Abstract
A fundamental challenge in vaccinology is learning how to induce durable antibody responses. Live viral vaccines induce antibody responses that last a lifetime but those induced with sub-unit vaccines wane rapidly. Studies in mice and humans have established that long-lived plasma cells (LLPCs) in the bone marrow are critical mediators of durable antibody responses. Here, we present data that adjuvanting a HIV-1 clade C 1086.C derived gp140 immunogen (Env) with a novel synthetic TLR-7/8 agonist named 3M-052 formulated in poly(lactic-co-glycolic) or PLGA nanoparticles (NP) or with alum, either alone or in combination with a TLR-4 agonist GLA, induces strikingly high and persistent (upto ~ 1 year) frequencies of Env specific LLPCs in the BM and serum antibody responses in rhesus macaques. Up to 36% and 18% of Env specific cells among total IgG secreting bone marrow resident plasma cells were detected at peak and termination respectively. In contrast, adjuvanting Env with Alum or GLA in NP induced significantly lower (~>100 fold) LLPC and antibody responses. Immune responses induced by 3M-052 were also significantly higher than those induced by a combination of TLR-7/8 (R848) and TLR-4 (MPL) agonists. Adjuvanting Env with 3M-052 also induced robust activation of blood monocytes, strong plasmablast responses in blood, germinal center (GC) B cells, T follicular helper (TFH) cells and persistent Env specific plasma cells in draining lymph nodes. Overall, these results demonstrate efficacy of 3M-052 in promoting high magnitude and durability of antibody responses via robust stimulation of innate immunity and BM resident LLPCs.
Summary:
Our study describes robust and durable induction of HIV-1 specific antibody as well as germinal center and plasma cellular responses in macaques.
INTRODUCTION
Vaccination has had a profound impact on preventing infectious diseases, but one major challenge remains in learning how to develop durable immunity against global pandemics such as HIV, malaria and TB (1). Although live attenuated virus based smallpox or yellow fever vaccines induce durable antibody (Ab) responses that can last a lifetime, waning immunity has been documented with inactivated and sub-unit vaccines against HIV, malaria, influenza, Bordetella pertussis, Salmonella enterica serovar Typhi, and Neisseria meningitidis, that represents a major shortcoming in inducing long-lasting protective immunity (2, 3). Specifically, in a phase III HIV vaccine clinical trial (the RV144 trial), vaccine efficacy of ~60% during the first year rapidly declined to ~31% by 42 months, again highlighting the lack of durable immunity(4–6). Therefore, a great challenge in vaccinology is learning how to program the immune system to yield long-lived Ab response and plasma cells (PCs) that last a lifetime (7).
During an immune response, activated antigen-specific B cells either differentiate into short-lived PCs (SLPCs), producing low-affinity antibodies, or initiate germinal center (GC) reactions(8) which produce high-affinity memory B cells(9) and long-lived plasma cells (LLPCs) that secrete high-affinity antibodies (10–12). LLPCs migrate to the bone marrow (BM) where they can reside for extended periods and produce antibodies, there maintaining the levels of antibodies in serum (13, 14). Hence, understanding immunological mechanisms that regulate the generation of LLPCs is key to developing vaccines that elicit durable protective humoral immunity.
Adjuvants are an important component of sub-unit vaccines and can substantially improve the magnitude and durability of Ab responses(15). It is well established that adjuvants activate the innate immune system, which in turn enhances magnitude and durability of antigen-specific T and B cell responses induced by vaccines (3, 15). In particular, recent research has highlighted a new generation of molecular adjuvants targeted to pathogen recognition receptors (PRRs) on dendritic cells (DCs). Specific agonists of various toll-like receptors (TLRs) hold much promise as vaccine adjuvants (3, 15, 16). In this regard, the live attenuated yellow fever vaccine YF-17D induces innate activation by signaling via multiple TLRs on distinct subsets of DCs, and this appears to be essential for its immunogenicity, highlighting the importance of TLR signaling in inducing effective immune responses (17). Our previous studies in mice have demonstrated that multiple TLR agonists delivered in PLGA nanoparticles (NPs) could induce strikingly enhanced germinal center (GC) and Ab responses (18, 19). In addition, we and others have reported the potency of TLR agonists as vaccine adjuvants in rhesus macaques, a clinically relevant non-human primate (NHP) model(18–26). The TLR-4 targeted monophosphoryl lipid A (MPL) formulated with alum has been licensed for clinical use with sub-unit vaccines against the human papillomavirus(27) as well as Herpes Zoster (Shingrix)(28). TLR agonists are also being evaluated for use in cancers(15, 29). However, there is currently a paucity of data on the longevity of the Ab responses induced by these adjuvants, and in particular in their capacity to induce LLPCs in humans or in NHPs.
Here, we describe data from two rhesus macaque studies involving a total of 90 animals highlighting the adjuvanticity of 3M-052, a novel synthetic TLR-7/8 agonist used by itself or in combination with a synthetic TLR-4 targeted agonist GLA. The synthetic imidazoquinolinone 3M-052, is structurally similar to resiquimod (R848), a small molecule TLR-7/8 agonist; however, 3M-052 has an 18-C fatty acyl chain which confers enhanced hydrophobicity, reduced systemic dissemination and hence improved bioavailability at the site of immunization and in draining lymphoid organs(30). Specifically, in the first study, we report strikingly superior immune responses with the 3M-052 adjuvant with or without GLA formulated in poly(lactic-co-glycolic) or PLGA based NP’s when used with 1086.C, a HIV-1 clade C derived gp140 immunogen (Env) in comparison with immune responses induced by GLA alone in NPs or by alum, an adjuvant approved for human use. The Env immunogen used here is currently a component as gp120 of the HVTN 702 ALVAC-bivalent gp120 efficacy trial (NCT02968849)(31). We also report a successful induction and maintenance of Env specific LLPCs for one year post vaccination with the 3M-052 adjuvant. Furthermore, these responses were also significantly superior to those induced by R848, or a combination of R848 and TLR-4 agonist monophosphoryl lipid A (MPL). Finally, we report reproducible innate and Env specific B cellular and serological responses in an independent RM study not only with PLGA NP formulated 3M-052 but also with a novel alum adsorbed 3M-052 formulation that is currently being developed for use in human clinical trials.
RESULTS
Study Design
Our first pre-clinical study involved a total of 60 rhesus macaques (RMs) distributed amongst six cohorts (n = 8 per cohort for longitudinal analysis and n=6 each in groups 1 and 4 allocated for necropsy at four weeks post last vaccination). All RMs were immunized with Env and adjuvants as detailed in Fig. 1A. Male and female RMs (n=30 each) were equally distributed among the experimental groups. Animals in group 1 were immunized with Env and alum, a benchmark adjuvant used in many licensed vaccines. Animals in groups 2, 3 and 4 were immunized with soluble Env and GLA, 3M-052 and a combination of GLA+3M-052 respectively where in all adjuvants were formulated in PLGA NP’s. Animals in groups 5 and 6 were immunized with soluble Env and R848 and a combination of MPL and R848 respectively also formulated in PLGA NP’s. All treatment groups with TLR agonists in nanoparticles will be referenced with the suffix NP for brevity. All animals received four immunizations at weeks 0,6,12 and 18. Blood and BM samples were analyzed for immune responses at various time points for upto 70 weeks after the final immunization (Fig. 1B).
Fig. 1. Study design.
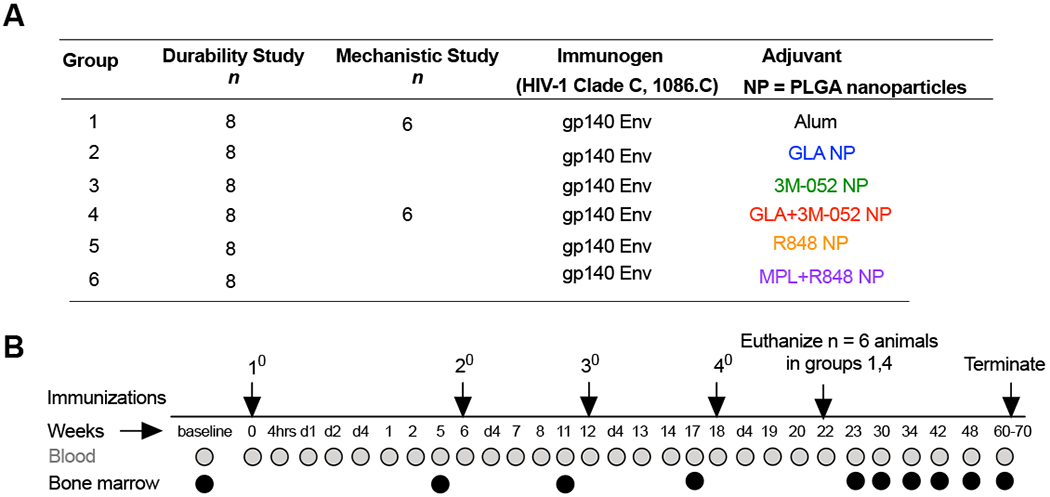
(A) Six groups of rhesus macaques (RMs) (n=8/group) were immunized with the HIV-1 Clade C derived 1086.C gp140 immunogen admixed with different adjuvants in groups 1-6 (referred to as durability arm of the study). An additional (n=6) animals were allocated to groups 1 and 4 (also referred to as mechanistic arm of the study). (B) All animals were vaccinated four times with 6 week intervals as shown in the experimental timeline schematic. Blood and bone marrow aspirates were collected from animals at time points indicated for various assays described in the study. n=6 animals in groups 1 and 4 were euthanized at ~ week 22 or one month after the final vaccination. Immune responses in all other animals were monitored for close to a year after the final vaccination before sacrifice at ~ week 70 in the study. Adjuvant names are color coded for clarity and consistency in all data plots described in the study.
The primary objective of this study was to investigate if immune responses stimulated by GLA NP, 3M-052 NP or the combination of GLA and 3M-052 in NP were superior to those induced by alum and to identify the best candidate adjuvant that promotes high magnitude and durable antibody responses to the Env immunogen. The secondary objective of the study was to investigate if immune responses stimulated by 3M-052 or the combination of GLA and 3M-052 were superior to those induced by the first generation of TLR-4 and TLR-7/8 agonists or MPL and R848 respectively. Hence, the statistical tests used to establish significance of differences in immune responses were designed to address these two study objectives as detailed in the methods section.
