropriate to order the test for the purpose of determining the presence of LVH in an asymptomatic patient; however, a limited echocardiogram for that purpose has been advocated. In addition to direct measurements of wall thickness and chamber dimensions of the left atrium and ventricle (Figure), echocardiography offers a measurement of LV performance (ejection fraction [EF]) as well as visualization of wall‐motion abnormalities. Diastolic filling abnormalities can be determined in advanced laboratories.
Figure 8.
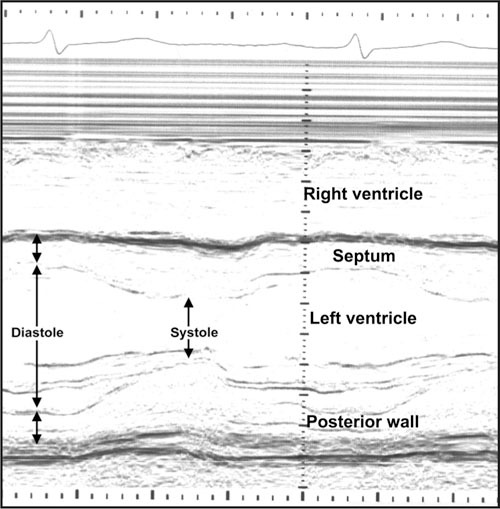
M‐mode echocardiogram; direct measures of wall thickness and chamber dimensions in diastole and systole are measured.
An increased LVM and relative wall thickness (>0.45) indicates concentric LVH. An abnormal LVM index is greater than 110 g/m 2 in women and 125 g/m2 in men. An increase in LVM indexed for height appears to bestow a greater risk for women than for men. The presence of concentric LVH amplifies the risk associated with epicardial CAD.
Treatment
Several meta‐analyses have reported superior regression of LV hypertrophy with angiotensin‐converting enzyme inhibitors than with other classes of drugs. In contrast, two prospective, double‐blind studies—the Treatment of Mild Hypertension Study and the Veterans Affairs Single Drug Comparison Study in Men—found that diuretics were associated with greater regression of LVM than other drug classes. The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study specifically was designed to study hypertensive patients 55‐80 years of age with ECG LVH, using the Cornell voltage‐duration product (>2440 mm × msec) or the Sokolow‐Lyon voltage criteria (SV1+ RV5/6 >38 mm). Atenolol and losartan were compared in this prospective, randomized, double‐blind study of 9193 patients. Enrollment BP was a sitting systolic between 160–200 mm Hg or a diastolic between 95–115 mm Hg. Additional antihypertensive drugs (except (β blockers, angiotensin receptor blockers, or angiotensin‐converting inhibitors) could be added to achieve the target BP after the dose of each drug was doubled and up to 25 mg of hydrochlorothiazide supplemented. The primary composite end point was death, MI, and stroke. Losartan was superior to atenolol by reducing the composite end point by 13% due to a 25% reduction in strokes. Among the enrolled diabetic patients, total mortality was reduced.
Both ECG LVH enrollment criteria improved more with the losartan‐ than the atenolol‐based treatment regimen. Regression in ECG LVH by each criteria reduced cardiovascular mortality, MI, and stroke. Approximately 10% of study participants had an echocardiogram performed. The greatest decline in LVM was observed between 12‐24 months. Echocardiographic LVH was present in 70% of patients at baseline and was reduced to 23% at 60 months. Associated with regression was a reduction in left atrial diameter and improvement diastolic filling parameters. Regression of LVM index on treatment was associated with a reduction in cardiovascular mortality, stroke, and all‐cause mortality.
HEART FAILURE
The incidence of HF is increasing. This seems somewhat paradoxical given the decline in ischemic heart disease and cerebrovascular events. However, the aging of the US population is contributing to the increased incidence and prevalence of HF. Hypertension and ischemic heart disease are the major factors that result in HF (Figure). LVH also contributes to the development of HF (Figure). Hypertension is antecedent in 75% of cases of HF. HF can be secondary to hyperthyroidism, hypothyroidism, renovascular hypertension, and chronic kidney disease.
Diastolic dysfunction can be present in both systolic (low EF) and diastolic (preserved EF) HF. Preserved EF HF is associated with impaired quality of life and increased all‐cause mortality (Figure). Diastolic dysfunction be due to an abnormality of LV 1) distensibility, 2) filling, or 3) relaxation. Distensibility of the ventricle can be limited by increased muscle (i.e., LVH), and collagen. To improve filling, the left atrial pressure increases and left atrial enlargement ensues.
