Abstract
Pulse wave velocity is a reliable marker of arterial compliance. Stiffness of large and elastic arteries leads to a faster propagation of pulse wave. The aim of this study was to evaluate changes in arterial distensibility using antihypertensive drugs. This treatment focused on the inhibition of the renin‐angiotensin‐aldosterone system and the changes produced in blood pressure. Measurements were taken at baseline and throughout 60 months in 66 previously untreated hypertensive patients (22 men and 44 women, aged 54±9.5 years, range 38–73 years at baseline). All patients received either angiotensin‐converting enzyme inhibitors or, in case of adverse effects, angiotensin receptor blockers. To control blood pressure, diuretics, calcium channel blocking agents, or â blockers were added when appropriate. Statistical analysis was performed by means of ANOVA with á=0.05. Systolic and diastolic blood pressure decreased during the first year without significant changes thereafter. There were no significant changes in pulse pressure. Pulse wave velocity showed a continuous and significant decrease throughout the follow‐up period, but its reduction since the third year was more evident than the decrease in systolic and diastolic blood pressure (p<0.0001 for both). This observation could be related to changes in arterial remodeling probably due to angiotensin‐converting enzyme inhibition or renin angiotensin system blockade. Further investigations are needed to establish this relationship.
Hypertension is an important risk factor in the setting of cardiovascular (CV) disease. Systolic blood pressure (SBP) and pulse pressure (PP) have become increasingly considered as CV risk factors in the past decade. 1 , 2 Aortic stiffness is the main determinant of SBP and, thus, of PP. There are several variables that affect aortic stiffness: aging, blood pressure (BP), body height, 3 heart rate, 4 and high insulin levels, 5 among others. Improving arterial wall composition with treatment is still controversial. Mechanical properties of the elastic arteries are currently the focus of clinical research in the setting of high BP. The velocity at which a pressure wave propagates through an arterial segment is called pulse wave velocity (PWV) and is partly dependent on the stiffness of the vessel wall, being that the higher the PWV, the higher the stiffness. 6 Aortic stiffness can be uninvasively evaluated by ultrasound techniques measuring the PWV along the aorta. Carotid‐femoral PWV is the easiest method to use in pharmacologic trials and gives information about the elastic properties of the thoracic and abdominal aorta. Moreover, this measurement is an independent predictor of CV mortality and primary coronary events among hypertensive patients. 7 , 8 , 9
Recent studies indicate that among CV risk factors, high PWV may be a stronger indicator than SBP. 7 , 8 , 9 , 10 Several years ago, Asmar and colleagues 11 assessed the effect of antihypertensive treatment on arterial stiffness during a short period of time, but it was not possible to know whether the observed decrease in PWV was due to BP changes, arterial wall modifications, or a combination of both factors. However, according to Laurent et al., 12 optimal pharmacologic treatment should fully normalize arterial stiffness by the combined effects of BP lowering and vascular structural changes. Pharmacologic intervention studies have demonstrated the improvement of PWV with different classes of drugs such as angiotensin‐converting enzyme (ACE) inhibitors, β blockers, diuretics, and calcium channel blockers during a short period of time. 13 Moreover, Benetos et al. 14 found that annual rates of progression in PWV in treated hypertensives were significantly higher than in normotensive subjects. However, these patients were not normotensive at the end of the study. To date, our study is the longest one that has evaluated PWV changes under antihypertensive treatment in a well controlled cohort of hypertensive patients.
METHODS
Study Design We enrolled a group of 66 Caucasian hypertensive patients previously untreated or without treatment for the previous 3 months seen in our ambulatory unit (22 men and 44 women aged 54±9.5 years, range 34–74 years at baseline) (Table I). All patients were nonsmokers, and none had diabetes. Pre‐study work‐up included medical history review and a complete clinical examination, including laboratory tests and radiologic and echocardiographic studies. Cardiac and vascular Doppler ultrasound evaluations and any other diagnostic tools were done in case of suspected secondary hypertension.
Table I.