Alum was used in the study at a dose of 500μg as recommended and used previously in RMs (19, 32). All TLR- targeted agonists were formulated in PLGA based NPs, (18) (Kasturi et al., 2017), (table. S1). 3M-052, a 18-C fatty acyl chain bearing hydrophobic imidazoquinoline was designed to facilitate improved incorporation of the molecule in emulsion or liposome based formulations or synthetic particles (30). As such, we observed increased loading efficiency of 3M-052 in NP’s (upto 100% of target loading) in comparison with previously reported ~50% loading of the R848 molecule (18, 19) (table. S1). Similar ~100% loading efficiency of hydrophobic purified or synthetic MPL in PLGA particles has been reported previously and used accordingly (33) (table. S1). Experimental dose of R848 (750μg) was similar to that used previously in RMs (21, 25). MPL dose (50μg) was chosen based on the dose used with the HPV vaccine (34). The initial dose of 3M-052 and GLA with primary immunization was also chosen to match the dose of R848 and MPL at 750μg and 50μg respectively. However, due to localized reactions at the injection site several weeks after the primary vaccination (swelling, redness and ulceration) in 2/8 and 4/14 total animals in groups 3 and 4 respectively, the doses of 3M-052 and GLA was scaled down 10 fold to 75ug and 5μg respectively for the 2nd, 3rd and 4th immunizations. Since these reactions were observed during the second immunization, a small group of animals in groups 2 (n = 4),3 (n = 2) and 4 (n = 2) received the original high dose before the 10 fold reduction of the GLA and 3M-052 doses for all subsequent doses. One animal included in the listed number above displayed a reaction only after this second high dose vaccination. Also, for the second immunization alone, vaccinations were administered in the contralateral flanks for animals receiving the 3M-052 adjuvant to avoid potential aggravation or induction of localized reaction. All animals were included in statistical analyses as no impact was observed in long term immune responses between animals within a single treatment regimen with these dose reductions.
Vaccination with Env and 3M-052 or GLA+3M-052 NP induces robust plasmablast responses and durable bone marrow LLPCs in RMs
Recall responses upon secondary vaccinations leads to rapid generation of plasmablasts in peripheral blood both in humans and RMs (19, 35). Here, we evaluated the kinetics of Env specific plasmablast responses induced by immunization with GLA and/or 3M-052, using an Env specific B cell ELISPOT assay (Fig. S1A). We observed a rapid and striking increase in numbers of Env specific plasmablasts four days after each of the booster vaccinations relative to pre-immunization time points in all treatment groups (Fig. 2A). While no significant difference was observed in the magnitude of these plasmablast responses was observed at day 4 after the second vaccination in animals receiving the 3M-052 adjuvant in comparison with alum or GLA adjuvants, significantly higher frequencies of Env specific plasmablasts were observed after the third and the fourth vaccinations [Mann Whitney test at four days post 4th vaccination, p= 0.0001 and 0.0003 for 3M-052 NP and p = <0.0001 and 0.0002 for GLA+3M-052 NP vs. alum and GLA NP respectively]. These responses were similar at day 4 time points across all animals receiving either the R848 or the 3M-052 adjuvants. However, plasmablast responses contracted rapidly by 7 days to less than 10 spots/million mononuclear cells in animals vaccinated with alum or GLA NP and also substantially decreased in animals vaccinated with R848 NP or MPL+R848 NP (Fig. 2A) (~8 fold change in median frequencies after 2nd and 3rd vaccinations vs. 10-23 fold change after the 4th vaccination) confirming previous observations (19). In contrast, a noticeably persistent Env specific plasmablast frequencies at day 7 (less than 3 fold contraction after the 3rd and 4th vaccination) was observed selectively in animals treated with 3M-052 NP or GLA+3M-052 NP adjuvants respectively (Fig. 2A). Responses at day 7 in animals were significant higher once again selectively only in animals vaccinated with 3M-052 NP [p=<0.0001 vs alum, 0.0002 vs. GLA NP and 0.0281 vs. R848 NP after fourth vaccination; Mann Whitney test] and GLA+3M-052 NP [p= <0.0001 vs. alum and GLA-NP and p= 0.0001 vs. MPL+R848 NP]. We have recently reported identical kinetics with changes in frequencies of Env specific IgA secreting plasmablasts (~2 fold lower in absolute magnitude) and very low levels of Env specific IgM induced in RMs when using the MPL+R848 NP adjuvant (19) and hence no Env specific IgM+ plasmablast responses were assayed in the current study.
Fig. 2. 3M-052 adjuvant formulated in PLGA NP’s with or without GLA induces high magnitude of Env specific plasmablasts in peripheral blood and bone marrow resident LLPCs that are durable for ~ 1 year.
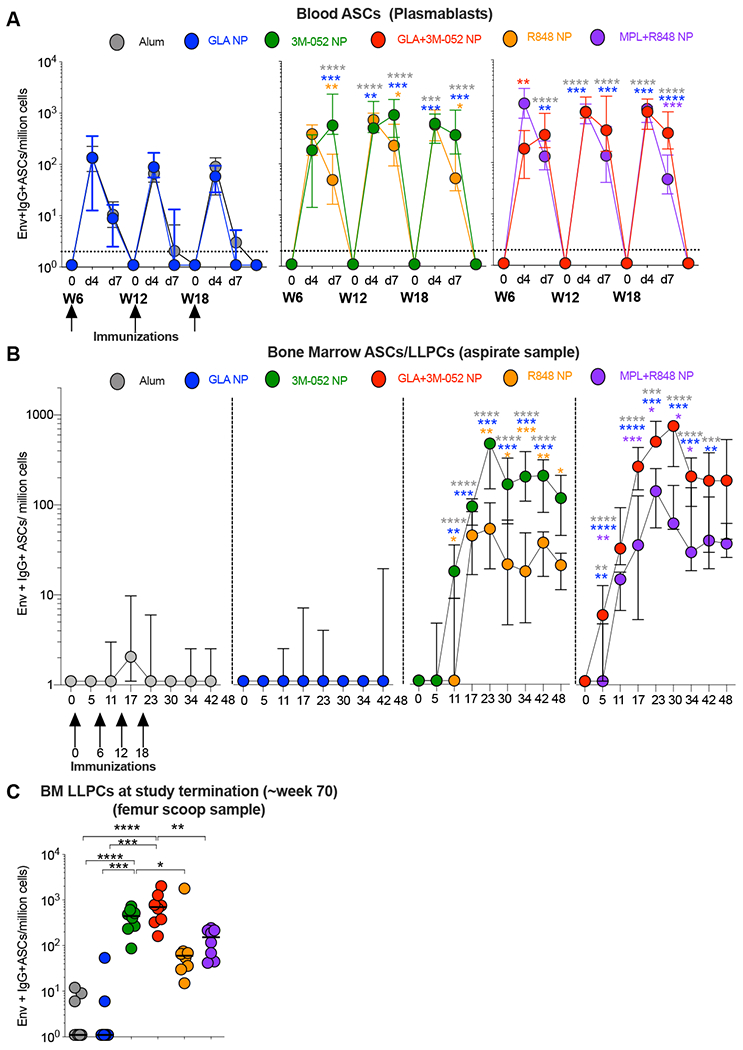
Env specific ASCs were enumerated using a B cell ELISPOT assay. (A) Line graphs indicate frequencies of plasmablast responses in peripheral blood at pre-boost, days 4 and 7 after the second, third and fourth vaccinations in the study. Y axis represents the numbers of Env specific IgG+ plasmablasts per million PBMCs. (B) Line graphs indicate frequencies of Env specific LLPCs per million bone marrow mononuclear cells (BMMCs) quantified in aspirates collected from Iliac crest at indicated time points. (C) Graph highlights frequencies of Env specific LLPCs per million BMMCs assayed in femur tissue scoop harvested at termination (~week 70) of the study. Statistical significance of differences in magnitude of immune responses was tested with a one-tailed non-parametric Mann-Whitney test for comparing groups 2,3 and 4 with group 1 as per the primary study objective. All other comparisons were performed using a two tailed non-parametric Mann-Whitney test using the Graph Pad Prism version 8.0 software. Asterisk’s are color coded to highlight the difference between two distinct treatment groups. **** p<0.0001: *** p <0.001: ** p<0.01, *p<0.05.
LLPCs in the BM are critical for sustained antibody responses (10, 11, 36). We assayed for Env specific LLPCs in BM aspirates collected over the duration of the study (Fig. 2B). While a transient yet significant increase in Env specific plasmablasts was observed in peripheral blood in RMs vaccinated with Env and alum or GLA-NP at four days after boost vaccinations, very minimal accumulation of Env specific LLPCs in the BM was observed when vaccinating with these adjuvants (Fig. 2, B and C). In contrast, a strikingly higher frequency of Env specific LLPCs was observed in animals vaccinated with 3M-052 NP or GLA+3M-052 NP with median frequencies per million mononuclear cells at 472.5 (min, max: 27, 729) and 513 (min, max: 63, 1782) respectively observed five weeks (at Week 23) post final vaccination. These responses were significantly higher in comparison with those induced by alum and GLA NP respectively [p=<0.0001 for 3M-052 NP vs. alum and p =0.0002 vs. GLA NP and p=0.0002 for GLA+3M-052 NP vs. alum and p = 0.0003 vs. GLA NP]. Env specific LLPCs remarkably persisted in these 3M-052 NP or GLA+3M-052 NP vaccinated animals at median frequencies per million mononuclear cells at 445.5 (min, max: 87, 729) and 702 (min, max: 162,2025) respectively at termination at week 70. These responses were again significantly higher in comparison with those induced by alum and GLA NP [p =<0.0001 for both 3M-052 NP and GLA+3M-052 NP vs alum and p = 0.0002 vs. GLA NP respectively]. Furthermore, we observed an additive to synergistic impact on enrichment of Env specific IgG+ LLPCs within all IgG secreting BM PCs (fig. S1 B and C). In particular, vaccination with GLA+3M-052 NP constituted a median frequency of 20.24 % (min, max: 5.6% and 36.70%) of all total IgG+ BM PCs at week 23 (peak after the final vaccination). This persisted at 6.7% (min, max: 1.7% and 18.12%) at ~ 1 year post the final vaccination. The LLPC response observed when vaccinating with GLA+3M-052 NP was only slightly improved than that seen when vaccinating with 3M-052 NP both at peak and termination time points. Finally, in addition to LLPCs in BM, we also detected persistent PCs responses in the primary draining Iliac LNs close to ~1 year post the final vaccination (fig. S1 D and E). In summary, these data demonstrate the potent adjuvanticity of 3M-052 in inducing superior magnitude and durability of blood plasmablasts and BM resident LLPCs in comparison with other adjuvants used in the study.