Figure 9.
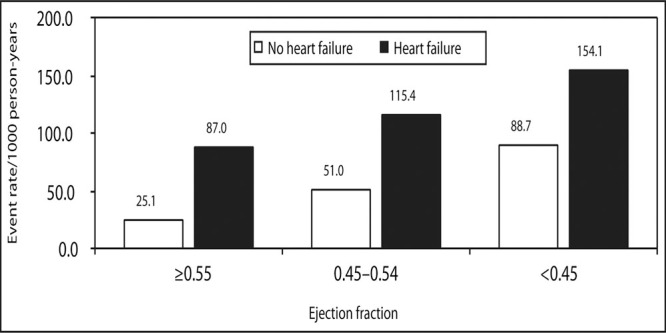
All‐cause mortality in elderly patients without and with symptoms of heart failure. According to left ventricular ejection fraction, patients with symptoms of preserved ejection fraction (>0.55) and symptoms of heart failure experience a similar event rate as asymptomatic patients with a depressed ejection fraction (<0.45). Data derived from Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137(8):631‐639.
Diastolic HF is common among the elderly with hypertension, especially women. Early historical findings include exertional fatigue and exertional dyspnea. Although rarely performed, assessing LV filling pressure invasively on presentation with HF is the “gold standard” for proving diastolic HF. With HF findings on physical examination or chest x‐ray that clear with diuresis, finding a preserved EF, absent valvular (acute aortic or mitral regurgitation), and nonexistent pericardial abnormalities (e.g., constrictive pericarditis) on echocardiogra‐phy is how most clinicians make this diagnosis.
A well‐trained cardiologist using cardiac Doppler ultrasound in an advanced echocardiography laboratory can provide better information. This requires a simultaneous assessment of mitral inflow, pulmonary venous flow, and Doppler tissue mitral annular motion. Many echocardiographic laboratories simply report diastolic dysfunction is present when the early mitral inflow (E wave) is less than the late (atrial contraction) mitral inflow (A wave). This E<A flow velocity is present with aging (probably due to increased collagen), as well as impaired relaxation (mild diastolic dysfunction). However, pseudonormalization can occur with moderate diastolic dysfunction, but there are changes in pulmonary venous flow and tissue mitral annular motion. Peak Valsalva mitral inflow distinguishes a pseudonormalized mitral inflow pattern by transforming it into an abnormal pattern. Alternatively, radio‐nuclide ventriculography can be used to determine filling parameters in diastole in patients who are in a sinus rhythm (Figure).
Figure 10.
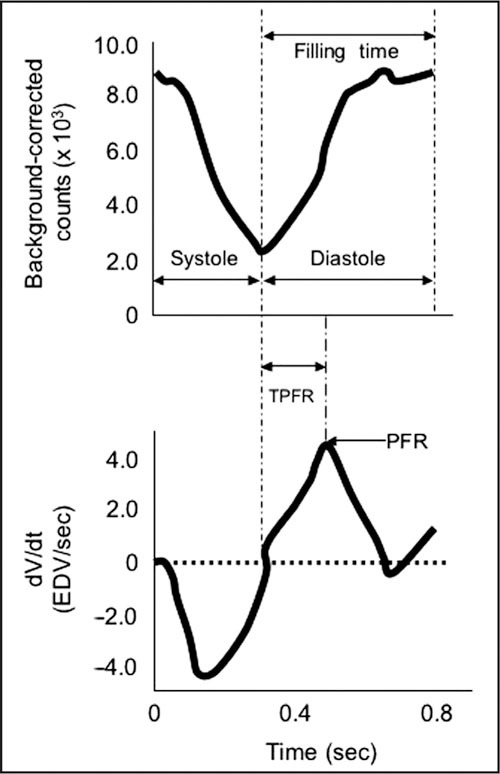
Radionuclide angiography is a highly reproducible method for determining ejection fraction for patients in a sinus rhythm. The top panel shows a background‐corrected time‐activity curve over a cardiac cycle. The bottom panel shows the first derivative of those counts: diastolic function is determined by evaluating the time‐activity curves during diastole; time‐to‐peak filling rate (TPFR) and the peak‐filling rate (PFR) are calculated as measures of diastolic dysfunction. dv/dt=the derivitave of ventricular volume over time; EDV=end‐diastolic volume
In randomized, double‐blind, hypertension outcomes trials, a diuretic‐based treatment regimen prevents the development of HF. Alternatively, HF trials with enalapril, (β blockers (bisoprolol fumarate,, metoprolol succinate, and carvedilol, but not metoprolol tartrate or atenolol), spironolactone, and candesartan cilexetil have been shown to reduce total mortality. There is only one outcome trial that addresses borderline preserved EF (>40%) HF, the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) study. As add‐on therapy to routine medications, candesartan reduced HF hospitalizations.