General Demographic Characteristics of Population at Baseline, 6 Months, and 60 Months (N=66)
| Baseline | 6 Months | 6o Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (yr) | Weight (kg) | Height (cm) | Waist (cm) | Hips (cm) | HR (bpm) | Weight (kg) | Waist (cm) | Hips (cm) | HR (bpm) | Weight (kg) | Waist (cm) | Hips (cm) | HR (bpm) | |
| Total | 66 | 54±9.6 | 67.8±12.6 | 163.6±9.6 | 84.6±10.5 | 97.6±7.1 | 71.2±7.9 | 66.6±11.7 | 83.1±9.7 | 96.5±5.6 | 70.8±7.7 | 67.9±9.7 | 87.5±11 | 97.9±7.8 | 68±7.0 |
| Men | 22 | 54±9.9 | 79.1 ± 12.1 | 172.1±7.8 | 92.9±11.0 | 98.8±7.8 | 73.1±8.7 | 77.2±11.8 | 89.6±8.4 | 97.2±5.2 | 70.3±9.2 | 74.4±7.5 | 91.4±9.1 | 98.1±7.5 | 69.7±7.9 |
| Women | 44 | 54±9.4 | 62.1±8.4 | 159.3±7.4 | 80.4±7.2 | 97.0±6.7 | 70.2±7.4 | 63.3±10.1 | 81.1±9.5 | 96.4±5.7 | 70.8±7.2 | 64.8±9.2 | 85.7±11.5 | 97.8±8.0 | 67.2±6.6 |
| Values are expressed as mean ± SD. HR=heart rate | |||||||||||||||
Height (cm), weight (kg), body mass index (BMI), waist and hip circumference (cm), and PWV were measured at baseline, after 2 months of treatment, and every 12 months during the study follow‐up period (60 months) (Table I). It is important to highlight the fact that we did not give the patients any special recommendation concerning diet or exercise, but we did advise them to reduce sodium intake.
To control high BP, all patients received either adequate doses of ACE inhibitors or angiotensin receptor blockers if adverse effects such as cough occurred. BP was maintained at ≤140/90 mm Hg by adding diuretics, β blockers, or calcium antagonists, when necessary (Table II).
Table TableII.
Distribution of Drugs Used and Their Combinations
| Drug Class | Single | DIUR | BB | CCA | DIUR+BB | DIUR+CCA | CCA+BB | TOTAL |
|---|---|---|---|---|---|---|---|---|
| ACEI | 9 | 17 | 4 | 7 | 3 | 1 | 1 | 42 |
| ARB | 11 | 5 | 3 | — | 3 | 2 | — | 24 |
| ACEI=angiotensin‐converting enzyme inhibitors; ARB=angiotensin receptor blockers; DIUR=diuretics; BB=β blockers; CCA=calcium channel antagonists | ||||||||
The Ethics Committee and Teaching and Research Committee of our institution approved the protocol. Each subject signed a consent form in which the nature of the research and its purpose were clearly explained.
BP Measurement At each examination, SBP and diastolic BP (DBP) were measured three times using a standard certified sphygmomanometer. SBP was defined as the first Korotkoff sound (phase 1), and the DBP level was stated at the disappearance of Korotkoff sounds (phase 5). The manometer was deflated at a rate of 2 mm Hg/sec. Before measurement, the patients rested in a seated position for 10 minutes. They had not consumed food, alcohol, or coffee or practiced exercise within the previous 30 minutes. A mean of the three readings was used for data analysis. Subjects with SBP ≥140 mm Hg, DBP ≥90 mm Hg, or both were considered to be hypertensive. PP was calculated as SBP minus DBP. Mean arterial pressure was defined as DBP plus one third of the PP.
Determination of PWV Aortic PWV was determined using a noninvasive automatic device, Complior (Colson AS, Paris, France), which allowed an online pulse wave recording and automatic calculation of PWV. The validation of this automatic method and its reproducibility have been previously published. 15 Briefly, common carotid artery and femoral artery pressure waveforms were recorded noninvasively using a TY‐306 pressure‐sensitive transducer (Fukuda, Tokyo, Japan). The waveforms digitized were sampled at 500 Hz. Both waveforms were stored in a memory buffer. Details of this procedure have been previously published. 11 , 15 When the operator observed a pulse wave of sufficient quality on the computer screen, digitization was suspended and calculation of the time delay between the two pressure upstrokes was initiated. Measurement was repeated over more than 30 different cardiac cycles, and the calculated mean value was used for the final analysis. The distance between the two measurement positions was measured over the body surface as the distance between the two recording sites (D), whereas pulse transit time (t) was automatically determined by the Complior device as PWV=D/t. Validation of this automatic method and its reproducibility have been described previously. 11 The concept of PWV as an indicator of arterial compliance was previously shown. 16 The same observer, who was blind to the previously assessed BP of the subject, performed all measurements.