Vaccination with Env and 3M-052 or GLA+3M-052 NPs induces enhanced and durable Tier-1 HIV-1 virus neutralizing Ab responses (nAb) in RMs
We next evaluated Env-specific nAb responses in serum. We observed a robust Tier 1A MW965.26 Clade C HIV-1 NAb response in animals immunized with 3M-052 NP or GLA+3M-052 NP that was durable for one year post the final vaccination (Fig. 3A). Specifically, we observed an early and significantly higher nAb response in animals immunized with Env + GLA+3M-052 NP at week 14, in comparison with animals immunized with Env + 3M-052 NP (6 fold higher, p = 0.017) or GLA NP [47 fold higher, p <0.001] (Fig. 3B). These results are consistent with our work and other studies previously reported in mice and mini pigs, highlighting an advantage of combining TLR-4 and TLR-7/8 adjuvants to induce higher Ab responses(18, 37, 38). However, at week 70, the magnitude of nAb response was highest and comparable in animals immunized with Env + GLA+3M-052 NP or 3M-052 NP (Fig. 3C). Breadth of Tier 1 HIV-1 virus neutralizing activity against MW965.26, MN.3 and SF162.LS was calculated as the average of the log10 nAb titer over the panel of isolates and plotted as magnitude-breadth (MB) plots as described previously(39) as shown in (Fig. 3 D and E). These combined analyses continued to highlight higher nAb responses in animals receiving 3M-052 or the combination of GLA+3M-052 NP adjuvants in comparison with alum, GLA NP, R848 and MPL+R848 NP adjuvants respectively. Finally, a significant correlation (Spearman r = 0.8064, p =0.0001) overall was observed between the frequencies of Env specific bone marrow LLPCs and Tier 1 MW965.26-specific nAb titers (Fig. 3F). These results highlight the adjuvanticity of 3M-052 to induce a high magnitude and durable nAb response in RMs.
Fig. 3. 3M-052 adjuvant formulated in PLGA NP’s with or without GLA improves rapidity and durability respectively of Tier-1A HIV nAb responses in serum upto ~ 1 year in comparison with the clinically used alum adjuvant.
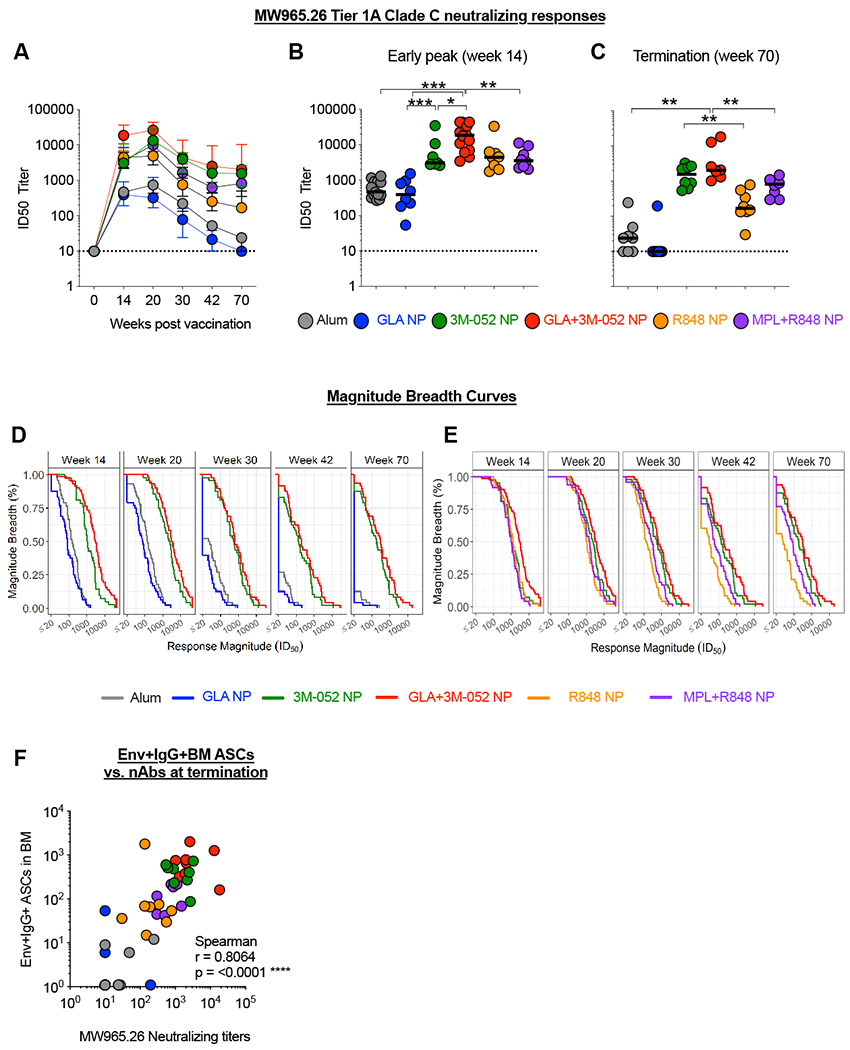
Tier1A HIV-1 pseudovirus neutralizing activity was assayed using a standardized TZM-bl assay. (A) Line graphs indicate the kinetics of Tier1A MW965.26 HIV-1 pseudovirus neutralizing activity in serum. (B) Scatter plots display peak Tier1A MW965.26 HIV-1 pseudovirus neutralization at 2 weeks post the third vaccination or week 14 in the study. (C) Scatter plots display durable Tier1A MW965.26 pseudovirus neutralization at week 70 or close to a year post the fourth vaccination. (D) Magnitude breadth curve of Tier-1A HIV-1 pseudovirus neutralizing antibody responses in treatment groups 1-4 is shown. (E) Magnitude breadth curve of Tier-1A HIV-1 pseudovirus nAb responses in groups 3-6 is displayed to highlight differences between adjuvanting with 3M-052 with or without GLA in comparison with R848 with or without MPL. (F) Graph shows a Spearman correlation of all combined Tier1A MW965.26 HIV-1 pseudovirus nAb response (all treatment groups) vs. corresponding frequencies of 1086.c Env gp140 specific LLPCs in the bone marrow. Statistical significance of differences in magnitude of immune responses was tested using a non-parametric Wilcoxon rank-sum test (also known as Mann-Whitney U test) using the R software version 3.6.1. **** p<0.0001: *** p <0.001: ** p<0.01, *p<0.05.
Vaccination with Env and 3M-052 or GLA+3M-052 NPs induced enhanced and durable HIV-1 Env binding Ab responses in RMs
An optimal panel of antigens comprised of Env gp140, gp120, gp41 and V1V2 antigenic domains have recently been down selected to guide mapping of Ab responses with HIV Env vaccinations(40). We assayed for HIV-1 specific IgG Ab responses against this panel of antigens using the binding antibody multiplex assay (BAMA assay) (Fig. 4 A, B, C and D). Vaccination with Env + 3M-052 NP or GLA+3M-052 NP induced significantly higher magnitude of binding Ab titers against the whole homologous 1086.C gp140 immunogen (Fig. 4A), the gp120 and gp41 antigenic domains (Fig. 4 B and C) and against the V1/V2 epitope (Figure 4D) at two weeks post final vaccination (week 20) [ p values between 0.01 and <0.0001 for all antigens assayed between groups 3,4 vs. 5,6 and 1,2 respectively as shown in the figures]. A striking aspect of this data was that the magnitude of persistent Env Ab responses in 3M-052 NP and GLA+3M-052 NP adjuvanted animals at week 70 was significantly higher than those induced by alum and GLA NP at week 20, the peak time point in the study (Fig. 4 A–D). Of note, one such example worth highlighting is the magnitude of Ab response against the homologous 1086.C V1V2 antigen (Fig. 4D). At week 70, Ab titer in animals vaccinated with 3M-052 and GLA+3M-052 NP was 30,681 (min, max; 15564, 43,804) and 34,813 (min, max; 17543, 57,919) higher than the median magnitude of Ab responses in animals receiving alum and GLA NP at week 20 which was 15653(min, max; 5279, 36,679) and 8043 (min,max; 1065, 16,825) respectively. Enhanced responses in 3M-052 adjuvanted animals was not only observed against the whole Env or its antigenic domains, but against a panel of HIV-1 antigens representing most circulating Clades of HIV-1 (Fig. 4E). Group average magnitude-breadth (MB) curves were also used to display the breadth of binding Ab activity against various gp140 (n=7) and gp120 (n=8) antigens (Fig. 4F). These data demonstrate that 3M-052 adjuvant by itself or in combination with GLA +3M052 in PLGA NPs induced enhanced and long-lived IgG binding antibody responses against a large panel of HIV-1 Env antigens.
Fig. 4. 3M-052 adjuvant formulated in PLGA NP’s with or without GLA significantly improves the magnitude and breadth of binding Ab responses against HIV-1 Env gp140, gp120 and V1V2 epitope bearing antigens in comparison with other adjuvants in the study.