ISCHEMIC HEART DISEASE
There are similarities between epicardial CAD and hypertensive heart disease (Figure). There are multiple potential reasons why hypertensive patients experience ischemia (Table I). Ischemic heart disease events are numerically the most common cardiovascular event among hypertensive patients. The presence of LVH increases the probability of a cardiovascular event (Figure).
Table I.
Potential Mechanisms of Ischemia With Hypertension
| Increased coronary arteriolar resistance |
| Impaired coronary flow reserve |
| Endothelial dysfunction |
| Epicardial coronary artery atherosclerosis |
| Compression of coronary arterioles by muscle and fibrosis |
| Arteriolar wall thickening |
| Coronary artery size mismatch |
| Increased blood viscosity |
| Abstracted from Hypertension. 1999;34:782–789. 5 |
Coronary flow reserve refers to the increment in coronary blood flow measured by an intracoronary Doppler catheter due to coronary vasodilators such as papaverine, dipyridamole, or adenosine. Impaired coronary reserve (ratio <3:1) refers to a less than expected increase in coronary blood flow. Coronary vasodilator reserve is lower and LVM index is higher in patients who have positive thallium stress test without significant CAD. Since MI is the most common potentially preventable cardiovascular event in hypertensive patients, it would be useful to have a noninvasive test to distinguish significant obstructive large conduit epicardial CAD from other causes of myocardial ischemia in hypertensive patients. However, there is no such test at present.
Routine exercise stress testing in hypertensive patients is associated with a 62% false‐positive rate and 53% false‐negative rate for significant obstructive CAD (Table II). Exercise radionuclide angiography has been shown to be associated with a 49% positive predictive accuracy in hypertensive compared with an 89% predictive accuracy in normotensive patients. Thallium (with dipyridamole if the target heart rate is not achieved) stress testing is useful as a test of exclusion in hypertensive patients, since the negative predictive value is 98% and the sensitivity is 94.4%. Newer, noninvasive tests, including dipyridamole and dobutamine echocardiography, have higher false‐negative rates and a high interobserver variability; whereas, that is not the case with exercise dipyridamole thallium or technetium‐99m sestamibi nuclear scans. These tests should be used to exclude those with significant obstructive epicardial CAD. Although coronary arteriography is considered the “gold standard” for defining anatomical coronary disease, there are problems evaluating the degree and significance of luminal stenosis by this method. Intravascular ultrasound with Doppler has been useful to elucidate a better understanding.
Table II.
Predictive Value of Various Noninvasive Tests in Hypertensive Patients
| Positive Predictive Value (%) | Negative Predictive Value (%) | Overall Accuracy (%) | |
|---|---|---|---|
| Bruce treadmill | 41 | 43 | 42 |
| Exercise radionuclide ventriculography | 46 | 56 | 49 |
| Planar exercise thallium (± dipyridamole) | 39 | 98 | 70 |
| SPECT technetium‐99m sestamibi | 67 | 94 | 71 |
| Dipyridamole echocardiography | 90 | 64 | 74 |
| Dobutamine echocardiography | 85 | 83 | 84 |
| SPECT=single‐photon emission computed tomography | |||
(β blockers and angiotensin‐converting enzyme inhibitors are usually recommended for patients with CAD. The recently completed International Verapamil‐Sustained Release Trandolapril Study (INVEST) examined over 22,000 hypertensive patients with stable CAD using verapamil‐sustained release or atenolol as initial therapy to control BP utilizing a prospective, open‐label, blinded end point design. Trandolapril and hydrochlorothiazide could be added to each group to achieve the target BP. There was no difference between the treatment approaches in achieving the composite end point of death, MI, or stroke. There were more new cases of diabetes mellitus in the atenolol group.