Statistical Methods. The values are expressed as mean ± SD. All statistical analyses were performed using absolute values and processed through analysis of variance for repeated measurements. Differences of mean values between baseline and each stage, and between different stages, were assessed by post hoc Scheffé test. A value of p<0.05 was considered statistically significant.
RESULTS
General characteristics of the studied population are shown in Table I. The observations of the study patients made during the follow‐up period showed different behaviors of the hemodynamic variables (SBP, DBP, PWV, and PP).
Both SBP and DBP were quickly and significantly reduced, achieving a normal average value after 2 months of treatment. Nevertheless, during the subsequent measurements, these variables stayed stable or with slight oscillations without statistical significance (Figure 1 and Table III). No differences in the results were observed either by gender, age, and/or using different antihypertensive drugs. The patients did not show any significant change in their heart rate, weight, or waist and hip circumference. Moreover, the variation in PP was not significant during the span of the study.
Figure.
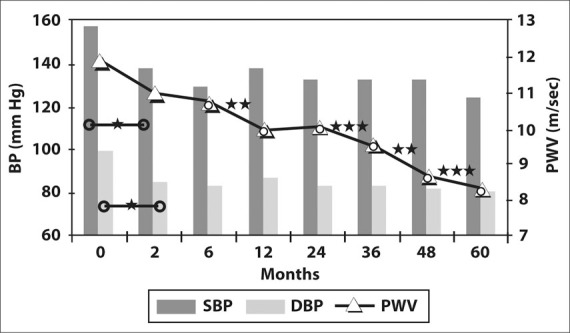
Variation of blood pressure (BP) and pulse wave velocity (PWV) over the follow‐up period. (Values are detailed in Table III.) SBP=systolic BP; DBP=diastolic BP. *p<0.0001; **p<0.001; ***p<0.01
Table III.
Comparative Variation of Blood Pressure and Pulse Wave Velocity Over the Follow‐Up Period
| Months | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 12 | 24 | 36 | 48 | 60 | |
| SBP (mm Hg) | 157.30 | 138.22* | 129.89 | 138.33 | 132.78 | 131.00 | 132.89 | 124.67 |
| DBP (mm Hg) | 99.78 | 85.41* | 83.63 | 87.89 | 84.63 | 83.96 | 82.59 | 81.07 |
| PWV (m/sec) | 11.90 | 10.97 | 10.75 | 9.97** | 10.06 | 9.57† | 8.72** | 8.35† |
| SBP=systolic blood pressure; DBP=diastolic blood pressure; PWV=pulse wave velocity; *p<0.0001; **p<0.001; † p<0.01 | ||||||||
Remarkably, PWV showed a very significant reduction from the beginning of the study, continuing with a further progressive reduction throughout the entire follow‐up period. During this period, we observed that BP levels were stable while PWV continued to decrease. In addition, PWV showed significant changes compared with previous measurements after 24 months of treatment (Figure 1 and Table III). These changes did not show differences by sex, age, and/or use of different antihypertensive drugs.
DISCUSSION
In this study, we found that arterial compliance assessed by means of carotid‐femoral PWV showed an improvement beyond BP control over several years of follow‐up in a cohort of 66 hypertensive patients who were well controlled with treatment. These data extend the observations published by Asmar et al. 11 —the trend of a greater PWV decrease than expected after a short period of time on antihypertensive treatment.
The observed improvement could be explained both by continued BP control and changes of arterial structure due to the effect of antihypertensive drugs.
The main objective of our follow‐up study was to obtain the normalization of BP using antihypertensive treatment based principally on renin‐angiotensin‐aldosterone system (RAAS) inhibition. Nevertheless, as usually happens, most patients needed a combination with other groups of drugs to be well controlled. For that reason, we cannot affirm that our results were due specifically to RAAS inhibition. The alteration of RAAS function is recognized as an important path for arterial remodeling during the natural history of hypertension. 17 , 18 , 19 , 20 The pathologic changes are linked to arterial stiffness, with the consequent risk of CV and cerebrovascular events.