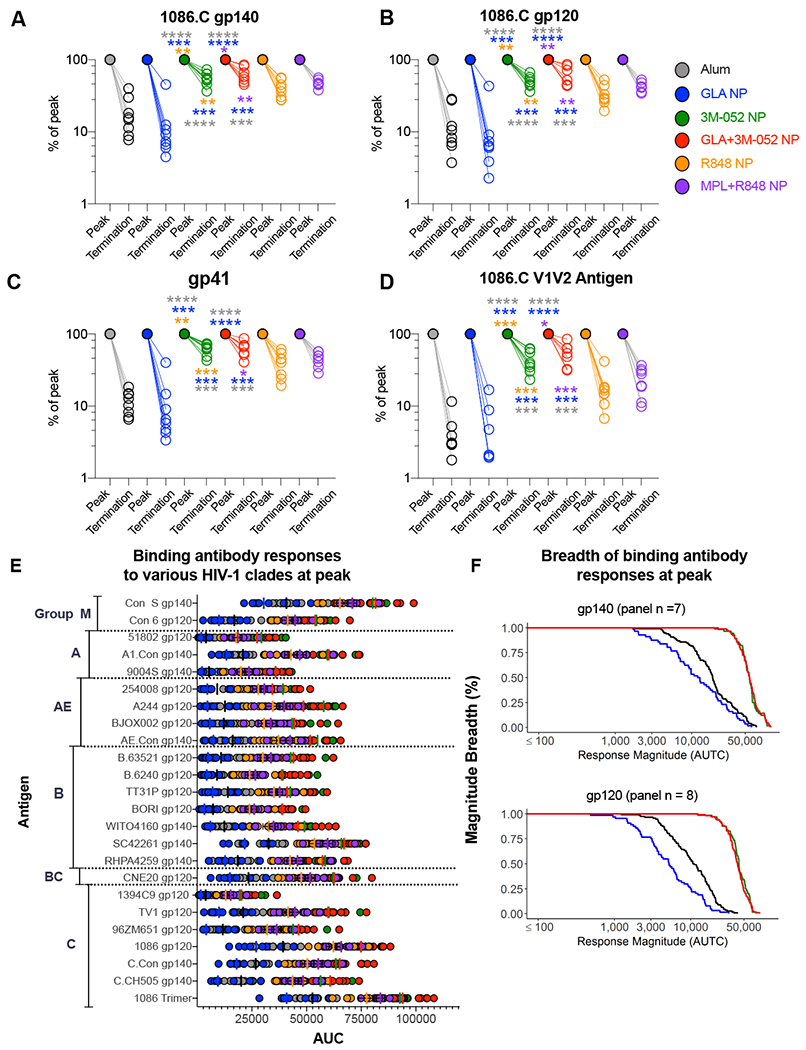
Env antigen specific IgG Ab responses were measured using a binding antibody multiplex (BAMA) assay. Antibody responses are represented as area under the serum titration curve (AUTC) detailed in the methods section. Scatter plots represent % change in binding Ab responses from peak (week 20) to termination (~week 70) time points against; (A) autologous 1086.C gp140 Env antigen, (B) 1086.C gp120 Env antigen, (C) gp41 antigen and (D) 1086.C V1V2 tag antigen. (E) Binding Ab responses to various HIV-1 Env antigens (n=24) are summarized by Clade groups. (F) Line graphs show group average magnitude breadth curves highlighting breadth of binding activity against Env gp140 (n=7) and Env gp120 (n=8). Statistical differences are color coded to indicate the respective group comparisons. Statistical significance of differences in magnitude of immune responses was tested using a non-parametric Wilcoxon rank-sum test (also known as Mann-Whitney U test) using the R software version 3.6.1. **** p<0.0001: *** p<0.001: ** p<0.01, *p<0.05.
Vaccination with Env and 3M-052 or GLA+3M-052 NPs induces enhanced and durable ADCC activity in RMs
Emerging evidence in both pre-clinical and clinical studies point to a role for non-neutralizing Fc receptor-mediated effector functions of HIV specific Abs as a complementary mechanism of protection to neutralization responses(41–44). We thus measured serum ADCC activity against the whole 1086.C gp120 immunogen coated target cells using a standardized GTL assay(45). Env specific ADCC activity was induced in RMs immunized with Env plus 3M-052 NP (Fig. 5 A–C). Similar to rapid induction of nAb titers observed in animals immunized with GLA+3M-052 NP, we noted enhanced ADCC responses were detected as early as week 8 in animals those were immunized with GLA+3M-052 NP in comparison with responses in animals receiving 3M-052 NP [2.9 fold higher, p = 0.13] or GLA NP [180 fold higher, p =0.049] alone (Fig. 5 A and B). These responses were also higher than those induced by alum (~21 fold higher) and the combination of MPL+R848 in NPs (~6 fold higher). Striking durability and enhanced ADCC titers (100% response rates) was observed for the entire duration of the study (Fig. 5 A and C) only when vaccinating with Env + 3M-052 NP or GLA+3M-052 NP. In contrast, a substantial contraction in responses was observed as early as three months after the final vaccination in animals immunized with alum or with GLA NP with only 37.5% and 0% animals responding at termination respectively (Fig. 5C).
Fig. 5. 3M-052 adjuvant formulated in PLGA NP’s with or without GLA significantly improves Ab dependent cellular cytotoxicity (ADCC) as well as avidity of Ab responses durable in serum upto ~ 1 year in comparison with the clinically used alum adjuvant.
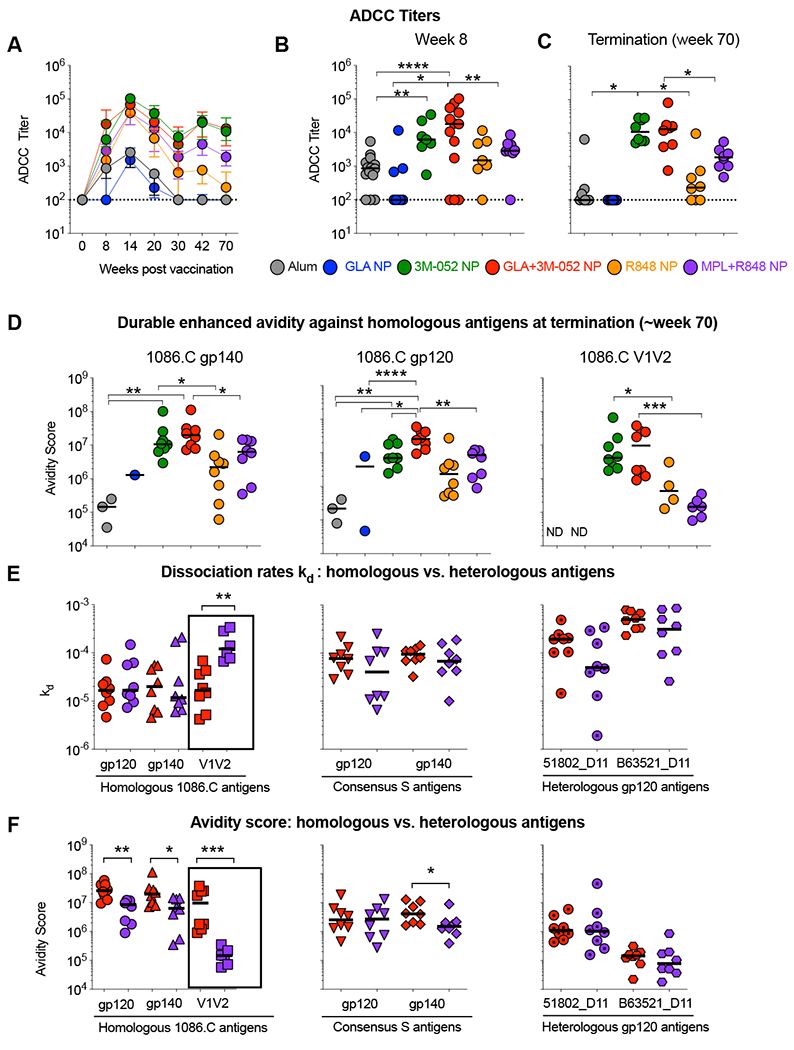
ADCC activity was assayed using an established Granzyme Toxi Lux or GTL assay. Avidity of polyclonal sera was assayed on purified serum immunoglobulin (IgG) using a BIAcore surface plasmon resonance (SPR) assay. (A) Line graphs show kinetics of ADCC titers observed in the study. (B) Scatter plots display an early peak ADCC activity observed after the 2rd vaccination in the study at week 8. (C) Scatter plots show persistent ADCC activity at termination of the study at week 70. (D) Scatter plots display avidity analysis at termination (week 70) against homologous 1086.C Env gp140, 120 and V1V2antigens. Avidity is calculated by dividing binding response units (BRU) by inverse of the dissociation rate constant or kOFF values. (E) Scatter plots display kOFF or dissociation rates against homologous, consensus and heterologous antigens. (F) Scatter plots display Avidity Scores against homologous, consensus and heterologous antigens. Statistical significance of differences in magnitude of immune responses was tested using a non-parametric Wilcoxon rank-sum test (also known as Mann-Whitney U test) using the R software version 3.6.1. . **** p<0.0001: *** p<0.001: ** p<0.01, *p<0.05.
In addition to ADCC activity, avidity of Env specific Ab responses has previously been correlated with their ability to protect against intrarectal SIV or SHIV challenges in macaques(46, 47). We performed a surface plasmon resonance (SPR) based comparison of avidity of Env specific Ab responses. Avidity was assayed against three homologous (1086.C gp120, gp140 or V1V2) antigens, consensus gp120 and gp140 and two heterologous gp120 antigens at weeks 20 (peak) and at week 70 in the study. We report both kd or dissociation rate which is a parameter independent of the magnitude of the response as well as the avidity-score which is dependent on the magnitude of the response at a given time point calculated as a ratio of binding response units (BRU) and kd. Consistent with significant differences observed in the magnitude of binding Ab responses in Fig. 4, we observed differences with avidity-scores of Ab responses in animals receiving 3M-052 or GLA+3M-052 adjuvants in comparison with alum or GLA NPs at week 70 where an Ab response is expected to have matured over time (Fig. 5D and fig. S2A). Specifically, avidity-score with responses against the homologous 1086.C gp120 was ~100 fold higher in animals receiving the GLA+3M-052 NPs in comparison with those receiving alum (Fig. 5D). Similar responses in these animals were observed against both the 1086.C gp140 as well as the 1086.C V1V2 antigens. Avidity-scores against the 1086.C V1V2 antigen in animals immunized with the 3M-052 adjuvants were also significantly higher than those achieved by R848 or MPL+R848 NP respectively [p=0.0283, 3M-052 vs. R848 and p=0.0007, GLA+3M-052 vs. MPL+R848] while no binding responses were detected when vaccinating with alum or GLA NP highlighting rapid decay. Furthermore, this striking difference in avidity-score was predominantly due to differences in kd values highlighting increased avidity independent of the magnitude of the response (fig. S2 C–D). Both avidity-score and kd responses were highest when stratifying responses against homologous antigens followed by consensus and heterologous antigens (Fig. 5 E and F). Overall, these data highlight the ability of 3M-052 to promote persistent high avidity responses against Env immunogens in RMs.