ARRHYTHMIAS
Hypertensive patients are more likely have both atrial and ventricular arrhythmias. Both hypertension and ECG LVH are potent risk factors for the development of AF (Figure). Systolic BP and pulse pressure are associated increased left atrial size. The echocardiographic findings of left atrial enlargement, LVH, and reduced EF are predictors of AF. Cardiac conditions predisposing to AF include HE, MI, and valve disease. Given the potential embolic risk for cerebrovascular events, prompt identification and treatment is mandatory.
Figure 11.
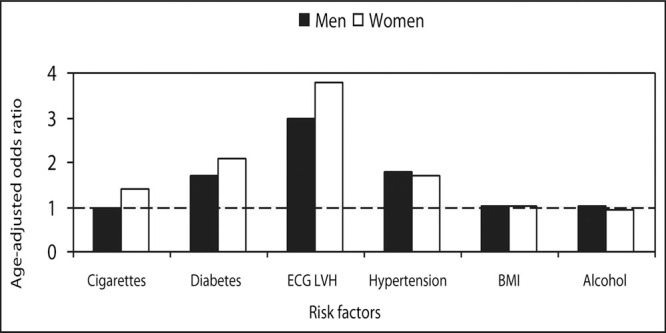
Risk factors for the development of atrial fibrillation: 38‐year follow‐up of the Framingham Study. Hypertension, electrocardiographs left ventricular hypertrophy, and diabetes were predictors for the development of atrial fibrillation; in contrast, body mass index, alcohol, and cigarettes in men were not. ECG LVH=electrocardiographic left ventricular hypertrophy; BMl=body mass index. Data derived from Kannel WB, Wolf PA, Benjamin EJ, et at. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N‐9N.
Even without LVH, ventricular arrhythmias are more common in hypertensive compared with normotensive patients. If LVH is present, then ventricular ectopy, ventricular tachycardia, and sudden cardiac death occur more frequently.
AORTA
Hypertension is a known predictor of proximal aortic dissection. The LIFE Study reported that aortic root dilatation in hypertensive patients with ECG LVH is associated with greater LVM, eccentric hypertrophy, and reduced circumferential end‐systolic stress/end‐systolic volume index. In the Framingham Study, echocardiographic aortic root size was determined primarily by age, height, weight, and gender. There was a weaker (but significant) direct association with mean arterial and diastolic BP and an inverse association with systolic BP and pulse pressure. More data are needed to better understand these relationships. Modern management of aortic dissection requires an emergency imaging study and cardiology and thoracic surgery consultations.
CONCLUSIONS
The various components of hypertensive heart disease increase overall morbidity and mortality. Careful attention and treatment of LVH, heart failure, ischemic heart disease, and AF will improve survival.
References
- •. Prisant LM, Frank MJ, Carr AA, et al. How can we diagnose coronary heart disease in hypertensive patients? Hypertension. 1987; 10: 467–472. [DOI] [PubMed] [Google Scholar]
- •. Kannel WB. Left ventricular hypertrophy as a risk factor: the Framingham experience. J Hypertens Suppl. 1991;9: S3–S8; discussion S8–S9. [DOI] [PubMed] [Google Scholar]
- 3. Frohlich ED, Apstein C, Chobanian AV, et al. The heart in hypertension. N Engl J Med. 1992; 327: 998–1008. [DOI] [PubMed] [Google Scholar]
- •. Prisant LM, Houghton JL, Bottini PB, et al. Hypertensive heart disease. How does blood pressure affect left ventricular mass? Postgrad Med. 1994;95:59–62, 66–76. [PubMed] [Google Scholar]
- •. Frohlich ED. State of the art lecture. Risk mechanisms in hypertensive heart disease. Hypertension. 1999; 34: 782–789. [DOI] [PubMed] [Google Scholar]
- •. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000; 102: 470–479. [DOI] [PubMed] [Google Scholar]
- •. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004; 292: 2343–2349. [DOI] [PubMed] [Google Scholar]
- •. Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004; 292: 2350–2356. [DOI] [PubMed] [Google Scholar]
- •. Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004; 351: 1097–1105. [DOI] [PubMed] [Google Scholar]
- •. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004; 350: 1953–1959. [DOI] [PubMed] [Google Scholar]