It is widely recognized that angiotensin II stimulates the production of collagen‐type fibers together with growth factors that lead to vascular remodeling and PWV increase. 21 , 22 Recently, Laurent et al. 23 proposed the structural and genetic basis of arterial stiffness. They showed that elastin and collagen are not only responsible for the elasticity and rigidity of the arterial wall but are also involved in the control of smooth muscle cell function. These findings have also provided evidence that the amount, density, and spatial organization of the wall material affect the arterial stiffness.
The increase of the aortic wall stiffness is the cause of the elevation of PWV along this vessel. The rise of PWV produces an earlier reflection of the wave, with the subsequent augmentation of aortic systolic pressure. 24
This understanding of the arterial changes related to aging provides an opportunity for more appropriate pharmacologic treatment for the pathologic processes associated with aging, such as isolated systolic hypertension, heart failure, and angina pectoris. In regard to this, it is important to identify drugs with more selective effects on aortic wall stiffness in older patients.
Pharmacologic intervention focused on blocking the RAAS, both with ACE inhibitors and angiotensin receptor blockers, has demonstrated that the chronic inhibition of this system is able to modify arterial stiffness. 25 , 26 Previous studies have demonstrated the efficacy of ACE inhibitors to improve arterial stiffness independent of BP hemodynamic changes in comparison with other antihypertensive agents, such as β blockers, which failed to produce improvement in vascular distensibility. 11 , 27 These observations support the idea that the inhibition or blockade of the RAAS results in a substantial benefit in reducing arterial stiffness. 28
The risk burden is directly linked to increased stiffness of the large arteries that leads to the rise of central SBP. This risk could be assessed by means of noninvasive methods such as PWV measurement.
Thus, this longitudinal study is in accordance with the fact that blockade of the RAAS leads to a quick fall in both SBP and DBP within the first months of treatment. A similar effect was observed with PWV. This last observation, however, could not be attributed to changes in arterial wall composition but to a direct effect of the BP fall, since no comparative treatment group was included. The continuous improvement in PWV observed thereafter, not accompanied by a further decrease in BP, seems to indicate a direct action of treatment on vascular structure (Figure 1 and Table III) beyond the BP reduction. Since the complete normalization of the vascular fiber composition with pharmacologic treatment has not been demonstrated, the PWV fall could be explained by the regenerative changes in the muscle layer of the arteries and/or the improvement of the endothelial function in peripheral arteries. In agreement with this, Ahimastos and colleagues 29 have observed that an ACE inhibitor promoted an elastogenic matrix profile, which may contribute to the improvement in arterial stiffness in cell culture.
Nevertheless, the accurate control of BP seems to be the most important objective to improve PWV. In regard to this, Benetos and associates 14 found an impairment of PWV after 6 years in poorly controlled treated hypertensive patients. The same impairment was found in normotensives with aging. Our results enhance the concept of the importance of BP control and, possibly, also the inhibition of the RAAS to delay arterial aging. The most relevant difference between our cohort and Benetos' patients was that the former included consistently treated patients, most of them with nearoptimal BP, who had a regular yearly follow‐up.
CONCLUSIONS
We think that the present long‐term observational study provides substantial evidence that reinforces the importance of both good BP control and perhaps also blockade of the RAAS for modulating vascular structures in patients with arterial hypertension. However, we believe further investigations will be needed to clarify the effect of treatment based on other antihypertensive agents because, so far, good BP control was the main evidence of the success of the treatment and PWV improvement could only be related to this.
Acknowledgmentse: We are indebted to Dr. Vicente Castiglia, from the Division of Scientific Advisory, Hospital de Clínicas José de San Martín, for the processing and analysis of the data and to Mrs. Maria Ines Castagnino for her advice and review of the manuscript.