Vaccination with Env + GLA+3M-052 NP induces an enhanced GC response in RM draining LNs in comparison with that induced by alum
Combination of TLR-4 and TLR-7 agonists significantly enhance GC responses in mouse draining LNs (18). Here, we investigated GC B cell and TFH responses in draining and non-draining LNs of animals immunized with Env + GLA+3M-052 NP or alum at ~4 weeks post fourth immunization (Fig. 1B). GC B and TFH cells were identified by flow cytometry as described previously(48, 49) (Fig. S3). A high frequency of GC B cells was induced in both the draining Iliac and proximal popliteal LNs of animals vaccinated with the GLA+3M-052 NP and this was significantly higher [p=0.0022 and p=0.0260 for right Iliac and popliteal LNs respectively] than that induced by Alum (Fig. 6B). A corresponding and significant increase in GC TFH [p = 0.0043, GLA+3M-052 vs. alum] was also observed in the draining Iliac LN (Fig. 6C). Visualization of these responses by immunohistochemistry revealed a remarkably high proportion of follicles with GCs in animals vaccinated with the GLA+3M-052 NP (Fig. 6D and fig. S4). These GC frequencies were significantly higher [p=0.0043] than those induced by alum (Fig. 6E).
Fig. 6. 3M-052 adjuvant formulated in PLGA NP’s with GLA induces significantly higher GC B cells, TFH and Env specific PC responses in RM draining lymph nodes (dLNs) in comparison with those induced by alum adjuvant.
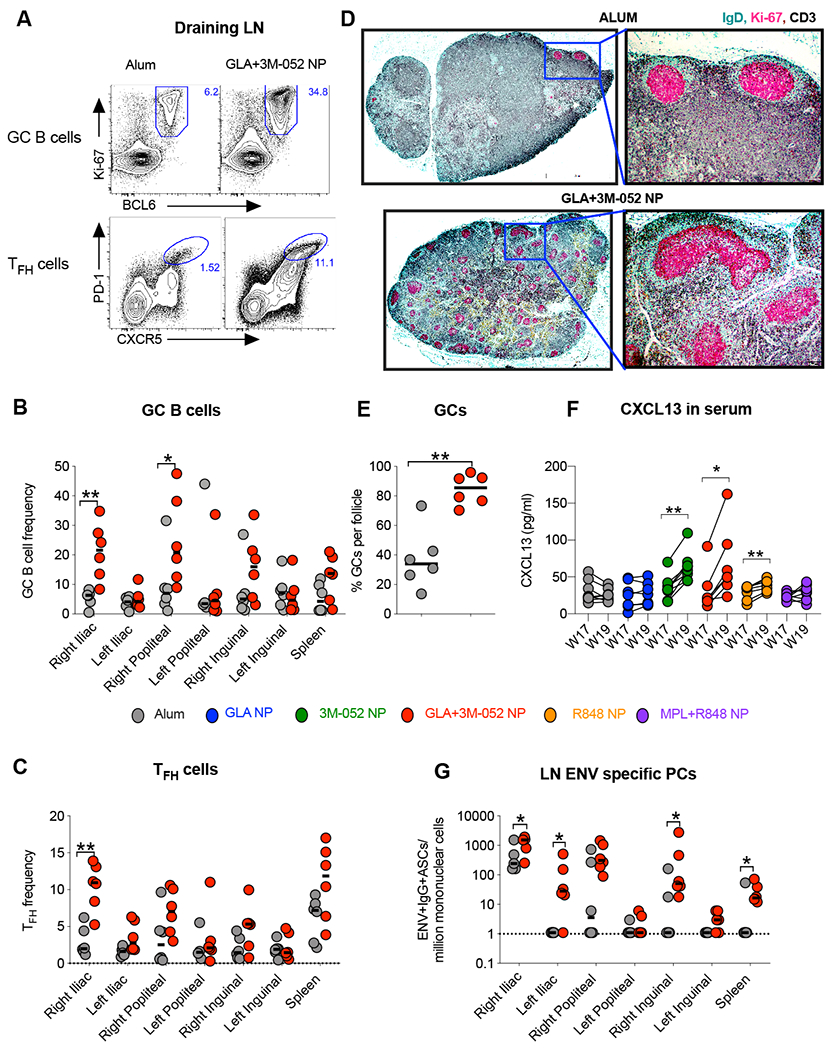
Data shown in the figure summarizes immune responses at ~week 22 or 4 weeks post final vaccination in n = 6 animals in the mechanistic arm, assigned to treatment groups 1 and 4 in the study (see study design in Figure 1). (A) Representative flow cytometry plots are shown highlighting GC B cells (live,CD3-CD20+Ki67+Bcl6+) and GC TFH cells (live,CD3+CD4+ PD-1 high and CXCR5 high). Bar graphs summarize (B) frequencies of GC B cells and (C) frequencies of GC TFH cells. (D) Immunohistochemistry was performed on draining Iliac LNs in n=6 animals from the alum and GLA+3M-052 NP adjuvant treated animals respectively. Green, magenta and black colors were used to represent staining for IgD, Ki67 and CD3 staining respectively. Figure represents low magnification and high magnification images from alum and GLA+3M-052 NP adjuvanted animals (see methods for details). (E) Graph summarizes percent of B cell follicles staining positive for GCs with immunohistochemistry (F) Graph shows relative increase in CXCL13 chemokine in RM plasma one week before and after the final vaccination. (G) Graph summarizes Env specific plasma cells ( B cell ELISPOT assay) per million live mononuclear cells in various draining and non-draining LNs. Statistical significance of differences in magnitude of immune responses was tested with a two tailed non-parametric Mann-Whitney test using the Graph Pad Prism version 8.0 software. ****p<0.0001: ***p<0.001: ** p<0.01,*p<0.05.
Since LNs post immunizations were only available from animals in groups 1 and 4, it was unclear if GC responses were also induced in other groups in the study. CXCL13 in plasma can be indicative of GC activity in LNs (50). This biomarker provided us an opportunity to assess GC activity without having to sacrifice animals. We assayed the concentration of CXCL13 in serum in all animals pre and post final vaccination. Significant increases in CXCL13 one week after final vaccination in plasma were observed in animals immunized with Env and 3M-052 NP or GLA+3M-052 NP or in animals immunized with Env and R848 or MPL+R848 NP (Fig. 6F). No appreciable increase in CXCL13 was observed in animals receiving alum or GLA NP consistent with the weaker antibody response in these animals.
A significantly higher frequency of Env specific PCs was observed in draining Iliac and popliteal LNs in animals vaccinated with Env plus GLA+3M-052 NP (Fig. 6G) highlighting differential drainage of the vaccine and priming of Env specific responses. Few Env specific plasma cells were observed in the spleen, highlighting a more localized response when vaccinating sub-cutaneously. Finally, a striking correlation was observed between GC B cells, TFH cells and BM plasma cell responses with the corresponding Tier 1A Clade C as well as heterologous Clade B neutralizing responses (fig. S5).
In addition to inducing a strong GC TFH response in draining LNs, both 3M-052 and GLA+3M-052 NP adjuvants induced enhanced Env specific CD4+ T cell responses in peripheral blood both after primary and booster vaccinations in comparison with other groups in the study (fig. S6). We have previously reported very low or no appreciable induction of CD8+ T cells in RMs when vaccinating with soluble protein immunogens and TLR-agonists (19). We also investigated any potential increases in frequencies of CCR5+CD4+ T cells in the rectal mucosa post vaccinations with Env and adjuvants in the study (fig. S7). In contrast with increased Env specific immune responses observed when vaccinating with Env and 3M-052 NP, no significant changes in frequencies of CCR5+CD4+ T cells were observed after each round of vaccination in comparison with baseline frequencies. Overall, these data suggest that the 3M-052 adjuvant with or without GLA in NPs is capable of orchestrating strong B cellular and serological responses in RMs without enhancing the frequency of target CD4+ T cells in the rectal mucosa.
Vaccination with Env + GLA+3M-052 NP induces persistent activation of monocytes (upto 2 weeks ) in peripheral blood
Immunization of RMs with TLR agonists has previously highlighted activation of DCs and monocytes in the blood (19, 21). Immunization with Env + 3M-052 NP or GLA+3M-052 NP induced a rapid increase in the proportion of CD14+CD16+ intermediate monocytes (fig. S8, S9A and B). In contrast, frequencies of classical and intermediate monocytes were minimally changed post vaccination with Env + alum or GLA NP. In addition, vaccination with 3M-052 NP or GLA+3M-052 NP induced enhanced expression of CD86 and/or CCR7 on all monocyte subsets that persisted for at least two weeks post primary vaccination (fig. S9C). We have also recently reported, transient increase in frequencies of pDCs and conventional DC subset #1 (BDCA-1 or CD1c expressing DC) at day 1 followed by increased and peak activation (CD86 and CCR7) of these DC subsets at day 2 post primary vaccination when vaccinating with TLR agonists in NP(19). Overall, these data highlight a critical role for TLR-7/8 agonists in activating innate immunity.
Vaccination with Env + Alum (3M-052), an adjuvant designed for clinical use, recapitulates induction of BM LLPCs and durability of antibody responses in RMs
Currently, there are no approved vaccine formulations with PLGA micro or NPs with challenges foreseen in scale up (51, 52). As such, we compared the immune responses stimulated by the 3M-052 or GLA+3M-052 NP with 3M-052 or GLA+3M-052 formulated in alum, which offers an easier path towards translation(53). Also, the first NHP study (described in Fig. 1A and B) used a dose of 750μg of the 3M-052 adjuvant (harmonized initially with the dose of R848) for the primary vaccination, followed by a reduced dose of 75 μg in all boost vaccinations. Hence, we wanted to evaluate if the long-lived cellular and serological responses were dependent on the higher priming dose of the adjuvant used. We performed a second experiment with five groups of n = 6 RMs each as detailed in Fig. 7A. (Of note, we have left out reporting data from a sixth group of n=6 animals due to an error with vaccinations encountered during the study). Animals in group 1 received vaccinations with 1086.C Env gp140 immunogen with alum. Animals in groups 2 and 3 received vaccinations with Env + alum formulated 3M-052 or GLA+3M-052 adjuvants respectively. Animals in groups 4 and 5 received vaccinations with Env + 3M-052 and GLA+3M-052 NPs respectively. The primary objective of this study was to compare immune responses induced by the Alum (3M-052) and 3M-052 NP formulations with those induced by alum only clinical adjuvant. The secondary objective of this study was to compare immune responses induced by combinations of GLA+3M-052 in alum and NP formulations respectively with those induced by 3M-052 adjuvant itself. Hence, the statistical tests used to establish significance of differences in immune responses were designed to address these two study objectives as detailed in the methods section.
Fig. 7. 3M-052 adjuvant formulated with alum induces robust and durable Env specific LLPCs in bone marrow comparable with those induced when formulating in PLGA NPs and also significantly higher than alum adjuvant.
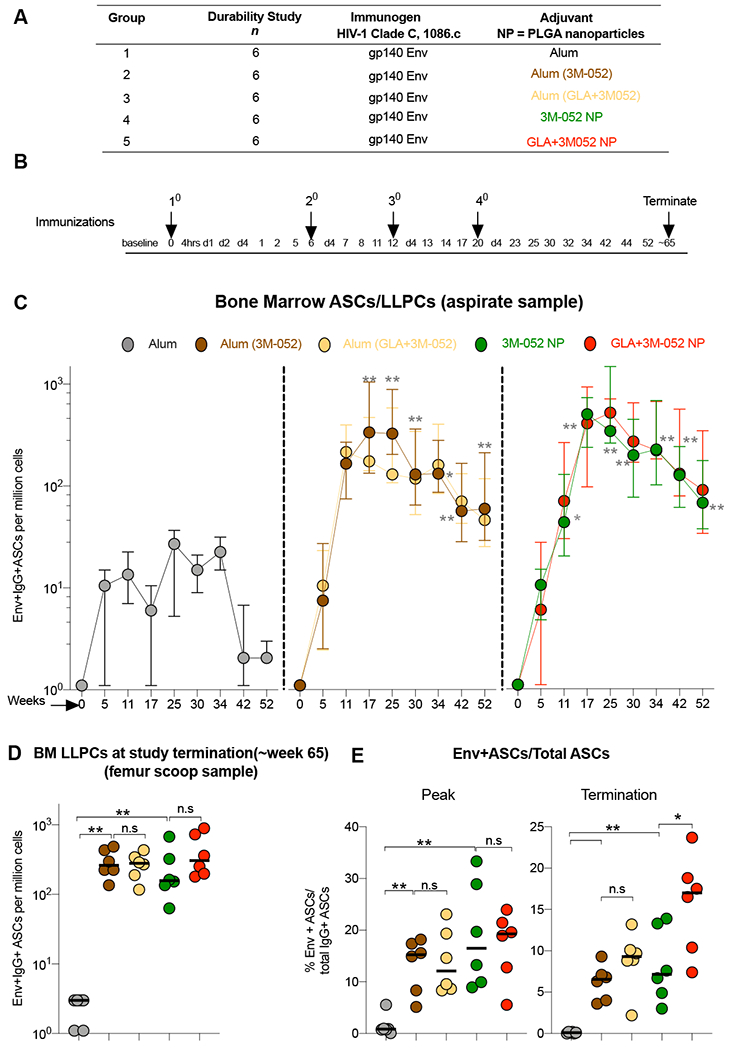
(A) Study design details treatment groups with various adjuvants and Env vaccinations in the second study. (B) Experimental timeline details blood and bone marrow collections as well the sacrifice time point in the study. (C) Env specific LLPC responses were enumerated using a B cell ELISPOT assay. Line graphs indicate frequencies of Env specific LLPCs per million bone marrow mononuclear cells (BMMCs) quantified in aspirates collected from Iliac crest at indicated time points. (D) Graph highlights frequencies of Env specific LLPCs per million BMMCs assayed in femur tissue scoop harvested at termination (~week 65). (E) Enrichment of Env specific IgG+ LLPCs in BM within total IgG+ ASCs is highlighted for the peak and termination time points. Statistical significance of differences in magnitude of immune responses was tested with a one tailed non-parametric Mann-Whitney test comparing groups 2, 3 vs. 1 and two tailed non-parametric Mann-Whitney test comparing the rest of the groups. using the Graph Pad Prism version 8.0 software. Asterisk’s are color coded to highlight the difference between two distinct treatment groups. **** p<0.0001: *** p<0.001: ** p<0.01, *p<0.05.
Consistent with our observations in the first study, we observed a robust induction and persistence (upto ~ 1 year) of BM resident Env specific LLPCs in RMs when vaccinating with Env and 3M-052 adjuvant with or without GLA in both NPs as well as the novel alum adsorbed formulations (Fig. 7C and D). Of note, while no differences were noted between frequencies of Env specific LLPCs in groups 2-5 (Fig. 7C and D). In contrast, similar to study# 1 (fig. S1C), enrichment of Env specific LLPCs among all IgG+ PCs was highest in animals vaccinated with GLA +3M-052 NP [p=0.041 vs. 3M-052 NP and p = 0.0043 vs. Alum (3M-052)] (Fig. 7E). We observed a reproducible induction and persistence of high magnitude and durability of nAb responses against MW965.26, a HIV-1 Tier-1 pseudovirus (fig. S10A). A significant correlation (Spearman r = 0.7840, p <0.0001) was observed between the presence of a range of frequencies with Env specific BM LLPCs (upon combining all treatment groups) and corresponding Clade C MW965.26 pseudovirus specific nAb responses (fig. S10B). Ab responses with higher ADCC activity and avidity were observed in animals in groups 2-5 in comparison with group 1 (fig. S10 C and D). Furthermore, we observed high magnitude and durability of binding Ab responses against an optimal panel of HIV-1 Env antigens (fig. S11) when vaccinating with all 3M-052 adjuvant formulations with or without GLA. Finally, we demonstrate reproducible changes in monocyte subsets and persistent activation with enhanced CD86 and CCR7 expression (in comparison with alum) when vaccinating with the novel Alum (3M-052) adjuvant identical to those induced by the 3M-052 NP adjuvant (fig. S12 A–C). These data are consistent with those described in fig. S9. Overall, these data highlight much promise for rapid testing of the 3M-052 adsorbed alum formulation with HIV-1 Env protein immunogens in human clinical trials.
Discussion
It is now well established that adjuvants substantially improve immunogenicity of protein based vaccines(15). Based on data in mice and more recent report in humans, induction of LLPCs is a critical objective in vaccine and/or adjuvant design(10, 11, 14). However, no studies with adjuvants so far have demonstrated long term (> 1 year ) persistence of BM resident HIV-1 vaccine specific LLPCs in RMs given that NHPs are used as the most relevant model for HIV vaccine testing. A key goal of our study here was to carefully downselect a single or combination of TLR-4 and TLR-7/8 targeted adjuvants and independent formulation approaches that could promote high magnitude and durable HIV-1 Env specific antibody responses and most critically LLPCs in RMs. Our results highlight a significant and superior ability of the 3M-052 adjuvant in stimulating HIV-1 Env specific antibody responses that are not only substantially higher at peak (~ 2 weeks) after four vaccinations but also remarkably persistent for a year at levels higher than that achieved by alum at peak (Fig. 2–7). Moreover, broad immune responses were strikingly durable against multiple antigenic components of the Env protein (gp41, V1V2 ) as shown in Figs. 4 and 5, including against the gp120 component previously reported to be immunosuppressive when interacting with both DCs and B cells (54, 55). Interestingly, both with alum, a benchmark adjuvant used in clinic, and GLA, a TLR-4 agonist formulated in PLGA NPs, we did observe robust Env specific plasmablast responses in blood (Fig. 2B) after each round of boost vaccination but failed to detect appreciable Env specific LLPCs in the BM.
Several immunological outcomes in groups 3-6 in comparison with groups 1-2 could have contributed to successful induction of LLPCs: 1) robust and more persistent (day 7 vs. day 4) plasmablast responses which suggests a threshold of frequencies above which the surviving emigrating cells could traffick to the BM, 2) the direct targeting of TLR-7 by 3M-052 or R848 in B cells resulting in distinct programming of ASCs helping them home to the BM 3) successful induction of GC B and GC TFH cells in addition to Th1 polarized CD4+ T cells and finally 4) robust differentiation and activation of monocyte subsets both contributing to PC differentiation. While the requirement and impact of direct targeting of TLRs on B cells for vaccine efficacy is unclear from work in mice(56, 57), we find consistent and robust induction of Env specific BM resident PCs only when vaccinating with TLR-7/8 agonists suggesting a critical role for B cell instrinsic TLR-7 in PC differentiation. Future studies investigating the direct impact of distinct adjuvants on B cells could identify mechanisms if any, that improves both memory and PC differentiation. We would also like to hypothesize that a use of an adjuvant capable of directly targeting memory B cells may be able to minimize the dependency on CD4+ T cells and hence reduce the generation of target cells for HIV-1(58) (fig. S7). Recent work also highlights a synergy between TLR-7/8 and IFN-γ signaling in B cells in promoting PC differentiation(59, 60). As a consequence, it was also reported that IFN-γ signaling increased responsiveness of B cells to IL-21 cytokine, predominantly secreted by TFH cells. In line with these observations, we report induction of significantly higher IFN-γ responding CD4+ T cells when vaccinating with 3M-052 NP (Fig. S5) and strikingly high GC TFH responses when vaccinating with both 3M-052 and GLA+3M-052 NP (Fig. 6 E and F), both which could have supported PC differentiation. Finally, we have previously reported robust plasmablast differentiation of B cells supported by the CD14+CD16++ intermediate monocytes that are largely expanded and activated in RMs when vaccinating with TLR-7/8 agonists(21, 61). Again, it is highly likely that robust differentiation and activation of intermediate monocytes induced by 3M-052 (Fig. S8) could have contributed to programming of LLPCs. Collectively all these data strongly suggest a critical role for Th1 immune response polarizing TLR-7/8 agonists in supporting and augmenting PC differentiation.