References
- 1. Domanski M, Mitchell G, Pfeffer M, et al. Pulse pressure and cardiovascular disease‐related mortality: follow‐up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 2002;287:2677–2683. [DOI] [PubMed] [Google Scholar]
- 2. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 3. Smulyan H, Marchais SJ, Pannier B, et al. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol. 1998;31:1103–1109. [DOI] [PubMed] [Google Scholar]
- 4. Haesler E, Lyon X, Pruvot E, et al. Confounding effects of heart rate on pulse wave velocity in paced patients with a low degree of atherosclerosis. J Hypertens. 2004;22:1317–1322. [DOI] [PubMed] [Google Scholar]
- 5. Hansen TW, Jeppesen J, Rasmussen S, et al. Relation between insulin and aortic stiffness: a population based study. J Hum Hypertens. 2004;18:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Lehmann ED, Hopkins KD, Gosling RG. Aortic compliance measurement using Doppler ultrasound: in vivo mechanical correlates. Ultrasound Med Biol. 1993;19:683–710. [DOI] [PubMed] [Google Scholar]
- 7. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 8. Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. [DOI] [PubMed] [Google Scholar]
- 9. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness in an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 10. Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 11. Asmar R, Topouchian J, Pannier B, et al., for the Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. Pulse wave velocity as endpoint in large‐scale intervention trial. The Complior study. J Hypertens. 2001;19:813–818. [DOI] [PubMed] [Google Scholar]
- 12. Laurent S, Kingwell B, Bank A, et al. Clinical applications of arterial stiffness: therapeutics and pharmacology. Am J Hypertens. 2002;15:453–458. [DOI] [PubMed] [Google Scholar]
- 13. Delerme S, Boutourye P, Laloux B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short and long term antihypertensive treatment: a meta‐analysis of individual data in 294 patients [abstract]. Hypertension. 1998;32:789. [DOI] [PubMed] [Google Scholar]
- 14. Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6‐year period. Circulation. 2002;105:1202–1207. [DOI] [PubMed] [Google Scholar]
- 15. Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. [DOI] [PubMed] [Google Scholar]
- 16. Bramweel JC, Hill AV, McSwiney BA. The velocity of the pulse wave in man in relation to age as measured by the hot‐wire sphygmograph. Heart. 1923;10:233–255. [Google Scholar]
- 17. Niarchos AP, Laragh JH. Renin dependency of blood pressure in isolated systolic hypertension. Am J Med. 1984;77:407–414. [DOI] [PubMed] [Google Scholar]
- 18. Safar ME, London GM. The arterial system in human hypertension. In: Swales JD, ed. Textbook of Hypertension. London, England: Blackwell Scientific; 1994:85–102. [Google Scholar]
- 19. Safar ME, Benetos A. Factors influencing arterial stiffness in systolic hypertension in the elderly: role of sodium and the renin‐angiotensin system. Am J Hypertens. 2003;16:249–258. [DOI] [PubMed] [Google Scholar]
- 20. Izzo JL Jr.. Systolic hypertension, arterial stiffness, and vascular damage: role of the renin angiotensin system. Blood Press Monit. 2000;5(suppl 2):S7–S11. [DOI] [PubMed] [Google Scholar]
- 21. Kato H, Suzuki H, Tajima S, et al. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens. 1991;9:17–22. [PubMed] [Google Scholar]
- 22. Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming grow factor‐β1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. [DOI] [PubMed] [Google Scholar]
- 24. Safar ME, Levy BI, Struijker‐Bourdier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 25. Levy BI, Curmi P, Poitevin P, et al. Modification of the arterial mechanical properties of normotensive and hypertensive rats without arterial pressure changes. J Cardiovasc Pharmacol. 1989;14:253–259. [DOI] [PubMed] [Google Scholar]
- 26. Albaladejo P, Bouaziz H, Duriez M, et al. Angiotensin converting enzyme inhibition prevents the increase in aortic collagen in rats. Hypertension. 1994;23:74–82. [DOI] [PubMed] [Google Scholar]
- 27. Asmar RG, London GM, O'Rourke ME, et al. Improvement in blood pressure, arterial stiffness and wave reflections with a very low dose perindopril‐indapamide combination in hypertensive patients: a comparison with atenolol. Hypertension. 2001;38:922–926. [DOI] [PubMed] [Google Scholar]
- 28. London GM, Asmar RG, O'Rourke MF, et al. Mechanism(s) of selective systolic blood pressure reduction after low‐dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–99. [DOI] [PubMed] [Google Scholar]
- 29. Ahimastos AA, Natoli AK, Lawler A, et al. Ramipril reduces large‐artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension. 2005;45:1194–1199. [DOI] [PubMed] [Google Scholar]