Many new adjuvants either in clinical testing or recently approved for use in humans are combination adjuvants(15, 29). Combination adjuvants such as with AS01 and AS04 (MPL and QS-21 in liposomes and alum + TLR-4 agonist, MPL) include components that perform additively or synergistically better than when used individually (15, 62). Here, we report that the combination of GLA + 3M-052 adjuvants improves the rapidity of neutralizing and ADCC activity as observed in Fig. 3B and 5B at early time points post vaccinations. However, no such advantage was observed with the combination of GLA and 3M-052 on the magnitude of reponses at termination (Fig. 2–7). One possible explanation is that higher responses induced early by the combination of GLA and 3M-052 or pre-existing vaccine induced immunity could have perhaps limited the boostability of the final vaccination. Studies with longer intervals in between vaccinations in general (63)can improve boostability when using such potent adjuvants. An alternate explanation is the use of NP or alum based formulations with GLA in this study. Squalene based stable emulsion (SE) has been reported to be superior in enhancing adjuvanticity of GLA(64) and it could very well enhance the efficacy of GLA in combination with 3M-052 as well. Here, we conclude that 3M-052 even in the absence of GLA as an adjuvant demonstrates potential to improve both peak and durability of key non-neutralizing effector functions desired in HIV specific Ab responses.
There were two goals in the second study. First, we wanted to investigate whether we could induce and sustain high frequencies of Env specific LLPCs with a reduced dose of 3M-052 (75 vs. 750μg) with a primary vaccination. Also, we aimed to address if formulation (NP vs. alum) impacted the adjuvanticity of 3M-052. We hypothesized that an alum adsorbed 3M-052 formulation has an accelerated path for testing in humans due to the previously reported challenges with the PLGA platform (53). Innate and Env specific Ab responses induced by alum formulated 3M-052 (Fig. 7, fig. S10,11) were not only identical to results from the first study but demonstrated striking similarity to that induced by distinct 3M-052 NP formulations. Hence, these data suggest a minimal dependency of the 3M-052 molecule on the delivery vehicles tested so far. Furthermore, now with reduced priming doses in comparison with the first study, we continued to observe a lack of impact on immune response longevity when combining GLA and 3M-052 in comparison with the 3M-052 adjuvant alone. One caveat worth highlighting is the route of vaccination used in our studies. We reasoned that sub-cutanoues (SC) vaccinations could recapitulate our findings of improved vaccine efficacy in mice when using protein immunogens and particulate adjuvants(18). However, most approved sub-unit vaccines are administered via the intramuscular (IM) route of vaccination in humans. Recent studies comparing SC vs. IM routes of vaccinations with HIV-1 immunogens and adjuvants have reported either improved immunogenicity or no impact on innate or adaptive immunity with SC vs. IM vaccinations(49, 65). Future studies in macaques comparing a) impact of SC vs. IM routes of vaccinations with 3M-052 adjuvant formulations and b) dose titration of the agonist in adjuvanting protein immunogens could significantly inform the choice of route and dose for human use. In summary, these results highlight the potential for 3M-052 as use as an adjuvant in vaccines against a variety of infections such as with HIV, malaria and influenza. Furthermore, these results have prompted a phase 1 clinical trial to assess the adjuvant potential of 3M-052 in the context of HIV Env antigens (http://clinicaltrials.gov NCT04177355).
Supplementary Material
Acknowledgements:
We thank Amy Weiner at the BMGF for her continuous support, encouragement and insights. We would like to thank all animal staff at the Yerkes National Primate Research Center at Emory University and specifically Christopher Souder, Robert Sheffield, John Wambua, Stephanie Ehnert in assisting with the RM study. We would also like to thank Drs. Elizabeth Strobert, Jennifer Wood and Sherrie Jean for exceptional assistance with animal health assessment with these adjuvant studies. We would like to acknowledge the NIH AIDS reagent program for consensus peptide pool for clade C envelope procured to run the T cell ICS assay reported in Fig S6.
Funding
This work was supported by a Center for AIDS Vaccine Discovery (CAVD) grant from the Bill and Melinda Gates Foundation (BMGF) to BP and a grant to BFH for Env protein production. SPK was additionally supported by a NIH K01 award OD023039-03. SC and CHD were supported by NIH NIAID R01 AI125068. Adjuvant formulation work at IDRI was supported by the Bill and Melinda Gates Foundation (BMGF) grant#OPP1055855. The CFAR Immunology/Emory Vaccine Center Flow cytometry core is supported by the NIH grant (P30 A050509). We also acknolwdege the support from grants NIH P51-RR000165 and P51-OD011132 to the Yerkes National Primate Research Center.
Competing interests: MT and JV are employees of 3M Drug Delivery Systems and are co-inventors of the synthetic TLR-7/8 agonist. CBF is a member of the Scientific Advisory Board of MaxHealth Biotechnology, LLC. CBF is an inventor on patent application (US 2019/0142935) held/submitted by IDRI that covers formulations of TLR ligands with lipid excepients and aluminum salts. RG has received consulting income from Juno Therapeutics, Takeda, Infotech Soft, Celgene, Merck and has received research support from Janssen Pharmaceuticals and Juno Therapeutics, and declares ownership in CellSpace Biosciences.
Footnotes
Data and materials availability
All raw data used in graphs in the manuscript are included in master excel file included in the supplementary materials. 3M-052 and GLA are available from CBF, and MT under a material agreement with IDRI and 3M Drug Delivery Systems. 1086.C Env is available from BFH under a material agreement with Duke.
REFERENCES
- 1.Plotkin SA, Vaccines We Need But Donť Have. Viral immunology 31, 114–116 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Gu XX, Plotkin SA, Edwards KM, Sette A, Mills KHG, Levy O, Sant AJ, Mo A, Alexander W, Lu KT, Taylor CE, Waning Immunity and Microbial Vaccines-Workshop of the National Institute of Allergy and Infectious Diseases. Clin Vaccine Immunol 24, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B, Ahmed R, Immunological mechanisms of vaccination. Nature immunology 12, 509–517 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, M.-T. Investigators, Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine 361, 2209–2220 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH, Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine 366, 1275–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Excler JL, Michael NL, Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med 66, 423–437 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B, Oh JZ, Nakaya HI, Ravindran R, Kazmin DA, Immunity to viruses: learning from successful human vaccines. Immunol Rev 255, 243–255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victora GD, Nussenzweig MC, Germinal centers. Annu Rev Immunol 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Weisel F, Shlomchik M, Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE, Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 43, 132–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slifka MK, Antia R, Whitmire JK, Ahmed R, Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Brynjolfsson SF, Persson Berg L, Olsen Ekerhult T, Rimkute I, Wick MJ, Martensson IL, Grimsholm O, Long-Lived Plasma Cells in Mice and Men. Frontiers in immunology 9, 2673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amanna IJ, Slifka MK, Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev 236, 125–138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slifka MK, Ahmed R, Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Current opinion in immunology 10, 252–258 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Reed SG, Orr MT, Fox CB, Key roles of adjuvants in modern vaccines. Nature medicine 19, 1597–1608 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Georg P, Sander LE, Innate sensors that regulate vaccine responses. Current opinion in immunology 59, 31–41 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B, Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. The Journal of experimental medicine 203, 413–424 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasturi SP, Kozlowski PA, Nakaya HI, Burger MC, Russo P, Pham M, Kovalenkov Y, Silveira EL, Havenar-Daughton C, Burton SL, Kilgore KM, Johnson MJ, Nabi R, Legere T, Sher ZJ, Chen X, Amara RR, Hunter E, Bosinger SE, Spearman P, Crotty S, Villinger F, Derdeyn CA, Wrammert J, Pulendran B, Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5alpha Restrictive Macaques. J Virol 91, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwissa M, Amara RR, Robinson HL, Moss B, Alkan S, Jabbar A, Villinger F, Pulendran B, Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. The Journal of experimental medicine 204, 2733–2746 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwissa M, Nakaya HI, Oluoch H, Pulendran B, Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood 119, 2044–2055 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling DJ, van Haren SD, Scheid A, Bergelson I, Kim D, Mancuso CJ, Foppen W, Ozonoff A, Fresh L, Theriot TB, Lackner AA, Fichorova RN, Smirnov D, Vasilakos JP, Beaurline JM, Tomai MA, Midkiff CC, Alvarez X, Blanchard JL, Gilbert MH, Aye PP, Levy O, TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2, e91020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petitdemange C, Kasturi SP, Kozlowski PA, Nabi R, Quarnstrom CF, Reddy PBJ, Derdeyn CA, Spicer LM, Patel P, Legere T, Kovalenkov YO, Labranche CC, Villinger F, Tomai M, Vasilakos J, Haynes B, Kang CY, Gibbs JS, Yewdell JW, Barouch D, Wrammert J, Montefiori D, Hunter E, Amara RR, Masopust D, Pulendran B, Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spangberg M, Juraska M, Wille-Reece U, Weiner A, Howard RF, Long CA, Duffy PE, Johnston L, O’Neil CP, Lore K, TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA, HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America 102, 15190–15194 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francica JR, Zak DE, Linde C, Siena E, Johnson C, Juraska M, Yates NL, Gunn B, De Gregorio E, Flynn BJ, Valiante NM, Malyala P, Barnett SW, Sarkar P, Singh M, Jain S, Ackerman M, Alam M, Ferrari G, Salazar A, Tomaras GD, O’Hagan DT, Aderem A, Alter G, Seder RA, Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv 1, 2329–2342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper DM, DeMars LR, HPV vaccines - A review of the first decade. Gynecol Oncol 146, 196–204 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Bharucha T, Ming D, Breuer J, A critical appraisal of ‘Shingrix’, a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Hum Vaccin Immunother 13, 1789–1797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffman RL, Sher A, Seder RA, Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD, Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 29, 5434–5442 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Wen Y, Trinh HV, Linton CE, Tani C, Norais N, Martinez-Guzman D, Ramesh P, Sun Y, Situ F, Karaca-Griffin S, Hamlin C, Onkar S, Tian S, Hilt S, Malyala P, Lodaya R, Li N, Otten G, Palladino G, Friedrich K, Aggarwal Y, LaBranche C, Duffy R, Shen X, Tomaras GD, Montefiori DC, Fulp W, Gottardo R, Burke B, Ulmer JB, Zolla-Pazner S, Liao HX, Haynes BF, Michael NL, Kim JH, Rao M, O’Connell RJ, Carfi A, Barnett SW, Generation and characterization of a bivalent protein boost for future clinical trials: HIV-1 subtypes CR01_AE and B gp120 antigens with a potent adjuvant. PloS one 13, e0194266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecchi S, Bufali S, Skibinski DA, O’Hagan DT, Singh M, Aluminum adjuvant dose guidelines in vaccine formulation for preclinical evaluations. J Pharm Sci 101, 17–20 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Kazzaz J, Singh M, Ugozzoli M, Chesko J, Soenawan E, O’Hagan DT, Encapsulation of the immune potentiators MPL and RC529 in PLG microparticles enhances their potency. J Control Release 110, 566–573 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Szarewski A, Cervarix(R): a bivalent vaccine against HPV types 16 and 18, with cross-protection against other high-risk HPV types. Expert Rev Vaccines 11, 645–657 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC, Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453, 667–671 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMillan R, Longmire RL, Yelenosky R, Lang JE, Heath V, Craddock CG, Immunoglobulin synthesis by human lymphoid tissues: normal bone marrow as a major site of IgG production. J Immunol 109, 1386–1394 (1972). [PubMed] [Google Scholar]
- 37.Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, Coblentz D, Reed SG, Carter D, A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J Nanobiotechnology 12, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay PF, King DF, Mann JF, Barinaga G, Carter D, Shattock RJ, TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes. PloS one 11, e0148984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Gilbert PB, Montefiori DC, Self SG, Simultaneous Evaluation of the Magnitude and Breadth of a Left and Right Censored Multivariate Response, with Application to HIV Vaccine Development. Stat Biopharm Res 1, 81–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yates NL, deCamp AC, Korber BT, Liao HX, Irene C, Pinter A, Peacock J, Harris LJ, Sawant S, Hraber P, Shen X, Rerks-Ngarm S, Pitisuttithum P, Nitayapan S, Berman PW, Robb ML, Pantaleo G, Zolla-Pazner S, Haynes BF, Alam SM, Montefiori DC, Tomaras GD, HIV-1 Envelope Glycoproteins from Diverse Clades Differentiate Antibody Responses and Durability among Vaccinees. J Virol 92, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes BF, Moody MA, Alam M, Bonsignori M, Verkoczy L, Ferrari G, Gao F, Tomaras GD, Liao HX, Kelsoe G, Progress in HIV-1 vaccine development. J Allergy Clin Immunol 134, 3–10; quiz 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL, Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155, 531–539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones AT, Shen X, Walter KL, LaBranche CC, Wyatt LS, Tomaras GD, Montefiori DC, Moss B, Barouch DH, Clements JD, Kozlowski PA, Varadarajan R, Amara RR, HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nature communications 10, 798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson HL, Non-neutralizing antibodies in prevention of HIV infection. Expert Opin Biol Ther 13, 197–207 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G, High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79, 603–612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL, Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. The Journal of infectious diseases 204, 164–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, Chennareddi L, Yu T, Kannanganat S, Ofielu L, Villinger F, Wyatt LS, Moss B, Amara RR, Robinson HL, GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 369, 153–167 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Havenar-Daughton C, Carnathan DG, Torrents de la Pena A, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, van der Woude P, Locci M, Le KM, de Taeye SW, Sok D, Mohammed AUR, Huang J, Gumber S, Garcia A, Kasturi SP, Pulendran B, Moore JP, Ahmed R, Seumois G Burton DR, Sanders RW, Silvestri G, Crotty S, Direct Probing of Germinal Center Responses Reveals Immunological Features and Bottlenecks for Neutralizing Antibody Responses to HIV Env Trimer. Cell Rep 17, 2195–2209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR, Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088 e1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, McGuire HM, Bothwell M, Vagefi PA, Scully E, Investigators IPCP, Tomaras GD, Davis MM, Poignard P, Ahmed R, Walker BD, Pulendran B, McElrath MJ, Kaufmann DE, Crotty S, CXCL13 is a plasma biomarker of germinal center activity. Proceedings of the National Academy of Sciences of the United States of America 113, 2702–2707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansen P, Martinez Gomez JM, Gander B, Development of synthetic biodegradable microparticulate vaccines: a roller coaster story. Expert Rev Vaccines 6, 471–474 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Jain S, O’Hagan DT, Singh M, The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev Vaccines 10, 1731–1742 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Fox CB, Orr MT, Van Hoeven N, Parker SC, Mikasa TJ, Phan T, Beebe EA, Nana GI, Joshi SW, Tomai MA, Elvecrog J, Fouts TR, Reed SG, Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: A formulation approach. J Control Release 244, 98–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan M, Klasse PJ, Banerjee K, Dey AK, Iyer SP, Dionisio R, Charles D, Campbell-Gardener L, Olson WC, Sanders RW, Moore JP, HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog 3, e169 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jelicic K, Cimbro R, Nawaz F, Huang da W, Zheng X, Yang J, Lempicki RA, Pascuccio M, Van Ryk D, Schwing C, Hiatt J, Okwara N, Wei D, Roby G, David A, Hwang IY, Kehrl JH, Arthos J, Cicala C, Fauci AS, The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-beta1 production and FcRL4 expression. Nature immunology 14, 1256–1265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasare C, Medzhitov R, Control of B-cell responses by Toll-like receptors. Nature 438, 364–368 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D, Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314, 1936–1938 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carnathan DG, Wetzel KS, Yu J, Lee ST, Johnson BA, Paiardini M, Yan J, Morrow MP, Sardesai NY, Weiner DB, Ertl HC, Silvestri G, Activated CD4+CCR5+ T cells in the rectum predict increased SIV acquisition in SIVGag/Tat-vaccinated rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America 112, 518–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, Rosal-Vela A, Botta D, Bradley JE, Wojciechowski W, Ptacek T, Danila MI, Edberg JC, Bridges SL Jr., Kimberly RP, Chatham WW, Schoeb TR, Rosenberg AF, Boss JM, Sanz I, Lund FE, IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone SL, Peel JN, Scharer CD, Risley CA, Chisolm DA, Schultz MD, Yu B, Ballesteros-Tato A, Wojciechowski W, Mousseau B, Misra RS, Hanidu A, Jiang H, Qi Z, Boss JM, Randall TD, Brodeur SR, Goldrath AW, Weinmann AS, Rosenberg AF, Lund FE, T-bet Transcription Factor Promotes Antibody-Secreting Cell Differentiation by Limiting the Inflammatory Effects of IFN-gamma on B Cells. Immunity 50, 1172–1187 e1177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, Pulendran B, Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell host & microbe 16, 115–127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coccia M, Collignon C, Herve C, Chalon A, Welsby I, Detienne S, van Helden MJ, Dutta S, Genito CJ, Waters NC, Deun KV, Smilde AK, Berg R, Franco D, Bourguignon P, Morel S, Garcon N, Lambrecht BN, Goriely S, Most RV, Didierlaurent AM, Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ Vaccines 2, 25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rerks-Ngarm S, Pitisuttithum P, Excler JL, Nitayaphan S, Kaewkungwal J, Premsri N, Kunasol P, Karasavvas N, Schuetz A, Ngauy V, Sinangil F, Dawson P, deCamp AC, Phogat S, Garunathan S, Tartaglia J, DiazGranados C, Ratto-Kim S, Pegu P, Eller M, Karnasuta C, Montefiori DC, Sawant S, Vandergrift N, Wills S, Tomaras GD, Robb ML, Michael NL, Kim JH, Vasan S, O’Connell RJ, Team RVS, Randomized, Double-Blind Evaluation of Late Boost Strategies for HIV-Uninfected Vaccine Recipients in the RV144 HIV Vaccine Efficacy Trial. The Journal of infectious diseases 215, 1255–1263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seydoux E, Liang H, Dubois Cauwelaert N, Archer M, Rintala ND, Kramer R, Carter D, Fox CB, Orr MT, Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses. J Immunol 201, 98–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ols S, Yang L, Thompson EA, Pushparaj P, Tran K, Liang F, Lin A, Eriksson B, Karlsson Hedestam GB, Wyatt RT, Lore K, Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Rep 30, 3964–3971 e3967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, Parks R, Chen H, Blinn JH, Lapedes A, Watson S, Xia SM, Foulger A, Hahn BH, Shaw GM, Swanstrom R, Montefiori DC, Gao F, Haynes BF, Korber B, Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J Virol 87, 4185–4201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.V. Excepients.
- 68.Monie A, Hung CF, Roden R, Wu TC, Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2, 97–105 (2008). [PMC free article] [PubMed] [Google Scholar]
- 69.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC, Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc 4, 372–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O’Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G, HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 88, 7715–7726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sambor A, Garcia A, Berrong M, Pickeral J, Brown S, Rountree W, Sanchez A, Pollara J, Frahm N, Keinonen S, Kijak GH, Roederer M, Levine G, D’Souza MP, Jaimes M, Koup R, Denny T, Cox J, Ferrari G, Establishment and maintenance of a PBMC repository for functional cellular studies in support of clinical vaccine trials. J Immunol Methods 409, 107–116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montefiori DC, Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485, 395–405 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Lynch HE, Stewart SM, Kepler TB, Sempowski GD, Alam SM, Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J Immunol Methods 404, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF, Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38, 176–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, Lawson B, Villinger F, Amara RR, Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol 193, 4527–4536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


